Abstract
In order to achieve an optimal biological activity and desired pharmacokinetic profiles, a dithiocyclopeptide linker was designed for in vivo release of protein domains from a recombinant fusion protein. This novel in vivo cleavable disulfide linker, based on a dithiocyclopeptide containing a thrombin-sensitive sequence and an intra-molecular disulfide bond, was inserted between transferrin and granulocyte colony-stimulating factor recombinant fusion protein domains. After expression of the fusion protein, G-C-T, from HEK293 cells, thrombin treatment in vitro generated a fusion protein linked via a reversible disulfide bond that was quickly cleaved in vivo, separating the protein domains. After release from the fusion protein, free G-CSF exhibited an improved biological activity in a cell proliferation assay. Although reversible disulfide bonds are commonly used in protein chemical conjugation methods, to our knowledge this report is the first example of the construction of a recombinant fusion protein with a disulfide linkage for the release of the functional domain. This linker design can be adapted to diverse recombinant fusion proteins where in vivo separation of protein domains is required to achieve an improved therapeutic effect, and a desirable pharmacokinetic profile and biodistribution, of the functional domain.
Keywords: recombinant fusion protein, disulfide linker, cleavable linker
Introduction
Recombinant fusion proteins have become an important class of molecules in numerous fields of biotechnology, including protein engineering and purification, drug targeting and delivery, and immunology. By genetically fusing two or more genes together, the resulting fusion protein can attain multiple functional properties derived from each of its components (1). Successfully constructed fusion proteins require not only the desired component proteins, but also suitable linkers to connect the protein domains. Linkers in fusion proteins generally consist of stable peptide sequences, including the glycine-serine linker (GGGGS)n and α-helix-forming peptide linkers, such as A(EAAAK)nA (n=2-5), which can provide structure flexibility, improve protein stability, or increase biological activity (2-5). However, stable peptide linkers do not allow for the separation of the two fusion protein domains in vivo, and have several limitations including steric hindrance between two functional domains, altered biodistribution and metabolism of the protein moieties due to interference with each other, and incorrect folding of the fusion protein (6).
To overcome some of these potential pitfalls in stable peptide linkers, we designed a fusion protein linkage utilizing the reversible nature of the disulfide bond (7). There are numerous examples of application of a disulfide linkage between two proteins in drug delivery, either by using cysteine amino acid residues in proteins, or by employing hetero- or homo-bifunctional linkers (8-10). This approach requires chemical conjugation of the two separate proteins, since recombinant technologies require expression of fusion proteins as one single polypeptide chain. However, the major obstacle for the chemical conjugation approach is that the composition and size of the final product can be heterogeneous, which is unacceptable for therapeutic use. In comparison, the novel recombinant fusion protein approach described in this report offers the significant advantage in the generation of a precisely constructed, homogenous product. This cleavable dithiocyclopeptide linker contains a thrombin-sensitive sequence, as well as an intra-molecular disulfide bond formed between two cysteine residues of the linker. Treatment in vitro with thrombin results in cleavage of the thrombin-sensitive sequence, while the disulfide linkage between the two domains of the recombinant fusion protein remains (Figure 1(a)). Conceivably, the disulfide linkage will be reduced in vivo, allowing for the protein domains to be separated and regain their individual characteristics such as biological activity, biodistribution and/or metabolism. In this paper, we use a fusion protein containing granulocyte colony stimulating factor (G-CSF) and transferrin (Tf) (11) to demonstrate the successful construction and the in vivo cleavability of a dithiocyclopeptide linker between two domains of a recombinant fusion protein.
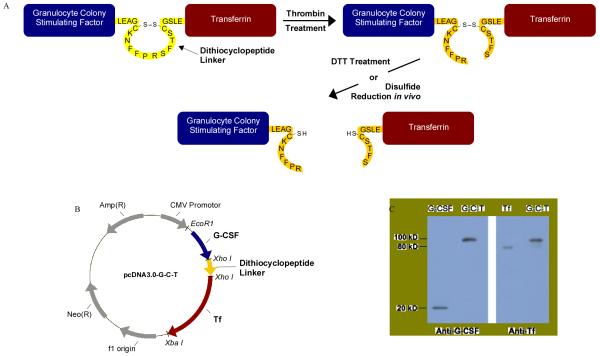
Figure 1. Construction and Expression of G-C-T Fusion Protein.
