Abstract
Androgen receptor (AR) signaling not only plays a pivotal role in the development of androgen-dependent prostate cancer but is also important in the growth and survival of castration-resistant prostate cancer (CRPC). The first line of treatment of androgen-dependent prostate cancer is the use of androgen deprivation therapy. However, most patients will eventually relapse due to development of CRPC. Thus, development of a strategy to target AR for treatment of CRPC is urgently needed. The authors have previously identified andrographolide as an inhibitor of interleukin-6, which can suppress tumor growth of prostate cancer cells by screening compounds from the Prestwick Natural compound library. In this study, they identified that andrographolide can inhibit AR expression and prostate cancer cell growth and induce apoptosis. Andrographolide is able to down-regulate AR expression at both mRNA and protein levels, prevents its nuclear translocation, and inhibits transactivation of its target genes. Andrographolide prevents the binding of Hsp90 to AR, resulting in proteasome-mediated AR degradation. Furthermore, andrographolide inhibits castration-resistant C4-2 cell growth by reducing AR expression and activity. Thus, andrographolide can be developed as a potential therapeutic agent for prostate cancer by inhibition of androgen receptor signaling.
Keywords: prostate cancer, androgen receptor, andrographolide
Introduction
Prostate cancer is the most frequently diagnosed cancer and the second-leading cause of cancer death in men in the United States. Androgen deprivation therapy (ADT) is currently a standard treatment for androgen-dependent prostate cancer. Unfortunately, all patients ultimately develop castration-resistant prostate cancer (CRPC) approximately 2 to 3 years after ADT. Patients with CRPC have a progressive disease with a median survival of 10 to 12 months without any treatment.1-3 Development of effective therapeutics for patients with CRPC is one of the major challenges for the management of this disease. Bicalutamide is a nonsteroidal antiandrogen that is often used with androgen deprivation therapy. Although bicalutamide treatment initially exhibits favorable responses, prostate cancers eventually become refractory and develop resistance to bicalutamide therapy, as shown by the limited response to secondary addition or withdrawal.4,5 Docetaxel was approved by the United States Food and Drug Administration (USFDA) in 2004 for the palliative management of men with CRPC who fail bicalutamide treatment, based on improved survival, tumor response, pain, and quality-of-life responses.6,7
Many studies have revealed that CRPC is commonly associated with increased androgen receptor (AR) gene expression, which can occur through AR gene amplification or other mechanisms.8-11 Multiple immunohistochemical studies have shown that AR protein is expressed at high levels in most cases of CRPC.10,12 Up-regulation of AR mRNA was found to occur in all transitions from hormone-sensitive to hormone-refractory disease in a mouse tumor-xenograft model of prostate cancer.13 These data suggest that the AR not only plays a central role in the development of androgen-dependent prostate cancer but is also very important for the growth and survival of CRPC,14-17 and targeting AR signaling is an effective therapeutic strategy for CRPC.
Andrographolide is a diterpenoid lactone isolated from Andrographis paniculata, an anti-inflammatory botanical medicine, which has been used for the treatment of various ailments, including respiratory infection, bacterial dysentery, and fever.18,19 Numerous studies demonstrate that andrographolide exhibits anticancer activity by inducing apoptosis and cell cycle arrest.20-22 Andrographolide possesses strong anti-inflammatory activity23 and triggers apoptosis via the caspase-8-dependent pathway in human cancer cells.24
Our previous study has demonstrated that andrographolide significantly inhibits DU145 and PC-3 cells in vitro and in vivo by blocking interleukin (IL)–6-stimulated Stat3 activation.25 In this study, we have identified andrographolide as a potent AR inhibitor that induces apoptosis and suppresses prostate cancer tumor growth. Andrographolide inhibits expression of AR at both mRNA and protein levels and decreases the expression of androgen target genes such as prostate-specific antigen (PSA). Inhibition of AR by andrographolide correlates with dose-dependent apoptosis induction and growth inhibition in C4-2 cells.
Results
Andrographolide Inhibits Androgen Receptor Expression
To determine whether andrographolide affects AR expression, C4-2 cells that express endogenous AR were treated with different concentrations of andrographolide. Total RNAs were isolated, and AR mRNA expression was measured by qRT-PCR. Andrographolide inhibits AR mRNA expression in a dose-dependent manner (Figure 1A). The levels of AR mRNA decreased initially by about 20% at 5 µM of andrographolide and continued to decrease by about 80% at 20 µM of andrographolide at 24 hours. The levels of AR mRNA started to decrease at about 6 hours and continued to decrease by about 80% and reached a plateau at 24 hours with 20 µM andrographolide treatment (Figure 1B). Consistent with mRNA inhibition, andrographolide inhibits AR protein expression in a dose- and time-dependent manner (Figure 1C). Similar to the C4-2 cells, andrographolide inhibits AR protein expression in LNCaP (Figure 1D) and CWR22Rv1 cells (Figure 1E). Interestingly, andrographolide does not affect vitamin D receptor (VDR) expression (data not shown).
Figure 1.
