Abstract
Background
Lymphedema (LE) may be a complication following surgical resection and radiation treatment in a number of cancer types, and is especially debilitating in regions where treatment options are limited. While upper and lower extremity LE may be effectively treated with manual lymphatic drainage (MLD) therapies and devices that use compression to direct proximal flow of lymph, head and neck LE is more challenging.
Methods and Results
Herein, we describe the compassionate use of an investigatory technique of near-infrared (NIR) fluorescence imaging to understand the lymphatic anatomy and function, help direct MLD, and use 3D surface profilometry to monitor response to therapy in a subject suffering head and neck LE following surgery and radiation treatment.
Conclusion
NIR fluorescence imaging provides a mapping of functional lymph vessels for direction of efficient MLD therapy in the head and neck. Additional studies are needed to assess efficacy of MLD therapy when directed by NIR fluorescence imaging.
Keywords: fluorescence imaging, lymphedema, manual lymph drainage
Introduction
Cancer-related lymphedema (LE) is a complication that arises following irradiation and/or surgical excision of lymph nodes. This acquired form of LE is typically associated with upper and lower extremities in survivors of breast, melanoma, cervical, endometrial, vulvar, prostate, penile, and soft tissue cancers1 in whom symptoms may present days to years after the initial insult. Sometimes the patient does not have any symptoms of LE until after a minor challenge such as a trivial injury, insect bite, or local infection. LE is manifested by irresolvable edema and, if left untreated, can progress to fibrosis, pain/paresthesias, extreme disfigurement, reduced immune responsiveness, and rarely to the development of lymphangiosarcoma.
Currently, the diagnosis of LE is mainly clinical. There are no routine imaging studies readily available to image the lymphatic vessels and function. Traditionally the gold standard to image the lymphatic vessels has been direct lymphangiography (DL) using an oil-soluble contrast agent. This test consists of the canulation of a distal lymphatic vessel under direct visualization through an incision made on the skin that allows for dissection and exposure of a lymphatic vessel. Many experts belief that this technique poses a risk of worsening LE in part because of the manipulation of the already diminished or compromised lymphatics and in part due to the high osmolarity of the contrast agent. Indirect lymphangiography (IL) is less invasive as it consists of the acquisition of X-Ray or MRI images after intradermal injection of a water-soluble contrast agent. This technique does not require the dissection and cannulation of the lymphatic vessel but requires intradermal administration of substantial volumes of contrast agent that is not currently available in the US. Nuclear lymphoscintigraphy can be of value in some patients but it does not typically enable resolution of the lymphatic anatomy. Near infrared (NIR) fluorescence imaging of the lymphatics is an emerging technology that has allowed us to simultaneously study the lymphatic structure, anatomy and function in patients with LE of the upper and lower extremities with small volumes and microdosages of contrast agents 2, 3 but has not been used previously to characterize LE of the head and neck.
Understanding the lymphatic anatomy can be important to help treat patients with LE. Currently, there is no cure for LE. The accepted method to manage LE in upper and lower extremities is through the use of complete decongestive therapy (CDT), which includes manual lymphatic drainage (MLD), compression bandaging, therapeutic exercise, and meticulous skin care. Pneumatic compression devices (PCDs) can also be used as part of CDT and typically consist of garments comprised of circumferential chambers that, when sequentially inflated and deflated along the length of the limb, tend to push lymph and extravascular fluids proximally towards functional draining basins 4. Therapeutic efficacy is typically measured by change in limb volume as indicated by circumferential limb measurements or limb water displacement. Unfortunately, when LE involves body areas other than the extremities, such as the cervical and facial areas as in the case of head and neck cancer survivors, several difficulties arise: (i) bandaging and PCD usage are not feasible; (ii) conventional measurements of reduction in tissue volume are difficult; and (iii) the lymphatic drainage pathways from the affected area are highly variable 5, presenting difficulties for efficient direction of MLD therapy.
Our collaborative basic science/technology (I-CT, JCR, KEA, MVM, and EMS) and clinical (EAM, LAS, CEF, RG) teams have combined to develop and deploy quantitative, near-infrared (NIR) fluorescence function imaging for assessment of normal and abnormal lymphatic function and architecture in limbs of normal and LE subjects. 2, Herein, we present a single case using NIR fluorescence imaging to map the lymphatic drainage of a patient suffering from head and neck LE and unique measurements to assess to the efficacy of treatment.