(a) Design of the recombinant fusion protein. The G-C-T fusion protein is linked via a dithiocyclopeptide linker based on the structure of somatostatin modified to contain a thrombin-specific sequence, PRS. Two cysteinyl-residues on somatostatin naturally form a disulfide bond. (b) Plasmid encoding the G-C-T fusion protein was constructed based on the mammalian expression vector pcDNA3.0. (c) Recombinant G-C-T was analyzed by western blot using anti-human G-CSF or serum Tf primary antibody, with horseradish peroxidase-conjugated anti-rabbit or anti-goat, respectively, IgG secondary antibody. The peroxidase activity was detected by enhanced chemiluminescence using the ECL substrate system.
Materials and Methods
Cell lines
HEK293 cells, purchased from ATCC (Manassas, VA), were grown as monolayers in Dulbecco’s Modified Eagle’s medium (DMEM) with 10% (vol/vol) fetal bovine serum (FBS) at 37 °C in 5% CO2. Protein-free, chemically defined CD293 medium, obtained from Invitrogen (Carlsbad, CA), was used for protein expression after transfection of HEK293 cells. Murine myeloblastic NFS60 cells, kindly provided by Dr. James Ihle (St. Jude Children’s Research Hospital, Memphis, TN), were grown in RPMI medium 1640 with 10% (vol/vol) FBS and 0.1 ng/mL recombinant mouse IL-3.
Animals
Male CF-1 mice (18-20 g) from Charles River Laboratories (Kingston, NY) were used for in vivo cleavability studies. The protocol of animal experiments in this study has been approved by the Institutional Animal Care and Use Committee (IACUC) at USC. The animals were handled in accordance with the “Guide for the Care and Use of Laboratory Animals” (NIH Publication No. 85-23, revised 1985). The animals were fed with a standard laboratory rodent diet (Purina Mills, St. Louis, MO) and housed on a 12-h light and 12-h dark cycle with room temperature maintained at 22 ± 3 °C and relative humidity at 50 ± 20%.
Construction of G-CSF-dithiocyclopeptide-Tf (G-C-T) plasmid
G-CSF-Tf fusion protein was constructed into the mammalian expression vector pcDNA3.0 (Invitrogen) as previously described (11). A dipeptide linker, Leu-Glu, was introduced between G-CSF and Tf due to the cloning site XhoI. The design of dithiocyclopeptide linker was based on the structure of the cyclopeptide, somatostatin, with the replacement of amino acids 8-10, WKT, by a thrombin-specific sequence, PRS. Two cysteine residues on somatostatin, Cys-3 and Cys-14, naturally form a disulfide bond. The oligonucleotides encoding the dithiocyclopeptide linker, synthesized by Invitrogen, were inserted between G-CSF and Tf by using the XhoI cloning site. The correct sequence was confirmed by DNA sequencing.
Expression of the recombinant G-C-T fusion protein
HEK293 cells were seeded into T175 cm2 cell culture flasks the day before transfection in order to achieve 60% - 80% confluence at the time of transfection. Serum-free DMEM medium was used during transfection. The plasmids encoding the G-C-T fusion protein were transfected into HEK293 cells by using linear polyethylenimine (Polysciences, Inc, Warrington, PA) as described (12). Following a 5-hour transfection, CD293 medium was used for protein expression for an additional 4 day incubation period. The conditioned medium containing the G-C-T fusion protein was centrifuged at 3000 g for 10 minutes to remove the cell debris. The medium was then concentrated by Tangential Flow Filtration (TFF) (Millipore, Billerica, MA) to a final volume of 20 mL. Buffer exchange was conducted by TFF to change the expression medium to the storage buffer (50 mg/mL mannitol, 0.1 mg/ml tween-80, 50 mM phosphate, pH 7). The fusion protein was stored at − 80 °C until use.