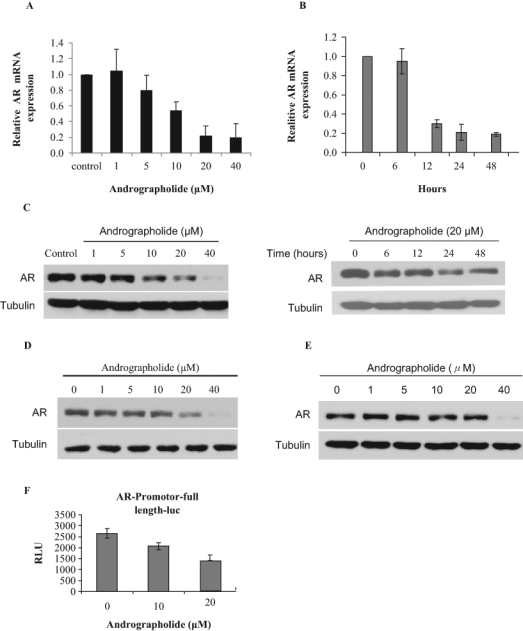
Andrographolide inhibits androgen receptor (AR) expression. (A) Dose-dependent inhibition by andrographolide. C4-2 cells were treated with different concentrations of andrographolide for 24 hours in fetal bovine serum (FBS) condition. Total RNAs were isolated for qRT-PCR analysis of AR mRNA expression. (B) C4-2 cells were treated with 20 µM andrographolide at different time points as indicated. Total RNAs were isolated for qRT-PCR analysis of AR mRNA expression. (C) Andrographolide inhibits AR protein expression. C4-2 cells were treated with different concentrations of andrographolide with different time points as indicated. Whole-cell lysates were collected and subjected to Western blot analysis using the antibodies as indicated. (D) Andrographolide inhibits AR protein expression in LNCaP cells. LNCaP cells were treated with different doses of andrographolide, and whole-cell lysates were subjected to Western blot analysis. (E) Andrographolide inhibits AR protein expression in CWR22Rv1 cells. (F) Andrographolide inhibits AR promoter luciferase activity. C4-2 cells were transfected with AR promoter-driven luciferase reporter and treated with different doses of andrographolide. Luciferase activity was measured.
Since andrographolide decreases the levels of AR mRNA, we tested whether andrographolide affects AR transcriptional initiation using a luciferase reporter containing the full-length promoter of AR. We transfected the full-length (~6 kb) AR promoter or the control vector into C4-2 cells and performed luciferase assays. Andrographolide decreased the activity of the AR promoter (Figure 1F). These data suggest that inhibition of AR expression by andrographolide is at least in part at the transcription level.
Andrographolide Reduces PSA Expression and Transactivation of the PSA Promoter
PSA is one of the best-characterized androgen-responsive genes. We used PSA as a model to examine the effects of andrographolide on AR signaling. C4-2 cells were treated with different concentrations of andrographolide, supernatants were collected for measurement of PSA protein by enzyme-linked immunosorbent assay (ELISA), and total RNAs were isolated for examining PSA mRNA expression. As shown in Figure 2A,B, andrographolide decreases PSA mRNA as well as protein expression. Similar results were observed showing that andrographolide reduces the levels of NKX3.1 mRNA expression (Figure 2C). It is reported that several tumor suppressor genes such as maspin and UGT2B15 are repressed by androgen and androgen receptor.26-28 Interestingly, andrographolide up-regulates basal transcription of both maspin and UGT2B15 in C4-2 cells (Figure 2D,E). DHT-mediated AR activation represses expression levels of both genes, which is counteracted by andrographolide (Figure 2D,E).
Figure 2.
