Abstract
Consciousness is typically construed as being explainable purely in terms of either private, raw feels or higher-order, reflective representations. In contrast to this false dichotomy, we propose a new view of consciousness as an interactive, plastic phenomenon open to sociocultural influence. We take up our account of consciousness from the observation of radical cortical neuroplasticity in human development. Accordingly, we draw upon recent research on macroscopic neural networks, including the “default mode,” to illustrate cases in which an individual's particular “connectome” is shaped by encultured social practices that depend upon and influence phenomenal and reflective consciousness. On our account, the dynamically interacting connectivity of these networks bring about important individual differences in conscious experience and determine what is “present” in consciousness. Further, we argue that the organization of the brain into discrete anti-correlated networks supports the phenomenological distinction of prereflective and reflective consciousness, but we emphasize that this finding must be interpreted in light of the dynamic, category-resistant nature of consciousness. Our account motivates philosophical and empirical hypotheses regarding the appropriate time-scale and function of neuroplastic adaptation, the relation of high and low-frequency neural activity to consciousness and cognitive plasticity, and the role of ritual social practices in neural development and cognitive function.
Keywords: plasticity, consciousness, resting-state networks, development, phenomenology, cognition, culture, intersubjectivity
Introduction
A constant theme in cognitive science is to define the explanandum of consciousness in terms of qualia or “phenomenal feels,” i.e., some ineffable, subjective “what-it-is-like” to experience the world. Moreover, it is often argued that consciousness requires either some kind of higher-order metarepresentation of first-order states (Gennaro, 2004) or that consciousness is itself localized to the pure phenomenal feels or “what-it-is-like” (Dretske, 1993). We contend that the prevailing theoretical spectrum begins from the incorrect assumption that both phenomenal feels and higher-order representations can be collapsed into a single phenomenon. In contrast, we argue that the qualities of phenomenal experience and a subject's higher-order representations of those qualities are separate explananda, while still contending that higher-order representations significantly change the “what-it-is-like” of human experience. This is in accordance with our thesis that reflective consciousness is something that develops in ontogeny and depends upon the plastic individual development of the sensorimotor system in interaction with the default mode network (DMN). Moreover, we contend that both phenomena are highly complex, reciprocally interact, and depend upon the organisms phylogenetic and ontogenetic history of structural coupling between body, brain, and culture.
What drives us to this conclusion? First, the phenomenological tradition, as exemplified by the work of Martin Heidegger and Maurice Merleau-Ponty, emphasizes that our experience of the world is primarily prereflective in nature. Accordingly, we would like to construct an account of mental life grounded by the basic insight that cognition is primarily embodied and embedded within an organized environment and social field rather than detached and spectatorial (Stern, 2009). Second, in light of recent evidence of the brain's radical, multisensory plasticity, we will argue that this profound adaptivity at the molecular, network, and systems levels underlies the development and intersubjective function of human consciousness. We will thus argue that both the long-term plasticity underlying skill development and cultural learning and “fast” sensory–motor plasticity underpin our conscious experience of the world and ourselves. Indeed, there are physiological reasons to suspect that both “primary” prereflective processing1 and “secondary” reflective processing are both dynamic and flexible in nature, grounded in the actual history of the system's encounter with the environment. Whether we are discussing neuron recycling underlying memory consolidation, synaptic reorganization following limb amputation, or alterations in the particular communicative balance between macroscopic neural networks, the old tropes of radical modularism and localization of function are no longer tenable.
Of course, any account of plasticity must face the unique challenge of explaining both “stop” and “go” mechanisms, i.e., the accelerators and “brakes” of cognitive development. While the human brain demonstrates profound plasticity in many domains, it is also the case that two individuals, ceteris paribus, will likely develop with visual, motor, and auditory cortices in roughly the same place with roughly the same function. Indeed, in many cases, the function of radical neuroplasticity may be a kind of gambler's bargain, in which multiple avenues to reproductive success are made available via profound neural plasticity. Any successful account of consciousness must demonstrate how the brain both adapts to and resists profound environmental, biological, and sociocultural changes.
What is the alternative to the radical representational model or pure sensory–motor account? What kind of system could cope with changing environmental demands with functionally specific responses? “A functional system of this sort would be based on a dynamic process aimed at achieving an invariant result across changing circumstances” (Reed, 1996, p. 72). On the picture we are endorsing, we must assume that the organism is dynamically reactive from the very beginning of its ontogenetic history in virtue of autonomous, self-organizing animacy, what Julian Jaynes called behavioral reactivity. Interactively learned reflective capacities then further enhance our capacities for action and consciousness while receiving a great deal of inheritance from sensory–motor practice and the phylogeny of the organism.
The role of the neural system then is to coordinate or regulate the animal's encounter so as to effectively utilize the resources of a changing environment by means of adaptive behavior. On this view, evolution acts upon the distributed functional system, leading to adaptive behavior units across a nested set of spatial–temporal scales. Of course, there could not have been cultural evolution if humans had not been able, for example, to see “fire” as something “useful” instead of something that only frightened them. The plasticity of the human mind subserves not only our ability to adapt in the face of shifting challenges, but also to resist them at times, putting the “breaks” on development in order to build individual and collective cognitive “niches.” Thus our eventual mastery of fire and other potent environment and mind-shaping tools opened up a world of new biological and cultural stimulants (e.g., nutrients, shelter, bone tools, and hunting rituals) that furthered our particular evolutionary development2.
This leads to a “transition from models of representation as mirroring or encoding to models of representation as control” (Clark, 1997, p. 47). As Maturana and Varela put it, the patterns of brain activity are not symbolic representations standing in for the stimulus, but rather, state transitions induced by the perturbations or triggering effects of the ecological information embedded within the stimulus. In other words, “Stimuli act upon the organism as control parameters, which upon reaching a certain critical threshold induce a global qualitative discontinuity in the organism (a bifurcation in phase space)” (Thompson, 2007, p. 69). This radical and transitory plasticity of processing is contrasted by the elements of human change that are also partly determined by our own innate biology (e.g., homeostasis, survival instincts), as well as acquired and subjective values (ethical, cultural, religious, etc.). Moreover, a human being does not always change. Sometimes (or even many times) he can resist, decide not to consider stimuli, see them under a different viewpoint, etc. Thus our brain–body systems have evolved to maximize the interaction of reflective and prereflective processes, as well as their interaction and embeddedness within a cultural–intersubjective field. Indeed it is often the case that learned culture and intersubjectivity are themselves the innate control factors restricting our development in particular ways, with examples ranging from “background” factors of social value (race, class, etc.) to the culturally-specific “rules of engagement” for everyday conversation.
Furthermore, we contend that human adults typically engage with the world of objects at a high level of abstraction and linguistic categorization. We are, as Dennett (1993) says, spiders constantly and instinctively spinning our experience into narrative webs that filter and constrain our sensory dynamics. Narrative elaboration appears to be both habitual and open to training at the neural level (Farb et al., 2007). Within eastern traditions, it is common to describe the subject as one who cannot help but assign narrative evaluations to the world of sensory-chaos.
In this paper we develop an account of consciousness that embodies the basic insight that our primary subjective engagement with the world is not constituted by the formal propositional reasoning of Good Old Fashioned Artificial Intelligence (GOFAI), otherwise known as the sense-represent-plan-act model (Wheeler, 2007). However, we do not deny that there are constants in both the primary and secondary processes or that representations play a critical role in cognition and reflective consciousness. Rather, we follow Clark's suggestion that a mature science of mind must invoke both representational information processing and notions of emergent sensorimotor and cultural dynamics (Clark and Toribio, 1994; Clark, 2003, 2008). A variety of phylogenetic factors ensure that through the “cloud” of sensory–motor disorder, certain variables remain more or less constant and transferable within the community by means of joint-attention, abstraction, and categorization.
We will argue that this basic intersubjectivity is critical for human development and underlies our most basic modes of social attunement. Indeed, when discussing the “breaks” on plasticity, it is worth noting that intersubjective practices play a primary role in delimiting an individuals’ progress through life. On our account, intersubjectivity is a primary motivating factor for learning through joint action, and depends crucially upon the radically neuroplastic nature of our social-cognitive capacities. Furthermore, we argue that embodied world-directedness and sociocultural cognition are reciprocally related. Research suggests that attending to external stimuli may actively inhibit the kinds of reflective, higher-order thought posited in so-called “theater models” of consciousness (Baars, 1997; Fox et al., 2009).
Accordingly, we contend that recent research on the gross functional connectivity of the human brain sheds light on the problem of understanding both phenomenal feels and meta-consciousness. This new paradigm underlines the importance of ontogenetic plasticity and social–cultural development for determining “what-it-is-like” to be human. We will thus argue that the intrinsic connectivity of the human brain, particularly its organization into discrete anti-correlated networks, supports a view of reflective consciousness as a sociocultural cognitive control mechanism, motivating cortical adaptation and helping to shoehorn individuals into their particular social-cognitive “niche.” Delimiting the functional role of the task-negative and positive networks is a step forward in understanding the brain's large-scale organization, and as this special issue suggests, it is not clear why something supervening on a dynamic substrate would not itself be dynamic in nature.
What is the Explanada of Consciousness?
Theorists are divided about the need to rigorously define the concept of consciousness before scientifically explaining it as a natural phenomenon. Some, like Koch (2004), think that “Until the problem is better understood, a more formal definition of consciousness is likely to be either misleading or overly restrictive, or both” (p. 12). Others, like Julian Jaynes, argue “We first have to start from the top, from some conception of what consciousness is, from what our own introspection is. We have to be sure of that, before we can enter the nervous system and talk about its neurology” (p. 18). Because the first approach is bound to improperly delimit the explanandum and thus prove explanatorily evasive, we will follow Jaynes in emphasizing the importance of phenomenologically driven definitions as a mutual constraint on scientific explanation3. It is our contention that an empirically sound and phenomenologically driven approach to cognition and consciousness will allow us to begin explaining the enigmatic nature of human subjectivity rather than explaining it away. Moreover, coming to terms with phenomenal experience is at the heart of the solving the mind–body problem and other issues related to the naturalization of subjectivity.
First off, we contend that it is necessary to develop an adequate mental taxonomy of reflective and prereflective experience before attempting naturalistic explanation of such phenomena. Mental taxonomies that distinguish between different levels of consciousness are important because they allow us to trace the phylogenetic and ontogenetic trajectory of reflective consciousness in relation to purely prereflective mentalities such as those driven solely by instinct, perceptual learning, and habit. Mental taxonomies come at a price, however, freezing a shifting phenomena into a static form. Thus we strive to denote specific situations in which alternative functional substrates swap between the “top” and “bottom” position depending upon the context and demands of any given situation (see Table 1, below). Even in this formulation it is clear that our innate tendency to explain consciousness in spatial, rather than with temporal or biodynamic metaphors, can be misleading. What sense does it make, for example, to explain conscious control solely in terms of top-down and/or bottom-up processes if both are in reciprocal relation with “bottom” intersubjectivity and “top” executive thought-elements? Before investigating dynamic inter-relations of these functional processes, we first distinguish between roughly two different types of mentality in order to better delimit these substrata: those driven purely by prereflective reactivity and those driven by this plus reflective consciousness4.
Table 1.