Case Report
The protocol used for this compassionate use study was approved under a single patient combinational investigational new drug application (IND 105,824) for the off-label use of indocyanine green (ICG) as a NIR fluorescent contrast agent. The HIPPA-compliant study was approved by the Institutional Review Boards (IRBs) at the University of Texas Health Science Center and Memorial Hermann Hospital, where the study was conducted. A 50-year-old African-American male subject was referred to the Memorial Hermann Center for Lymphedema Management for his LE, provided informed consent, and was enrolled for imaging in July 2009. He has since provided permission for his medical history and images to be presented herein. The subject's past medical history was significant for right mandibular gingival and left ventral tongue squamous cell carcinoma, T4N0M0 and T2N0M0 respectively. He had been treated at another institution with surgery, including hemimandibulectomy and hemiglossectomy, radiation and chemotherapy in 2005 and 2008. Patient was diagnosed with recurrence of his cancer soon after completion of the study. Our team was not involved in any part of his cancer treatments and discussion of the cancer management is not within the scope of this paper. Over the ensuing six months after radiation he developed radiation fibrosis of the neck with significant facial edema. His LE involved the eyelids, tongue, face and cheeks. Dysphagia necessitated the placement of a feeding tube. The facial edema was minimally reduced by MLD, but intraoral swelling was not improved. Surgical scars and fibrosis appeared to obstruct most obvious lymphatic outflow tracts from the face. It was postulated that lymphatic mapping might allow MLD therapy to be focused on patent channels thus improving his response to therapy.
Immediately after four intradermal injections of ICG of 25 μg in 0.1 cc each (demarked as injections (1) through (4) in Figure 1), NIR fluorescence images were simultaneously acquired on the head and neck using the imaging system described elsewhere 2,3. Five subsequent injections at sites (5) through (9) occurred approximately 1.5 hours later. Injection sites were covered with black vinyl tape to avoid oversaturation of the camera system by ICG. MLD using the technique developed by Vodder 6 was performed 60 minutes after the start of imaging by a certified LE therapist during the imaging sections; neither oils nor lotions were used during MLD therapy. Profilometry was performed to produce three-dimensional (3D) color facial surface images (3dMDface™ System - 3dMD LLC, Atlanta, GA) after NIR imaging. These images provided a precise roadmap of the facial structure and scar around the neck (dashed lines in Figure 1) and in the four subsequent weeks, were used to document a change in facial volumes to track therapeutic efficacy as part of regular standard of care.
Figure 1.
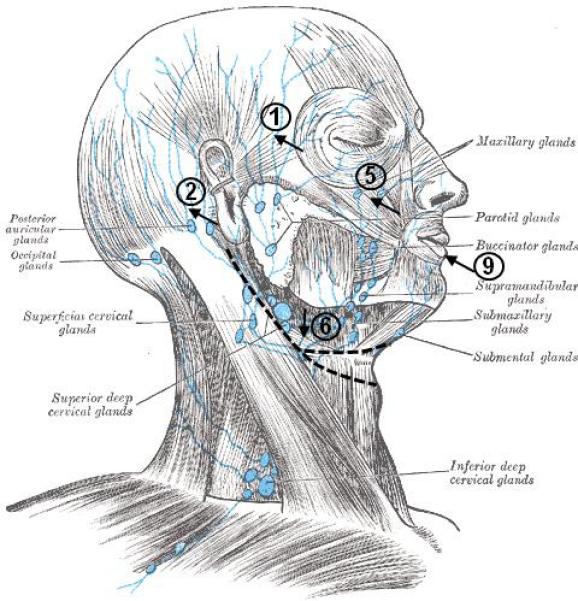
Injection sites of fluorescence contrast agent on the right side. Injection sites (3) and (4), not showing in the figure, are on the left side and symmetrical to (1) and (2): (7) and (8) are symmetrical to (5) and (6). Also the incision lines from previous surgeries are marked. Adapted from Gray's anatomy of the human body (http://www.bartleby.com/107/illus602.html)
No adverse events were associated with the imaging agent or devices. The subject wore eye protection against the unlikely event of damage from the <1.9 mW/cm2 laser diode illumination. Owing to the advanced stage of LE, the subject was unable to open his mouth wide enough for imaging lymphatics within the oral cavity.