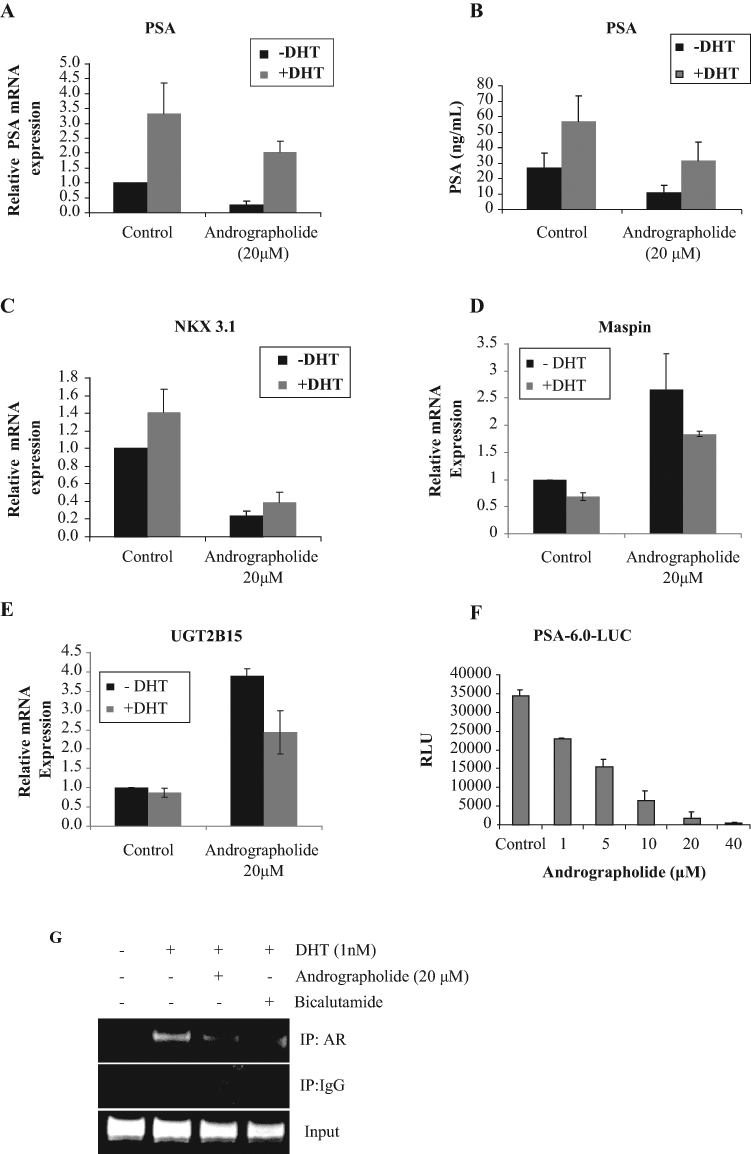
Andrographolide inhibits androgen receptor (AR) activity and transcription of AR target genes. (A, B) Andrographolide inhibits prostate-specific antigen (PSA) mRNA (A) and protein expression (B). C4-2 cells were cultured in charcoal-stripped fetal bovine serum (FBS) and treated with 20 µM andrographolide in the presence and absence of 1 nM DHT for overnight. Total RNAs were isolated for qRT-PCR analysis of PSA mRNA, and supernatants were collected for enzyme-linked immunosorbent assay (ELISA) analysis for PSA protein. (C) NKX3.1 mRNA levels were also analyzed by qRT-PCR. (D) Andrographolide increases maspin mRNA expression. (E) Andrographolide increases UGT2B15 mRNA expression. (F) Andrographolide inhibits PSA transcriptional activity. C4-2 cells were transfected with PSA-6.0-Luc plasmids and treated with different doses of andrographolide overnight. Luciferase activity was measured. (G) Andrographolide inhibits AR recruitment to androgen-responsive genes. C4-2 cells were treated with either andrographolide or bicalutamide. ChIP assays were performed using AR antibodies.
To determine the effect of andrographolide on PSA promoter activity, C4-2 cells were transfected with PSA enhancer-promoter-luciferase (pGL3-PSA-6.0-Luc) followed by treatment with different concentrations of andrographolide for 24 hours. As shown in Figure 2F, PSA promoter activity was decreased upon andrographolide treatment.
To determine whether andrographolide modulates recruitment of AR to the PSA promoter, ChIP assays were performed. As shown in Figure 2G, DHT enhanced AR recruitment to the ARE site, which was blocked by andrographolide.
Andrographolide Inhibits the Nuclear Translocation of AR
To examine whether andrographolide affects the nuclear translocation of AR, nuclear extracts from andrographolide-treated C4-2 cells grown in CS-FBS in the presence of 1 nM DHT were analyzed by immunoblotting with anti-AR antibodies. DHT treatment increased the levels of AR protein in the nucleus, which were reduced by treatment with andrographolide (Figure 3A), but there was apparently no change in the cytoplasmic AR. To further confirm these results, C4-2 cells treated with andrographolide were processed for immunofluorescent staining with AR antibodies. AR was confined mainly to the cytoplasmic compartment in control cells in CS-FBS conditions, whereas stronger nuclear staining of AR was observed in cells cultured in 1 nM DHT, which was blocked by andrographolide treatment (Figure 3B). These results suggest that andrographolide inhibits the nuclear translocation of AR.
Figure 3.
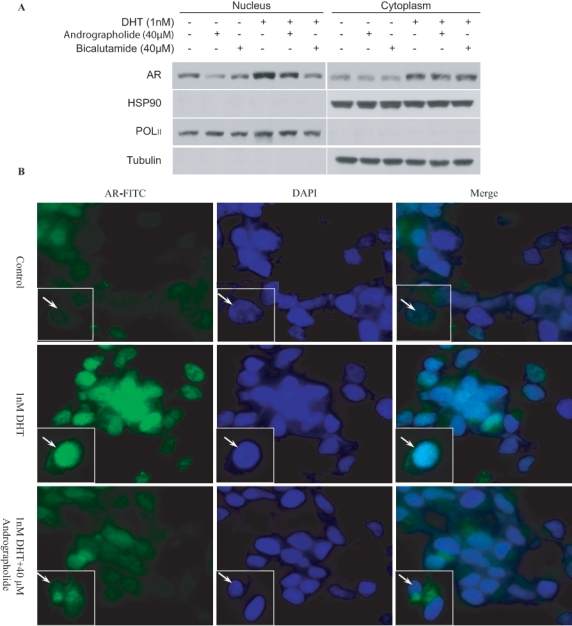
Andrographolide inhibits androgen receptor (AR) nuclear translocation in C4-2 cells. (A) C4-2 cells were treated with 40 µM andrographolide or bicalutamide in CS-FBS with or without 1 nM DHT for 6 hours. Cytoplasmic and nuclear proteins were isolated and immunoblotted with AR, Hsp90, PolII, and tubulin antibodies. PolII and tubulin were used as loading controls for nuclear and cytoplasmic proteins, respectively. (B) C4-2 cells were treated with control, 1 nM DHT, and 40 µM andrographolide in CS-FBS for 6 hours. Cells were processed for immunofluorescent staining with AR-FITC, and nuclei were stained with DAPI.