Qualitative differences between prereflective and reflective consciousness, and their interaction.
| General characteristics | Role of plasticity | Time-scale of operation | “What-it-is-like” | Inherits features primarily from | |
|---|---|---|---|---|---|
| PR | Innate, automatic, embodied, habitual, resistant to perturbations, “online” | Rapid synaptic turn-over, functional adaptation, major development 0–4 years | Short (milliseconds) | Autopilot, flow, seamless, external absorption, effortless, intuitive, extended through tools and the body | Phylogeny |
| R | Partially learned, socially embedded, can be arrested, sensitive to intersubjectivity, “offline” | Learned Behavior; individual differences, alteration of macroscopic connectome. Major development 4–20 years, throughout life | Long (seconds, minutes, even days and years) | Narrotological, reflective, action-controlling, detached, interiority, folk psychological, calculative, deliberate | Ontogeny |
| PR × R | Intersubjective interaction, individual differences in perception and cognition. Neither “offline” nor “online.” Structures PR and R (“pre-noetic”) | Influence of local field potentials on global connectivity, rest-stimulus interaction. Develops from birth on | Variable; integration of experience with self-narrative, influence of culture on perception | Smooth expertise without zoning out, integration between online and offline cognition | Both |
Phenomenological reflection indicates that disengaged contemplation of the world's sensory richness is not the foundation of our consciousness. Rather we are typically enacting a phenomenal world that is both shifting and stable in nature. Individuals across cultures share a remarkable degree of perceptual and intersubjective features, yet also display a profound individualism between one another. Thus the challenge for any sociocultural or neurodynamic account of consciousness is to situate our profound adaptivity with our innate phylogenetic inheritance. Thus we observe that across cultures, evolution produces human beings with highly similar gross neuroanatomy, basic perceptual constraints, rearing practices, and ontogenetic development. Yet we must also explain the radical plasticity that has enabled humanity to thrive in the face of drastic environmental and cultural–technological shifts5. Thus we are able to shift processing from one cortical area to another given minor brain injury or sensory deprivation (Bavelier and Neville, 2002), or retool motion tracking areas for visual word-perception through acquisition of literacy (Dehaene et al., 2010). Moreover, when distinguishing prereflective and reflective consciousness, Merleau-Ponty at times emphasized the dependence of the former on the latter:
The sensible quality, far from being coextensive with perception, is the peculiar product of an attitude of curiosity or observation. It appears when, instead of yielding the whole gaze to the world, I turn toward the gaze itself, and when I ask myself what precisely it is I see. (Merleau-Ponty, 2006, p. 263)6
In some cases, my meta-reflective capacities fundamentally inform my basic experience of the world. We find in other places, also, rich examples where my reflective goals and intentions pre-noetically structure what is present in my lived practice, as when Merleau-Ponty describes the footballer in action:
The field is not given to him, but present as the immanent term of his practical intentions; the player becomes one with it and feels the direction of the “goal,” for example, just as immediately as the vertical and the horizontal planes of his own body. (Merleau-Ponty, 2006, p.169)
Thus, although prereflective reactivity in adult humans is often characterized by subpersonal, task-driven “flow” states7 in which reflective consciousness recedes into the background, we can also find cases in which these states are profoundly impacted by or even co-occur with reflective processes. Julian Jaynes details a complementary case, in which the prereflective engagement actively co-occurs with a detached reflective process:
My hand, foot, and head behavior...are almost in a different world. In touching something, I am touched; in turning my head, the world turns to me; in seeing, I am related to a world I immediately obey in the sense of driving on the road and not on the sidewalk. And I am not [reflectively] conscious of any of this. And certainly not logical about it. I am caught up, unconsciously enthralled, if you will, in a total interacting reciprocity of stimulation that may be constantly threatening or comforting, appealing or repelling, responding to the changes in traffic and particular aspects of it with trepidation or confidence, trust or distrust, while my [narrative] consciousness is still off on other topics. (Jaynes, 2000, p. 85, bracketed comments and italics added)
These “zombie” skills suggest that our cognitive system can automatically carry out intentions without the need for meta-conscious oversight, while also demonstrating the subtle dynamics of embedding “top” reflective intentions within active practice. The point is not that automobile drivers are asleep while they drive. Rather, the driver often steers automatically while his or her reflectively conscious mind is ruminating on something else. Thus, their prior and ongoing reflective intentions structure and guide their experience of driving. Similarly, we can see how intersubjective, prereflective elements structure the reflective observation of and interaction with a tool (as in Heidegger), or in Husserl's famous comparison of the first experiences of a Scandic anthropologist in Greenland and those of a naïve tourist. We literally “see intentions” and “experience thoughts” and these explanada are intimately interwoven within one another.
Furthermore, prereflective reactivity is subjective, and through development and interaction comes to be structured by the cultural–linguistic constructs such as the self, the mind, and other folk psychological narratives (Hutto, 2008). We argue that the conceptual categorization afforded by self-reflective folk psychological narratives greatly enhance our capacity for self-reflective action within an internal “mind-space.” We thus agree with accounts of language as a form of highly evolved tool use or extended cognition (Tylen et al., 2010). As Andy Clark argues,
“[T]hinking about thinking” is a good candidate for a distinctively human capacity – one not evidently shared by the non-language using animals that share our planet. Thus, it is natural to wonder whether this might be an entire species of thought in which language plays the generative role – a species of thought that is not just reflected in (or extended by) our use of words but is directly dependent on language for its very existence. (Clark, 1997, p. 209)8
In contrast to a continual absorption in realtime temporal dynamics, average human adults with “narratively driven” metacognition are capable of going “offline” to engage in lingual thought-monitoring, deliberative thinking/moral judgment, conscious impulse control, self-consciousness (thoughts about self-image, future, past, etc.), daydreaming, abstract problem solving, reconstructive imagination (visual imagery, internal sketchpad), subvocal rehearsal, rumination, etc. Self-reflexive “ego functions” such as these have been studied by psychologists for decades under headings such as inner speech (Morin, 2005), working memory, thought-monitoring (Frith, 2005), and more recently, mind-wandering (Smallwood and Schooler, 2006; Christoff et al., 2009). From this point forward, we will reserve the term “reflective consciousness” for such offline, decoupled activity. This is done to preserve the phenomenological distinction between low-level sensorimotor cognition and higher-order narrative-driven consciousness. We keep this strong distinction to primarily aid their modeling as dynamic interacting phenomena, not to suggest a fundamental separation or antagonism of their function. Although we review evidence that there exists a fundamental anti-correlation between the neural substrates of online and offline processing, we examine evidence that the strong hypothesis of “pure anti-correlation” is unlikely. Ultimately, we aim to show that while this distinction is useful, it is the dynamic interaction of reflection and action that primarily underpins human consciousness.
Reflective Versus Prereflective Consciousness
Perhaps the best way to understand the functional role of reflective consciousness in the human cognitive economy is to begin with its counterpart: the fast and efficient attention–salience reactivity system. This vast and intricately connected coordination system has been investigated since the inception of psychology under the rubric of what William James called “automaton theory” and what was later to become taken up and refined as classic behaviorism under Watson and Skinner. In our own time, we have seen the rise of dynamic systems and 4EA theory9 as an explanatory model for how these automatic, subpersonal processes function so as to regulate our changing response to the environment. A rapidly growing body of research highlights a myriad of “cognitive” functions that are served or even dominated by everyday behavior, the body, intersubjective/interactive processes, and cultural forces. In many cases, these are underwritten by subpersonal body schemas that run automatically and without metarepresentational consciousness of their function10. One could say that our mental landscape is pervaded by a prereflective consciousness regardless of whether reflective consciousness is totally absent, co-occurring, or intimately present in the action. Historically, some philosophers (e.g., Huxley) have argued that reflective consciousness as such is epiphenomenal, an “inert spectator” that plays no causal role in the control architecture and can be compared to the steam of a train whistle.
One problem with this view is that it fails to explain the inverse relationship between reflective consciousness and action. As Julian Jaynes observed, “If [reflective] consciousness is the mere impotent shadow of action, why is it more intense when action is most hesitant? And why are we least conscious when doing something most habitual?” (Jaynes, 2000, p. 11, bracketed comment added). Indeed, this question cannot be answered unless we acknowledge the phenomenological reality of effortless prereflective reactivity and the effortful deliberations of metacognitive control wherein behavior and attention is modulated by higher-order reflection and narratization. Prereflective flow states, wherein we are “lost” or “absorbed” in the moment of action, are ubiquitous among athletes and other experts who have honed and automatized their skills through continuous training. This training serves a dual purpose: first, to refine automatic processing schemas, second, to enable greater strategic control via adept representational and metarepresentational thought. Thus the human phenomenon of flow is not all or nothing. Even in athletic flow, there are conscious, reflective thoughts and varying degrees of prereflective and reflective awareness of the body, the goal, the action, and so on. Indeed one could give examples in which one is wholly absorbed in a reflective thought, and it is likely that ritualistic mental–social training regimens such as sports and pedagogy are aimed at refining both levels of consciousness as well as their dynamic communication.
Prereflective cognitive processes depend upon ongoing structural transformations in the nervous system which give rise to both simple and complex behaviors across nested sets of temporal scales. According to the 4EA tradition in the philosophy of mind, these subpersonal perception–action cycles are carried out without the use of explicit symbol tokens or “second-order” or “metarepresentational” consciousness. This means that the prereflective, “first order” sensorimotor network is not a Physical Symbol System, but rather, a dynamic, behaviorally reactive and recursive network of sensorimotor connectivity. On this perspective, the system conserves computational energy by using the actual world as a kind of “external memory source” (O'Regan, 1992) to be consulted on-the-fly in response to realtime information needs. As Rodney Brooks famously put it, “It turns out to be better to use the world as its own model” (Brooks, 1991, p. 139).
This has an important upshot for deflating the mind/body problem in regards to how “inner” experience corresponds to an “external world.” Strictly speaking, dynamics systems theory claims that for the prereflective system there is no epistemological (or experiential) distinction between “inner” and “outer” because the brain is not modeling the environment in low-level perceptual guidance, but rather, responding to it in terms of its ontogenetic history of structural coupling as a brain–body system. As Maturana and Varela (1987) argued, simple surgical experiments on the ontogenetic development of control assemblies suggest that in regard to automatic, task-oriented behavior such as a frog catching prey “there is no such thing as up and down, front and back, in reference to an outside world, as it exists for the observer doing the study. There is only an internal correlation between the place where the retina receives a given perturbation and the muscular contractions that move the tongue, the mouth, the neck, and, in fact, the frog's entire body” (Maturana and Varela, 1987, pp. 125–126) Accordingly, we can reject the Cartesian assumption that first-order behavioral dynamics depend on the sense-represent-plan-act model (Wheeler, 2007). Instead, dynamic systems theory claims that
…intentions are [best] seen as grounded in neural patterns....Decisions are precisely the brain's falling into one pattern or another, a falling that is modeled as the settling into a basin of attraction that will constrain neural firing in a pattern. There is no linear causal chain of input, processing, and output. Instead there is a continual looping as sensory information feeds into an ongoing dynamic system, altering, or reinforcing pattern formation. (Protevi, 2009, p. 18)11
Strictly speaking, the continual operation of first-order cognitive dynamics is subpersonal and not conceptually structured by mentalistic metaphors such as the inside/outside, subject–object, mind–body schema (Lakoff and Johnson, 1980, 1999)12. In other words, primary prereflective processes are unaccompanied by metacognitive reflection or the experience of a private “introcosm” or “theater” inside our heads13. Based on neuropsychological evidence, we argue that this inside/outside, subject–object schema operationalizes whenever we engage in “introspective gazing” or “reflection”14. Metaphorically speaking, reflective consciousness operationalizes15 whenever we sharply focus our mind's eye on experiential and sensory qualities rather than being mindlessly “absorbed” into the usability of affordances.
