Abstract
We have recently demonstrated that nutritional bioactives (fish oil and pectin) modulate microRNA molecular switches in the colon. Since integrated analysis of microRNA and mRNA expression at an early stage of colon cancer development is lacking, in this study, four computational approaches were utilized to test the hypothesis that microRNAs and their posttranscriptionally regulated mRNA targets, i.e., both total mRNAs and actively translated mRNA transcripts, are differentially modulated by carcinogen and diet treatment. Sprague-Dawley rats were fed diets containing corn oil ± fish oil with pectin ± cellulose and injected with azoxymethane or saline (control). Colonic mucosa was assayed at an early time of cancer progression, and global gene set enrichment analysis was used to obtain those microRNAs significantly enriched by the change in expression of their putative target genes. In addition, cumulative distribution function plots and functional network analyses were used to evaluate the impact of diet and carcinogen combination on mRNA levels induced via microRNA alterations. Finally, linear discriminant analysis was used to identify the best single-, two-, and three-microRNA combinations for classifying dietary effects and colon tumor development. We demonstrate that polysomal profiling is tightly related to microRNA changes when compared with total mRNA profiling. In addition, diet and carcinogen exposure modulated a number of microRNAs (miR-16, miR-19b, miR-21, miR26b, miR27b, miR-93, and miR-203) linked to canonical oncogenic signaling pathways. Complementary gene expression analyses showed that oncogenic PTK2B, PDE4B, and TCF4 were suppressed by the chemoprotective diet at both the mRNA and protein levels.
Keywords: n-3 polyunsaturated fatty acids, fish oil, pectin, epigenetics
microRNAS are a diverse class of highly conserved small noncoding RNAs (∼22 nucleotides long) shown to play a critical role in fundamental biological processes including cellular differentiation, apoptosis, cell proliferation, and development. Currently, >800 human and mouse microRNAs have been identified (23). MicroRNAs regulate protein expression by acting through perfect or imperfect complementation to 3′-untranslated regions (UTRs) of their target mRNAs, resulting in posttranscriptional repression of gene expression (19, 68). With respect to chronic diseases, it has been shown that the microRNA gene-silencing pathway influences the processes of carcinogenesis and immune modulation (19, 83). With regard to mechanisms of oncogene activation, loss of microRNA complementary sites due to widespread shortening of 3′-UTRs by alternative cleavage and polyadenylation selectively activates oncogenes and drives malignant transformation (43). Along these lines, several studies have shown that dysregulation of microRNAs and their mRNA targets contribute to the initiation and progression of colon carcinogenesis (5, 65). For example, microRNA (miR)-145 and miR-143 have been shown to act as tumor suppressors in the colon, while miR-21 can act as an oncogene by repressing several tumor suppressor genes including PTEN, Pdcd4, and TPM1 (4, 87).
Several clinical and experimental studies have demonstrated that diets rich in n-3 polyunsaturated fatty acids (PUFAs) such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), found in fish oil, are protective against colon tumorigenesis (10, 38, 58, 81). In contrast, n-6 PUFAs such as linoleic acid and arachidonic acid, found in vegetable oils, have been shown to enhance both the initiation and promotion of colon cancer (17, 57, 82). These findings are supported by a growing list of clinical and epidemiological studies indicating a functional link between dietary fat intake and colon cancer risk (2, 3, 71). In addition, it has been reported that the consumption of fiber, a source of butyrate in the intestinal lumen, may confer protection against colorectal cancers (8). Intriguingly, the protective effect of n-3 PUFAs with respect to colon tumor development is enhanced when a highly fermentable fiber, pectin, rather than a poorly fermentable fiber, cellulose, is added to the diet (11, 14). With respect to noncoding RNAs, we recently reported that these chemoprotective dietary agents favorably modulate carcinogen-directed noncoding microRNA signatures in the rat colon (17). Several recent studies have also documented the chemoprotective effects of other dietary agents such as folate, retinoids, and curcumin (diferuloylmethane) on microRNA expression in different cancers (36, 69, 80). Collectively, these data suggest a pervasive effect of diet in microRNA-mediated oncogenic transformation.
It has been shown that several tumor suppressors and proto-oncogenes can influence the formation of mature ribosomes and thereby regulate the activity of translation factors. Translational regulation plays an important role in repression/activation of gene expression during malignant transformation. In addition, some microRNAs that target mRNAs have been shown to be associated with polysomes and shuttle in the polysomal spectrum as a consequence of microRNA regulation (45, 48, 53–56, 72). To date, a genome-wide perspective of the effects of microRNAs on actively translated (polysomal) mRNA populations in the colon has not been performed.
To elucidate the biological function of intestinal microRNAs, it is necessary to infer microRNA activity by combining gene expression with microRNA target prediction. Since microRNAs interact with complementary mRNAs (posttranscriptional level), resulting in either degradation or translational repression of their mRNA targets (7, 26), it is reasonable to expect that genome-wide profiling of gene expression and microRNAs will allow for the investigation of genomic changes in cancer development. Therefore, when mRNA and microRNA levels are measured in the same sample, an integrative analysis can be performed to compare both profiles and determine their interactions. Various computational algorithms are currently used to predict the target genes of microRNAs. However, since a single microRNA can directly target >200 genes and each mRNA may be regulated by several microRNAs (6, 40), computational challenges in microRNA-mediated regulation persist. As a result, there have been several approaches taken to analyze microRNA and gene expression data, such as performing cluster analysis or computing correlation coefficients for microRNA and mRNA target expression (34, 37, 50, 68).
In the absence of comprehensive human data, the azoxymethane (AOM) chemical carcinogenesis model serves as one of the most definitive means of assessing human colon cancer risk (1, 57). Therefore, in this study, we determined the effect of carcinogen and diet on targets of colonic microRNAs in rats at 10 and 34 wk postinitiation. microRNA expression was quantified using a qPCR approach, and mRNA expression was quantified using a Codelink microarray platform. For the purpose of determining the effect of cancer progression and dietary chemoprevention on genomic profiles, we specifically examined the effect of carcinogen, n-6 vs. n-3 fatty acid effects, and fat × fiber interaction on global colonic microRNA and mRNA expression profiles. Four complementary computational approaches were utilized to test the hypothesis that microRNAs and their posttranscriptionally regulated mRNA targets, i.e., both total mRNAs and actively translated mRNA transcripts (in polyribosome complexes), are differentially modulated by carcinogen and diet treatment. Specifically, gene enrichment analysis was used to obtain those microRNAs significantly enriched by the change in expression of their putative target genes across carcinogen and diet comparisons. This was complemented with canonical pathway analyses to further assess microRNA and mRNA interaction. In addition, cumulative distribution function (CDF) plots were used to evaluate the impact of diet/carcinogen on mRNA levels induced via microRNA alterations. For validation purposes, a subset of the gene targets was also examined at the protein level. Lastly, linear discriminant analysis (LDA) was used to identify the best single-, two-, and three-microRNA combinations for classifying diet effects and colon tumor development.
MATERIALS AND METHODS
Animals
Fifty-four weanling male Sprague-Dawley rats (Harlan, Houston, TX) were acclimated for 2 wk in a temperature- and humidity-controlled facility on a 12 h light/dark cycle. The animal use protocol was approved by the University Animal Care Committee of Texas A&M University. The study was a 2 × 2 × 2 factorial design with two types of dietary fat (n-6 PUFA or n-3 PUFA), two types of dietary fiber (cellulose or pectin), and two treatments (injection with the colon carcinogen, AOM, or with saline). Animals (n = 6 per group) were terminated 10 wk after AOM injection. For generation of colonic tumors, a second group of rats (n = 6 per treatment) were continued on diet for 34 wk postinjection. All tumors were independently classified as adenocarcinomas by a board-certified pathologist.
Experimental Diets
Refer to Supplemental Methods for details.1
MicroRNA Analysis
Total RNA enriched with microRNA was isolated using mirVana microRNA Isolation Kit (Ambion, Austin, TX). Expression of 368 mature microRNAs was determined using TaqMan Human MicroRNA Panel Low Density Arrays (Applied Biosystems). microRNA expression was normalized to RNU6B expression. We disqualified 215 microRNAs due to low (close to background level) expression. The resultant readings were quantile normalized within each experimental condition, and one-way ANOVA analysis (t-test) was performed followed by false discovery rate correction. Adjusted P and q values were obtained, and significantly altered microRNAs (q < 0.05) with a fold change >1.3 or <0.7 were selected for further analysis.
mRNA Analysis
Polysome and total RNA were isolated from diet and carcinogen-treated animals as previously described (16). Refer to Supplemental Methods for details. Only genes with a G flag (good quality) from the microarrays were selected for further analysis. Expression values were median normalized per array. Similar to the case of microRNA, both total and polysomal RNA expression values were quantile normalized within each experimental condition. The normalization step was followed by one-way ANOVA, and significant (P < 0.05) differentially expressed mRNAs were identified.
MicroRNA Target Prediction
Since routine identification of an overlap between microRNA target prediction algorithms is discouraged (59), Target Scan (http://www.targetscan.org/) was used to identify putative microRNA targets. This program predicts biological targets of microRNAs by searching for conserved 8-mer and 7-mer sites that match the seed region of the microRNA (39). The predictions were ranked based on the putative efficacy of targeting as calculated using context scores of the sites (24). Specifically, the algorithm generated PCT scores for the probability of any seed match being selectively maintained due to microRNA targeting. The higher the PCT score, the greater the probability that a microRNA could target a particular gene. In addition, the DIANA-microT 3.0 algorithm was utilized to verify Target Scan output (41).
Computational Analyses
Overview of analysis pipeline.