Architecture and flow of lymphatics in head and neck
NIR fluorescence images provided the visualization of tortuous, abnormal lymphatic structures in the head and neck region. Figure 2 shows a tortuous lymphatic channel traversing across the midline and connecting lymph basins on the right and left sides of the neck. This vessel may have formed through the process of lymphangiogenesis in response to lymphatic obstruction or damage associated with the surgery or irradiation. Unfortunately, imaging was not conducted simultaneously with the agent administration; therefore, the direction from which the vessel filled with ICG could not be identified. This finding is interesting and, if found on normal subjects, it could be of crucial importance as it has been traditionally believed that lymphatic drainage is within strictly demarcated watersheds delineated by an imaginary horizontal line at the level of the umbilicus and crossed by the midline vertically. For the first time we are able to demonstrate lymphatic drainage crossing from one watershed into another which supports the frequently refuted theory that massaging across the midline during MLD is of no use. Demonstration that lymph travels to areas that are not typically considered the natural draining pattern might have serious implications in the staging of cancer as one could hypothesize that spreading of a tumor could occur directly into contralateral lymphatic basin. Further studies are warranted by our results.
Figure 2.
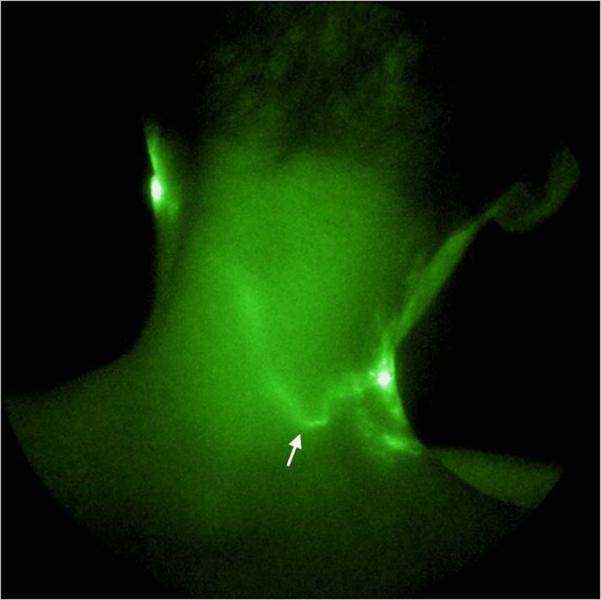
NIR fluorescence image of back of neck showing a lymphatic channel (indicated by arrow) across the midline.
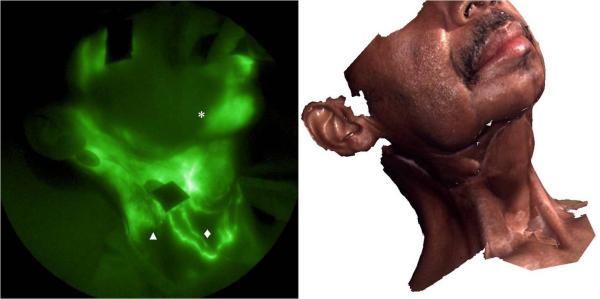
Figure 3 shows the lymphatic structure on the right side of the subject's face and neck. Propulsive lymphatic flow was observed in the lymphatic channel draining from the injection site (5) below the eye toward the ear (refer to supplement video 1) as we have previously detected in the limbs of normal subjects. However, dense capillary networks and extravascular accumulation of ICG were observed in the region between ear and upper neck. Well-defined but tortuous lymphatic channels traversed the line of the surgical incision on the neck and drained to the jugular notch. This is also a finding that could change the way MLD is performed as it has been taught as a dogma that lymph vessels do not cross scars and that thus massaging across a scar was of no benefit in order to decongest a lymphedematous area. Unfortunately, we have not conducted similar imaging on the head and neck of normal subjects and cannot yet make comparison to normal lymphatic architecture and propulsive function. However, in subjects with LE of the limbs, we have likewise observed tortuous functional vessels as well as ICG dye accumulation away from the site of injection which may be associated with “leaky” lymphatic vessels.
Figure 3.
NIR fluorescence image of right face and neck. Lymphatic capillary networks (▲), extravascular lymph leakage (*), and some well-defined lymphatic channels (◆) were seen. 3D facial image taken immediately after NIR fluorescence imaging is shown on the right for anatomical reference.