Andrographolide Prevents the Binding of Hsp90 to AR
Hsp90 is a ubiquitously expressed molecular chaperone that has been found in complexes with a variety of proteins, including steroid hormone receptors such as AR, dioxin receptor, actin, v-src, and other kinases.29,30 Binding of Hsp90 protein to the AR and recruitment of other co-chaperones to form a mature complex stabilizes AR.29,30 To test whether andrographolide affects the association of AR to Hsp90, co-immunoprecipitation was performed. As shown in Figure 4, the levels of AR-bound Hsp90 protein were decreased after treatment with andrographolide without affecting the amount of total cellular Hsp90 protein.
Figure 4.

Andrographolide inhibits androgen receptor (AR) binding to Hsp90. C4-2 cells were treated with 20 µM andrographolide for 24 hours, and whole-cell lysates were immunoprecipitated with AR or IgG antibodies and probed for Hsp90 antibody.
Andrographolide Inhibits AR-Positive Prostate Cancer Cell Growth and Induces Apoptosis
Since AR plays a central role in the development and progression of CRPC, we tested whether knockdown of AR expression by its specific shRNA affects the growth of C4-2 cells in vitro. C4-2 cells were transfected with AR shRNA or vector control for 72 hours and cell numbers were counted. Knockdown of AR expression resulted in cell growth inhibition (Figure 5A,B), consistent with previous reports.1,14,16 Since andrographolide inhibits AR expression, we tested whether andrographolide affects the growth of AR-positive C4-2 cells. As shown in Figure 5C, andrographolide inhibits C4-2 cell growth in a dose-dependent manner. Similar growth inhibition was observed in other prostate cancer cells such as CWR22Rv1 (Figure 5C). It should be noticed that PZ-HPV7 benign prostate epithelial cells are not growth inhibited by andrographolide.25 Furthermore, andrographolide-induced cell growth inhibition was associated with induction of apoptosis (Figure 5D).
Figure 5.

Andrographolide inhibits cell growth and induces apoptosis in prostate cancer cell lines. (A) Knockdown of androgen receptor (AR) expression in C4-2 cells inhibits cell growth. C4-2 cells were transiently transfected with either control shRNA or AR-specific shRNA. Cell numbers were counted after 2 days following transfection. Whole-cell lysates were isolated and subjected to Western blot analysis of AR protein (B). (C) Andrographolide inhibits the growth of C4-2 and CWR22Rv1 prostate cancer cells. C4-2 and CWR22Rv1 cells were treated with different concentrations of andrographolide and cell numbers were counted at 48 hours. (D) Andrographolide induces apoptosis. Mono- and oligo-nucleosomes in the cytoplasmic fraction of C4-2 cells were analyzed by cell death detection enzyme-linked immunosorbent assay (ELISA) as described in Materials and Methods.
Discussion
Because the growth of prostate cancer cells depends on the presence of androgens, androgen deprivation therapy has been the primary treatment for patients with metastatic prostate cancer since the seminal recognition of the disease as androgen sensitive by Huggins and Hodges in 1941.31 Almost all patients with advanced prostate cancer respond initially to androgen deprivation therapy. However, virtually every patient will relapse due to the growth of castration-resistant cancer cells. One of the greatest challenges facing prostate cancer therapy is its evolution to castration resistance. Accumulated data emphasize the presence of residual androgens and persistent activation of the androgen receptor signaling axis in castration-resistant prostate tumors despite castration. CRPC cells often continue to express androgen-responsive genes such as PSA and often express AR,32,33 suggesting that the AR becomes activated by a castration-resistant mechanism in AR-positive CRPC cells. The AR can also be activated in the absence of or very low levels of androgen by cross-talk with other signaling pathways.34-40 Mutations, deletions, and amplification of the AR gene or alterations of the interactions between AR and some of its coregulators may enhance sensitivity or promiscuity of the AR to very low levels of androgen and other steroids.41-45 In addition, levels of intraprostatic androgens are at concentrations sufficient to activate the AR and stimulate tumor growth.46-49 Thus, abnormal activation of the AR plays a major role in the development of castration resistance.
