Abstract
Hummingbird tongues pick up a liquid, calorie-dense food that cannot be grasped, a physical challenge that has long inspired the study of nectar-transport mechanics. Existing biophysical models predict optimal hummingbird foraging on the basis of equations that assume that fluid rises through the tongue in the same way as through capillary tubes. We demonstrate that the hummingbird tongue does not function like a pair of tiny, static tubes drawing up floral nectar via capillary action. Instead, we show that the tongue tip is a dynamic liquid-trapping device that changes configuration and shape dramatically as it moves in and out of fluids. We also show that the tongue–fluid interactions are identical in both living and dead birds, demonstrating that this mechanism is a function of the tongue structure itself, and therefore highly efficient because no energy expenditure by the bird is required to drive the opening and closing of the trap. Our results rule out previous conclusions from capillarity-based models of nectar feeding and highlight the necessity of developing a new biophysical model for nectar intake in hummingbirds. Our findings have ramifications for the study of feeding mechanics in other nectarivorous birds, and for the understanding of the evolution of nectarivory in general. We propose a conceptual mechanical explanation for this unique fluid-trapping capacity, with far-reaching practical applications (e.g., biomimetics).
Keywords: biomechanics, fluid dynamics, nectar trapping, surface tension
Phenomena driven by surface tension are important in a variety of biological systems (1, 2), and in recent years the importance of working with living organisms to test theoretical biophysical models [e.g., trees (3, 4), arthropods (5–8), and birds (9, 10)] has become evident. Exploration of natural solutions to specific fluid dynamics challenges has provided conceptual tools fostering practical advances in a wide array of fields (11, 12). Discovery of new biophysical mechanisms opens doors to new applied research lines [e.g., biomimicry (13, 14)]. We report here on a previously undescribed mechanism of fluid capture and transport in nature, performed by the tongue of hummingbirds.
The tetrapod tongue evolved to facilitate feeding on land, and in many taxa its primary function is to transport captured food to where it can be swallowed (15). Nectarivores, however, have evolved specialized tongues that function as their primary food-capturing device (Fig. 1A). Hummingbirds are the most specialized nectar-feeding vertebrates (16, 17); thus, we would expect them to possess a highly efficient liquid extraction system.
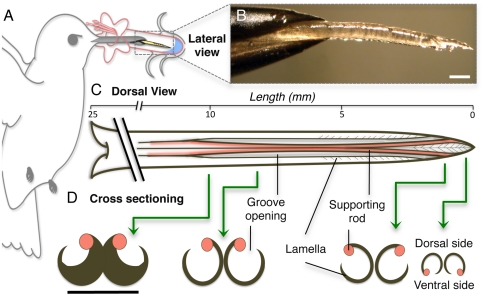
Fig. 1.
Hummingbird tongues. (A) Nectarivores use their tongue (yellow) as their primary food-gathering tool. (B) Lateral picture of a post mortem Ruby-throated Hummingbird (Archilochus colubris) tongue tip protruding from the bill tip. (C) Dorsal view of the morphology of a hummingbird tongue (approximate dimensions for A. colubris) showing length of the entire tongue, open-sided grooves, and the fringed (lamellar) region of the tip (distal approximately 6 mm). Base of the tongue is on the left; tip on the right. (D) Cross-sectioning shows the structural arrangement along the distal region of the tongue; green arrows identify the placement of the cross-sections. Black lines indicate the same structures in dorsal and cross-sectional views. Note the change in position of supporting rods from the base of the grooves to the tongue tip. Unlabeled scale bars, 0.5 mm.
Since first proposed in 1833, it has been believed that the tongue tips of hummingbirds are loaded with nectar by means of capillary rise (18). Detailed biophysical models of nectar-feeding strategies developed almost 30 y ago were based on this idea (19, 20). Since then, all models of foraging strategy (including those predicting concentration preferences) and energy balance in hummingbirds have calculated calorie intake rate under the assumption that the tongue tip is in the form of a pair of semicylindrical grooves that fill passively via capillarity when in contact with the nectar (21–26). The notion that fluid is drawn toward the mouth from the tongue tip and along the lingual grooves through the action of capillarity is currently widely accepted (19–28). However, if capillarity were responsible for tongue loading, the aid of gravity should increase nectar-uptake rates at pendulous (downward facing) flowers, yet empirical work in recent years has failed to demonstrate any consistent correlation between nectar extraction rates and flower position (26, 29). Similarly, according to the parameters of the capillarity models (19, 20), maximum energy intake is predicted to occur with nectar at low sugar concentrations [20–40% (mass/mass)]. Nonetheless, in experimental studies, hummingbirds offered a range of nectar concentrations (spanning those found in wild flowers) preferred higher values [45–65% (21, 24, 30–32)]. Such inconsistencies suggest that a mechanism other than capillarity is involved during tongue loading.