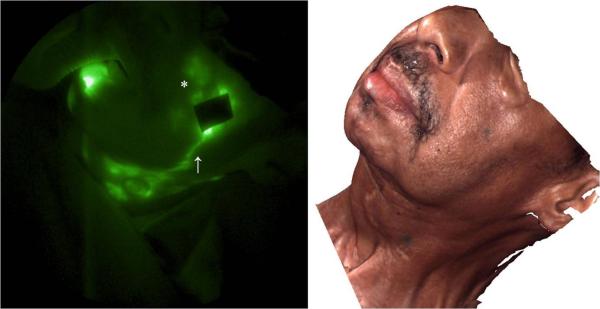
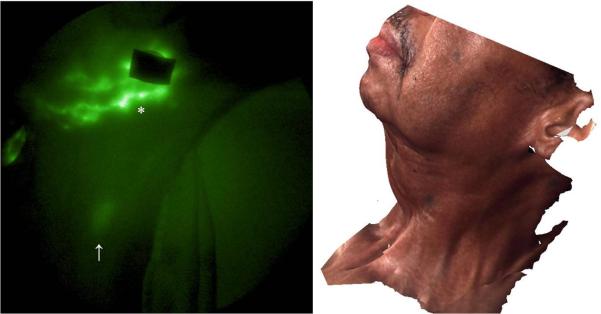
Figure 4 shows the lymphatic structure on the left side of the face and neck. A lymphatic channel draining lymph from the injection site (6) below the eye toward the neck was observed and propulsive flow was imaged in a lymphatic channel draining from the injection site (3) toward ear (refer to supplement video 2). Extravascular accumulation of ICG was seen from injection site (4) to the neck as shown in Figure 5 and a cervical node was identified from the image. The lack of symmetric, functionally lymphatic architecture may have resulted from the process of lymphangiogenesis or co-opting of otherwise non-functional lymph channels in response to obstruction. Longitudinal imaging studies would be necessary to understand the etiology of the lymphatic structure and architecture observed following surgery and radiation therapy.
Figure 4.
NIR fluorescence image of the left side of the face and neck. Extravascular lymph leakage (*)and a well-defined lymphatic channel (↑) were observed. The corresponding 3D facial image is shown on the right.
Figure 5.
NIR fluorescence image of right side of neck. Extravascular lymph leakage (*) and a cervical lymph node (↑) were observed. The corresponding 3D facial image is shown on the right.
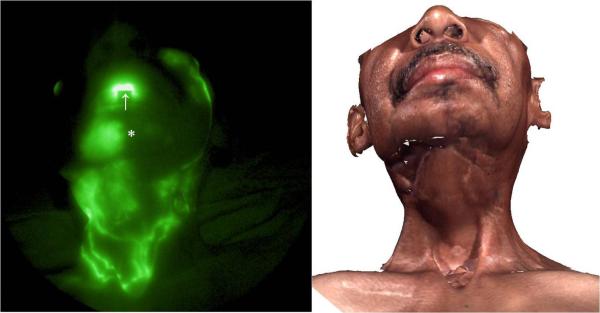
In the absence of NIR imaging, lymph flow from this subject's face was assumed to drain toward the back of the neck and toward the axilla because of the disruption owing to surgery and radiation therapy in the front of the neck. Figure 6 shows the frontal view of the lymphatic structure in the cervical region as well as extravascular accumulation of ICG at the chin and right side of the upper neck and in a smaller area on the left side of the upper neck. Several well-defined channels below the incision line on the lower neck near jugular notch were also observed, suggesting that drainage across the scar lines did occur in cervical regions. No fluorescence was visualized in the axilla by 3 hours after ICG injection, suggesting that drainage from the injection sites occurred primarily in the cervical regions during this time. Consequently, the mapping of functional cervical lymphatic vessels enabled MLD therapy moving fluid toward these functional vessels and cervical and subclavian lymph nodes (Figure 7).
Figure 6.
NIR fluorescence image of frontal view of face and neck. Extravascular lymph leakage (*) was observed along with well-defined lymphatic channels at the lower portion of the neck. The arrow points to the injection site on the lower lip. The corresponding 3D facial image is shown on the right.
Figure 7.
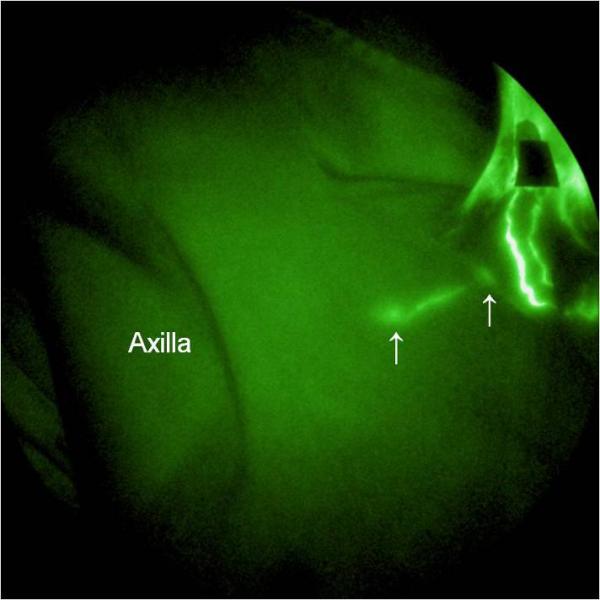
NIR fluorescence image of right clavicular region. A lymphatic channel across the collar bone from a cervical node toward a subclavian node was seen. The lymph nodes are indicated by arrows.