Down-regulation of AR expression has been suggested as one of the therapeutic strategies to inhibit prostate cancer growth and progression to a castration-resistant stage. Edwards et al.50 reported that a significant increase in AR gene amplification rates is seen in the transition from hormone-sensitive to hormone-resistant disease. Real-time RT-PCR demonstrated that even one additional copy of the AR gene may increase AR expression, and the elevated AR expression is sufficient to confer resistance to antiandrogen.10,13 Several studies demonstrated that knockdown of AR expression inhibited the proliferation of C4-2 and CWR22Rv1 cells and tumor growth in vivo.16,51 Consistent with these results, we showed that knockdown of AR expression using AR shRNA inhibited C4-2 cell growth in vitro. We also demonstrated that andrographolide inhibits expression of AR at both mRNA and protein levels and decreases the expression of androgen target genes such as PSA. Inhibition of the AR pathway by andrographolide in C4-2 cells was found to occur through 4 different mechanisms: andrographolide (1) directly inhibits AR expression at both mRNA and protein levels, (2) induces dissociation of AR from Hsp90 and subsequently degradation by the proteasome, (3) inhibits AR nuclear translocation, and (4) prevents AR recruitment to the promoters of androgen-responsive genes.
The anticancer activity of andrographolide has been demonstrated in several types of cancers. Andrographolide inhibits invasive ability of A549 cells via down-regulation of PI3K/Akt signaling and inactivation of c-Jun/c-Fos followed by a reduction in MMP-7 expression.52 Andrographolide enhances chemosensitivity of tumor cells to doxorubicin through inhibition of STAT3 activity, suggesting a potential therapeutic strategy using andrographolide in combination with conventional chemotherapeutic agents for treatment of cancer.53 Andrographolide induces cell cycle arrest21 and triggers apoptosis in human cancer cells.54 We have previously demonstrated that andrographolide inhibits DU145 cell growth in vitro and in vivo via suppression of the IL-6 signaling pathway.25 In this study, we found that andrographolide inhibits castration-resistant C4-2 cell via down-regulation of AR expression and activity.
In summary, we have identified andrographolide as a potent AR inhibitor that induces apoptosis and suppresses prostate cancer tumor growth. Andrographolide inhibits expression of AR at both mRNA and protein levels and decreases the expression of androgen target genes such as PSA. Inhibition of AR by andrographolide correlates with dose-dependent apoptosis induction and growth inhibition in C4-2 cells. These data suggest that inhibition of AR expression using andrographolide may have therapeutic effects on prostate cancer and that andrographolide, an herbal medicine used in China and India, may be developed as a potential therapeutic agent to treat prostate cancer.
Materials and Methods
Reagents and cell culture
C4-2, LNCaP, and CWR22Rv1 cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and 100 units/mL penicillin and 0.1 mg/mL streptomycin. The cells were maintained at 37°C in a humidified incubator with 5% CO2. Andrographolide was purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in DMSO.
Real-time PCR analysis
Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA) reagent. Then, 1 µg of total RNA was digested with RQ1 DNase (Promega, Madison, WI) and reverse transcribed with random primers and Im-Prom II Reverse transcriptase (Promega). The cDNA was used to perform qRT-PCR using specific primers for PSA, NKX3.1, and β-actin as described previously.55 Quantitative real-time RT-PCR was performed in a 25-µL reaction mixture using SYBR Green IQ supermix (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. Expression levels of PSA and NKX3.1 were normalized to β-actin. The experiments were repeated 3 times with duplicates.
Preparation of nuclear and cytosolic extracts
Cells were cultured in medium containing CS-FBS for 3 days and treated with 1 nmol/L DHT in the absence or presence of 40 µM andrographolide or bicalutamide for 6 hours. Cells were harvested, washed with PBS twice, and resuspended in low-salt buffer (10 mmol/L HEPES-KOH [pH 7.9], 1.5 mmol/L MgCl2, 10 mmol/L KCl, and 0.1% NP40) supplemented with protease inhibitors (Roche, Basel, Switzerland) and incubated on ice for 30 minutes. Nuclei were precipitated by centrifugation at 3000 g for 10 minutes at 4°C. The supernatants were collected as the cytosolic fraction. After washing once with the low-salt buffer, nuclei were lysed in high-salt lysis buffer (50 mmol/L Tris-HCl [pH 8], 150 mmol/L NaCl, 1% Triton X-100) followed by mechanical disruption at 4°C for 30 minutes. The nuclear lysate was cleared by 10,000 rpm centrifugation at 4°C for 15 minutes. Protein concentration was determined using the Coomassie Plus protein assay kit (Pierce, Rockford, IL).
Western blot analysis
Whole-cell protein extracts were resolved on 10% SDS-PAGE, and proteins were transferred to nitrocellulose membranes. After blocking for 1 hour at room temperature in 5% milk in 1× PBS/0.1% Tween-20, membranes were incubated overnight at 4°C with the indicated primary antibodies. Following secondary antibody incubation, immunoreactive proteins were visualized with an enhanced chemiluminescence detection system (GE Healthcare, Piscataway, NJ).