Our approach is thus similar to higher-order perception (HOP) and higher-order thought (HOT) theories. However, we differ significantly insofar as we do not invoke higher-order representations to explain phenomenal feels, but rather, to explain narrative consciousness and self-reflexive cognition. In contrast to higher-order theorists, we do not think higher-order representations are needed to explain phenomenal consciousness (the “what-it-is-like” of an organism). Instead, we think all organisms have a “what-it-is-like” insofar as they are living, embodied beings. However, we do contend that higher-order representations change the what-it-is-like of human cognition to such an extent as to radically change the phenomenal qualities of experience, giving rise to new forms of narratological subjectivity.
Accordingly, we contend that this special psychological interiority or “mind-space” does not correspond to a metaphysical substance (or ghostly process) as assumed by Cartesian dualists, but rather, to a virtual (i.e., temporary and easily dissoluble), analogically constructed “workspace” or “global theater” which acts as a “facility for accessing, disseminating, and exchanging information, and for exercising global coordination and control” (Baars, 1997, p. 7). Focused introspection upon pure sensation by means of reflective consciousness is itself a metacognitive skill that fundamentally changes our “what-it-is-like,” uniting the dynamic sensory–motor processing with the profoundly cultural reflective lens. It is in these unique cases in which the vibrant individual differences in consciousness and metacognition are most manifest. We thus contend that a dynamic systems approach coupled with well-established “theater models” of reflective consciousness gives reasoned answers to philosophical quandaries concerning qualia and subjectivity. Phenomenology suggests that complete absorption into task-oriented, world-directed mental states is often unaccompanied by introspective thought-monitoring or autobiographical memory storage. Instead of being “self-present,” we often seem to be “away from ourselves” and “empty minded” when absorbed in the world at large.
Examples like the truck driver or trained meditation practitioner illustrate the oscillatory nature of self-other, reflective–prereflective processing networks. When absorbed in the world, we often find ourselves “coming back” from selfless states unaware of the temporal gaps in consciousness. Indeed, “[narrative] consciousness knits itself over its time gaps and gives the illusion of continuity” (Jaynes, 2000, p. 25, bracketed comment added). Furthermore, research on change blindness suggests that we are often deluded about the level of detail available for report in our episodic memory. In reality, narrative consciousness is able to access only a fraction of what stirs beneath it16. In other words, our reflective experience of perceptual detail is a top-down17, virtual construction based on autobiographical memory. As Alva Noë puts it, “To experience detail virtually, you do not need to have all the detail in your head. All you need is quick and easy access to the relevant detail when you need it” (Noë, 2004, p. 50).
Nevertheless, we delude ourselves into thinking that we have a rich picture “inside” our heads when perceiving the world. We suggest that this is a side-effect of language turning experience itself into an object of understanding, amenable to folk psychological metaphors steeped with dualistic presuppositions about the continual presence of consciousness for the control of “rational” thought and action. Reflective consciousness seems to pervade our experience because our mental metaphors are structured by the concept of rational access and control, i.e., the “I.” Indeed, our entire autobiographical language is centered around a culture in which the ineffable “self” is both container and director of our experiences. But as Jaynes says, “[Narrative] consciousness is a much smaller part of our mental life than we are conscious of, because we cannot be conscious of what we are not conscious of” (Jaynes, 2000, p. 23, bracketed comment added). This limitation of access is built into the basic structure of conscious introspection, but it is not a burden. Rather, our ability to package prereflective states into increasingly complex and useful representational and metarepresentational “tools” represents a decisive factor in our sudden departure from prelinguistic animals (Tylen et al., 2010).
We contend that appreciation of such facts suggests that the subjective experience of non-human animals is subjective in the same way that the empty mindedness of long distance truck drivers is subjective. Although the truck driver may drive “mindlessly,” engaging only the basic sensorimotor subroutines needed to drive safely, it is her automatic conscious rumination that may remind her of an important forgotten task or keep her attention at the road regardless of her fatigue. The real trick then, is to understand how our reflective, linguistic consciousness of the world structures our prereflective engagement with the world. Without the possibility of making mental experience an explicit object of attention by means of a linguistic, self-reflexive “tag” with special experiential associations of interiority, most non-human animals are unable to create the necessary psychological distance from their actions to construct the reflective “introcosm” familiar to humans when they turn inwards upon the “hidden hermitage where we may study out the troubled book of what we have done and yet may do” (Jaynes, 2000, p. 1).
As we have reviewed, an inspection of phenomenology reveals a few basic structural elements of both reflective and prereflective experience. Crucially, consciousness is both hierarchical and dynamic: we are not always reflective or prereflective in nature, but rather, constantly shifting between these poles of reference. In examining the kinds of cases explored by Merleau-Ponty and Heidegger, we are presented with examples in which particular actions or stances are mediated by corporeal states, prior intentions, future thinking, and present-oriented action. For humans moving through the world then, consciousness is not any one static state or achievement, but rather a coordinated movement through various interrelated states of representation and dynamic world-body exchange. As we will show in the next section, the neurobiology of social interaction plays a crucial role in the development of these conscious capacities.
The Neurophenomenology of Sociocultural Consciousness
Does this phenomenological taxonomy have any grounding in cognitive neuroscience? Recently there has been a great deal of work examining the properties of macroscopic neural networks when subjects are left “task-free” in a brain-scanning environment (Raichle et al., 2001; Fox et al., 2009). These so-called “resting state” functional magnetic resonance imaging experiments (rsfMRI) consistently find statistically coherent relationships within and between gross neuroanatomical networks that are correlated with, and anti-correlated with, a variety of functions relevant for the development and functioning of consciousness. These networks include the DMN, salience network (SAL), and central-executive network (CEN). As we will primarily focus on the function and anatomy of the DMN, it is helpful to briefly review the latter two networks.
Originally described simply as the “task-positive network,” attention system, or executive network, traditional, and rsfMRI have lead to the description of two independent but closely related exogenous neural networks. These are deemed “task-positive” due to their consistent tendency to activate during cognitively demanding tasks and deactivate at rest. The CEN refers to the top-down dorsal attention network associated with the online control of behavior, and includes the dorsolateral prefrontal cortex, frontal eye fields, dorsal medial-prefrontal cortex (MPFC), intraparietal sulcus, and superior parietal lobule. The SAL refers to a more ventral network of regions involved in the automatic the detection of error, somatosensory awareness, and the detection of salient non-target stimulus. The SAL network is made up of the dorsal anterior cingulate cortex, frontoinsular cortices, amygdala and ventral midbrain (Buckner and Vincent, 200718; Carhart-Harris and Friston, 201019). Collectively these networks show some degree of integration, both being anti-correlated with the DMN at rest, yet also retaining a significant degree of functional non-overlap.
To better understand the relevance of rsfMRI, let us briefly review its historical development. In the mid-1990s, during the initial development and boom of social-cognitive neuroscience, researchers consistently observed deactivations of the MPFC during “task-positive” conditions (Damoiseaux and Greicius, 2009; Fox et al., 2009). In other words, it appeared that whenever subjects were required to complete tasks requiring focused attention and continual cognitive-executive effort, the MPFC would deactivate. Early controversy revolved around whether this deactivation was merely relative to task-induced positive activations or a “true” deactivation of this area from baseline. In the decade since these early discoveries, a great body of neurophysiological research has gone underway, revealing that these deactivations are not the product of relative activation ratios, but rather, are likely to reflect true task-induced neurophysiological decoupling, interaction, and deactivation depending upon the nature of the task or resting “state” (Greicius and Menon, 2004; Esposito et al., 2006; Buckner and Vincent, 2007; Jerbi et al., 2010). Why are deactivations in the MPFC relevant for the study of both prereflective and reflective consciousness?
First, the MPFC has been implicated in particular types of mental representation (Frith and Frith, 2006), theory-of-mind (Frith, 2007), and narrative processing (Mar, 2004; Mano et al., 2009). The MPFC has long been implicated in social cognition tasks, and can be further subdivided into areas associated with cognitive (posterior region of rostral MFC), affective (anterior rostral MFC), and task outcomes associated with punishment or reward (orbital MFC; Amodio and Frith, 2006). The MPFC is thus uniquely situated to mediate between top-down cognition and bottom-up reward-salience cues, and has been hypothesized as a necessary area for the representation of information in a conscious, socially communicable format (Frith and Frith, 2007). The MPFC remains highly plastic throughout childhood and adolescence, reaching biological maturity during late adolescence (Gogtay et al., 2004; Blakemore, 2008). Further, the development of theory-of-mind is considered a significant landmark in cognitive development that can be accelerated or inhibited by environmental factors (Jenkins and Astington, 1996).
Due to the prominent role of the DMN in these domains as well as within episodic memory, early researchers hypothesized that executive function tasks might require an inhibition of “stimulus-irrelevant thoughts” (SITs) or mind-wandering. Simply put, given the boring nature of sitting prone within a magnet, experimental participants might naturally engage in social-cognitive mind-wandering between the rigorous experimental trials. As task conditions appear to inhibit these networks, it was hypothesized that mind-wandering might constitute a DMN (Raichle et al., 2001). Initially, this formulation was controversial as it is not entirely clear what it might mean if laying in the scanner doing nothing was to be considered a “true baseline” for all cognitive tasks20.
More methodologically, many researchers complained that due to the slow wave (<0.1 Hz, or about one cycle every 10 s) nature of resting-state connectivity, cardiovascular, and respiratory noise could not be excluded as causes of the apparent resting-state networks (RSNs). Given that initial RSN findings did not adequately control for these confounding variables, and the fact that it remains unclear exactly how the BOLD signal maps onto neural activity, many expressed doubt that these low-frequency fluctuations (LFFs) represented actual neural phenomenon with a functional counterpart. These concerns have since been largely dissuaded due to growing evidence that RSNs are not confounded by physiological noise and do in fact represent cross-culturally replicable21, robust phenomena of neurophysiological origin (Mantini et al., 2007; Van Dijk et al., 2010). Recent research has demonstrated that the slow-wave oscillations indexed in fMRI that characterize the DMN can be verified as neurophysiological deactivation during task performance via intracerebral EEG (Jerbi et al., 2010). Although it is still unclear exactly what role the RSNs play in cognition, memory, and perception, researchers have begun to converge around some common theories of DMN function.
Since Raichle's early exposition of the DMN, most researchers have moved beyond describing the resting state as a “true baseline.” Raichle and Fox, two prominent researchers, have independently stated that the spatiotemporal coherence exhibited by spontaneous DMN activity is unlikely to reflect a baseline, but rather, is indicative of deep, almost architectural features of reflective consciousness. This “Mariana's Trench” view of the DMN supports recent theories that relate hyper or hypoactivity connectivity within the network to thought disorders like schizophrenia and OCD. Thus it is not our claim that the DMN is the direct neural correlate of consciousness, as it is likely that the experiences of ruminative reflection and sensory–motor consciousness do not reduce to these low-frequency oscillations. Further, significant evidence of relatively developed (albeit significantly altered) DMN-like connectivity in the brains of pre-term infants (Fransson et al., 2007), and anesthetized adults (Greicius, 2008) lend credence to the pre-noetic view of DMN function. Rather, our claim is that it is the interaction of these networks with the “task-positive” environment and history of the organism out of which social-cognitive consciousness arises. We thus predict that the functions of the DMN are crucial for the conscious integration of experience in self-narrative and interaction, setting the spatiotemporal and intersubjective associations between experiences, both in terms of autobiographical and episodic memory and pre-potent prereflective tendencies. This mapping is further reflected by the distributed set of functions represented by individual nodes of the DMN.