Here, we provide evidence for a different nectar-uptake mechanism and offer a biophysical hypothesis for our observations of tongue–nectar interactions. We found that, contrary to the capillarity models, hummingbird tongue tips dynamically trap nectar by rapidly changing their shape during feeding (Fig. 2 and Movies S1 and S2). High-speed video observations show that an entire tongue transformation cycle occurs in as little as 1/20th of a second (cf. ref. 33). This oscillating transformation is driven by fluid and atmospheric forces acting directly on morphological elements of the tongue tips. This description of a (highly efficient) dynamic liquid collecting mechanism has implications for the development of capillary-driven self-assembly of flexible structures (34, 35), and may be useful in microfluidic (36, 37) and microelectromechanical (34, 38) systems with a broad range of applications [e.g., micropliers (39)].
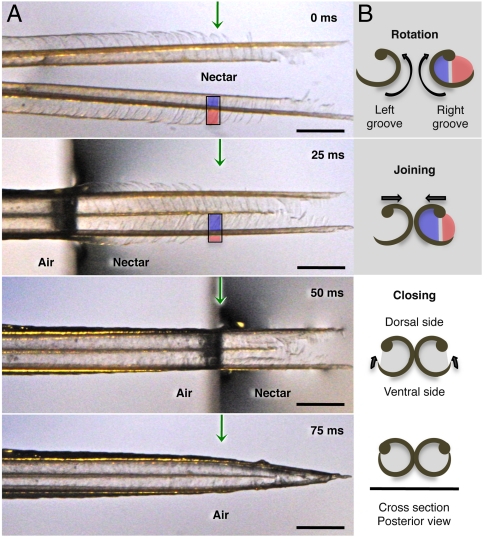
Fig. 2.
Hummingbird tongue trapping nectar. (A) Dorsal view of a post mortem tongue tip (A. colubris) leaving nectar, from totally immersed (Top photograph) at 0 milliseconds (ms), to outside the liquid (Bottom photograph). Green arrows mark the same reference point on the tongue in each image. (B) Cross-sectional diagrams (right margin) indicate the changes in position of lamellae at the reference point over time. From top to bottom: inside rotation of the entire structure (blue and red colors represent portions of visible lamellae along each side of the rod), tongue tips joining, and lamellae closing. In the first two diagrams, lamellae are inside the nectar; in the last two, lamellae have been withdrawn but contain nectar trapped inside the grooves. Scale bars, 0.5 mm.
Results
Hummingbird Tongue Morphology.
Earlier studies have shown that the distal portion of a hummingbird tongue is bifurcated, with each side forming a groove (by the sides furling inward) when the structures are wet, and that the tongue tips have membranous edges that are fringed with lamellae (18, 40–42). We provide here previously uncharacterized morphological details. We examined the fringed (lamellar) region of the tongue tip of 120 specimens in 20 species of hummingbirds (Table S1). We found that the last approximately 6 mm of the tongue (regardless of its total length) is structured in a previously undocumented arrangement (Fig. 1 B–D). The lamellae are supported longitudinally by rods (cf. ref. 40), and we found that these structures change their relative position both anatomically (along the tongue’s length; Fig. 1 C and D) and dynamically (during the process of feeding; Fig. 2). The change in orientation of the supporting rods in resting position, from the dorsal (proximally) to the ventral side (distally, at the tips) of the tongue (Fig. 1 C and D), allows the rotation of the tongue tips when they are withdrawn from the nectar (Fig. 2B and Movie S3), which in turn could improve liquid collection in shallow nectar layers (a common condition in horizontal flowers).
Mechanics.
We used high-speed video, at rates up to 2,400 frames/s, to document the mechanics of whole, unaltered hummingbird tongues moving in and out of nectar. We filmed 30 free-living birds (10 species; Table S1) attracted to a modified feeder; hereafter, we refer to these results as in vivo observations. To improve visualization of the mechanics, and to assess the degree of control of the mechanism that birds might exert via tongue muscles, we also used 20 tongues removed from salvaged carcasses of dead hummingbirds (4 species; Table S1). We emulated position and movements of the tongue and air–nectar interface under controlled laboratory conditions. The results from these salvaged specimens are hereafter referred to as post mortem observations.