Characterization of the G-C-T fusion protein by Western blotting
To determine the presence of G-CSF and Tf domain in the fusion protein, anti-G-CSF and anti-Tf Western blotting were performed. Antibody against human G-CSF (ProSci, San Diego, CA) was used as primary antibody and horseradish peroxidase-conjugated anti-rabbit IgG antibody (Sigma, St. Louis, MO) was used as secondary antibody. Antibody against human serum Tf (Sigma) was used as primary antibody and horseradish peroxidase-conjugated anti-goat IgG antibody (Sigma) was used as secondary antibody. The peroxidase activity was detected by enhanced chemiluminescence using the ECL substrate system (GE Healthcare, Piscataway, NJ).
Characterization of the dithiocyclopeptide linker by Western blotting
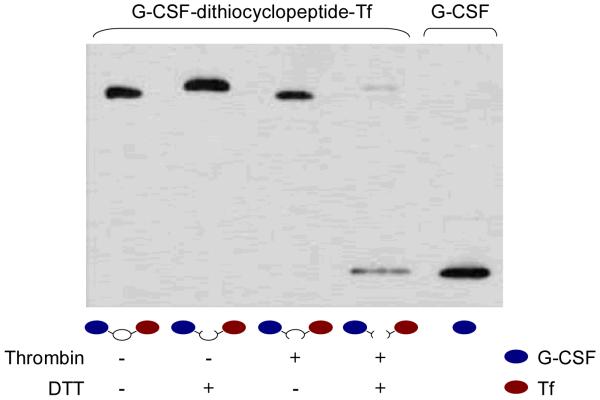
To test whether the correct dithiocyclopeptide linker formed in G-C-T fusion protein as design, the fusion protein was first treated by thrombin in vitro. Thrombin from bovine plasma (Sigma) was added to the fusion protein at 10 units/mL, and incubated at 20 °C for 16 hrs to cleave the dithiocyclopeptide linker. The thrombin-treated fusion protein, which has a disulfide bond linker, is denoted as G-S-S-T. The protein samples, with or without thrombin treatment, were boiled in reducing or non-reducing loading buffer. The reducing loading buffer contains 100 mM dithiothreitol (DTT) to reduce the disulfide bond linkage in the fusion protein. These samples were subjected to anti-G-CSF Western blotting analysis as described above.
NFS-60 proliferation assay for in vitro biological activity
In order to release free G-CSF from Tf, the G-C-T fusion protein was first treated by thrombin in vitro as described above, and then incubated with 25 mM DTT at 37 °C for 15 minutes. The reduced fusion protein was then subjected to a 100-fold dilution in RPMI 1640 medium with 10% FBS to neutralize the DTT. The thrombin and DTT treated fusion protein, as well as the untreated fusion protein, were further diluted to the desired concentration and was used to stimulate the proliferation of the NFS-60 cells as previously described (11). Briefly, NFS-60 cells were washed by RPMI 1640 medium with 10% FBS for 3 times to wash off the IL-3 that was contained in the culture medium.
The cells were then seeded to 96-well plates at a density of 105 cells per well. Following the addition of serially diluted fusion protein samples, the cells were incubated at 37 °C in a 5% CO2 incubator for 48 hours. An MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was subsequently performed incubating the cells in phenol red-free RPMI medium 1640 with 1 mg/mL MTT for 4 hours. The formazan crystals that formed were then dissolved in isopropanol, and the absorbance was measured at 570 nm using a TECAN GENios Plus microplate reader.
In vivo release of free G-CSF from G-S-S-T
The G-C-T fusion protein was pre-treated with thrombin as described above, and the samples were subjected to a 4-fold dilution prior to administration. G-S-S-T or G-C-T was administered intravenously to CF1 mice via the tail vein at a dose of 4 mg/kg. After administration, blood was collected from the saphenous vein as described by Hem et al. (13) at the desired time points. The collected blood was mixed with heparin to prevent clotting, and was subsequently centrifuged at 500 g for 20 minutes to remove the blood cells. The collected plasma samples from 3 mice were pooled together and then analyzed by non-reducing SDS-PAGE followed by anti-G-CSF Western blot as described above.