To infer the regulatory activities of microRNAs in the colon, microarray expression data was integrated with microRNA target prediction. For this purpose, three main biological comparisons were examined: 1) carcinogen effects (tumor vs. saline), 2) dietary fat effects in the presence of carcinogen (corn oil + AOM vs. fish oil + AOM), and 3) dietary fat × fiber interaction in the presence of carcinogen [corn oil + cellulose + AOM (CCA) vs. fish oil + pectin + AOM (FPA)]. The global impact of diet and carcinogen on microRNAs and mRNA tissue profiles was systematically elucidated using four complimentary computational methods. In the first approach, Gene Set Enrichment Analysis (GSEA) (http://www.broad.mit.edu/gsea/index.jsp) was utilized. GSEA incorporates knowledge of known gene networks/pathways and identifies significantly enriched microRNA putative target lists based on the change in putative target expression. Analysis of the biological role of putative targets of microRNAs was performed using Database for Annotation, Visualization and Integrated Discovery (DAVID) (http://david.abcc.ncifcrf.gov/) (refer to Supplemental Methods for details). In the second approach, highly abundant microRNAs exhibiting an inverse trend with their mRNA targets based on fold change were examined. Treatment effects were examined relative to significantly altered total and polysomal mRNAs (P < 0.05). Next, microRNAs predicted to target mRNAs based on Target Scan were selected. Finally, the list of significantly altered total and polysomal mRNAs predicted to be targeted by several microRNAs was intersected with the list of significantly altered highly abundant microRNAs to obtain noncoding RNAs that were inversely related to their significantly altered target total and polysomal mRNAs. Network-eligible molecules were also combined into canonical pathways that maximize connectivity. Specifically, datasets containing significant differentially expressed genes and microRNAs (fold changes) for the three comparisons were uploaded into the Ingenuity Pathway Analysis (Ingenuity Systems, http://www.ingenuity.com) knowledge base. Separate core analyses were performed using microRNA and total mRNA datasets. The miRWalk (previously known as Argonuate) algorithm was used to predict the association of microRNAs with gene targets (63). Molecules from each dataset that met the 1.5-fold change cut-off and were associated with biological functions and/or diseases in the Ingenuity's Knowledge Base were used to generate networks. Right-tailed Fisher's exact test was used to calculate P values determining the probability that biological functions and/or diseases assigned to each data set were not due to chance alone. For a general overview of this analysis, refer to Supplemental Fig. S1. In the third approach, we examined the global influence of microRNA differential expression across the various diet/carcinogen treatments using CDF plots of fold change for putative mRNA target lists (refer to Supplemental Methods for details). In the final approach, LDA classification was performed using microRNAs as features to discriminate between the conditions described in the comparisons 1), 2), and 3) above (refer to Supplemental Methods for details). The general overview of the analysis pipeline is shown in Supplemental Fig. S1.
Immunoblotting.
To generate colonic protein extracts, the mucosa was scraped and homogenized in ice-cold buffer (50 mM Tris·HCl, pH 7.2, 250 mM sucrose, 2 mM EDTA, 1 mM EGTA, 50 mM sodium fluoride, 100 mM sodium orthovanadate, 1% Triton X-100, 100 μM activated sodium orthovanadate, 40 μl/ml protease cocktail, and 10 mM β-mercaptoethanol). Samples were processed and subjected to polyacrylamide gel electrophoresis in precast 4–20% Tris-glycine mini gels (Invitrogen). After electrophoresis, proteins were transferred onto a polyvinylidene fluoride membrane at 400 mA for 120 min. After the transfer, membrane was incubated in blocking solution (5% nonfat dry milk and 0.1% Tween 20 in PBS) at room temperature for 1 h with shaking. Following blocking, the membrane was incubated overnight at 4°C with rabbit TCF4 at 1:1,000 dilution (Cell Signaling Technology), rabbit PDE4B at 1:200 dilution (Abcam), rabbit PTK2B at 1:200 dilution (Santa Cruz Biotechnology), rabbit IGF1R 1:200 dilution (Santa Cruz Biotechnology) or mouse β-actin at 1:8,000 dilution (Sigma-Aldrich). Membranes were washed with PBS containing 0.1% Tween 20 and incubated with peroxidase conjugated anti-rabbit IgG secondary antibody at 1:8,000 dilution (Jackson Immuno Research) or goat anti-mouse at 1:30,000 (Kirkegaard & Perry, Gaithersburg, MD) as per manufacturer's instructions. Bands were developed using Pierce SuperSignal West Femto maximum sensitivity substrate and quantified with a Fluor-S Max MultiImager system (Bio-Rad, Hercules, CA). β-Actin was used as a loading control. The effect of treatment was assessed by nonparametric Mann-Whitney test using SPSS. P < 0.05 was considered to be statistically significant.
RESULTS
miR-34a, miR-190, miR-193a, miR-214, and miR-215 are Enriched Based on Target (Total and Polysomal) mRNA Genome-wide Expression Analysis in Carcinogen-treated Rats
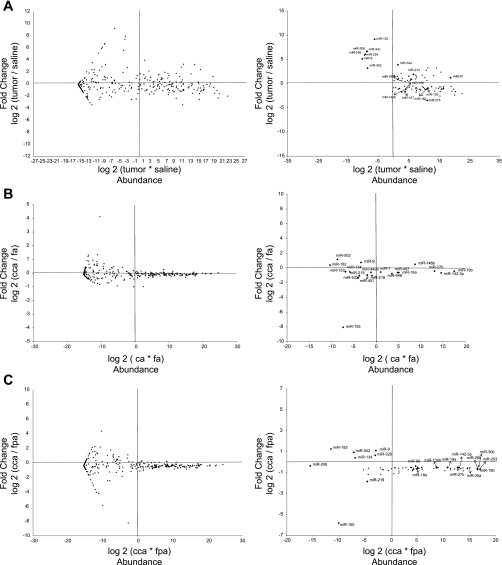
We have recently demonstrated that several colonic microRNAs are significantly altered in carcinogen (AOM) compared with saline-treated rats (17). To further elucidate the regulation of these microRNAs, we determined whether they targeted total mRNA and/or polysome mRNAs. Utilizing a computational approach, GSEA, which incorporates knowledge of known gene networks/pathways and identifies significantly enriched microRNAs based on the change in expression of putative targets, we identified five enriched microRNAs, miR-34a, miR-190, miR-193a, miR-214, miR-215, out of 46 candidate microRNAs (Table 1A) in tumor vs. saline-treated rats. Examples of enrichment plots are shown in Fig. 1. These noncoding RNAs were significantly enriched based on the change in expression of their total and polysomal mRNA targets. By plotting the data as a ratio intensity profile map, miR-34a, miR-190, miR-193a, miR-214, and miR-215 were shown to be highly abundant in the colon (Fig. 2A).
Table 1.
Summary of colonic microRNAs significantly enriched according to GSEA
| Total/Polysomal | microRNA | Size | ES | NES | P Value |
|---|---|---|---|---|---|
| A) Tumor vs. Saline Treatments | |||||
| Total T | miR-193a | 17 | 0.511 | 1.688 | 0.025 |
| Total T | miR-190 | 13 | −0.545 | −1.600 | 0.035 |
| Poly T | miR-214 | 23 | −0.482 | −1.689 | 0.014 |
| Poly T | miR-34a | 13 | −0.516 | −1.591 | 0.022 |
| Poly T | miR-215 | 1 | −0.965 | −1.312 | 0.029 |
| B) Corn oil/AOM vs. Fish Oil/AOM Treatments | |||||
| Total CA | miR-132 | 15 | 0.450 | 1.519 | 0.035 |
| Poly CA | miR-218 | 55 | −0.466 | −1.592 | 0.030 |
| Poly CA | miR-146b | 2 | −0.980 | −1.517 | 0.008 |
| Poly CA | miR-206 | 3 | −0.809 | −1.470 | 0.032 |
| Poly CA | miR-192 | 9 | −0.602 | −1.463 | 0.047 |
| C) Corn Oil/Cellulose/AOM vs. Fish Oil/Pectin/AOM Treatments | |||||
| Total CCA | miR-206 | 8 | 0.675 | 1.650 | 0.017 |
| Total CCA | miR-93 | 17 | 0.512 | 1.549 | 0.029 |
| Total CCA | miR-130b | 25 | 0.440 | 1.485 | 0.046 |
| Total CCA | miR-19a | 109 | 0.329 | 1.398 | 0.028 |
| Total FPA | miR-98 | 6 | 0.786 | 1.692 | 0.015 |
| Poly CCA | miR-26b | 13 | 0.674 | 1.559 | 0.029 |
| Poly FPA | miR-206 | 4 | −0.768 | −1.480 | 0.029 |
| Poly FPA | miR-30b | 3 | −0.830 | −1.438 | 0.034 |
Data represent significantly enriched microRNAs based on the differential expression of their putative targets in either total or polysomal mRNA datasets for the 3 comparisons: 1) tumor (T) vs. saline (S), 2) corn oil + azoxymethane (AOM) (CA) vs. fish oil + AOM (FA), and 3) corn oil + cellulose + AOM (CCA) vs. fish oil + pectin + AOM (FPA). GSEA, gene set enrichment analysis; size, number of differentially expressed mRNA putative targets; ES, enrichment score; NES, normalized enrichment score.
Fig. 1.
Enrichment plots of microRNAs in total vs. polysome mRNA datasets. Polysome and total mRNA enrichment plots are shown for the three comparisons: tumor (T) vs. saline (S), corn oil + azoxymethane (CA) vs. fish oil + azoxymethane (FA) and corn oil + cellulose + azoxymethane (CCA) vs. fish oil + pectin + azoxymethane (FPA). Horizontal bars in graded color from left to right represent mRNA targets ranked from high expression in tumor vs. saline (A), dietary fat effects (B), and fat × fiber (C) interactions. At bottom of each panel is shown the biological processes in which the enriched genes play a role. The vertical gray lines represent the projection onto the ranked gene list of the targets for each microRNA (T over S, CA over FA, and CCA over FPA). The top curve corresponds to the calculation of the enrichment score (ES). The horizontal line indicates the 0 value for the ES. The greater the enrichment curve is shifted to the upper left of the graph, the higher the gene set enrichment in the T, CA, and CCA treatments. P values for the ES scores are shown in the ES plots along with the number of significantly enriched genes.
Fig. 2.
Ratio intensity (RI) profile maps for colonic microRNAs from rats fed specific diets and injected with carcinogen or saline (control). Treatment effects on the expression of microRNAs are shown. The x-axis shows the intensity (abundance) of each microRNA, and the y-axis shows the fold change across treatments. MicroRNA expression is normalized to RNU6B. Each graph is divided into 4 quadrants where the top right quadrant contains those microRNAs that are highly abundant and exhibit a high fold change. For each comparison, there are 2 graphs: left, documenting all 384 microRNAs, and right, showing selected microRNAs with a fold change >1.3 and <0.7.