The functions of the individual nodes of the DMN are many, but do appear to share a common, self-referential theme. Comprised primarily of the MPFC, posterior cingulate cortex (PCC), and areas of the inferior parietal lobule (IPL), these regions are implicated in theory-of-mind, belief-understanding, and episodic memory generation. In line with this hypothesis, a recent quantitative meta-analysis of the DMN implicated the network in tasks involving autobiographical narrative, prospective memory, and theory-of-mind (Spreng et al., 2009). A vast array of studies have now linked particular patterns of resting connectivity between these nodes and psychopathologies including ADHD, OCD, depression, and schizophrenia, commonly construed as self-related reflective thought disorders (Greicius, 2008). It has become clear from this research that an individual's particular “connectomics” amongst default mode and other macroscopic neural networks is crucial for the functioning of bottom-up and top-down conscious processes.
Although a precise pattern has yet to emerge from these studies, a common theme throughout is hyper or hypoconnectivity of the DMN at rest, often correlated with measures of ruminative thinking, obsessive urges, or severity of symptoms and abnormalities in DMN deactivation during task-positive experimental conditions. It would thus appear that the “defaultness” of the DMN lies in its comprehensive involvement in tasks involving either the regulation of reflective thinking, the temporal structure of these thoughts, or their somatic references. It is our argument that this reflective, regular, and oftentimes unintentional narrative stream organizes experience into cohesive memories, facilitates action planning, and coordinates the joint action necessary for successful cognition and the ontogenetic development of consciousness.
Today, many researchers have moved beyond discussions centering on the “default” conception, moving instead to the “resting-state network” terminology. This new paradigm highlights the importance of an individual's “functional connectome” for the emergence of a particular consciousness (Buckner, 2010). The connectome approach emphasizes the relative contribution of a given functional localization, denoting that these “task-induced” activities are in a fine-grained dynamic relationship with the ongoing slow-wave activity of the macroscopic brain networks. This is to say that, while certain kinds of reflective and online experience evoke “fast” neural activity, over time the pattern of these excitations leaves its mark upon the “slow” wave, altering how the network processes future information. Indeed task-evoked activity in the medial-frontal gyrus (MFG) during a visual face/place categorization task has been shown to predict subsequent MFG to occipital place areas (PA) low-frequency coupling. In this experiment, the degree of post-task MFG–PA coupling significantly predicted post-scan memory performance (Stevens et al., 2010).
Experiments in animals and humans have revealed similar “rest-stimulus” interactions in which the degree of task-evoked activity can be predicted by prior LFFs or vice versa (See Northoff et al., 2010 for review). Thus, our gradual neuroplastic enculturation predicts reflective processing and our effortful attention to the environment. A great body of research has thus revealed that deactivations of the DMN are intrinsically anti-correlated with the CEN and SAL networks (Sridharan and Levitin, 2008; Carhart-Harris and Friston, 2010). Setting aside considerations of the “defaultness” of the DMN, the finding of these distributed networks and their anti-correlation is immediately interesting for the neurophenomenology of both prereflective and reflective consciousness.
We contend that given the MPFC's crucial role in social-cognitive tasks, including social priming and desirability, the DMN is clearly highly susceptible to stimulation by means of ostensive social cues and social interaction (Schilbach et al., 2006) while also contributing to the reflective stream of conscious thought (Christoff et al., 2009). The MPFC and TPJ sub-nodes of the DMN have been repeatedly implicated in the processing of emotional faces, threat behavior, ToM cartoons, and other task involving the processing of social-cognitive cues (see Frith and Frith, 2007 for review). Given the dual role of the DMN in constraining exogenous neural activity and processing these cues, we hypothesize that the network is involved in both the reflective regulation of social behavior and the bottom-up processes that determine what is “salient” for the prereflective consciousness.
Although the actual detection of and pre-potent response to rapid salient cues is likely mediated and directly processed within the salience and CEN networks, as is the online control of behavior, we argue that it is the MPFC that sets the social “frame” for what is salient and preloads the repertoire of socially appropriate gestures and concepts needed in an online interaction. From a narrative perspective of folk-psychology, this is akin to the interpretation of ones place as an agent within an unfolding interaction. Thus it is the role of DMN-supported self-rumination to evaluate and determine my place within a social hierarchy, to communicate what “set” or context I am to the SAL and CEN networks, and to play an active role in “writing” my own current and future position within a social folk psychological narrative.
In interaction, my ruminative reflection is thus extremely important for the regulation and evaluation of the unfolding scene; yet I must also be detached and authentic, fully automatic in my response. It is the smooth, sometimes anti-correlated interaction of the DMN with the SAL and CEN that enables our seamless interaction with others, underpinning intersubjective behavioral learning (see Figure 1, below). Further, we argue that this highly embedded social-cognitive nature (Schilbach et al., 2008) and high neural plasticity of the DMN through development (Gogtay et al., 2004; Blakemore, 2008) highlights an important feature of the human cognitive-executive-salience system: social interaction and enculturation are central motivators of plastic brain adaptation.
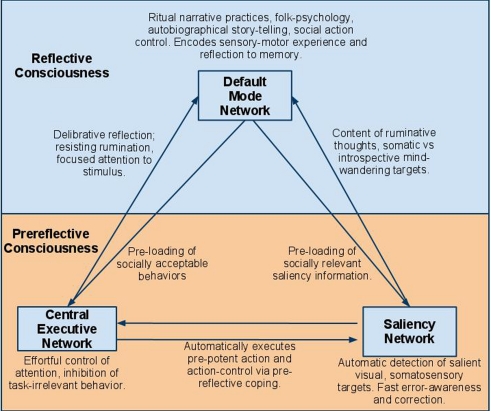
Figure 1.
Diagram of resting-state network mappings to prereflective and reflective consciousness. Arrows represent interactions between networks. DMN includes medial-prefrontal cortex, the posterior cingulate cortex, the inferior parietal lobule, the lateral and inferior temporal cortex, and the medial temporal lobes. CEN includes dorsolateral prefrontal cortex, frontal eye fields, dorsal medial-prefrontal cortex, intraparietal sulcus, and superior parietal lobule. The Saliency Network (SAL) includes the dorsal anterior cingulate cortex, frontoinsular cortices, amygdala, and ventral midbrain.
Plasticity: Crucial for Sociocultural Consciousness
The brain is an evolving, dynamic system. Human brains have often been described as proportionally larger than those of our simian counterparts. Recent research, however, appears to overturn this claim: the human neocortex is not larger than that of chimpanzees when body-size is controlled for. Comparative studies find that prefrontal white matter alone differentiates apes and humans (Schoenemann et al., 2005). White matter is highly plastic, with myelination increases occurring within as few as 11 h of body-awareness training (Tang et al., 2010) and remains highly variable throughout development (Blakemore, 2008) and the lifespan22. Finally, local connective tissue appears to possess the greatest level of plasticity, with mappings between synaptic terminals potentially undergoing complete remodeling in as few as 4 weeks (Stettler et al., 2006).
As cortical neuroplasticity in healthy human adults gradually begins to gain widespread acceptance in the scientific community23, we have begun to realize that a wide variety of psychopathologies can be described in terms of their impact on cortical connectivity. Through the newly developed methods of diffusion-tensor imaging and functional connectivity analysis, diseases that previously resisted neuroimaging classification have begun to reveal the complex way in which the human connectome shapes cognition. To give a simple example, imagine the physiology of focal stress. Given recent connectivity research, the experience of a stressful event can no longer be described as a focal disorder of, for example, stimulus processing, or ruminative thoughts. This is precisely because stress is not localized to any single region or function of the brain. Indeed, the neural stress response supervenes on a distributed system including multiple brain areas, the body, social roles, and the hormone system. The experience of social and mental stress depends upon both the sociocultural history of the actor and the subsequent entrainment of an agent's macroscopic resting networks.
What evidence is there regarding the kind of radical neuroconnective plasticity we are arguing for? By radical neuroconnectivity, we refer to fast adaptations at the cellular, molecular, functional, or anatomical level in response to training and experience. We do not deny that much of neural development is shared across cultures and persons; ceteris paribus two individuals will typically develop nearly identical gross neural anatomy24. Yet, the connective plasticity of the brain is so radically dynamic, that primary sensory cortices can “take-over” one another given damage (Bavelier and Neville, 2002) or reverse function to incorporate a 180° flip in vision (Shimojo and Nakajima, 1981). Cutting edge neurobiological imaging technologies have now revealed synaptic button turn-over rates of close to 7% per week (Stettler et al., 2006). If this rate holds constant across the axon, the entire synaptic-connective model of a given neural pathway could be remodeled within 3–4 weeks! As little as 4 h of high frequency trans-cranial magnetic stimulation (TMS) of the auditory cortex caused significant thickening of the auditory cortex, both ipsi and contralateral to the sight of the stimulation (May et al., 2007). This thickening, correlated with increased performance on an auditory discrimination task, vanishes 3 days without stimulation.
More recently, research by Tang et al. (2010) reveals that as little as 11 h of body-focused meditation results in significantly increased fractional anisotropy25 (FA) of anterior cingulate connective pathways, a critical pathway for interaction between CEN and SAL network functions such as behavioral inhibition and action selection. Finally, voxel-based morphometry before and after medical school exams reveals increased neural density in hippocampal learning-associated areas (Draganski et al., 2006), and 3 weeks of Tetris training in young girls revealed functional and structural differences in working memory and spatial processing areas (Haier et al., 2009). Given that working memory and spatial processing are clear predictors of successful language and career learning, and given the implicit impact of executive function networks upon the DMN and vice versa, a mere 3 weeks learning to play Tetris is clearly enough to improve communication between the strategic and sensory–motor elements common to the game. Clearly the brain adapts to its training, but to what degree is this effect realized in the social-cognitive and default mode areas? Do the rigorously ritualistic group-actions we engage in produce systematic alterations in brain structure?
In a landmark study, Gogtay et al. (2004) constructed “movies” of pediatric brain development through the use of MRI repeated every 2 years on 13 healthy children, aged 4–21, over a period of 8–10 years. Analysis of gray matter density changes across the whole brain revealed a striking pattern of neural pruning, with primary association areas being the first to mature, followed by secondary association, frontal, temporal, orbitofrontal, and dorsolateral prefrontal cortex. It is interesting to note that social-cognition associated areas reached maturity prior to classical executive function areas (e.g., DLPFC, rACC) suggesting that these more metacognitive functions may rely on the establishment of social-cognitive mechanisms.
Follow up studies conducted at multiple sites further establish white matter increases throughout the frontal and parietal cortices, coupled with regionally specific increases and/or decreases in gray matter throughout adolescence (Blakemore, 2008). The MPFC undergoes significant changes throughout adolescence, demonstrating altered response to faces and social cues (Blakemore, 2008). Studies of the default mode reveal that although LFF networks can be found even at birth (Fransson et al., 2007) and although they are generally well connected between hemispheres, they remain sparsely interconnected throughout childhood and into early adolescence, gaining maximum connectivity during early adult hood before declining again through late development (Fair et al., 2008). Within elderly populations, differences in DMN structure and function differentiate those with mild Alzheimer's from healthy age-matched controls (Greicius et al., 2004).