Results and Discussion
G-C-T fusion protein was successfully constructed and expressed
In contrast to the rapid progress in recombinant fusion protein application, the rational design of linkers in fusion proteins seems to lag behind. It has become more and more evident that with the suitable design of linkers, fusion proteins can achieve optimal biological activity and preferred structures. In order to address these concerns, we produced a recombinant disulfide-linked fusion protein by insertion of a dithiocyclopeptide linker in between G-CSF and Tf protein domains, resulting in a fusion protein, G-C-T, containing both a thrombin-sensitive cleavage site and a disulfide bond (Fig 1(b)). Following transfection and concentration of G-C-T from HEK293 cells, Western blotting analysis demonstrated that the 100 kDa protein could be detected by anti-G-CSF and anti-Tf antibody, confirming the fusion protein contained both G-CSF and Tf domains (Fig 1(c)).
The expression level of this dithiocyclopeptide-linked fusion protein was about 50% of that of the fusion protein without a linker, 1.2 mg/L vs. 2.5 mg/L as a typical yield. However, in human growth hormone-transferrin fusion protein (14), we found that this dithiocyclopeptide linker had a comparable expression level to the non-linker fusion protein (data not shown). Therefore, it is likely that the effect of the cyclopeptide linker on the expression level of a fusion protein may depend on the nature of the protein domains as observed in other peptide linkers (2). In the case of G-CSF-Tf, the expression level is only slightly decreased.
The dithiocyclopeptide linker is correctly formed in G-C-T fusion protein
To test the correct formation of the dithiocyclopeptide linker in G-C-T, the fusion protein was treated with thrombin and/or DTT to cleave the thrombin-sensitive and disulfide linkage, respectively. As shown in Fig. 2, free G-CSF was only released from G-C-T after treatment with both thrombin and DTT, demonstrating the formation of both linkages. Furthermore, fusion protein aggregates were not detected in the Western blot, indicating that there were only intramolecular, but not intermolecular, disulfide bonds formed. Therefore, these data show that the disulfide bond was spontaneously formed between the two cysteine residues on the linker, but not with those in the protein domains. In addition, the thrombin-sensitive sequence on the cyclic linker was recognized and cleaved by thrombin in vitro to break the stable peptide bond. By this design, we were able to create a disulfide linkage between two functional domains using recombinant fusion protein technology, a feat previously requiring the use of chemical conjugation methods of two separate proteins. We were also able to successfully generate another dithiocyclopeptide linked fusion protein, growth hormone-transferrin, which showed similar results (data not shown).
Figure 2. Characterization of recombinant G-C-T.
G-C-T was treated with thrombin (10 units/mL) and incubated at 20 °C for 16 hrs to generate G-S-S-T. The protein samples, with or without thrombin treatment, were boiled in reducing (containing 100 mM DTT), or non-reducing loading buffer, and analyzed by anti-G-CSF Western blotting as described in the Materials and Methods section.
G-C-T has improved in vitro biological activity with the release of free G-CSF
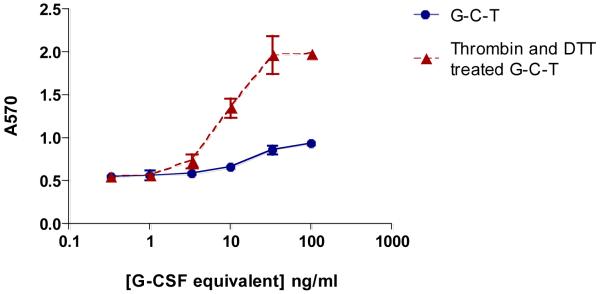
The in vitro G-CSF biological activity of the fusion protein was determined by NFS-60 cell proliferation assay (11). The cells were incubated with untreated G-C-T, and with G-C-T pre-treated with thrombin and reduced by DTT to mimic the expected in vivo released free G-CSF. The cleaved and reduced fusion protein exhibited a 2-fold increase of in vitro biological activity, with an EC50 of 10.12 ng/mL, compared to the non-cleaved, non-reduced fusion protein, i.e., EC50 = 23.69 ng/mL. In addition, the cleaved and reduced fusion protein exhibited a higher maximal response (Fig 3). Therefore, the biological activity of the fusion protein was greatly improved with the release of G-CSF from Tf. It is known that the receptor binding site of G-CSF is located at both N- and C-terminus (15). Therefore, the increased activity is likely due to the removal of Tf blockage at C-terminus following reduction. The in vitro activity results provide a proof of concept that due to the in vivo cleavability of the linker, the fusion protein with this disulfide linker may have better in vivo biological activity than a comparable fusion protein with a non-cleavable linker.