Diet Influences microRNA Enrichment in Carcinogen-injected Rats
GSEA analysis was also used to further elucidate the impact of diet and carcinogen treatment on microRNAs. With respect to the dietary lipid source comparison in the presence of carcinogen (CA vs. FA), five microRNAs (miR-132, miR-146b, miR-192, miR-206, miR-218) were significantly enriched based on the change in the expression of their total and polysomal mRNA targets (Table 1B). Of these, only miR-146b was highly abundant (Fig. 2B). With regard to the effect of dietary fat × fiber interaction in the presence of carcinogen (CCA vs. FPA), five microRNAs (miR-19a, miR-93, miR-98, miR-130b, miR-206) were significantly enriched based on the change in expression of their total and polysomal mRNA targets (Table 1C). In addition, three microRNAs (miR-26b, miR-30b, miR-206) were significantly enriched based on the change in the expression of their actively translated (polysome) mRNA targets (Table 1C). Of these, miR-26b and miR-30b were shown to be highly abundant in the colon (Fig. 2C). Collectively, GSEA identified 16 microRNAs which were significantly enriched in three comparisons (Table 1), and nine microRNAs targeted polysomal mRNA. These data indicate that both total and polysomal mRNAs are important for prediction of microRNA regulation with respect to experimental colon carcinogenesis. Interestingly, miR-206 was significantly enriched in both the dietary comparisons.
Global Assessment of microRNA Targets Using CDF Analysis
Using Target Scan, we partitioned the putative targets of differentially expressed microRNAs based on the microRNA-mRNA seed pairing, or lack thereof, in the 3′-UTR. For each diet/carcinogen comparison and for both total and polysomal mRNA, we compared CDF plots of fold change for each subset of the partitioned mRNA targets. This provides a visual examination of the global effects of colonic microRNA differential expression for the various diet/carcinogen comparisons. Three relevant examples, miR-214 for tumor vs. saline, miR-18a for corn oil + AOM vs. fish oil + AOM, and miR-19a for CCA vs. FPA are shown in Supplemental Fig. S2. For the majority of microRNAs, the lengths of the partitioned target lists were not sufficiently large to allow for statistically detectable differences between the associated CDFs. Overall, the qualitative visual representation of global microRNA provided from the CDF analysis was confirmatory of the GSEA findings.
Identification of Coherent Colonic microRNA-mRNA Modules
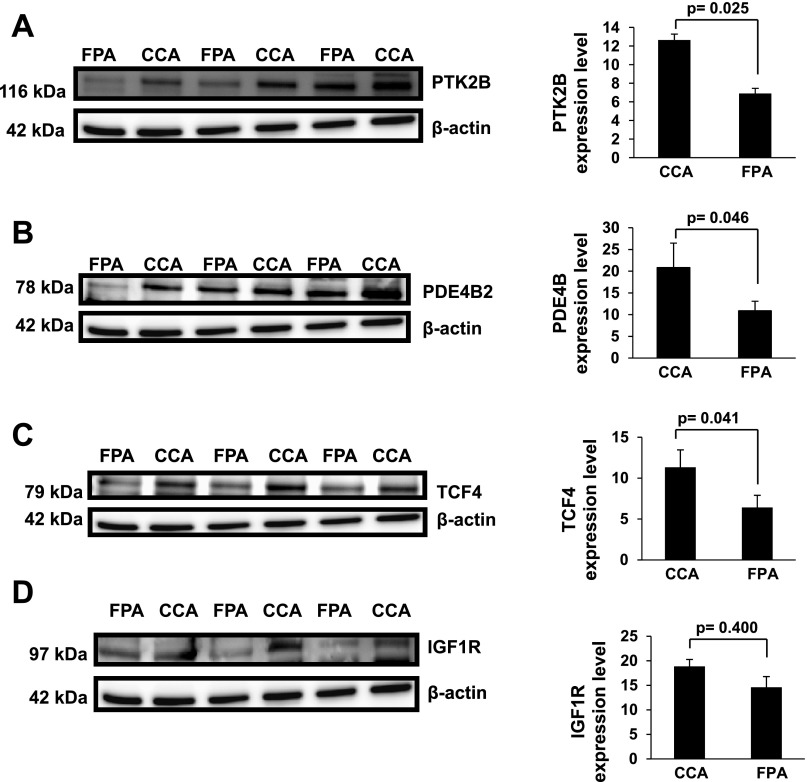
microRNAs have been shown to have an inverse relationship (coherent) with their mRNA targets (26). To infer the relative activity of colonic microRNAs, we examined expression changes in total and polysome mRNA target transcripts. For 1) T vs. S, 2) CA vs. FA, and 3) CCA vs. FPA treatment comparisons, microRNAs were selected based on three criteria: 1) significant fold change (Tables 2 and 3), 2) high abundance (Fig. 2), and 3) a shared coherent relationship (demonstrating inverse trends based on fold change) with their mRNA targets (Tables 2 and 3). With the aid of the Target Scan prediction algorithm, we identified a subset of microRNAs whose levels were inversely altered relative to their putative mRNA targets (Tables 2 and 3 and Supplemental Table S1). With respect to total and polysomal mRNA data, we selected only those mRNAs that satisfied the above criteria. In the case of carcinogen-treated animals (T vs. S), miR-15b, miR-16, miR-103, miR-107, miR-141, miR-146b, miR-148b, miR-183, miR-193a, miR-195, miR-204, and miR-497 exhibited coherent responses relative to their putative targets (Table 2). Furthermore, miR-15b, miR-16, miR-103, and miR-107 all target RASSF5; while miR-141, miR-183, and miR-204 target TCF12, both of which are linked to the total mRNA datasets. miR-103 and miR-107 are derived from the same gene (cluster) and share similar sequences. In contrast, from the polysome mRNA dataset, YWHAB can be targeted by miR-148b; SLC11A2 is a putative target of both miR-195 and miR-497 (miR-195 cluster), FLl1 and GR1D1 can be targeted by miR-193 and miR-146b, respectively. With respect to the effect of dietary lipid source (CA vs. FA), miR-18a, miR-19b, miR-27b, miR-93, and miR-497 exhibited a coherent response at the total and polysomal mRNA levels (Table 3). With regard to fat × fiber interaction in the presence of carcinogen (CCA vs. FPA), miR-19b, miR-26b, and miR-203 were inversely associated with the total mRNA expression levels of PTK2B, IGF2R, PDE4B, and TCF4, respectively (Table 3). To further assess the biological processes impacted by carcinogen and diet, gene ontology (GO) analysis was carried out and the top terms for the GO categories for each of the microRNA target gene sets was determined (Supplemental Table S3). The fold changes and significance of the microRNA target gene sets are listed in Supplemental Table S1. We also processed protein samples from carcinogen-exposed animals fed corn oil + cellulose (control) or fish oil + pectin (chemoprotective) diets to determine if some of the predicted targets were altered at the protein level. Specifically, we selected highly expressed targets with known proto-oncogene functions, which were significantly modulated by fat × fiber interaction. For example, PTK2B and PDE4B, both of which are predicted targets of miR-19b (Table 3); TCF4, a predicted target of miR-203 (Table 3); and IGF1R, a predicted target of miR-19b (Table 3) were assessed by immunoblotting. As expected, based on steady-state mRNA levels, PTK2B, PDE4B2, and TCF4 were upregulated (P < 0.05) by about twofold in CCA compared with FPA treatment (Fig. 3). In contrast, no significant (P > 0.05) change was observed in IGF1R levels.
Table 2.
Selection of colonic microRNAs based on the inverse trend in fold change of putative targets in tumor- vs. saline-treated rats: tumor vs. saline effects
| Treatment | microRNA | Fold Change microRNA | P Value microRNA | Significant Target Genes | Fold Change target Genes | P Value Target Genes | Pathway | miRNA Family/Cluster |
|---|---|---|---|---|---|---|---|---|
| Total T-S | miR-103 | 0.51 | <0.001 | RASSF5 | 1.32 | 0.023 | apoptosis | miR-103/miR107 family |
| Total T-S | miR-107 | 0.27 | <0.001 | RASSF5 | 1.32 | 0.023 | apoptosis | miR-103/miR107 family |
| Total T-S | miR-15b | 0.60 | <0.001 | RASSF5 | 1.32 | 0.023 | apoptosis | miR-15b-miR 16-2 cluster |
| Total T-S | miR-16 | 0.69 | <0.001 | RASSF5 | 1.32 | 0.023 | apoptosis | miR-15b-miR 16-2 cluster |
| Total T-S | miR-183 | 0.68 | 0.002 | TCF12 | 1.45 | 0.048 | Wnt signaling pathway | miR-96/182/183 cluster |
| Total T-S | miR-204 | 0.29 | <0.001 | TCF12 | 1.45 | 0.048 | Wnt signaling pathway | miR-204 family |
| Total T-S | miR-141 | 0.32 | <0.001 | TCF12 | 1.45 | 0.048 | Wnt signaling pathway | miR-200c/141 cluster |
| Poly T-S | miR-148b | 0.44 | <0.001 | YWHAB | 1.14 | 0.037 | apoptosis, cell cycle signaling | miR-148/152 family |
| Poly T-S | miR-195 | 1.83 | <0.001 | SLC11A2 | 0.68 | 0.034 | intestinal iron absorption | miR-195 cluster |
| Poly T-S | miR-497 | 1.92 | <0.001 | SLC11A2 | 0.68 | 0.034 | intestinal iron absorption | miR-195 cluster |
| Poly T-S | miR-193a | 2.46 | 0.001 | FLI1 | 0.64 | 0.001 | Wnt signaling pathway | miR-193 family |
| Poly T-S | miR-146b | 9.17 | <0.001 | GRID1 | 0.54 | 0.001 | glutamic acid signaling | miR-146 family |
MicroRNAs were selected based on 3 criteria: 1) a significant fold change, 2) high abundance, and 3) inverse association with putative targets of microRNAs.