Shifting Networks and Individual Differences
As argued previously, if we want to maximize our explanatory power, then reflective consciousness must be distinguished from prereflective consciousness. We thus argue for a view of consciousness as the embodied, ontogenetic development of balanced interactions between exteroceptive targeted sensory–motor processes and interoceptive mental representational and cultural forces that are particular to individuals and their sociocognitive history. This distinction is supported by the organization of the brain into endogenously anti-correlated neurological networks for exogenous and endogenous processing, as well as their particular interactions and tendency toward plastic adaptation to the social environ.
What does it mean for neural networks to be endogenously anti-correlated? Although the exact nature of LFF anti-correlations remains unclear, recent experiments have demonstrated both that natural anti-correlations (i.e., systematic complementary deactivations and activations between RSNs) have a neural underpinning (Jerbi et al., 2010) and are consistently predicted by task-difficulty and stimulus demands. However, other fMRI research on experience sampling during sustained attention and introspection tasks (Christoff et al., 2009) reveals that “pure anti-correlation” is less likely than the task and context-dependent distribution of these network's activity, in that these networks are probably not strictly antagonistic, but rather are distributed in their allocation depending on task and context. Thus in some cases the DMN and CEN may exhibit clear anti-correlation (for example during extremely attention-demanding tasks) whereas for various types of social-cognitive or introspective tasks, the DMN may actually coactivate with the CEN or SAL networks depending on task demands.
Given the particularly dynamic and individual nature of these networks, we hypothesize that human consciousness is crucially dependent upon an individual's particular balance of intrinsic and extrinsic brain networks. We do not concede that consciousness is reducible or localizable with these networks, but rather exists only when actively coupled with an individual's sociolinguistic and ontogenetic history. Both prereflective and reflective consciousnesses are, on our account, multiply realizable and constrained by individual differences. The natural plasticity and functional adaptability of both brains and human social networks support this view. We are not born “ready-to-go,” but rather, must undergo specialized socially interactive brain training in order to fulfill the massive distribution of niche-specializations that is unique to contemporary society. A growing body of evidence highlights a primary role for social interaction in health and brain development (Dickerson and Kemeny, 2004; Holt-Lunstad et al., 2010). To further elucidate the relationship between prereflective and reflective consciousness in terms of RSNs, consider the following examples.
Any given individual's morphogenetic history will be fundamentally shaped by both interaction with others and genetic inheritance. Interaction serves multiple neurologically formative purposes: the imitation of motor behaviors, the recitation of heuristic habitual behaviors, and the linguistic navigation of social encounters. Human behavior is characterized by a fundamental reliance on highly ritualized skills that are essentially designed to adapt the brain to any given environmental niche. Thus, contemporary society demands that children must be repetitively taught to brush their teeth, pick up after themselves, make their beds, eat well, be nice to others, adhere to appropriate gender norms, and other traits of polite society. In adolescence we require initiation ceremonies, participation in sports and group activity, exercise of the body, courtship behaviors, ritualized practice of mathematical and scientific reasoning, and so on.
It is this rich intersubjective history that allows us to make meaningful choices, deciding to shape our lives through the gradual enculturation of our malleable neural cortex. Yet this plasticity also has a consequence: the socioeconomic factors and small non-conscious habits we ritualistically ascribe to (e.g., “I don't know why, I just don't enjoy X without Y”) have lasting consequences on our future possibilities for action. The choices we make may not be “free,” yet they are highly consequential for our development and are grounded in our social lives. Thus the most important choices in life do not revolve around the decision to brush ones’ teeth or take a meal; these things are entrained such that the truly important (and fundamentally social) decisions, like becoming vegetarian or pursuing a new career far from home come into focus, both in our cultural practices and most cherished memories. Throughout adulthood we require the training of a highly specialized set of ritual behaviors and speech patterns, whether those be preparing sales reports or flipping burgers. The social developmental of functional units of behavior is fundamentally geared toward creating particular types of brains. As John Protevi puts it, “subjectivity [is] an emergent capacity of bodies when they are placed in the appropriate subjectification practices” (Protevi, 2009, p. 31).
A common refusal here might be to say that all of these behaviors require the mere learning of scripts, and do not need to invoke neuroplasticity beyond that required for the development of a functioning language module. This view fails on two counts. Given that activities like studying for exams, learning to juggle, playing a videogame, or learning to meditate all induce structural and functional brain alterations across a wide variety of cognitive–sensory domains26, it seems unlikely that developmental plasticity is entrained solely within linguistic learning modules. Second, the goal of sociocultural training is not in fact the reflective representation or meta-conscious analysis of a given task-set. Rather, the training to brush ones’ teeth, drive to work, or perform courtship rituals is in fact aimed at training the interaction and tempo of interaction between the prereflective sensorimotor and reflective-narrative systems. Successful parenting means that a child will not need to remember to brush her teeth; rather she will automatically do so every night at a given time.
That is to say, regardless of if she is reflectively conscious of needing to brush her teeth, if she goes long-enough without doing it, her prereflective salience network will at some point trigger the default mode related action-controlling ruminative thought, “I should really get on with brushing my teeth”27. Although skill learning likely begins with the recitation of ritualized action narratives, embodied practice ensures that in time the individual no longer needs to maintain top-down control. Conversely, over time one may gain sufficient metacognitive experience to listen to the body and determine that one has made some ill mistake, perhaps forgotten to turn the teakettle down. The ability to translate intuitive “gut” feelings into meaningful, reliable decisions requires both a keen introspective practice and a sufficiently well developed self-theory-of-mind. Thus we do not always listen to the cues of our bodies, instead repeating mistakes again and again. This is a kind of delicate balance between our sensory–motor and reflective consciousness, as the two are in a constant reciprocal connection.
If consciousness is related to sensory–motor history, one could ask how they differ from one another in consciousness. How does any one individual differ from, for example a female, an older person, a nun, a juggler, etc.? The answer lies within both the prereflective and reflective consciousness. Prereflectively, these individuals will have different potentials for action; reflectively, they will have subtly different self-metaphors. The juggler may notice his every move, while the nun feels that she is a direct extension of God, with little awareness of her body. A woman may be more or less likely to assert herself depending on the permissiveness of her local culture. Reflectively, the older person will experience herself and her world through the lens of a fundamentally different viewpoint from that of the child, perhaps with the knowledge of long months spent in a war trench, or the exoneration of a successful business venture. It is not the case that we differ merely in memory or the contents of consciousness, as these elements are intricately interwoven into the conscious experience of the individual, preconfiguring our perception of the world.
We contend further that social interaction plays a specific mechanistic role in the development of self-narrative and action-control. At one level, the constant reminders from a mother to her child seek to control his attention and teach new skills. The human prefrontal cortex is extremely sensitive to cues from others. Recent meta-analysis found that “threat to social identity” and “loss of social control” was the greatest elicitors of hypothalamic–pituitary–amygdala cortisol secretion (Dickerson and Kemeny, 2004). Cortisol leads to hyper-activation of the prefrontal cortex and repeated stress-induced cortisol exposure results in thinning of prefrontal density and fronto-amygdalar connectivity. This is, of course, only one among many similar mechanisms and hormones that are sensitive to social interaction and influence development; there are critical periods such as the spurt in theory-of-mind development between ages 3 and 4 and it seems certain that everything from a mother's congratulating smile to video games and school sports will have an impact on neural development across the brain. Still, we believe there is sufficient evidence of the central importance of intersubjectivity in infant cognitive development. For example, infants are highly responsive to social cues at an early age (Senju and Csibra, 2008), and even new-born twins demonstrate coordinated, coupled social interaction (Castiello et al., 2010). Clearly the brain is equipped from the very beginning to learn about the world from others.
Given the extreme plasticity of the social-cognitive and executive prefrontal networks in the first two decades of life, we contend that interactive and social-cognitive mechanisms play a crucial role in the development of consciousness. It is thus not our claim that social–cortisol response is the only mechanism of plastic adaptation, but rather, one (highly important) mechanism for the kind of sociocultural adaptation under discussion. For the purpose of length, we have not discussed other equally important milestones in the development of (for example) language and motor function, yet these also are likely to depend upon intersubjective plasticity mechanisms to some degree. For this paper, we restrict our review to related mechanisms of systematic anti-correlation between RSNs.
Another piece of evidence for the DMN's susceptibility toward interaction-induced plasticity inducing comes from recent research by Schilbach et al. (2006), demonstrating that a non-cognitive, interactive joint-attention task with virtual avatars actually activates DMN areas while deactivating action-salience systems. Thus what is actually “task-positive” may depend upon the social context within which it occurs; if I am to process an engaging, dynamic, interactive person than my DMN could be quite important for that interaction. On the other hand, if my task is to respond rapidly and accurately to eyeball distractors, the DMN might simply get in the way of this “social” task.
We can now take these analyses and combine them. The DMN exhibits task-free, slow-wave, spontaneous activity that is associated with narrative processing, self-relatedness, reflective consciousness, and ruminative thinking. This association is not strictly conscious; the DMN retains coherence under anesthesia28 (Peltier et al., 2005; Raichle and Snyder, 2007; Greicius et al., 2008), while connectivity of the DMN remains in locked-in (but not “brain dead”) patients (Boly et al., 2009; Vanhaudenhuyse et al., 2010). However, DMN activity does correlate with individual differences and deactivations relate to specific psychopathological traits and personality measures (Sheng et al., 2010).
Furthermore, Lewis et al. (2009) have demonstrated that learning on a visual–motor task, predicts alterations in frontoparietal and visual cortex resting connectivity. We thus propose that the DMN grounds the sometimes-reflective iterative rehearsal of social, self, and action narratives. More globally, the resting-connectome forms a crucial part of the pre-noetic structure29 of our sensorimotor consciousness, determining what is passed from salience, to reflective rumination, to pre-potent action control. Through specific patterns of neural entrainment, recitation of these ritualized themes brings about alterations in connectivity in these resting networks and alters task-elicited functional specializations (Northoff et al., 2010). In this way the gradual build-up of experience is synthesized in a subjective format and stored for future recollection.
Thus over time the specific sociocultural niche, including socioeconomic factors, access to quality education, parenting style, and even local pollutants (Chen and Schwartz, 2009) contribute to the precise individual balance of these networks. In short, we suggest that sociocultural learning entrains the “what” and “how” of information transfer between the DMN, Salience, and Control networks. Through repetition (reflective and otherwise) certain themes are entrained within the DMN. That is to say, the specifics of ones’ cultural context become a constant theme within the overall autobiographical narrative. In this way the reflective consciousness is linked to the particulars of one's culture, and will be shared or different between cultures depending upon the degree of overlap between them. The sensory–motor consciousness (and neural substrate), while highly plastic through early development and capable of recovery in response to injury, ultimately produces an extremely similar outcome regardless of ones’ locale.
The greatest area for difference, then, is the particular interaction of the automatic and reflective networks, i.e., those areas where small differences in gesture can have a vast impact on a group, or where careful reflective attention is absolutely necessary for ultimate sensory–motor control. Thus, the information that is available to the salience and CEN networks (e.g., what appears in visual consciousness, and the action-systems repertoire of acceptable pre-potent’ responses), will be modulated by whatever social set, context, or role is being primed by the social-cognitive default network. These are in turn set about by the developmental trajectory of the interactive agent.
Are Metacognition and Social Cognition Trainable “Skills”?