Co-immunoprecipitation
C4-2 cells were lysed in high-salt buffer. Equal amounts of the lysates (500 µg) were immunoprecipitated using 1 µg of AR antibodies (C19; Santa Cruz Biotechnology, Santa Cruz, CA) with 30 µL of protein A/G agarose with constant rotation overnight. The immunoprecipitates were washed with 10 mM HEPES (pH 7.9), 1 mM EDTA, 150 mM NaCl, and 1% Nonidet P-40 twice with 1 mL each. The precipitated proteins were eluted with 30 µL of SDS-PAGE sample buffer by boiling for 10 minutes. The eluted proteins were electrophoresed on 8% SDS-PAGE, transferred to nitrocellulose membranes, and probed with anti-Hsp90 and anti-AR antibodies.
Chromatin immunoprecipitation (ChIP) assays
C4-2 cells were cultured in phenol red–free RPMI 1640 supplemented with 10% CS-FBS for 3 days, and cells were treated with 20 µM andrographolide or 20 µM bicalutamide with or without 1 nM DHT for 24 hours. DNA-AR protein complexes were cross-linked inside the cells by the addition of 1% formaldehyde. Whole-cell extracts were prepared by sonication, and an aliquot of the cross-linked DNA-protein complexes was immunoprecipitated by incubation with the AR-specific antibody (AR441; Santa Cruz Biotechnology) overnight at 4°C with rotation. Chromatin-antibody complexes were isolated from solution by incubation with protein A/G agarose beads for 1 hour at 4°C with rotation. The bound DNA-protein complexes were washed and eluted from beads with elution buffer (1% SDS and 0.1 mol/L NaHCO3), cross links were reversed, and DNA was extracted. The resulting chromatin preparations were analyzed by PCR using primers spanning the ARE I/II and ARE III regions in the PSA promoter. Isotype-matched IgG was used as control.
Immunofluorescence
In 6-well chamber slides, 2 × 104 C4-2 cells were plated and treated with 40 µM andrographolide with or without 1 nM DHT for 6 hours. Cells were fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100, and incubated with 1% BSA to block nonspecific binding. Cells were incubated with anti-AR antibodies (AR441; Santa Cruz Biotechnology) overnight, intracellular AR was visualized with FITC-conjugated secondary antibodies, and nuclei were visualized with DAPI.
Cell growth assay
C4-2, CWR22Rv1, and LNCaP cells were seeded on 12-well plates at a density of 1 × 105 cells/well in RPMI 1640 media containing either 10% FBS or 10% CS-FBS. The cells were treated as indicated, and total cell numbers were counted.
Cell death ELISA
Cells were seeded on 12-well plates (1 × 105 cells/well) and treated with vehicle alone or different doses of andrographolide for 48 hours. Mono- and oligonucleosomes in the cytoplasmic fraction were measured by the Cell Death Detection ELISA kit (Roche) according to the manufacturer’s instructions. Briefly, floating and attached cells were collected and homogenized in 400 µL of incubation buffer. The wells were coated with antihistone antibodies and incubated with the lysates, horseradish peroxidase–conjugated anti-DNA antibodies, and the substrate, and absorbance was read at 405 nm.
Measurement of PSA
PSA levels were measured in the culture supernatants using ELISA (United Biotech, Inc., Mountain View, CA) according to the manufacturer’s instructions.
Luciferase assays
C4-2 cells were transfected with pGL3-PSA6.0-Luc and pGL4-AR-prom-Luc (~6 kb) reporters and treated with drugs as indicated in the figures in FBS or CS-FBS. Cell lysates were subjected to luciferase assays using the Luciferase Assay System (Promega).
Statistical analysis
Data are shown as mean ± SD. Multiple-group comparison was performed by one-way analysis of variance (ANOVA) followed by the Scheffé procedure for comparison of means. P < 0.05 was considered statistically significant.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
This work was supported in part by grants from the National Institutes of Health (CA109441 and CA 140468), a VA merit award (1I01 BX000526), the National High Technology Joint Research Program of China (No. 2006DAI02A02), and a grant from the Hi-tech Research and Development Program of China (No. 2006AA02401).