Table 3.
Diet effects on colonic microRNAs and their inversely associated gene targets in carcinogen-injected rats
| Treatment | microRNA | Fold Change microRNA | P Value microRNA | Significant Target Genes | Fold Change Target Genes | P Value Target Genes | Pathway | miRNA Family/Cluster |
|---|---|---|---|---|---|---|---|---|
| Corn Oil vs. Fish Oil Effects | ||||||||
| Total CA-FA | miR-19b | 0.45 | <0.001 | IGF1R | 1.48 | <0.001 | apoptosis | miR-17-92 cluster |
| Total CA-FA | miR-19b | 0.45 | <0.001 | RUNX3 | 1.34 | 0.041 | Runt-related transcription factor 3 | miR-17-92 cluster |
| Total CA-FA | miR-27b | 0.75 | 0.043 | ATP2B1 | 1.42 | 0.024 | stem cell pathway | miR-23b/27b/24-1 cluster |
| Total CA-FA | miR-27b | 0.75 | 0.043 | LIMK2 | 1.23 | 0.032 | actin cytoskeleton reorganization | miR-23b-24-1 cluster |
| Total CA-FA | miR-27b | 0.75 | 0.043 | PARD6B | 1.21 | 0.044 | asymmetrical cell division and cell polarization | miR-23b-24-1 cluster |
| Total CA-FA | miR-27b | 0.75 | 0.043 | ZADH2 | 1.22 | 0.017 | metabolic processes (oxidation reduction) | miR-23b-24-1 cluster |
| Total CA-FA | miR-497 | 0.64 | <0.001 | CASR | 1.37 | 0.050 | calcium homeostatsis | miR-195 cluster |
| Total CA-FA | miR-93 | 0.76 | <0.001 | NPAS2 | 1.23 | 0.024 | signal transduction | miR-106b-25 cluster |
| Poly CA-FA | miR-18a | 0.65 | <0.001 | LAT | 1.37 | 0.016 | Ras protein signal transduction | miR-17-92 cluster |
| Poly CA-FA | miR-27b | 0.75 | 0.043 | SLC6A6 | 1.35 | <0.001 | amino acid transporter | miR-23b-24-1 cluster |
| Poly CA-FA | miR-27b | 0.75 | 0.043 | GATA2 | 1.17 | 0.045 | transcription regulation | miR-23b-24-1 cluster |
| Poly CA-FA | miR-27b | 0.75 | 0.043 | ATP2B1 | 1.42 | 0.024 | stem cell pathway | miR-23b-24-1 cluster |
| Fat × Fiber Effects | ||||||||
| Total CCA-FPA | miR-19b | 0.64 | 0.035 | PTK2B | 1.772 | 0.032 | cell proliferation | miR-17-92 cluster |
| Total CCA-FPA | miR-19b | 0.64 | 0.035 | IGF2R | 1.664 | 0.034 | apoptosis | miR-17-92 cluster |
| Total CCA-FPA | miR-26b | 0.65 | <0.001 | PDE4B | 2.43 | 0.050 | apoptosis | miR-26 family |
| Total CCA-FPA | miR-203 | 0.61 | <0.001 | TCF4 | 2.16 | 0.043 | apoptosis | |
| Total CCA-FPA | miR-27b | 0.75 | 0.047 | ATP2B1 | 1.42 | 0.025 | stem cell pathway | miR-23b-24-1 cluster |
Refer to Table 2 for legend details.
Fig. 3.
Suppression of PTK2B, PDE4B, and TCF4 in rats fed a chemoprotective diet. Expression of PTK2B (A), PDE4B (B), TCF4 (C), and IGF1R (D) in carcinogen-injected rats fed diets containing either (control) corn oil-cellulose or (chemoprotective) fish oil-pectin was analyzed by immunoblotting, n = 5 animals per treatment.
Together, these data indicate that 25 highly abundant microRNAs exhibited a significant (P < 0.05) inverse relationship with their putative polysome and total mRNA targets following diet and carcinogen exposure (Tables 2 and 3 and Supplemental Table S1). Furthermore, microRNAs that were not highly abundant (low expressors), miR-219 and miR-9, also exhibited a significant (P < 0.05) inverse relationship with their putative total and polysomal mRNA targets (Supplemental Table S2).
Functional Analysis of Differentially Expressed microRNAs
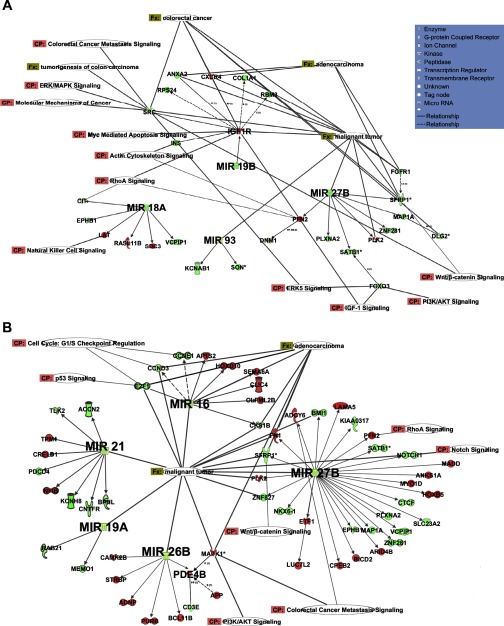
To explore the functional relevance of select microRNA species and predicted targets in the context of colorectal cancer, microRNA and total mRNA data were analyzed using Ingenuity interactive pathway analysis (Ingenuity Systems, http://www.ingenuity.com) software. With respect to the effect of dietary fish oil treatment on carcinogen-injected rats, the top associated (P < 0.05) networks were NFAT, adenocarcinoma, Wnt/beta-catenin signaling, phospholipase C, and Notch signaling. Moreover, fatty acid metabolism in mitochondria was the top ranked canonical pathway. With respect to the fat × fiber comparison, p38 Mapk signaling, RhoA signaling, and notch signaling genes were modulated (P < 0.03). In addition, Wnt/beta-catenin, carcinoma, and phospholipase C were the top ranked associated network functions (P < 0.01). For the dietary lipid (CA vs. FA) comparison, fish oil feeding downregulated microRNAs (miR-18a, miR-19b, miR-27b, and miR-93) targeted several differentially expressed targets that are involved in pathways related to colorectal cancer, e.g., ERK-MAPK, Wnt/β-catenin, PTEN, and apoptosis (Fig. 4A). These microRNAs also shared a coherent relationship with their predicted total mRNA targets (Table 3). Moreover, the association of miR-19b and IGF1R shown by IPA analysis was also observed in the coherent analysis (Table 3). Upregulation of IGF1R can lead to downregulation of genes such as SRC, INS, COL1A1, CXCR4, all of which have been associated with colorectal cancer (25, 61). With regard to fat × fiber interaction in the presence of carcinogen (CCA vs. FPA), the fish oil-pectin combination downregulated miR-16, miR-21, miR-26b, and miR-27b, which targeted several differentially expressed genes associated with adenocarcinomas, colorectal cancer, mTOR signaling, PI3K/AKT signaling, and apoptosis (Fig. 4B). Fat × fiber effects on miR-26b and miR-27b were also observed in GSEA and coherent analyses (Tables 1 and 3).
Fig. 4.
Functional analyses of differentially expressed predicted targets of significantly altered microRNAs. A: microRNAs miR-18a, miR-19b, miR-27b, and miR-93 were downregulated in CA vs. FA treatments (expression of CA divided by expression in FA). B: microRNAs- miR-16, miR-21, miR-26b, and miR-27b were downregulated in the CCA vs. FPA (expression of CCA divided by expression in FPA treatments). Their differentially expressed predicted targets (by mirWalk) are also shown. Signaling networks identified by IPA software are based on significant fold changes of the differentially expressed microRNAs. The intensity of the node (gene/microRNA) color indicates the degree of upregulation (red) or downregulation (green). Nodes are displayed using various shapes that represent the functional class of the gene product. Nodes without any color were not present in the input dataset but were present as a part of the network. Solid lines between genes/microRNAs represent a direct interaction based on experimental proven associations. Dashed line represent predicted interactions (indirect interactions) based on experimental evidence. Fx, functions and diseases; CP, canonical pathway. Symbols describing cell functions are shown in A.
microRNA Expression and Treatment Classification
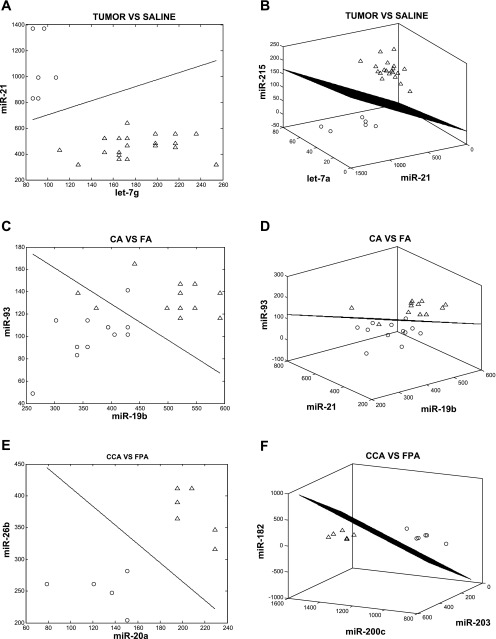
For the purpose of classifying colon tumor development using microRNAs, we applied LDA. The number of microRNAs (features) for each linear classifier was limited to three, which allowed for an exhaustive search while avoiding errors associated with small-sample setting feature extraction. Using this approach, the top 10 best single-, two-, and three-microRNA classifiers were queried as a means to distinguish phenotype; 1) T vs. S, 2) CA vs. FA, and 3) CCA vs. FPA. We noted several cases where single microRNAs can provide good (in terms of the error estimate) classification (Tables 4 and 5). As expected, when considering these features as part of two- or three-gene classifiers, a significant decrease in the classification error was noted (Table 5). To identify sets of microRNAs that perform in a multivariate manner to provide strong classification, we specifically looked for pairs of microRNAs that performed better than either of the microRNAs individually, and also triplets of microRNAs that performed well and substantially better than the best-performing pair among the three. To estimate the improvements of the classification performance, we introduced two quantities for each feature set: εbolstered and Δ(εbolstered). εbolstered denotes the bolstered resubstitution error for the LDA classifier for the respective feature set of size n (n = 1, 2, 3), and Δ(εbolstered) denotes the decrease in error with respect to the highest ranked of its subset of features (in the list of features of size n −1, n = 2, 3). The feature sets were ranked based on the value of εbolstered. The top T vs. S (control) single-gene classifier (one-feature), miR-21 (ranked 1st in the list of single-miR classifiers) had an estimated classification error of 0.001. Interestingly, when this microRNA was combined with a poorly performing single-microRNA classifier, let-7g (ranked >20 in the list of single-gene classifiers), it resulted in a two-noncoding gene classifier with a low estimated error, <0.001 (Table 5). In addition, the let-7a, miR-141, and miR-375 triple classifier exhibited unique properties, as all three microRNAs performed poorly as single classifiers (ranked >15 in the list of single-gene classifiers). This shows that when combined, these microRNAs increase the power of classification with a low estimated error, <0.001 (Table 4). Moreover, let-7a and let-7c appeared frequently in the top 10 triple classifiers. For example, let-7 family members let-7a and let-7c performed well as classification features when combined with miR-215 and miR-21 (Table 4), well-known colon cancer microRNAs (4, 67). Interestingly, miR-215 was also significantly enriched as assessed by GSEA (Table 1). miR-141, found to share a coherent relationship with its putative targets, also classified with the let-7 family. In summary, these microRNAs performed poorly as single classifiers, but when combined, they decreased the classification error (Fig. 5, A and B).