Here is an obvious truism: some people are better at social cognition than others. Politicians, lawyers, secret service agents, and other trained professionals depend upon highly sharpened belief–desire prediction models. Academics in the humanities must spin long, extremely complicated and obtuse narratives entertaining hundreds if not thousands of years of sociocultural development. And yet, throughout the social-cognitive neurosciences, there exists almost no objective measures of social-cognitive competence. This has made the exploration of plasticity within the medial-prefrontal node of the default mode somewhat more difficult, as there exists no metric by which to evaluate training-related social-cognitive gains. However, in the face of a lack of direct evidence, we can conclude from several sources that these processes are also highly plastic, both intrinsically and in relation to the salience and control networks.
First, developmentally speaking, the medial-prefrontal and temporoparietal regions are among the very last to reach full developmental maturity (Gogtay et al., 2004) with neural development in the human neocortex being marked by massive Hebbian reinforcement (white matter and synaptic connectivity increases) and neural pruning (gray matter decreases). As neural maturity is achieved via “back to front” development, the neural substrates of theory-of-mind (e.g., MPFC, TPJ, etc) remain open to experiential plasticity and training well into early adulthood (see Blakemore and Choudhury 2006). As we have argued, a primary causal locus for the development of consciousness is the small-group ritualization and enculturation of young brains. Although we are in some sense born with the sensorimotoric equivalent of our early hominid ancestors, we must rapidly entrain ourselves within the highly complex and interwoven social-narrative tapestries that regulate action and prescribe behavior across nested spatiotemporal scales.
Before moving on, we need to briefly mention the notion of temporal receptivity. The given plasticity of a cognitive circuit should at least in part depend upon its window of temporal receptivity. That is to say, it does not make much sense for my social-narrative brain system to be sensitive to the fine tuned sensory–motor dynamics that entrain action-oriented networks. Simply put, blindfolding a participant for an hour of perceptual-motor training should not directly create neuroplastic adaptation in the medial-prefrontal network. Rather, these adaptations are likely to be localized to the high-speed window of the visual–motor system. Indeed, recent research suggests a differential topography of temporal receptive windows throughout the human neocortex: areas associated with reflective cognition tend to have much slower windows (3–36 s and upward) whereas visual cortex responds to information at higher frequency (<1 s; Hasson et al., 2008). We suggest that the greater temporal period of the temporoparietal junction and MPFC correspond more directly to the frequency of slow-wave behavioral–narrative interaction.
Take, for example, a conference dinner. We can here identify multiple temporal scales for relevant information processing. The fast-wave phenomenon like group shifts, behavioral chameleon phenomenon, and embodied mirroring occur far too fast to be tracked exhaustively by the reflective representational system. Rather, it is only the slowly aggregated summation of these events that enter into my narratological, metarepresentational processing, which is itself constrained by the acoustic temporal dynamics and rhythms of spoken interaction. Spiraling this concept outward, we can deduce that narrative processing also includes many slow-wave phenomena, entailing high-speed tasks such as the online identification of misplaced utterances, but also incorporating the gradual updating of self and social narratives. It is not the case that I rapidly and constantly update my self-narrative; rather it is only the aggregate sum of significant interactive events that eventually enters into my narrative. Thus it is the sum of my social interactions that engineer particular types of consciousness through the gross plasticity of our neural system. Our hypothesis is that this gradual integration of experience, led by social engagement with the world, is entrained by the default mode and in turns structures the phenomenal salience of both prereflective and reflective perception.
Consciousness, Anti-Correlation, and the Topography of Mind
Although we have argued for a bifurcated mental taxonomy between the prereflective and the reflective, we contend that a full understanding of the human mind must move beyond simplistic dualisms of any form, instead embracing the view that the constructs we denote as mental are in shifting, interrelated positions. Thus, although we discuss anti-correlations and their implications for the reflective/prereflective dimension, it is important to note that the “whole story” is likely to be far less clean than this distinction implies.
We are certainly not alone in surmising that the anti-correlation finding minimally suggests a unique form of informational interaction between the action-oriented salience/control and social-cognitive domains (see Carhart-Harris and Friston, 2010). However, basic phenomenology here reveals a few caveats. It is not the case that in prereflective interaction I am no longer able to engage in detached, metarepresentational processing. Nor is it fully the case that in my detached navel-gazing I am shielded from the sensory–motor fluctuation of my body in its environment. Rather, in both cases there exists a fine tuned spatiotemporal distribution of processing and resource allocation between these functional domains.
To illustrate, consider the classical example of “cocktail party coping.” To clarify and keep this example quite familiar, let us say the party in question is a post-conference dinner. I, the subject in question, having just entered the room, am immediately presented with the multitude of faces, voices, and explorative eyeball saccades that fixate in wild fluctuation across the room. Further constraining my interaction are the intersubjective power narratives that hang like spectral ether across the room; the “who's whos” and veritas of any social gathering. Entering into conversation, I must not only attend to the complex linguistic content of my new dyad, but also the randomly wavering eye-gazes, body postures, and other embodied semantic content determining the mood of the room. Should I continue speaking, or perhaps take my place at the dinner table? Has my conversational partner become bored, or should I continue our discussion? As we interact, I must continuously update the narrative coming from my mouth and my memory with the information given back to me by my partner. This process will be continually structured by salient target information as well as cultural representational values and is likely to only be minimally “conscious” in the traditional (i.e., intentional, reflective, self-identical) sense of the term.
Consider further the relationship between power narrative and embodied dynamics that unfold in this particular scene. Surely I am not constantly meta-conscious of the continuously unfolding social dynamics. To be so would be almost schizophrenic, and certainly I might suffer social-anxiety should I try to iteratively track all these possible variables. Rather, in line with the reputation costs associated with embodied social behavior, I simply act. I respond automatically to belief states, embodied gestures, and a host of constantly unfolding social-cognitive dynamics. My eyes and face must automatically track my partner's, lest I fail in engaging the chameleon effects that seem so crucial for smooth interpersonal interaction. Clearly we have a situation where my narrative processing is automatically guiding my tracking of salient social cues, and also in the inhibition of action: the social-narrative stream pouring forth from my mouth is consistently inhibited. Simply put, the power-dynamics of my social context are modulating both my behavior (compare ones’ posture in a work setting to that of a bar or amongst close friends) and my default speech. If I am to be socially successful, I will inhibit whatever dirty jokes I might tell otherwise. Human social interaction is completely pervaded by these information intensive interchanges of narrative and embodied coping (Gallagher and Hutto, 2008). Pure anti-correlation cannot obtain. Rather, my executive-control systems and visual salience networks must be in constant reciprocal communication with my social-representational-moral DMN.
Conclusion
As is often the case in the cognitive sciences, we have tried to demonstrate that there are multiple areas of critical overlap between a variety of currently disparate research trends. We have reviewed new research findings and theoretical developments in neuroplasticity, cognitive neuroscience, development, joint action and social cognition, phenomenology, and the philosophical investigation of consciousness. As a definitive integration of these areas is obviously beyond the scope of the present paper, we present these findings in hopes of convincing the reader that there is a crucial role for social interaction and plasticity in the ontogeny of human consciousness.
Our account motivates several conclusions. First, an explanation of human consciousness is not primarily a matter of explaining pure phenomenal feels. This is not to deny the importance of phenomenal feels for grounding our experience as living, embodied organisms. Rather, we have argued that to fully explain consciousness is to also give an account of the unique capacity for metacognition and metarepresentation demonstrated through what has been called offline intelligence. We have argued that a mature science of consciousness must be careful in avoiding the conflation of online intelligence with offline intelligence. Second, a mature explanation of the human mind must take into account the radical multisensory plasticity underlying both online and offline intelligence.
Further than just integration, we have hoped to advance an account that places the DMN, and the larger paradigm of human connectomics, as a central mechanism for the development of consciousness. We have thus proposed the following:
Human consciousness depends upon the ruminative, self-specific narrative stream of reflective thought.
This narrative stream is central for the production and control of action, and isspecifically encultured in the DMN by social interaction.
Consciousness appears to emerge from the complex interplay of reflective-narrative process and the sensorimotor control/salience dynamic.
It is the vast connective plasticity of the human frontoparietal cortex that enables the rapid development and acquisition of 10,000 plus years of evolved tool use, upon which our extended consciousness depends.
There are of course some worries generated by our account. One initial worry might be that we have “over intellectualized” consciousness and lost sight of the original explanandum of “what-it-is-like” to be an organism. However, although philosophical accounts of consciousness as pure phenomenal subjectivity might be initially appealing, we have argued that they are insufficient to account for the rich narratological capacities of contemporary human consciousness and fail to capture what makes us uniquely human. Accordingly, we avoid the theoretical pitfall of denying phenomenal states to non-human organisms while still retaining a robust sense of consciousness that goes beyond embodied sensorimotor experience. Such an account is desirable precisely because it can do justice to the subjectivity of animals while acknowledging the unique cultural–linguistic conditioning underlying the cognitive prowess of adult humans.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to thank Shaun Gallagher, Andreas Roepstorff, Chris Frith, Leonhard Schilbach, Jon Cogburn, and John Protevi for comments on early versions of this paper. We also thank the LSU Philosophy Department, the Danish Center for Functionally Integrative Neuroscience, and the Interacting Minds Project for supporting this research.
Footnotes
1Otherwise known as “online,” realtime, adaptive processing.
2Our account thus shares several features with other social–cultural accounts of the ontogeny and functioning of consciousness, see Donald (2001) or Steinberg (2006) for representationally or culturally weighted accounts, respectively.
3See Varela et al. (1991).
4Hobson (2009), Lycan (1997), Schooler (2002), Armstrong (1997), Dewey (1958), and James (1950) all make a distinction along similar lines.
5See for example, cases in which an innate function such as basic auditory processing may migrate to or co-opt visual processing areas or even vision itself given brain damage (see Bavelier and Neville, 2002 for review) or a consistent alteration in a sensory–motor association (Shimojo and Nakajima, 1981).
6On our interpretation, Merleau-Ponty's notion of “sensible quality” is more than just a pure phenomenal quality, but rather, refers to the quality of what-it-is-like to reflect on the sensory stream from a detached perspective.
7i.e., those states typical of automatically executed instincts, habits, and skills (Dreyfus, 2002).
8Accordingly, we believe that unless you are engaging in second-order dynamics, you are not reflectively conscious at that moment. This is not to suggest that we are somehow asleep when we are not self-reflexive. It just means that there is a qualitative difference between phenomenal feelings and metacognitive reflection on phenomenal feelings.
9i.e., the “embodied, embedded, extended, enactive, affective” tradition.
10See, for example, Gallagher (2005).
11See Freeman (2005).
12It is crucial to note that subpersonal cognition is still “subjective” insofar as there is something-it-is-like to experience first-order cognitive dynamics, but this subjectivity is not structured by narrative consciousness and autobiographical memory.
13Or hearts, solar plexus, or whatever other folk metaphor of the time might be in play.
14Recent lines of research (see Clark (2008), ch. 3 for an overview) suggest the construction of attentional objects such as our own thoughts and perceptions, as well as those of others, directly influences our reflective cognitive system, and vice-versa. Self-reflexive linguistic cognition grounded in lexical metaphors opens up the possibility for a psychological distance between ourselves and the immediate actions of our body that gives rise to novel modes of self-regulation based on the interaction between conscious narratization, imagination, executive control, and working memory (especially the episodic buffer).
15To operate literally means “to perform a function; to exert power or influence.” When consciousness operationalizes, it exerts influence over the rest of the brain. This is meant to indicate how consciousness is temporary and easily dissoluble.