Longitudinal assessment of edema
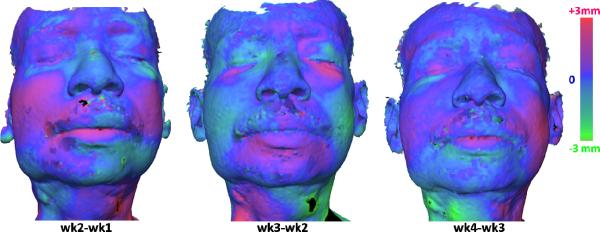
Results of longitudinal assessment of edema using profilometry on the face are illustrated in Figure 8. By taking a difference of the tissue surface maps, an increase in swelling is denoted by a positive difference (rendered in magenta color) while a reduction is indicated by a negative distance (rendered by green color). Increased swelling occurred in the cheek from week 1 to week 2, but subsequently decreased by week 3 possibly owing to therapy. The swelling returned in the cheek in week 4, commensurate with a relapse of metastatic disease within the oral cavity. At this point, the case study was suspended.
Figure 8.
Results of longitudinal assessment of edema using profilometry on the face. The color rendering shows the distances between the surfaces obtained from two consecutive weeks, (a) week 2 compared to week 1, (b) week 3 compared to week 2, and (c) week 4 compared to week 3. Increased edema is presented in magenta and decreased edema is presented in green.
Discussion
Herein, we present a compassionate use case of NIR fluorescence imaging to provide mapping of functional lymph vessels for direction of efficient MLD therapy in the head and neck. Unfortunately, the subject encountered recurrent disease, not allowing long-term follow-up. Nonetheless, the ability to track functional vessels to direct therapy has been demonstrated.
Although we were unable to visualize newly recruited lymphatic vessels in the single imaging session, we were able to track changes in edema using surface profilometry. Given the small doses of ICG employed and its normally rapid clearance from the body, repeated dosing for multiple, longitudinal NIR fluorescence imaging sessions could be used to track progress in increasing contractile lymphatic activity and recruitment of vessels. Additional patients need to be imaged in the manner described herein in order to evaluate the reproducibility and incidence of our findings. In expanded studies, the imaging technique may also provide new insights on the impact of cancer metastasis and its treatment on regional lymphatic structure and function.
Supplementary Material
Supplemental Material
Video 1: Pulsing at right eye.
Video 2: Pulsing at left eye.
Acknowledgment
This work was supported in parts by the Longaberger Foundation through an American Cancer Society Research Scholar Grant (RSG-06-213-01-LR) and the National Institutes of Health (R01 HL092923 and U54 CA136404). The authors acknowledge Ms. Lisa Kinder from Memorial Hermann Center for Lymphedema Management for performing MLD in this study.
References
- 1.Rockson SG, Rivera KK. Estimating the population burden of lymphedema. Ann N Y Acad Sci. 2008;1131:147–154. doi: 10.1196/annals.1413.014. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen JC, Tan IC, Marshall MV, Fife CE, Sevick-Muraca EM. Lymphatic imaging in humans with near-infrared fluorescence. Curr Opin Biotechnol. 2009 Feb;20(1):74–82. doi: 10.1016/j.copbio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall MV, Rasmussen JC, Tan I-C, et al. Near-Infrared Fluorescence Imaging in Humans with Indocyanine Green:A Review and Update. The Open Surgical Oncology Journal. 2010:2. doi: 10.2174/1876504101002010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayrovitz HN. Interface pressures produced by two different types of lymphedema therapy devices. Phys Ther. 2007 Oct;87(10):1379–1388. doi: 10.2522/ptj.20060386. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds HM, Smith NP, Uren RF, Thompson JF, Dunbar PR. Three-dimensional visualization of skin lymphatic drainage patterns of the head and neck. Head Neck. 2009 Oct;31(10):1316–1325. doi: 10.1002/hed.21089. [DOI] [PubMed] [Google Scholar]
- 6.Ko DS, R Lerner, G Klose, Cosimi AB. Effective treatment of lymphedema of the extremities. Arch Surg. 1998 Apr;133(4):452–458. doi: 10.1001/archsurg.133.4.452. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material
Video 1: Pulsing at right eye.
Video 2: Pulsing at left eye.