Table 4.
Classification of colonic microRNAs altered in tumors vs. saline-treated rats
| microRNA | εbolstered | ▵(εbolstered) | ||
|---|---|---|---|---|
| miR-21 | 0.001 | |||
| let-7c | 0.001 | |||
| miR-200c | 0.001 | |||
| miR-215 | 0.001 | |||
| miR-200a | 0.001 | |||
| miR-30c | 0.001 | |||
| miR-30e-3p | 0.001 | |||
| miR-30b | 0.001 | |||
| miR-422a | 0.001 | |||
| miR-200b | 0.020 | |||
| let-7 g | miR-21 | <0.001 | <0.001 | |
| let-7a | miR-200c | <0.001 | <0.001 | |
| let-7a | miR-215 | <0.001 | <0.001 | |
| let-7a | miR-200b | <0.001 | <0.001 | |
| let-7c | miR-141 | <0.001 | <0.001 | |
| let-7c | miR-200c | <0.001 | <0.001 | |
| let-7c | miR-215 | <0.001 | <0.001 | |
| let-7c | miR-200a | <0.001 | <0.001 | |
| let-7c | miR-30c | <0.001 | <0.001 | |
| let-7c | miR-30b | <0.001 | 0.020 | |
| let-7a | let-7c | miR-30c | <0.001 | <0.001 |
| let-7a | let-7c | miR-30b | <0.001 | <0.001 |
| let-7a | miR-21 | miR-215 | <0.001 | <0.001 |
| let-7a | let-7c | miR-215 | <0.001 | <0.001 |
| let-7a | let-7c | miR-200c | <0.001 | <0.001 |
| let-7a | let-7c | miR-375 | <0.001 | <0.001 |
| let-7a | miR-141 | miR-30b | <0.001 | <0.001 |
| let-7a | let-7b | miR-200c | <0.001 | <0.001 |
| let-7a | let-7b | miR-215 | <0.001 | <0.001 |
| let-7a | let-7b | miR-30b | <0.001 | <0.001 |
Top 10 single, pair-wise, and triplet-wise microRNA linear discriminant analysis (LDA) classifiers are shown. εbolstered denotes bolstered resubstitution error for the respective classifier. The classifiers are ranked according to the error measurement. ▵(εbolstered) denotes the decrease in error for each feature set relative to its highest ranked subset of features.
Table 5.
Classification of microRNAs altered by dietary effects in carcinogen-treated rats
| microRNA | εbolstered | ▵(εbolstered) | ||
|---|---|---|---|---|
| Corn Oil vs. Fish Oil Effects | ||||
| miR-19b | 0.133 | |||
| miR-93 | 0.152 | |||
| miR-497 | 0.181 | |||
| miR-18a | 0.218 | |||
| miR-101 | 0.238 | |||
| miR-532 | 0.240 | |||
| miR-199b | 0.266 | |||
| miR-146b | 0.268 | |||
| miR-92 | 0.277 | |||
| miR-106b | 0.285 | |||
| miR-19b | miR-21 | 0.117 | 0.016 | |
| miR-19b | miR-15b | 0.137 | 0.014 | |
| miR-19b | miR-93 | 0.138 | 0.042 | |
| miR-19b | miR-30c | 0.147 | 0.071 | |
| miR-18a | miR-497 | 0.154 | 0.084 | |
| miR-19b | miR-92 | 0.154 | 0.085 | |
| miR-93 | miR-25 | 0.157 | 0.108 | |
| miR-93 | miR-15b | 0.157 | 0.110 | |
| miR-19b | miR-106b | 0.158 | 0.119 | |
| miR-93 | miR-106b | 0.161 | 0.124 | |
| miR-19b | miR-21 | miR-25 | 0.107 | 0.010 |
| miR-19b | miR-22 | miR-15b | 0.109 | 0.027 |
| miR-19b | miR-23 | miR-92 | 0.110 | 0.028 |
| miR-19b | miR-24 | miR-106b | 0.112 | 0.034 |
| miR-19b | miR-25 | miR-30c | 0.126 | 0.028 |
| miR-19b | miR-30c | miR-15b | 0.132 | 0.021 |
| miR-19b | miR-21 | miR-93 | 0.136 | 0.021 |
| miR-93 | miR-324-5p | miR-15b | 0.140 | 0.017 |
| miR-93 | miR-106b | miR-15b | 0.140 | 0.018 |
| miR-19b | miR-93 | miR-106b | 0.141 | 0.020 |
| Fat × Fiber Effects | ||||
| miR-27b | <0.001 | |||
| miR-30d | 0.015 | |||
| miR-20a | 0.018 | |||
| miR-200c | 0.033 | |||
| miR-11 | 0.037 | |||
| miR-26b | 0.048 | |||
| miR-93 | 0.060 | |||
| miR-30e-3p | 0.066 | |||
| miR-195 | 0.069 | |||
| miR-196b | 0.072 | |||
| miR-200c | miR-26a | <0.001 | <0.001 | |
| miR-27b | miR-30e-3p | 0.001 | 0.014 | |
| miR-20a | miR-30d | 0.001 | 0.017 | |
| miR-200c | miR-203 | 0.001 | 0.031 | |
| miR-27b | miR-182 | 0.003 | 0.033 | |
| miR-30d | miR-196b | 0.004 | 0.044 | |
| miR-146a | miR-27b | 0.004 | 0.055 | |
| miR-27b | miR-186 | 0.004 | 0.061 | |
| miR-301 | miR-20a | 0.005 | 0.064 | |
| miR-20a | miR-26b | 0.005 | 0.066 | |
| miR-146a | miR-27b | miR-30e-3p | <0.001 | <0.001 |
| miR-200c | miR-20a | miR-203 | <0.001 | <0.001 |
| miR-200c | miR-26a | miR-148a | <0.001 | 0.001 |
| miR-200c | miR-26a | miR-203 | <0.001 | 0.001 |
| miR-200c | miR-26a | miR-15b | <0.001 | 0.003 |
| miR-200c | miR-203 | miR-25 | <0.001 | 0.004 |
| miR-17-3p | miR-20a | miR-30d | <0.001 | 0.004 |
| miR-107 | miR-20a | miR-30d | <0.001 | 0.004 |
| miR-200c | miR-26a | miR-200b | <0.001 | 0.004 |
| miR-200c | miR-203 | miR-182 | <0.001 | 0.005 |
Top 10 single, pair-wise, and triplet-wise microRNA LDA classifiers are shown. Refer to Table 4 for legend details.
Fig. 5.
Linear discriminant analysis phenotype classification using microRNAs. Panels represent examples of the top 2- and 3-microRNA classifiers. For example, tumor vs. saline comparison, miR-21 and let-7g (A), corn oil + AOM vs. fish oil + AOM comparison, miR-93 and miR-19b (C), and corn oil + cellulose + AOM vs. fish oil + pectin + AOM comparison, miR-26b and miR-20a (E) are the best performing 2-microRNA feature sets. Classification is between tumor, corn oil, corn oil cellulose (○) and saline, fish oil, fish oil pectin (▵)-treated rats (refer to Tables 4 and 5 for additional details). The axes represent the normalized intensity values of the indicated microRNAs. Note the clear separation between the 2 groups in each comparison. The best-performing 3-microRNA feature sets are shown: tumor vs. saline comparison, miR-215, miR-21, and let-7a (B), corn oil + AOM vs. fish oil + AOM comparison miR-21, miR-93, and miR-19b (D), and corn oil + cellulose + AOM vs. fish oil + pectin + AOM comparison, miR-182, miR-200c, and miR-203 (F). The 3-dimensional LDA hyperplane discriminates between the tumor, corn oil, corn oil cellulose (○)- and saline, fish oil, fish oil pectin (▵)-treated rats.
With respect to the classification of the n-3 PUFA-enriched chemoprotective diet, comparison of the FA vs. CA treatments revealed that miR-19b, miR-21, and miR-93 appeared at the top of the single, double, and triple classifier lists (Table 5; Fig. 5, C and D). These data, combined with the fact that miR-19b and miR-93 exhibited a significant coherent relationship with their putative targets (Table 3), suggest an important role for these noncoding RNA. In addition, miR-106b when combined with miR-93 and miR-19b produced a classifier with a low estimated error. With regard to the fat × fiber interaction, for the CCA vs. FPA comparison, miR-200c appeared repeatedly in the single εbolstered = (0.033), double (0.001), and triple (< 0.001) LDA classifier lists (Table 5; Fig. 5, E and F). In addition, miR-93 and miR-182 exhibited significant coherent relationships with their putative targets (Table 3).