Both the in vivo and post mortem observations reveal that before entering the fluid the tongue is wet (with some nectar inside) and the lamellae are tightly furled in a flattened tube-like conformation, with the tongue tips adhering to each other, forming a pointed, unitary structure (Fig. 1 B and C). Upon contact with fluid, the lamellae immediately unfurl and the tips separate (shown in vivo in Movies S1 and S2). At full immersion, the tongue tips are completely bifurcated and the lamellae entirely extended (Fig. 2, 0 ms). As the tongue is withdrawn from the fluid, the lamellae roll inward, trapping the nectar (shown post mortem in Movie S3). In vivo observations were wholly consistent with the higher-resolution visualization provided by manipulated post mortem tongues.
Post mortem observations were particularly useful in observing the details of the tongue furling process because they could be made under the highest magnification and the highest filming rate. As the tongue is withdrawn from the nectar, each lamella begins closing just before it passes the air–nectar interface, and is fully closed by the interface itself (shown post mortem in Movie S4). This implies that physical forces at the nectar surface are involved in the liquid collection (Fig. 3). We also noted that the progressively smaller lamellae toward the tongue tip (Figs. 1D and 2A) impart a conical shape, distally closed, at the furled tip when the tongue is withdrawn from the nectar (shown post mortem in Movie S3). We surmise that this creates a “lingual seal,” preventing fluid from dripping out of the tongue during the transit from the nectar chamber to the interior of the beak; avoiding nectar leakages could be especially important at high licking rates [approximately 17 Hz (33)] when inertial forces would tend to dislodge fluid from the tongue tip.
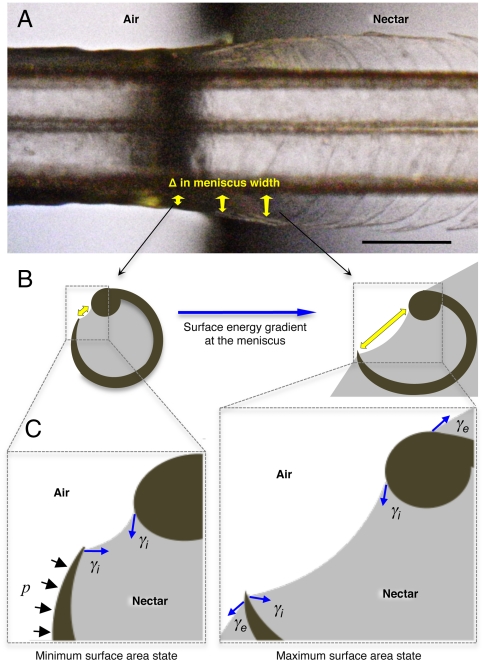
Fig. 3.
Conceptual hypothesis of the forces involved in lamellar closing. Blue arrows indicate the force exerted by surface tension (γ). Black arrows represent the Laplace pressure (p). (A) Dorsal view of a post mortem tongue (A. colubris) interacting with the air–nectar interface, showing the change in lamellar position with respect to the change in meniscal width (sagittally inclined yellow arrows). (B) Cross-section diagrams indicating the surface energy gradient on the internal menisci outside the nectar (Left) and at the beginning of the interface (Right). Yellow arrows depict meniscal width matching the points in the Upper panel. (C) Conceptual representation of the main forces acting on each lamella. Note that the minimum surface area state is achieved outside the nectar (Left) and the maximum surface area state is reached at the beginning of the interface (Right). When the tongue is leaving the nectar and the fluid no longer covers the outer wall of the lamella, the external component of the surface tension (γe) stops operating on the structure, Laplace pressure (p) begins to act and the surface area tends to be reduced by the internal component of surface tension (γi). The net result is the bending of the flexible lamella over the stiffer rod. Scale bar, 0.5 mm.
Our in vivo videos show that hummingbirds maintain a wider opening between the bill tips while retracting their nectar-loaded tongues than during protrusion (compare Fig. 4A vs. Fig. 4E; cf. ref. 33). We have observed in live birds that during tongue protrusion the bill is opened only at the tip, and apparently only enough to allow the tongue to squeeze past the upper and lower bill tips (cf. ref. 33 and Movie S1). These observations confirm that the distal portion of the tongue is furled, and compressed dorsoventrally during tongue protrusion, and that the compression is caused by the bill tips that are held closer together at this time (Fig. 4 A and B, frames in first column) than during retraction (Fig. 4 D and E).