Figure 3. Evaluation of G-CSF in vitro activity.
G-S-S-T was incubated with 25 mM DTT at 37 °C for 15 minutes. The thrombin and DTT treated fusion protein, as well as the untreated fusion protein, were used to stimulate the proliferation of NFS-60 cells as described in the materials and methods section. The DTT and thrombin treated fusion protein samples were subjected to a ≥100-fold dilution in RPMI 1640 medium with 10% FBS prior to cell treatment to eliminate the effects of DTT and thrombin on the proliferation assay. An increased absorbance at 570 nm is indicative of cell proliferation.
The disulfide linkage in thrombin pre-treated G-C-T is cleavable in vivo
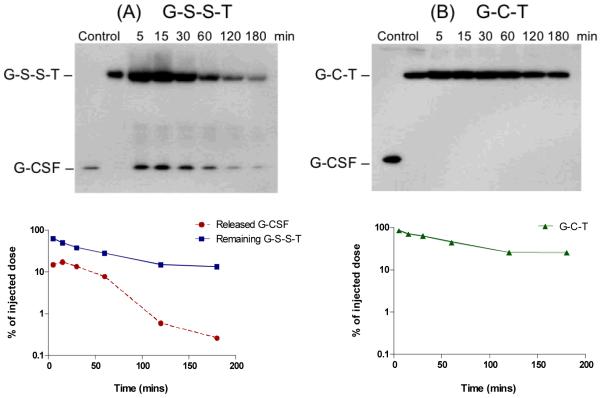
Next, we investigated the in vivo cleavage of the thrombin pre-treated, disulfide linked fusion protein (designated as G-S-S-T). G-S-S-T or G-C-T was administered intravenously to CF1 mice, and the plasma samples were analyzed by non-reducing SDS-PAGE followed by anti-G-CSF Western blot. As shown in Fig. 4, free G-CSF could be detected in the blood as early as 5 min after injection of G-S-S-T, with a peak at about 15 min post injection. In contrast, no detectable amount of free G-CSF was present in the blood of CF1 mice treated with G-C-T. At the last time point of 180 min, the remaining percentage of injected dose of the G-C-T fusion protein was about 2-fold higher than the G-S-S-T fusion protein. These results demonstrate that the disulfide linkage in G-S-S-T could indeed be cleaved in vivo.
Figure 4. Evaluation of in vivo release of free G-CSF.
G-S-S-T (a) or G-C-T (b) was administered intravenously to CF1 mice via the tail vein at a dose of 4 mg/kg. After administration, the collected blood from 3 mice were pooled together and analyzed by non-reducing SDS-PAGE followed by anti-G-CSF Western blot as described in the Materials and Methods section. The amount of protein in each band was quantified using Quality One® software (Bio-Rad, Hercules, CA), and the percentage was calculated based on the injected dose.