DISCUSSION
We have recently demonstrated that n-3 PUFA uniquely modulate carcinogen-directed noncoding microRNA signatures in the colon (17). In this study, to further elucidate the biological effects of chemoprotective diets on microRNAs, we used an integrated global approach to assess total and polysomal mRNA targets. The large-scale prediction of targets across the whole genome allowed us to achieve a higher degree of specificity of prediction and to infer the regulatory activities of microRNAs in the colon. To date, a number of studies have utilized statistical/systems biology methodologies to identify microRNAs that may target genes in response to a pathophysiological state (31, 44, 88). However, there is a paucity of integrative microRNA and mRNA expression studies that focus on early-stage colon cancer development and chemoprevention. Since mammalian microRNAs predominantly act to decrease target mRNA levels (26), our analyses focused on total and polysomal mRNA targets during the early promotional phase of malignant transformation.
We utilized four complementary approaches to elucidate the global effects of diet and carcinogen on microRNAs and total/polysomal mRNA profiles within the colon. Initially, GSEA was used to probe the effects of three biological conditions: 1) carcinogen (tumor vs. saline), 2) dietary fat effects in the presence of carcinogen (CA vs. FA), and 3) dietary fat × fiber interaction in the presence of carcinogen (CCA vs. FPA). We subsequently identified 18 microRNAs that were significantly enriched in the three treatments based on the change in the expression of total or polysomal mRNA targets. Out of 18 microRNAs, eight were significantly enriched, as assessed by their total mRNA targets, while nine microRNAs were significantly enriched with respect to their polysomal mRNA targets. In addition, miR-206 was significantly enriched with respect to both its polysomal and total mRNAs targets. These data suggest that polysome trafficking of transcripts and microRNAs plays an important role in experimental colon carcinogenesis. The notion that microRNAs localize to polyribosomes is consistent with the translational downregulation of specific mRNAs (35, 42). We argue, therefore, that studies employing polysome purification/ribosome profiling and microarray analysis are needed to fully elucidate the mechanisms of translational deregulation associated with colon tumor development.
For the purpose of inferring the relative activity of each microRNA, we also examined the expression level of target transcripts, assuming that if the target mRNA is downregulated by treatment, then the microRNA activity is likely to be enhanced. With the aid of microRNA-mRNA target prediction algorithms Target Scan and DIANA-microT, we identified the microRNA-mRNA coherent pairs using only those microRNAs that were highly abundant, were significantly altered, and shared a coherent relationship with their mRNA targets. We avoided using the intersection of overlapping computational algorithms because enrichment is weak at best (59, 62). In addition, Target Scan performs well in predicting targets for microRNAs (28, 79). With respect to carcinogen effects on colonic microRNAs, miR-15b, miR-16, miR-107, miR-141, miR-204 have been also shown to be downregulated in human colon cancer (18, 49, 77, 86). Although there is no literature regarding the association of miR-146 and miR-148b with colorectal cancer, they are altered in central nervous system tumor-derived cell lines (21) and thyroid tumors (51).
Consistent with the inverse trend of putative targets in carcinogen-modulated microRNAs (Table 2), miR-15b/miR-16 and miR-107 have been linked with RASSF5 (ras-association domain family) in other cancers (27, 64). RASSF5 is a member of the RASSF family, which has been shown to be a tumor suppressor and is proapoptotic (30, 78). Our data also suggest that TCF12, which is upregulated in tumor samples, may be targeted by miR-141, miR-183, and miR-204. TCF12 is a transcription factor abundantly expressed in Paneth cells of the small intestine (70). Interestingly, the activation of TCF12 may play a role in Paneth cell differentiation in colonic neoplasms (29). Therefore, TCF12 targeting by miR-141, miR-183, and miR-204 may mediate the differentiation of the stem cell niche, and disruption in this mechanism may contribute to colonic neoplasia. Recently, it has been reported that miR-204 represses SIRT1 whose expression is regulated during embryonic stem cell differentiation (60). We also noted a downregulation of FLI1 and upregulation of its targeting miR-193a, which may participate in tumor progression by activating β-catenin transcription (47). Next, we applied LDA to classify treatment effects on colonic microRNAs. The number of gene features for each linear classifier was limited to three, which allowed for an exhaustive search. Using this technique, we found a number of microRNAs to be strong single classifiers, including miR-21, a well-known oncomir in colon cancer (4). In contrast, several microRNAs did not classify well; however, when paired with other poor single classifiers, the pair-wise combination was strong with low estimated errors.
With respect to the effect of dietary lipid source (CA vs. FA), miR-18a, miR-19b, miR-27b, miR-93, and miR-497 were downregulated in corn oil + AOM- compared with fish oil + AOM-treated rats, reaffirming that n-3 PUFA modulate the effect of carcinogen with respect to both microRNA and target gene levels (17). With respect to disease progression, several studies have reported a bivalent role for the miR-17–92 cluster, i.e., it has both tumor suppressor and oncogenic properties (13, 52, 73). miR-18a and miR-19b are a part of the miR-17–92 cluster, and repression of miR-18a leads to an increase in cell proliferation and promotes anchorage independent growth in HT-29 colon adenocarcinoma cell line (74). Interestingly, even though miR-18a has been shown to be upregulated in several cancers, studies have indicated that the expression level of the members of miR-17–92 cluster depends on the cell type (9, 20, 76). Furthermore, miR-19b was reported to be downregulated in Crohn's disease (84). Other notable observations include miR-27b, which shared a coherent relationship with total mRNAs ATP2B1, LIMK2, PARD6B, and ZADH2 and polysomal mRNAs SLC6A6, GATA2, and ATP2B1. Interestingly, n-3 PUFA-fed animals injected with carcinogen exhibited an increase in miR-19b levels and decreased IGF1R levels, which would favorably modulate apoptosis and cell cycle activity within the colon. These findings are consistent with previous reports that fish oil blocks AOM-induced colon tumorigenesis by increasing apoptosis and suppressing cell proliferation (11, 15). Overall, the data are noteworthy, because in the absence of comprehensive human data, the AOM chemical carcinogenesis model serves as a highly relevant means of assessing human colon cancer initiation and progression (1, 85).
With regard to dietary combination chemotherapy, i.e., fat × fiber interaction in the presence of carcinogen (CCA vs. FPA), we observed a coherent relationship between miR-15b, miR-16, miR-18a, miR-19b, miR-20a, miR-26b, miR-27b, miR-93, miR-98, miR-107, miR-130b, miR-182, miR-183, miR-195, miR-196b, miR-203, and miR-497, and their respective mRNA targets (Supplemental Table S1). Of these, miR-19b, miR-26b, miR-27b, and miR-203 showed a strong coherent response with respect to total mRNAs, PTK2B, IGF2R, PDE4B, ATP2B1, and TCF4, respectively. All four microRNAs were downregulated in CCA- compared with FPA-treated rats. Furthermore, we examined a subset of these targets at the protein level and verified that PTK2B, PDE4B2 and TCF4 were also upregulated by about twofold in CCA compared with FPA treatment, while the expression of their microRNAs were downregulated (Fig. 3). This is noteworthy, because TCF4 is a well-known transcription factor involved in Wnt signaling, and mutations in TCF4 are linked to colon cancer development (22). We have previously demonstrated that colonic β-catenin signaling, an upstream mediator of TCF4, is suppressed in fish oil-/pectin-fed, AOM-injected rats (75). Therefore, dietary fish oil + pectin is capable of blocking the AOM-mediated downregulation of miR-203, thereby suppressing TCF4 and preventing the further subversion of Wnt signaling. PTK2B protein tyrosine kinase, a predicted target of miR-19b, has been shown to be involved in several signaling cascades and is overexpressed in malignant gastrointestinal stromal tumors (33). The level of PTK2B was upregulated in CCA compared with FPA at both the mRNA and protein level. PDE4B2 (variant of PDE4B), which is a predicted target of miR-26b, was also upregulated in CCA animals. This phosphodiesterase limits cAMP-associated apoptosis in a number of cancers (46, 66). In the integrative computational analysis, we also noted that IGF1R and IGF2R were predicted targets of miR-19b. Interestingly, there was no significant change in IGF1R at the protein level in CCA vs. FPA comparison. Collectively, these findings support the claim that pleiotropic bioactive components generated by fermentable fiber (butyrate) and fish oil (DHA and EPA) work coordinately to protect against colon tumorigenesis (12, 32).
In summary, we have documented the combined effects of dietary bioactive agents and carcinogen on microRNA and mRNA expression profiles in the rat colon. The fact that global microRNA expression patterns in human and rat AOM-induced tumors are similar (17) supports the utility of this model. Four complementary computational approaches were utilized to demonstrate that microRNAs and their putative mRNA targets, i.e., both total mRNAs and actively translated mRNA transcripts in polyribosome complexes, are differentially modulated by carcinogen and chemoprotective diets. Furthermore, immunoblot analyses were carried out, and oncogenic targets of miR-19b, miR-26b, and miR-203 were downregulated by the chemoprotective fish oil + pectin-containing diet. These microRNAs will be the focus of future experiments involving knockdown and overexpression strategies. We also conclude that polysomal profiling is tightly correlated with microRNA changes when compared with total mRNA profiling. To our knowledge, this represents the first integrated analysis of microRNA and mRNA expression at an early stage of cancer development.