16See Wegner (2002).
17Or better still, top-top (see Roepstorff and Frith, 2004).
20See Morcom and Fletcher (2007) for a review of rational against the “baseline” hypothesis.
21See “the human connectome project” (Biswal et al., 2010) for recent findings collected from approximately 1,800 individuals across 32 international neuroimaging centers.
22It is worth noting here, though we will not explore the issue further in this paper, that plasticity can be sub-divided into mechanisms corresponding to effortful learning (active) and those associated with recovery from damage or maturation (passive). Here we shall refer to both as “plasticity” and discuss a variety of mechanisms related to both sub-divisions.
23See Rubin (2009) for an excellent historical review of neurogenesis and plasticity.
24Although do note that how “nearly identical” gross neural morphology is between subjects and populations is a primary assumption in many imaging analysis techniques (Friston et al., 1994) and is a topic of heated debate, including evidence that over-prevalent “WEIRD” sampling in neuroimaging has led to a systematic bias in results due to the exclusion of Asian and African-American participants in many studies (Isamah et al., 2010).
25A measure of white matter integrity related to axonal myelination.
26See Draganski and May (2008) for a review of these and similar findings.
27Or perhaps a simple “stare” or gaze from the mother will motivate this behavior.
28At first glance this finding may seem contradictory with our hypothesis regarding the involvement of DMN activity in reflective consciousness. That the DMN remains intrinsically coupled during total anesthesia indicates only however that the function of the network is highly automatic. Research in this area indicates the differential dosages of anesthesia induce differential degrees of deactivation and decoupled activity in the DMN. It is our account that DMN in interaction with frontoparietal action-salience systems that underlies consciousness.
29Literally, “before consciousness,” e.g., that which structures but is not present within consciousness (Gallagher and Zahavi, 2008). What is pre-noetic for consciousness? The body, culture, and the neural connectome are all relevant examples.
References
- Amodio D., Frith C. (2006). Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 7, 268–277 [DOI] [PubMed] [Google Scholar]
- Armstrong D. (1997). “What is consciousness?” in The Nature of Consciousness: Philosophical Debates, eds Block N., Flanagan O., Güzeldere G. (Cambridge, MA: MIT Press; ), 721–728 [Original edition, 1978]. [Google Scholar]
- Baars B. J. (1997). In the Theater of Consciousness. New York: Oxford University Press [Google Scholar]
- Bavelier D., Neville H. (2002). Cross-modal plasticity: where and how? Nat. Rev. Neurosci. 3, 443–452 [DOI] [PubMed] [Google Scholar]
- Biswal B. B., Mennes M., Zuo X.-N., Gohel S., Kelly C., Smith S. M., Beckmann C. F., Adelstein J. S., Buckner R. L., Colcombe S., Dogonowski A.-M., Ernst M., Fair D., Hampson M., Hoptman M. J., Hyde J. S., Kiviniemi V. J., Kötter R., Li S.-J., Lin C.-P., Lowe M. J., Mackay C., Madden D. J., Madsen K. H., Margulies D. S., Mayberg H. S., McMahon K., Monk C. S., Mostofsky S. H., Nagel B. J., Pekar J. J., Peltier S. J., Petersen S. E., Riedl V., Rombouts, Serge A. R. B., Rypma B., Schlaggar B. L., Schmidt S., Seidler R. D., Siegle G. J., Sorghh C., Teng G.-J., Veijola J., Villringer A., Walter M., Wang L., Weng X.-C., Whitfield-Gabrieli S., Williamson P., Windischberger C., Zang Y.-F., Zhang H.-Y., Castellanos F. X., Milham M. P. (2010). Toward discovery science of human brain function. Proc. Natl. Acad. Sci. U.S.A. 107, 4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S. (2008). The social brain in adolescence. Nat. Rev. Neurosci. 9, 267–277 [DOI] [PubMed] [Google Scholar]
- Blakemore S., Choudhury S. (2006). Development of the adolescent brain: implications for executive function and social cognition. J. Child Psychol. Psychiatry 47, 296–312 [DOI] [PubMed] [Google Scholar]
- Boly M., Tshibanda L., Vanhaudenhuyse A., Noirhomme Q., Schnakers C., Ledoux D., Boveroux P., Garweg C., Lambermont B., Phillips C., Luxen A., Moonen G., Bassetti C., Maquet P., Laureys S. (2009). Functional connectivity in the default network during resting state is preserved in a vegetative but not in a brain dead patient. Hum. Brain Mapp. 30, 2393–2400 10.1002/hbm.20672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks R. (1991). Intelligence without representation. Artif. Intell. 47, 139–159 [Google Scholar]
- Buckner R. L. (2010). Human functional connectivity: new tools, unresolved questions. Proc. Natl. Acad. Sci. U.S.A. 107, 10769–10770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R. L., Vincent J. L. (2007). Unrest at rest: default activity and spontaneous network correlations. Neuroimage 37, 1091–1096 10.1016/j.neuroimage.2007.01.010 [DOI] [PubMed] [Google Scholar]
- Carhart-Harris R., Friston K. (2010). The default-mode, ego-functions and free-energy: a neurobiological account of Freudian ideas. Brain 133, 1265–1283 10.1093/brain/awq010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiello U., Becchio C., Zoia S., Nelini C., Sartori L., Blason L., D'Ottavio G., Bulgheroni M., Gallese V. (2010). Wired to be social: the ontogeny of human interaction. PLoS ONE 5, e13199. 10.1371/journal.pone.0013199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.-C., Schwartz J. (2009). Neurobehavioral effects of ambient air pollution on cognitive performance in US adults. Neurotoxicology 30, 231–239 10.1016/j.neuro.2008.12.011 [DOI] [PubMed] [Google Scholar]
- Christoff K., Gordon A. M., Smallwood J., Smith R., Schooler J. W. (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc. Natl. Acad. Sci. U.S.A. 106, 8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. (1997). Being There: Putting Brain, Body, and World Together Again. Cambridge, MA: MIT Press [Google Scholar]
- Clark A. (2003). Natural-Born Cyborgs: Minds, Technologies, and the Future of Human Intelligence. New York: Oxford University Press; 14624429 [Google Scholar]
- Clark A. (2008). Supersizing the Mind: Embodiment, Action, and Cognitive Extension. New York: Oxford University Press [Google Scholar]
- Clark A., Toribio J. (1994). Doing without representing? Synthese 101, 401–431 10.1007/BF01063896 [DOI] [Google Scholar]
- Damoiseaux J., Greicius M. (2009). Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct. Funct. 213, 525–533 [DOI] [PubMed] [Google Scholar]
- Dehaene S., Pegado F., Braga L. W., Ventura P., Filho G. N., Jobert A., Dehaene-Lambertz G., Kolinsky R., Morais J., Cohen L. (2010). How learning to read changes the cortical networks for vision and language. Science 330, 1359–1364 10.1126/science.1194140 [DOI] [PubMed] [Google Scholar]
- Dennett D. (1993). Consciousness Explained. London: Penguin [Google Scholar]
- Dewey J. (1958). Experience and Nature. New York: Dover Publications; [Original edition, 1925]. [Google Scholar]
- Dickerson S. S., Kemeny M. E. (2004). Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 130, 355–391 [DOI] [PubMed] [Google Scholar]
- Donald M. (2001). A Mind So Rare: The Evolution of Human Consciousness. New York: WW Norton & Company [Google Scholar]
- Draganski B., Gaser C., Kempermann G., Kuhn H. G., Winkler J., Büche C., May A. (2006). Temporal and spatial dynamics of brain structure changes during extensive learning. J. Neurosci. 26, 6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B., May A. (2008). Training-induced structural changes in the adult human brain. Behav. Brain Res. 192, 137–142 [DOI] [PubMed] [Google Scholar]
- Dretske F. (1993). Conscious experience. Mind 102, 263–283 10.1093/mind/102.406.263 [DOI] [Google Scholar]
- Dreyfus H. L. (2002). Intelligence without representation – Merleau-Ponty's critique of mental representation the relevance of phenomenology to scientific explanation. Phenomenol. Cogn. Sci. 1, 367–383 [Google Scholar]
- Esposito F., Bertolino A., Scarabino T., Latorre V., Blasi G., Popolizio T., Tedeschi G., Cirillo S., Goebel R., Di Salle F. (2006). Independent component model of the default-mode brain function: assessing the impact of active thinking. Brain Res. Bull. 70, 263–269 [DOI] [PubMed] [Google Scholar]
- Fair D A., Cohen A. L., Dosenbach N. U. F., Church J. A., Miezin F. M., Barch D. M., Raichle M. E., Petersen S. E., Schlaggar B. L. (2008). The maturing architecture of the brain's default network. Proc. Natl. Acad. Sci. U.S.A. 105, 4028–4032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb N. A. S., Segal Z. V., Mayberg H., Bean J., McKeon D., Fatima Z., Anderson A. K. (2007). Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Soc. Cogn. Affect. Neurosci. 2, 313–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M., Zhang D., Snyder A. (2009). The global signal and observed anticorrelated resting state brain networks. J. Neurophysiol. 101, 3270–3283 10.1152/jn.90777.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P., Skiöld B., Horsch S., Nordell A., Blennow M., Lagercrantz H., Åden U. (2007). Resting-state networks in the infant brain. Proc. Natl. Acad. Sci. U.S.A. 104, 15531–15536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman W. J. (2005). A field-theoretic approach to understanding scale-free neocortical dynamics. Biol. Cybern. 92, 350–359 10.1007/s00422-005-0563-1 [DOI] [PubMed] [Google Scholar]
- Friston K. J., Holmes A. P., Worsley K. J., Poline J.-P., Frith C. D., Frackowiak R. S. J. (1994). Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 2, 189–210 10.1002/hbm.460020402 [DOI] [Google Scholar]
- Frith C. (2005). The self in action: lessons from delusions of control. Conscious. Cogn. 14, 752–770 [DOI] [PubMed] [Google Scholar]
- Frith C. (2007). The social brain? Philos. Trans. R. Soc. Lond. B. Biol. Sci. 362, 671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith C., Frith U. (2006). How we predict what other people are going to do. Brain Res. 1079, 36–46 10.1016/j.brainres.2005.12.126 [DOI] [PubMed] [Google Scholar]
- Frith C., Frith U. (2007). Social cognition in humans. Curr. Biol. 17, R724–R732 [DOI] [PubMed] [Google Scholar]
- Gallagher S. (2005). How the Body Shapes the Mind. New York: Oxford University Press [Google Scholar]
- Gallagher S., Hutto D. (2008). “Understanding others through primary interaction and narrative practice,” in The Shared Mind: Perspectives on Intersubjectivity, ed. Zlatev J. (Amsterdam: John Benjamins Publishing Co.), 17–38 [Google Scholar]
- Gallagher S., Zahavi D. (2008). The Phenomenological Mind: An Introduction to Philosophy of Mind and Cognitive Science. Abingdon, Oxon: Routledge Press [Google Scholar]
- Gennaro R. J. (ed.). (2004). Higher-Order Theories of Consciousness: An Anthology. Philadelphia: John Benjamins [Google Scholar]
- Gogtay N., Giedd J. N., Lusk L., Hayashi K. M., Greenstein D., Vaituzis A. C., Nugent T. F., III, Herman D. H., Clasen L. S., Toga A. W., Rapoport J. L., Thompson P. M. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U.S.A. 101, 8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. (2008). Resting-state functional connectivity in neuropsychiatric disorders. Curr. Opin. Neurol. 21, 424–430 [DOI] [PubMed] [Google Scholar]