References
- 1. Andersen RJ, Mawji NR, Wang J, et al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell. 2010;17(6):535-46 [DOI] [PubMed] [Google Scholar]
- 2. Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl J Med. 2004;351(15):1488-90 [DOI] [PubMed] [Google Scholar]
- 3. Jiang J, Huang H. Targeting the androgen receptor by Taxol in castration-resistant prostate cancer. Mol Cell Pharmacol. 2010;2(1):1-5 [PMC free article] [PubMed] [Google Scholar]
- 4. Hodgson MC, Astapova I, Hollenberg AN, Balk SP. Activity of androgen receptor antagonist bicalutamide in prostate cancer cells is independent of NCoR and SMRT corepressors. Cancer Res. 2007;67(17):8388-95 [DOI] [PubMed] [Google Scholar]
- 5. Scher HI, Steineck G, Kelly WK. Hormone-refractory (D3) prostate cancer: refining the concept. Urology. 1995;46(2):142-8 [DOI] [PubMed] [Google Scholar]
- 6. Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26(2):242-5 [DOI] [PubMed] [Google Scholar]
- 7. Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502-12 [DOI] [PubMed] [Google Scholar]
- 8. Mostaghel EA, Page ST, Lin DW, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67(10):5033-41 [DOI] [PubMed] [Google Scholar]
- 9. Koivisto P, Kononen J, Palmberg C, et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57(2):314-9 [PubMed] [Google Scholar]
- 10. Linja MJ, Savinainen KJ, Saramaki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61(9):3550-5 [PubMed] [Google Scholar]
- 11. Gregory CW, He B, Johnson RT, et al. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 2001;61(11):4315-9 [PubMed] [Google Scholar]
- 12. Vis AN, Schroder FH. Key targets of hormonal treatment of prostate cancer: Part 1. The androgen receptor and steroidogenic pathways. BJU Int. 2009;104(4):438-48 [DOI] [PubMed] [Google Scholar]
- 13. Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33-9 [DOI] [PubMed] [Google Scholar]
- 14. Haag P, Bektic J, Bartsch G, Klocker H, Eder IE. Androgen receptor down regulation by small interference RNA induces cell growth inhibition in androgen sensitive as well as in androgen independent prostate cancer cells. J Steroid Biochem Mol Biol. 2005;96(3-4):251-8 [DOI] [PubMed] [Google Scholar]
- 15. Yuan X, Li T, Wang H, et al. Androgen receptor remains critical for cell-cycle progression in androgen-independent CWR22 prostate cancer cells. Am J Pathol. 2006;169(2):682-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Compagno D, Merle C, Morin A, et al. SIRNA-directed in vivo silencing of androgen receptor inhibits the growth of castration-resistant prostate carcinomas. PLoS One. 2007;2(10):e1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gregory CW, Johnson RT, Jr, Mohler JL, French FS, Wilson EM. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 2001;61(7):2892-8 [PubMed] [Google Scholar]
- 18. Poolsup N, Suthisisang C, Prathanturarug S, Asawamekin A, Chanchareon U. Andrographis paniculata in the symptomatic treatment of uncomplicated upper respiratory tract infection: systematic review of randomized controlled trials. J Clin Pharm Ther. 2004;29(1):37-45 [DOI] [PubMed] [Google Scholar]
- 19. Iruretagoyena MI, Tobar JA, Gonzalez PA, et al. Andrographolide interferes with T cell activation and reduces experimental autoimmune encephalomyelitis in the mouse. J Pharmacol Exp Ther. 2005;312(1):366-72 [DOI] [PubMed] [Google Scholar]
- 20. Geethangili M, Rao YK, Fang SH, Tzeng YM. Cytotoxic constituents from Andrographis paniculata induce cell cycle arrest in Jurkat cells. Phytother Res. 2008;22(10):1336-41 [DOI] [PubMed] [Google Scholar]
- 21. Shi MD, Lin HH, Lee YC, Chao JK, Lin RA, Chen JH. Inhibition of cell-cycle progression in human colorectal carcinoma Lovo cells by andrographolide. Chem Biol Interact. 2008;174(3):201-10 [DOI] [PubMed] [Google Scholar]
- 22. Rajagopal S, Kumar RA, Deevi DS, Satyanarayana C, Rajagopalan R. Andrographolide, a potential cancer therapeutic agent isolated from Andrographis paniculata. J Exp Ther Oncol. 2003;3(3):147-58 [DOI] [PubMed] [Google Scholar]
- 23. Xia YF, Ye BQ, Li YD, et al. Andrographolide attenuates inflammation by inhibition of NF-kappa B activation through covalent modification of reduced cysteine 62 of p50. J Immunol. 2004;173(6):4207-17 [DOI] [PubMed] [Google Scholar]
- 24. Kim TG, Hwi KK, Hung CS. Morphological and biochemical changes of andrographolide-induced cell death in human prostatic adenocarcinoma PC-3 cells. In Vivo. 2005;19(3):551-7 [PubMed] [Google Scholar]
- 25. Chun JY, Tummala R, Nadiminty N, et al. Andrographolide, an herbal medicine, inhibits interleukin-6 expression and suppresses prostate cancer cell growth. Genes Cancer. 2010;1(8):868-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang M, Magit D, Sager R. Expression of maspin in prostate cells is regulated by a positive Ets element and a negative hormonal responsive element site recognized by androgen receptor. Proc Natl Acad Sci U S A. 1997;94(11):5673-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. He ML, Jiang AL, Zhang PJ, et al. Identification of androgen-responsive element ARE and Sp1 element in the maspin promoter. Chin J Physiol. 2005;48(3):160-6 [PubMed] [Google Scholar]