GRANTS
This study was supported by National Institute of Health Grants CA-129444, CA-59034, and P30ES-09106 and US Department of Agriculture CSREES Special Grant Designing Foods for Health 2009-34402-19195.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Ahnen DJ. Are animal models of colon cancer relevant to human disease. Dig Dis Sci 30: 103S–106S, 1985. [DOI] [PubMed] [Google Scholar]
- 2. Anti M, Armelao F, Marra G, Percesepe A, Bartoli GM, Palozza P, Parrella P, Canetta C, Gentiloni N, De Vitis I, Gasbarrini G. Effects of different doses of fish oil on rectal cell proliferation in patients with sporadic colonic adenomas. Gastroenterology 107: 1709–1718, 1994. [DOI] [PubMed] [Google Scholar]
- 3. Anti M, Marra G, Armelao F, Bartoli GM, Ficarelli R, Percesepe A, De Vitis I, Maria G, Sofo L, Rapaccini GL, Gentiloni N, Miggiano G. Effect of omega-3 fatty acids on rectal mucosal cell proliferation in subjects at risk for colon cancer. Gastroenterology 103: 883–891, 1992. [DOI] [PubMed] [Google Scholar]
- 4. Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, Allgayer H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 27: 2128–2136, 2008. [DOI] [PubMed] [Google Scholar]
- 5. Aslam MI, Taylor K, Pringle JH, Jameson JS. MicroRNAs are novel biomarkers of colorectal cancer. Br J Surg 96: 702–710, 2009. [DOI] [PubMed] [Google Scholar]
- 6. Barbato C, Arisi I, Frizzo ME, Brandi R, Da Sacco L, Masotti A. Computational challenges in miRNA target predictions: to be or not to be a true target? J Biomed Biotechnol 2009: 803069, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Behm-Ansmant I, Rehwinkel J, Doerks T, Stark A, Bork P, Izaurralde E. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev 20: 1885–1898, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bingham SA, Day NE, Luben R, Ferrari P, Slimani N, Norat T, Clavel-Chapelon F, Kesse E, Nieters A, Boeing H, Tjonneland A, Overvad K, Martinez C, Dorronsoro M, Gonzalez CA, Key TJ, Trichopoulou A, Naska A, Vineis P, Tumino R, Krogh V, Bueno-de-Mesquita HB, Peeters PH, Berglund G, Hallmans G, Lund E, Skeie G, Kaaks R, Riboli E. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet 361: 1496–1501, 2003. [DOI] [PubMed] [Google Scholar]
- 9. Bonauer A, Dimmeler S. The microRNA-17–92 cluster: still a miRacle? Cell Cycle 8: 3866–3873, 2009. [DOI] [PubMed] [Google Scholar]
- 10. Chang NW, Huang PC. Effects of the ratio of polyunsaturated and monounsaturated fatty acid to saturated fatty acid on rat plasma and liver lipid concentrations. Lipids 33: 481–487, 1998. [DOI] [PubMed] [Google Scholar]
- 11. Chang WC, Chapkin RS, Lupton JR. Predictive value of proliferation, differentiation and apoptosis as intermediate markers for colon tumorigenesis. Carcinogenesis 18: 721–730, 1997. [DOI] [PubMed] [Google Scholar]
- 12. Chapkin RS, McMurray DN, Lupton JR. Colon cancer, fatty acids and anti-inflammatory compounds. Curr Opin Gastroenterol 23: 48–54, 2007. [DOI] [PubMed] [Google Scholar]
- 13. Cloonan N, Brown MK, Steptoe AL, Wani S, Chan WL, Forrest AR, Kolle G, Gabrielli B, Grimmond SM. The miR-17–5p microRNA is a key regulator of the G1/S phase cell cycle transition. Genome Biol 9: R127, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crim KC, Sanders LM, Hong MY, Taddeo SS, Turner ND, Chapkin RS, Lupton JR. Upregulation of p21Waf1/Cip1 expression in vivo by butyrate administration can be chemoprotective or chemopromotive depending on the lipid component of the diet. Carcinogenesis 29: 1415–1420, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davidson LA, Nguyen DV, Hokanson RM, Callaway ES, Isett RB, Turner ND, Dougherty ER, Wang N, Lupton JR, Carroll RJ, Chapkin RS. Chemopreventive n-3 polyunsaturated fatty acids reprogram genetic signatures during colon cancer initiation and progression in the rat. Cancer Res 64: 6797–6804, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davidson LA, Wang N, Ivanov I, Goldsby J, Lupton JR, Chapkin RS. Identification of actively translated mRNA transcripts in a rat model of early-stage colon carcinogenesis. Cancer Prev Res (Phila Pa) 2: 984–994, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davidson LA, Wang N, Shah MS, Lupton JR, Ivanov I, Chapkin RS. n-3 Polyunsaturated fatty acids modulate carcinogen-directed non-coding microRNA signatures in rat colon. Carcinogenesis 30: 2077–2084, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Earle JS, Luthra R, Romans A, Abraham R, Ensor J, Yao H, Hamilton SR. Association of microRNA expression with microsatellite instability status in colorectal adenocarcinoma. J Mol Diagn 12: 433–440, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer 6: 259–269, 2006. [DOI] [PubMed] [Google Scholar]
- 20. Fontana L, Fiori ME, Albini S, Cifaldi L, Giovinazzi S, Forloni M, Boldrini R, Donfrancesco A, Federici V, Giacomini P, Peschle C, Fruci D. Antagomir-17–5p abolishes the growth of therapy-resistant neuroblastoma through p21 and BIM. PLoS One 3: e2236, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gaur A, Jewell DA, Liang Y, Ridzon D, Moore JH, Chen C, Ambros VR, Israel MA. Characterization of microRNA expression levels and their biological correlates in human cancer cell lines. Cancer Res 67: 2456–2468, 2007. [DOI] [PubMed] [Google Scholar]
- 22. Gregorieff A, Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev 19: 877–890, 2005. [DOI] [PubMed] [Google Scholar]
- 23. Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res 36: D154–158, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell 27: 91–105, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gunter MJ, Hayes RB, Chatterjee N, Yeager M, Welch R, Schoen RE, Yakochi L, Schatzkin A, Peters U. Insulin resistance-related genes and advanced left-sided colorectal adenoma. Cancer Epidemiol Biomarkers Prev 16: 703–708, 2007. [DOI] [PubMed] [Google Scholar]
- 26. Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466: 835–840, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hanlon K, Rudin CE, Harries LW. Investigating the targets of MIR-15a and MIR-16–1 in patients with chronic lymphocytic leukemia (CLL). PLoS One 4: e7169, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang JC, Morris QD, Frey BJ. Bayesian inference of MicroRNA targets from sequence and expression data. J Comput Biol 14: 550–563, 2007. [DOI] [PubMed] [Google Scholar]
- 29. Joo M, Shahsafaei A, Odze RD. Paneth cell differentiation in colonic epithelial neoplasms: evidence for the role of the Apc/beta-catenin/Tcf pathway. Hum Pathol 40: 872–880, 2009. [DOI] [PubMed] [Google Scholar]
- 30. Khokhlatchev A, Rabizadeh S, Xavier R, Nedwidek M, Chen T, Zhang XF, Seed B, Avruch J. Identification of a novel Ras-regulated proapoptotic pathway. Curr Biol 12: 253–265, 2002. [DOI] [PubMed] [Google Scholar]
- 31. Kim S, Choi M, Cho KH. Identifying the target mRNAs of microRNAs in colorectal cancer. Comput Biol Chem 33: 94–99, 2009. [DOI] [PubMed] [Google Scholar]
- 32. Kolar SS, Barhoumi R, Callaway ES, Fan YY, Wang N, Lupton JR, Chapkin RS. Synergy between docosahexaenoic acid and butyrate elicits p53-independent apoptosis via mitochondrial Ca2+ accumulation in colonocytes. Am J Physiol Gastrointest Liver Physiol 293: G935–G943, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koon N, Schneider-Stock R, Sarlomo-Rikala M, Lasota J, Smolkin M, Petroni G, Zaika A, Boltze C, Meyer F, Andersson L, Knuutila S, Miettinen M, El-Rifai W. Molecular targets for tumour progression in gastrointestinal stromal tumours. Gut 53: 235–240, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kort EJ, Farber L, Tretiakova M, Petillo D, Furge KA, Yang XJ, Cornelius A, Teh BT. The E2F3-Oncomir-1 axis is activated in Wilms' tumor. Cancer Res 68: 4034–4038, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kren BT, Wong PY, Shiota A, Zhang X, Zeng Y, Steer CJ. Polysome trafficking of transcripts and microRNAs in regenerating liver after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol 297: G1181–G1192, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kutay H, Bai S, Datta J, Motiwala T, Pogribny I, Frankel W, Jacob ST, Ghoshal K. Downregulation of miR-122 in the rodent and human hepatocellular carcinomas. J Cell Biochem 99: 671–678, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lanza G, Ferracin M, Gafa R, Veronese A, Spizzo R, Pichiorri F, Liu CG, Calin GA, Croce CM, Negrini M. mRNA/microRNA gene expression profile in microsatellite unstable colorectal cancer. Mol Cancer 6: 54, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Latham P, Lund EK, Johnson IT. Dietary n-3 PUFA increases the apoptotic response to 1,2-dimethylhydrazine, reduces mitosis and suppresses the induction of carcinogenesis in the rat colon. Carcinogenesis 20: 645–650, 1999. [DOI] [PubMed] [Google Scholar]
- 39. Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120: 15–20, 2005. [DOI] [PubMed] [Google Scholar]
- 40. Lindow M, Jacobsen A, Nygaard S, Mang Y, Krogh A. Intragenomic matching reveals a huge potential for miRNA-mediated regulation in plants. PLoS Comput Biol 3: e238, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maragkakis M, Reczko M, Simossis VA, Alexiou P, Papadopoulos GL, Dalamagas T, Giannopoulos G, Goumas G, Koukis E, Kourtis K, Vergoulis T, Koziris N, Sellis T, Tsanakas P, Hatzigeorgiou AG. DIANA-microT web server: elucidating microRNA functions through target prediction. Nucleic Acids Res 37: W273–W276, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Maroney PA, Yu Y, Fisher J, Nilsen TW. Evidence that microRNAs are associated with translating messenger RNAs in human cells. Nat Struct Mol Biol 13: 1102–1107, 2006. [DOI] [PubMed] [Google Scholar]