The in vivo experiment in CF1 mice supported our hypothesis that the disulfide linkage created by the dithiocyclopeptide linker is cleavable in vivo. We observed a rapid release of G-CSF from the fusion protein, and a quick elimination of free G-CSF due to its short in vivo half-life (Fig. 4). The reversible nature of the disulfide bond has been widely applied for drug delivery and drug targeting (16). A well-known example for disulfide-linked conjugate is immunotoxin, in which the disulfide bond is reduced in the cytosol to release the active toxin domain. However, following administration of immunotoxin, it was found to be broken down in the circulation due to the instability of the disulfide bond in vivo (17-19). The site and the mechanism of the in vivo reduction of the disulfide linkage in the fusion protein have not been fully elucidated. It has been suggested that the disulfide bond was possibly reduced in the liver. It has been demonstrated that a disulfide linked insulin-transferrin conjugate could release insulin upon its incubation with rat liver slices, but not when the latter has been depleted of glutathione (20). Since the liver is the major organ for glutathione production (21), the higher local glutathione concentration in the liver may facilitate the reduction. In addition, a redox enzyme, protein disulfide isomerase (PDI) has been identified on hepatocyte plasma membranes in addition to its major site of localization in ER (22). The membrane associated PDI is required for the activation of diphtheria toxin by reducing the disulfide bridge between the catalytic subunit and receptor-binding subunit inside the endosome (23), and may have similar function to reduce the disulfide bond in our fusion protein.
In this report, we take advantage of the reversible nature of the disulfide bond to design an in vivo cleavable linker in a recombinant fusion protein to ensure the release of the active domain into the blood circulation. To our knowledge, this is the first example of the construction and characterization of a recombinant fusion protein linked through the use of a reducible disulfide bond. This linker can be utilized for a wide range of fusion proteins where the in vivo separation of the domains is required for achieving optimal activity or desirable biological functions.
Acknowledgments
This work was supported partially by NIH grant R01GM063647. WCS is John A. Biles Professor of Pharmaceutical Sciences.
Footnotes
The authors declare no competing interests.
References
- 1.Uhlen M, Forsberg G, Moks T, Hartmanis M, Nilsson B. Fusion Proteins in Biotechnology. Curr Opin in Biotech. 1992;3:363–369. doi: 10.1016/0958-1669(92)90164-e. [DOI] [PubMed] [Google Scholar]
- 2.Amet N, Lee HF, Shen WC. Insertion of the designed helical linker led to increased expression of tf-based fusion proteins. Pharm Res. 2009;26:523–528. doi: 10.1007/s11095-008-9767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai Y, Shen WC. Improving the oral efficacy of recombinant granulocyte colony-stimulating factor and transferrin fusion protein by spacer optimization. Pharm Res. 2006;23:2116–2121. doi: 10.1007/s11095-006-9059-5. [DOI] [PubMed] [Google Scholar]
- 4.Wriggers W, Chakravarty S, Jennings PA. Control of protein functional dynamics by peptide linkers. Biopolymers. 2005;80:736–746. doi: 10.1002/bip.20291. [DOI] [PubMed] [Google Scholar]
- 5.Arai R, Ueda H, Kitayama A, Kamiya N, Nagamune T. Design of the linkers which effectively separate domains of a bifunctional fusion protein. Protein Eng. 2001;14:529–532. doi: 10.1093/protein/14.8.529. [DOI] [PubMed] [Google Scholar]
- 6.Yao Z, Dai W, Perry J, Brechbiel MW, Sung C. Effect of albumin fusion on the biodistribution of interleukin-2. Cancer Immunol Immunother. 2004;53:404–410. doi: 10.1007/s00262-003-0454-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen WC. Emerging targeting concepts membrane-associated protein thiol-disulfide interchange activity: A potential target for anti-viral and anti-tumor drug design. J Drug Target. 1999;6:387–389. doi: 10.3109/10611869908996845. [DOI] [PubMed] [Google Scholar]