- Greicius M. D., Srivastava G., Reiss A. L., Menon V. (2004). Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc. Natl. Acad. Sci. U.S.A. 101, 4637–4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. D., Kiviniemi V., Tervonen O., Vainionpää V., Alahuhta S., Reiss A. L., Menon V. (2008). Persistent default mode network connectivity during light sedation. Hum. Brain Mapp. 29, 839–847 10.1002/hbm.20537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. D., Menon V. (2004). Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J. Cogn. Neurosci. 16, 1484–1492 [DOI] [PubMed] [Google Scholar]
- Haier R. J., Karama S., Leyba L., Jung R. E. (2009). MRI assessment of cortical thickness and functional activity changes in adolescent girls following three months of practice on a visual-spatial task. BMC Res. Notes 2, 174. 10.1186/1756-0500-2-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U., Yang E., Vallines I., Heeger D. J., Rubin N. (2008). A hierarchy of temporal receptive windows in human cortex. J. Neurosci. 28, 2539–2550 10.1523/JNEUROSCI.5487-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson J. A. (2009). REM sleep and dreaming: towards a theory of protoconsciousness. Nat. Rev. Neurosci. 10, 803–813 [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J., Smith T. B., Layton J. B. (2010). Social relationships and mortality risk: a meta-analytic review. PLoS Med. 7, e1000316. 10.1371/journal.pmed.1000316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutto D. D. (2008). Folk Psychological Narratives. Cambridge, MA: MIT Press [Google Scholar]
- Isamah N., Faison W., Payne M. E., MacFall J., Steffens D. C., Beyer J. L., Ranga Krishnan K., Taylor W. D. (2010). Variability in frontotemporal brain structure: the importance of recruitment of African Americans in neuroscience research. PLoS ONE 5, e13642. 10.1371/journal.pone.001364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. (1950). Principles of Psychology, Vol. I Mineola: Dover; [Original edition, 1890]. [Google Scholar]
- Jaynes J. (2000). The Origin of Consciousness in the Breakdown of the Bicameral Mind. Boston: Houghton Mifflin; [Original edition, 1976]. [Google Scholar]
- Jenkins J., Astington J. (1996). Cognitive factors and family structure associated with theory of mind development in young children. Dev. Psychol. 32, 70–78 [Google Scholar]
- Jerbi K., Vidal J. R., Ossandon T., Dalal S. S., Jung J., Hoffmann D., Minotti L., Bertrand O., Kahane P., Lachaux J.-P. (2010). Exploring the electrophysiological correlates of the default-mode network with intracerebral EEG. Front. Syst. Neurosci. 4:27. 10.3389/fnsys.2010.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C. (2004). The Quest for Consciousness: A Neurobiological Approach. Englewood, CO: Roberts & Company Publishers [Google Scholar]
- Lakoff G., Johnson M. (1980). Metaphors We Live By. Chicago: University of Chicago Press [Google Scholar]
- Lakoff G., Johnson M. (1999). Philosophy in the Flesh: the Embodied Mind and its Challenge to Western Thought. New York: Basic Books [Google Scholar]
- Lewis C. M., Baldassarre A., Committeri G., Romani G. L., Corbetta M. (2009). Learning sculpts the spontaneous activity of the resting human brain. Proc. Natl. Acad. Sci. U.S.A. 106, 17558–17563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycan W. G. (1997). “Consciousness as internal monitoring,” in The Nature of Consciousness: Philosophical Debates, eds Block N., Flanagan O., Güzeldere G. (Cambridge, MA: MIT Press; ), 755–771 [Google Scholar]
- Mano Y., Harada T., Sugiura M., Saito D. N., Sadato N. (2009). Perspective-taking as part of narrative comprehension: a functional MRI study. Neuropsychologia 47, 813–824 10.1016/j.neuropsychologia.2008.12.011 [DOI] [PubMed] [Google Scholar]
- Mantini D., Perrucci M., Del Gratta C. (2007). Electrophysiological signatures of resting state networks in the human brain. Proc. Natl. Acad. Sci. U.S.A. 104, 13170–13175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar R. (2004). The neuropsychology of narrative: story comprehension, story production and their interrelation. Neuropsychologia 42, 1414–1434 10.1016/j.neuropsychologia.2003.12.016 [DOI] [PubMed] [Google Scholar]
- Maturana H. R., Varela F. J. (1987). The Tree of Knowledge: The Biological Roots of Human Understanding. Boston: New Science Library [Google Scholar]
- May A., Hajak G., Gänbauer S., Steffens T., Langguth B., Kleinjung T., Eichhammer P. (2007). Structural brain alterations following 5 days of intervention: dynamic aspects of neuroplasticity. Cereb. Cortex 17, 205–210 [DOI] [PubMed] [Google Scholar]
- Merleau-Ponty M. (2006). Phenomenology of Perception. New York: Routledge and Kegan Paul; [Original edition, 1945]. [Google Scholar]
- Morcom A., Fletcher P. (2007). Does the brain have a baseline? Why we should be resisting a rest. Neuroimage 37, 1073–1082 10.1016/j.neuroimage.2006.09.013 [DOI] [PubMed] [Google Scholar]
- Morin A. (2005). Possible links between self-awareness and inner speech theoretical background, underlying mechanisms, and empirical evidence. J. Conscious. Stud. 12, 115–134 [Google Scholar]
- Noë A. (2004). Action in Perception. Cambridge, MA: MIT Press [Google Scholar]
- Northoff G., Qin P., Nakao T. (2010). Rest-stimulus interaction in the brain: a review. Trends Neurosci. 33, 277–284 10.1016/j.tins.2010.02.006 [DOI] [PubMed] [Google Scholar]
- O'Regan J. K. (1992). Solving the “real” mysteries of visual perception: the world as an outside memory. Can. J. Psychol. 46, 461–488 [DOI] [PubMed] [Google Scholar]
- Peltier S. J., Kerssens C., Hamann S. B., Sebel P. S., Byas-Smith M., Hu X. (2005). Functional connectivity changes with concentration of sevoflurane anesthesia. Neuroreport 16, 285. [DOI] [PubMed] [Google Scholar]
- Protevi J. (2009). Political Affect: Connecting the Social and the Somatic. Minneapolis: University of Minnesota Press [Google Scholar]
- Raichle M., MacLeod A., Snyder A. (2001). A default mode of brain function. Proc. Natl. Acad. Sci. U.S.A. 98, 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M., Snyder A. (2007). A default mode of brain function: a brief history of an evolving idea. Neuroimage 37, 1083–1090 10.1016/j.neuroimage.2007.02.041 [DOI] [PubMed] [Google Scholar]
- Reed E. (1996). Encountering the World: Toward and Ecological Psychology. New York: Oxford University Press [Google Scholar]
- Roepstorff A., Frith C. (2004). What ís at the top in the top-down control of action? Script-sharing and top-top control of action in cognitive experiments. Psychol. Res. 68, 189–198 [DOI] [PubMed] [Google Scholar]
- Rubin B. (2009). Changing brains: the emergence of the field of adult neurogenesis. Biosocieties 4, 407–424 10.1017/S1745855209990330 [DOI] [Google Scholar]
- Schilbach L., Wohlschlaeger A. M., Kraemer N. C., Newen A., Shah N. J., Fink G. R., Vogeley K. (2006). Being with virtual others: neural correlates of social interaction. Neuropsychologia 44, 718–730 10.1016/j.neuropsychologia.2005.07.017 [DOI] [PubMed] [Google Scholar]
- Schilbach L., Eickhoff S. B., Rotarska-Jagiela A., Fink G. R., Vogeley K. (2008). Minds at rest. Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious. Cogn. 17, 457–467 [DOI] [PubMed] [Google Scholar]
- Schoenemann P., Sheehan M., Glotzer L. (2005). Prefrontal white matter volume is disproportionately larger in humans than in other primates. Nat. Neurosci. 8, 242–252 [DOI] [PubMed] [Google Scholar]
- Schooler J. W. (2002). Re-representing consciousness: dissociations between experience and meta-consciousness. Trends Cogn. Sci. 6, 339–344 [DOI] [PubMed] [Google Scholar]
- Senju A., Csibra G. (2008). Gaze following in human infants depends on communicative signals. Curr. Biol. 18, 668–671 [DOI] [PubMed] [Google Scholar]
- Sheng T., Gheytanchi A., Aziz-Zadeh L. (2010). Default network deactivations are correlated with psychopathic personality traits. PLoS ONE 5, e12611. 10.1371/journal.pone.0012611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojo S., Nakajima Y. (1981). Adaptation to the reversal of binocular depth cues: effects of wearing left-right reversing spectacles on stereoscopic depth perception. Perception 10, 391–402 10.1068/p100391 [DOI] [PubMed] [Google Scholar]
- Smallwood J., Schooler J. (2006). The restless mind. Psychol. Bull. 132, 946. [DOI] [PubMed] [Google Scholar]
- Spreng R., Mar R., Kim A. (2009). The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci. 21, 489–510 [DOI] [PubMed] [Google Scholar]
- Sridharan D., Levitin D. (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. U.S.A. 105, 12569–12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg D. (2006). Consciousness Reconnected: Missing Links Between Self, Neuroscience, Psychology, and the Arts. Abingdon: Ratcliffe Press [Google Scholar]
- Stern D. (2009). Pre-reflexive experience and its passage to reflexive experience: a developmental view. J. Conscious. Stud. 16, 307–331 [Google Scholar]
- Stettler D. D., Yamahachi H., Li W., Denk W., Gilbert C. D. (2006). Axons and synaptic boutons are highly dynamic in adult visual cortex. Neuron 49, 877–887 10.1016/j.neuron.2006.02.018 [DOI] [PubMed] [Google Scholar]
- Stevens W. D., Buckner R. L., Schacter D. L. (2010). Correlated low-frequency BOLD fluctuations in the resting human brain are modulated by recent experience in category-preferential visual regions. Cereb. Cortex 8, 1997–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y.-Y., Lu Q., Geng X., Stein E. A., Yang Y., Posner M. I. (2010). Short-term meditation induces white matter changes in the anterior cingulate. Proc. Natl. Acad. Sci. U.S.A. 107, 15649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson E. (2007). Mind in Life: Biology, Phenomenology, and the Sciences of Mind. Cambrige, MA: Harvard University Press [Google Scholar]
- Tylen K., Weed E., Wallentin M., Roepstorff A., Frith C. D. (2010). Language as a tool for interacting minds. Mind Lang. 25, 3–29 [Google Scholar]
- Van Dijk K. R., Hedden T., Venkataraman A., Evans K. C., Lazar S. W., Buckner R. L. (2010). Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J. Neurophysiol. 103, 297–321 10.1152/jn.00783.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaudenhuyse A., Noirhomme Q., Tshibanda L. J., Bruno M. A., Boveroux P., Schnakers C., Soddu A., Perlbarg V., Ledoux D., Brichant J. F., Moonen G., Maquet P., Greicius M. D., Laureys S., Boly M. (2010). Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain 133, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela F., Thompson E., Rosch E. (1991). The Embodied Mind: Cognitive Science and Human Experience. Cambridge, MA: MIT Press [Google Scholar]
- Wegner D. M. (2002). The Illusion of Conscious Will. Cambridge, MA: MIT Press [Google Scholar]
- Wheeler M. (2007). Reconstructing the Cognitive World: The Next Step. Cambridge, MA: MIT Press [Google Scholar]