- 43. Mayr C, Bartel DP. Widespread shortening of 3′UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell 138: 673–684, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mendes ND, Freitas AT, Sagot MF. Current tools for the identification of miRNA genes and their targets. Nucleic Acids Res 37: 2419–2433, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nakamoto M, Jin P, O'Donnell WT, Warren ST. Physiological identification of human transcripts translationally regulated by a specific microRNA. Hum Mol Genet 14: 3813–3821, 2005. [DOI] [PubMed] [Google Scholar]
- 46. Narita M, Murata T, Shimizu K, Nakagawa T, Sugiyama T, Inui M, Hiramoto K, Tagawa T. A role for cyclic nucleotide phosphodiesterase 4 in regulation of the growth of human malignant melanoma cells. Oncol Rep 17: 1133–1139, 2007. [PubMed] [Google Scholar]
- 47. Navarro D, Agra N, Pestana A, Alonso J, Gonzalez-Sancho JM. The EWS/FLI1 oncogenic protein inhibits expression of the Wnt inhibitor DICKKOPF-1 gene and antagonizes beta-catenin/TCF-mediated transcription. Carcinogenesis 31: 394–401, 2010. [DOI] [PubMed] [Google Scholar]
- 48. Nelson PT, Hatzigeorgiou AG, Mourelatos Z. miRNP:mRNA association in polyribosomes in a human neuronal cell line. RNA 10: 387–394, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ng EK, Tsang WP, Ng SS, Jin HC, Yu J, Li JJ, Rocken C, Ebert MP, Kwok TT, Sung JJ. MicroRNA-143 targets DNA methyltransferases 3A in colorectal cancer. Br J Cancer 101: 699–706, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nicolas FE, Pais H, Schwach F, Lindow M, Kauppinen S, Moulton V, Dalmay T. Experimental identification of microRNA-140 targets by silencing and overexpressing miR-140. RNA 14: 2513–2520, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nikiforova MN, Chiosea SI, Nikiforov YE. MicroRNA expression profiles in thyroid tumors. Endocr Pathol 20: 85–91, 2009. [DOI] [PubMed] [Google Scholar]
- 52. O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature 435: 839–843, 2005. [DOI] [PubMed] [Google Scholar]
- 53. Olsen PH, Ambros V. The lin-4 regulatory RNA controls developmental timing in Caenorhabditis elegans by blocking LIN-14 protein synthesis after the initiation of translation. Dev Biol 216: 671–680, 1999. [DOI] [PubMed] [Google Scholar]
- 54. Orom UA, Lund AH. Experimental identification of microRNA targets. Gene 451: 1–5, 2010. [DOI] [PubMed] [Google Scholar]
- 55. Petersen CP, Bordeleau ME, Pelletier J, Sharp PA. Short RNAs repress translation after initiation in mammalian cells. Mol Cell 21: 533–542, 2006. [DOI] [PubMed] [Google Scholar]
- 56. Pillai RS, Bhattacharyya SN, Artus CG, Zoller T, Cougot N, Basyuk E, Bertrand E, Filipowicz W. Inhibition of translational initiation by Let-7 MicroRNA in human cells. Science 309: 1573–1576, 2005. [DOI] [PubMed] [Google Scholar]
- 57. Reddy BS. Chemoprevention of colon cancer by dietary fatty acids. Cancer Metastasis Rev 13: 285–302, 1994. [DOI] [PubMed] [Google Scholar]
- 58. Reddy BS, Burill C, Rigotty J. Effect of diets high in omega-3 and omega-6 fatty acids on initiation and postinitiation stages of colon carcinogenesis. Cancer Res 51: 487–491, 1991. [PubMed] [Google Scholar]
- 59. Ritchie W, Flamant S, Rasko JE. Predicting microRNA targets and functions: traps for the unwary. Nat Meth 6: 397–398, 2009. [DOI] [PubMed] [Google Scholar]
- 60. Saunders LR, Sharma AD, Tawney J, Nakagawa M, Okita K, Yamanaka S, Willenbring H, Verdin E. miRNAs regulate SIRT1 expression during mouse embryonic stem cell differentiation and in adult mouse tissues. Aging (Albany NY) 2: 415–431, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sengupta P, Xu Y, Wang L, Widom R, Smith BD. Collagen alpha1(I) gene (COL1A1) is repressed by RFX family. J Biol Chem 280: 21004–21014, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sethupathy P, Megraw M, Hatzigeorgiou AG. A guide through present computational approaches for the identification of mammalian microRNA targets. Nat Meth 3: 881–886, 2006. [DOI] [PubMed] [Google Scholar]
- 63. Shahi P, Loukianiouk S, Bohne-Lang A, Kenzelmann M, Kuffer S, Maertens S, Eils R, Grone HJ, Gretz N, Brors B. Argonaute–a database for gene regulation by mammalian microRNAs. Nucleic Acids Res 34: D115–D118, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sinha AU, Kaimal V, Chen J, Jegga AG. Dissecting microregulation of a master regulatory network. BMC Genomics 9: 88, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Slaby O, Svoboda M, Michalek J, Vyzula R. MicroRNAs in colorectal cancer: translation of molecular biology into clinical application. Mol Cancer 8: 102, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Smith PG, Wang F, Wilkinson KN, Savage KJ, Klein U, Neuberg DS, Bollag G, Shipp MA, Aguiar RC. The phosphodiesterase PDE4B limits cAMP-associated PI3K/AKT-dependent apoptosis in diffuse large B-cell lymphoma. Blood 105: 308–316, 2005. [DOI] [PubMed] [Google Scholar]
- 67. Song B, Wang Y, Titmus MA, Botchkina G, Formentini A, Kornmann M, Ju J. Molecular mechanism of chemoresistance by miR-215 in osteosarcoma and colon cancer cells. Mol Cancer 9: 96, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sood P, Krek A, Zavolan M, Macino G, Rajewsky N. Cell-type-specific signatures of microRNAs on target mRNA expression. Proc Natl Acad Sci USA 103: 2746–2751, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sun M, Estrov Z, Ji Y, Coombes KR, Harris DH, Kurzrock R. Curcumin (diferuloylmethane) alters the expression profiles of microRNAs in human pancreatic cancer cells. Mol Cancer Ther 7: 464–473, 2008. [DOI] [PubMed] [Google Scholar]
- 70. Tanigawa Y, Yakura R, Komiya T. The bHLH transcription factor Tcf12 (ME1) mRNA is abundantly expressed in Paneth cells of mouse intestine. Gene Expr Patterns 7: 709–713, 2007. [DOI] [PubMed] [Google Scholar]
- 71. Tavani A, Pelucchi C, Parpinel M, Negri E, Franceschi S, Levi F, La Vecchia C. n-3 polyunsaturated fatty acid intake and cancer risk in Italy and Switzerland. Int J Cancer 105: 113–116, 2003. [DOI] [PubMed] [Google Scholar]
- 72. Thermann R, Hentze MW. Drosophila miR2 induces pseudo-polysomes and inhibits translation initiation. Nature 447: 875–878, 2007. [DOI] [PubMed] [Google Scholar]
- 73. Trimarchi JM, Lees JA. Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol 3: 11–20, 2002. [DOI] [PubMed] [Google Scholar]
- 74. Tsang WP, Kwok TT. The miR-18a* microRNA functions as a potential tumor suppressor by targeting on K-Ras. Carcinogenesis 30: 953–959, 2009. [DOI] [PubMed] [Google Scholar]
- 75. Vanamala J, Glagolenko A, Yang P, Carroll RJ, Murphy ME, Newman RA, Ford JR, Braby LA, Chapkin RS, Turner ND, Lupton JR. Dietary fish oil and pectin enhance colonocyte apoptosis in part through suppression of PPARdelta/PGE2 and elevation of PGE3. Carcinogenesis 29: 790–796, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Venturini L, Battmer K, Castoldi M, Schultheis B, Hochhaus A, Muckenthaler MU, Ganser A, Eder M, Scherr M. Expression of the miR-17–92 polycistron in chronic myeloid leukemia (CML) CD34+ cells. Blood 109: 4399–4405, 2007. [DOI] [PubMed] [Google Scholar]
- 77. Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA 103: 2257–2261, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Vos MD, Clark GJ. RASSF family proteins and Ras transformation. Methods Enzymol 407: 311–322, 2006. [DOI] [PubMed] [Google Scholar]
- 79. Wang H, Huang S, Shou J, Su EW, Onyia JE, Liao B, Li S. Comparative analysis and integrative classification of NCI60 cell lines and primary tumors using gene expression profiling data. BMC Genomics 7: 166, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang Y, Lee AT, Ma JZ, Wang J, Ren J, Yang Y, Tantoso E, Li KB, Ooi LL, Tan P, Lee CG. Profiling microRNA expression in hepatocellular carcinoma reveals microRNA-224 up-regulation and apoptosis inhibitor-5 as a microRNA-224-specific target. J Biol Chem 283: 13205–13215, 2008. [DOI] [PubMed] [Google Scholar]
- 81. West NJ, Clark SK, Phillips RK, Hutchinson JM, Leicester RJ, Belluzzi A, Hull MA. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut 59: 918–925, 2010. [DOI] [PubMed] [Google Scholar]
- 82. Whelan J, McEntee MF. Dietary (n-6) PUFA and intestinal tumorigenesis. J Nutr 134: 3421S–3426S, 2004. [DOI] [PubMed] [Google Scholar]
- 83. Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 11: 228–234, 2009. [DOI] [PubMed] [Google Scholar]
- 84. Wu H, Ye C, Ramirez D, Manjunath N. Alternative processing of primary microRNA transcripts by Drosha generates 5′ end variation of mature microRNA. PLoS One 4: e7566, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yamada Y, Yoshimi N, Hirose Y, Kawabata K, Matsunaga K, Shimizu M, Hara A, Mori H. Frequent beta-catenin gene mutations and accumulations of the protein in the putative preneoplastic lesions lacking macroscopic aberrant crypt foci appearance, in rat colon carcinogenesis. Cancer Res 60: 3323–3327, 2000. [PubMed] [Google Scholar]
- 86. Yamakuchi M, Lotterman CD, Bao C, Hruban RH, Karim B, Mendell JT, Huso D, Lowenstein CJ. P53-induced microRNA-107 inhibits HIF-1 and tumor angiogenesis. Proc Natl Acad Sci USA 107: 6334–6339, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res 18: 350–359, 2008. [DOI] [PubMed] [Google Scholar]
- 88. Zinovyev A, Morozova N, Nonne N, Barillot E, Harel-Bellan A, Gorban AN. Dynamical modeling of microRNA action on the protein translation process. BMC Syst Biol 4: 13, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.