- 28. Bao BY, Chuang BF, Wang Q, et al. Androgen receptor mediates the expression of UDP-glucuronosyltransferase 2 B15 and B17 genes. Prostate. 2008;68(8):839-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schulte TW, Blagosklonny MV, Ingui C, Neckers L. Disruption of the Raf-1-Hsp90 molecular complex results in destabilization of Raf-1 and loss of Raf-1-Ras association. J Biol Chem. 1995;270(41):24585-8 [DOI] [PubMed] [Google Scholar]
- 30. Beliakoff J, Whitesell L. Hsp90: an emerging target for breast cancer therapy. Anticancer Drugs. 2004;15(7):651-62 [DOI] [PubMed] [Google Scholar]
- 31. Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22(4):232-40 Reprint [DOI] [PubMed] [Google Scholar]
- 32. Hobisch A, Culig Z, Radmayr C, Bartsch G, Klocker H, Hittmair A. Distant metastases from prostatic carcinoma express androgen receptor protein. Cancer Res. 1995;55(14):3068-72 [PubMed] [Google Scholar]
- 33. van der Kwast TH, Schalken J, Ruizeveld de Winter JA, et al. Androgen receptors in endocrine-therapy-resistant human prostate cancer. Int J Cancer. 1991;48(2):189-93 [DOI] [PubMed] [Google Scholar]
- 34. de Miguel F, Lee S, Onate S, Gao A. Stat3 enhances transactivation of steroid hormone receptors. Nuclear Receptor. 2003;1(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abreu-Martin MT, Chari A, Palladino AA, Craft NA, Sawyers CL. Mitogen-activated protein kinase kinase kinase 1 activates androgen receptor-dependent transcription and apoptosis in prostate cancer. Mol Cell Biol. 1999;19(7):5143-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sadar MD. Androgen-independent induction of prostate-specific antigen gene expression via cross-talk between the androgen receptor and protein kinase A signal transduction pathways. J Biol Chem. 1999;274(12):7777-83 [DOI] [PubMed] [Google Scholar]
- 37. Chen T, Wang LH, Farrar WL. Interleukin 6 activates androgen receptor-mediated gene expression through a signal transducer and activator of transcription 3-dependent pathway in LNCaP prostate cancer cells. Cancer Res. 2000;60(8):2132-5 [PubMed] [Google Scholar]
- 38. Wen Y, Hu MC-T, Makino K, et al. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 2000;60(24):6841-5 [PubMed] [Google Scholar]
- 39. Hobisch A, Eder IE, Putz T, et al. Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer Res. 1998;58(20):4640-5 [PubMed] [Google Scholar]
- 40. DeMiguel F, Lee SO, Lou W, et al. Stat3 enhances the growth of LNCaP human prostate cancer cells in intact and castrated male nude mice. Prostate. 2002;52(2):123-9 [DOI] [PubMed] [Google Scholar]
- 41. Tilley WD, Buchanan G, Hickey TE, Bentel JM. Mutations in the androgen receptor gene are associated with progression of human prostate cancer to androgen independence. Clin Cancer Res. 1996;2(2):277-85 [PubMed] [Google Scholar]
- 42. Linja MJ, Savinainen KJ, Saramaki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61(9):3550-5 [PubMed] [Google Scholar]
- 43. Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68(13):5469-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hu R, Dunn TA, Wei S, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69(1):16-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yeh S, Miyamoto H, Shima H, Chang C. From estrogen to androgen receptor: a new pathway for sex hormones in prostate. Proc Natl Acad Sci U S A. 1998;95(10):5527-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mohler JL, Gregory CW, Ford OH, III, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10(2):440-8 [DOI] [PubMed] [Google Scholar]
- 47. Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68(11):4447-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marks LS, Mostaghel EA, Nelson PS. Prostate tissue androgens: history and current clinical relevance. Urology. 2008;72(2):247-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mostaghel EA, Nelson PS. Intracrine androgen metabolism in prostate cancer progression: mechanisms of castration resistance and therapeutic implications. Best Pract Res. 2008;22(2):243-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Edwards J, Krishna NS, Grigor KM, Bartlett JM. Androgen receptor gene amplification and protein expression in hormone refractory prostate cancer. Br J Cancer. 2003;89(3):552-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Agoulnik IU, Vaid A, Bingman WE, III, et al. Role of SRC-1 in the promotion of prostate cancer cell growth and tumor progression. Cancer Res. 2005;65(17):7959-67 [DOI] [PubMed] [Google Scholar]
- 52. Lee YC, Lin HH, Hsu CH, Wang CJ, Chiang TA, Chen JH. Inhibitory effects of andrographolide on migration and invasion in human non-small cell lung cancer A549 cells via down-regulation of PI3K/Akt signaling pathway. Eur J Pharmacol. 2010;632(1-3):23-32 [DOI] [PubMed] [Google Scholar]
- 53. Zhou J, Ong CN, Hur GM, Shen HM. Inhibition of the JAK-STAT3 pathway by andrographolide enhances chemosensitivity of cancer cells to doxorubicin. Biochem Pharmacol. 2010;79(9):1242-50 [DOI] [PubMed] [Google Scholar]
- 54. Zhou J, Zhang S, Ong CN, Shen HM. Critical role of pro-apoptotic Bcl-2 family members in andrographolide-induced apoptosis in human cancer cells. Biochem Pharmacol. 2006;72(2):132-44 [DOI] [PubMed] [Google Scholar]
- 55. Nadiminty N, Lou W, Sun M, et al. Aberrant activation of the androgen receptor by NF-kappaB2/p52 in prostate cancer cells. Cancer Res. 2010;70(8):3309-19 [DOI] [PMC free article] [PubMed] [Google Scholar]