- 8.Shah D, Shen WC. Transcellular delivery of an insulin-transferrin conjugate in enterocyte-like Caco-2 cells. J Pharm Sci. 1996;85:1306–1311. doi: 10.1021/js9601400. [DOI] [PubMed] [Google Scholar]
- 9.Widera AS, Kim KJ, Crandall ED, Shen WC. Transcytosis of GCSF-transferrin across rat alveolar epithelial cell monolayers. Pharm Res. 2003;20:1231–1238. doi: 10.1023/a:1025005232421. [DOI] [PubMed] [Google Scholar]
- 10.Widera A, Bai Y, Shen WC. The Transepithelial Transport of a G-CSF-Transferrin Conjugate in Caco-2 Cells and Its Myelopoietic Effect in BDF1 Mice. Pharm Res. 2004;21:278–284. doi: 10.1023/b:pham.0000016240.81059.ec. [DOI] [PubMed] [Google Scholar]
- 11.Bai Y, Ann DK, Shen WC. Recombinant granulocyte colony-stimulating factor-transferrin fusion protein as an oral myelopoietic agent. P Natl Acad Sci USA. 2005;102:7292–7296. doi: 10.1073/pnas.0500062102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aricescu AR, Lu W, Jones EY. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr D Biol Crystallogr. 2006;62:1243–1250. doi: 10.1107/S0907444906029799. [DOI] [PubMed] [Google Scholar]
- 13.Hem A, Smith AJ, Solberg P. Saphenous vein puncture for blood sampling of the mouse, rat, hamster, gerbil, guinea pig, ferret and mink. Lab Anim. 1998;32:364–368. doi: 10.1258/002367798780599866. [DOI] [PubMed] [Google Scholar]
- 14.Amet N, Wang W, Shen WC. Human growth hormone-transferrin fusion protein for oral delivery in hypophysectomized rats. J Control Release. 2010;141:177–182. doi: 10.1016/j.jconrel.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamada T, Honjo E, Maeda Y, Okamoto T, Ishibashi M, Tokunaga M, Kuroki R. Homodimeric cross-over structure of the human granulocyte colony-stimulating factor (GCSF) receptor signaling complex. P Natl Acad Sci USA. 2006;103:3135–3140. doi: 10.1073/pnas.0511264103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito G, Swanson JA, Lee KD. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: role and site of cellular reducing activities. Adv Drug Deliv Rev. 2003;55:199–215. doi: 10.1016/s0169-409x(02)00179-5. [DOI] [PubMed] [Google Scholar]
- 17.Thorpe PE, Wallace PM, Knowles PP, Relf MG, Brown AN, Watson GJ, Knyba RE, Wawrzynczak EJ, Blakey DC. New coupling agents for the synthesis of immunotoxins containing a hindered disulfide bond with improved stability in vivo. Cancer Res. 1987;47:5924–5931. [PubMed] [Google Scholar]
- 18.Letvin NL, Goldmacher VS, Ritz J, Yetz JM, Schlossman SF, Lambert JM. In vivo administration of lymphocyte-specific monoclonal antibodies in nonhuman primates. In vivo stability of disulfide-linked immunotoxin conjugates. J Clin Invest. 1986;77:977–984. doi: 10.1172/JCI112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arpicco S, Dosio F, Brusa P, Crosasso P, Cattel L. New coupling reagents for the preparation of disulfide cross-linked conjugates with increased stability. Bioconjug Chem. 1997;8:327–337. doi: 10.1021/bc970025w. [DOI] [PubMed] [Google Scholar]
- 20.Xia CQ, Wang J, Shen WC. Hypoglycemic Effect of Insulin-Transferrin Conjugate in Streptozotocin-Induced Diabetic Rats. J Pharmacol Exp Ther. 2000;295:594. [PubMed] [Google Scholar]
- 21.DeLeve LD, Kaplowitz N. Glutathione metabolism and its role in hepatotoxicity. Pharmacol Ther. 1991;52:287–305. doi: 10.1016/0163-7258(91)90029-l. [DOI] [PubMed] [Google Scholar]
- 22.Noiva R. Protein disulfide isomerase: the multifunctional redox chaperone of the endoplasmic reticulum. Semin Cell Dev Biol. 1999;10:481–493. doi: 10.1006/scdb.1999.0319. [DOI] [PubMed] [Google Scholar]
- 23.Mandel R, Ryser HJ, Ghani F, Wu M, Peak D. Inhibition of a reductive function of the plasma membrane by bacitracin and antibodies against protein disulfide-isomerase. P Natl Acad Sci USA. 1993;90:4112–4116. doi: 10.1073/pnas.90.9.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]