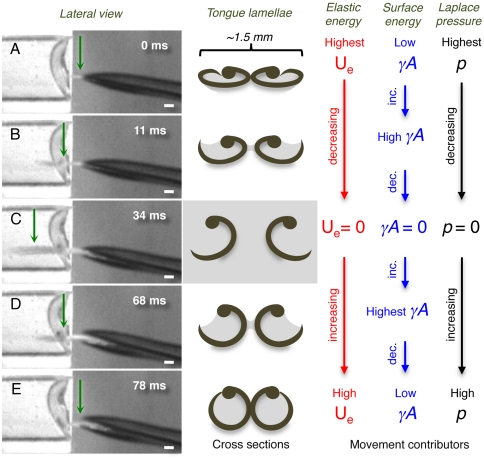
Fig. 4.
Conceptual hypothesis for lamellar movements during the licking (tongue) cycle. (Left column) Frames from the high-speed videos showing a lateral view of the bill tip and the tongue of a living Indigo-capped Hummingbird (Amazilia cyanifrons). Green arrows identify the cross-sections denoted in the middle column. (Center column) Cross-sections of the tongue tip showing the shape of the lamellae on each frame in the Left column. (Right columns) Conceptual depiction of the hypothesized relative contributions of the most important contributors to lamellar movements on each shape of the lamellae. Red stands for elastic potential energy (Ue), blue for surface energy (γA), and black for Laplace pressure (p). (A) The cycle begins when the tongue is protruded through a narrow space left when the bill tips are separating from each other. (B) Tongue penetrating the nectar located in the artificial feeder on the Left. (C) Maximum protrusion distance of the tongue in this licking cycle. (D) Tongue leaving the fluid while being retracted inside the bill. (E) Tongue almost fully retracted inside the bill; when the bill closes the cycle starts again. Scale bars, 1 mm.
Discussion
Our observation of rapid lamellar unfurling rules out the idea that the hummingbird tongue tip acts as a set of static capillary tubes during nectar feeding (18–28, 41). The tongue does not passively draw floral nectar up into the grooves via capillarity when its tips contact the liquid; rather, it is dynamically trapping nectar within the lamellae while the tips leave the fluid. Our work with dead specimens demonstrates that neither the unfurling nor the furling of the lamellae requires any muscular work; the process of nectar trapping results purely from the structural configuration of the tongue tips. We are unaware of any other biological mechanism for fluid trapping that is similarly dynamic, yet requires no energy expenditure to drive the opening and closing of the fluid trap.
Discovery of this dynamic nectar-trapping mechanism defies a consensus almost two centuries old, and has broad implications for our understanding of the evolution (16, 23, 43), energy budgets (24, 29, 44), foraging behavior (25, 26, 45), feeding mechanics (33, 41, 42), and morphology of the feeding apparatus (18, 46, 47) of hummingbirds. Our morphological survey documented the existence of the structures necessary for dynamic nectar trapping in species of hummingbirds representing all nine main clades in the family (cf. ref. 48). Thus, it is reasonable to assume, on the basis of the anatomical evidence, that the dynamic nectar-trapping mechanism documented here is present in every species of hummingbird. We suggest that dynamic nectar trapping is likely to be a component of the feeding mechanics of other nectarivorous birds with convergent tongue morphologies (26, 28, 41, 49, 50). Mechanistically, dynamic trapping appears likely to be functionally superior to simple capillarity in two ways: (I) the tongue-loading rate is not limited by the nectar displacement inside the tongue grooves (which makes it potentially faster) and, perhaps more importantly, (ii) the tongue tip can capture fluid successfully (filling its entire capacity) even in thin layers of nectar. This should allow hummingbirds to take full advantage of even the smallest quantity of resource offered in the shortest amount of time, which also has implications for the minimum volume of nectar a flower must offer in order to attract pollination services.
From a practical point of view, further understanding of this highly efficient liquid collecting mechanism may be useful in bionics (or biomimicry); for instance, in the development of low-energy mechanisms for trapping, transporting, and depleting fluids at high production rates, including surface interactions at the microscale (e.g., refs. 34–39) with industrial (e.g.. refs. 36 and 37) and biomedical (e.g., ref. 51) applications. But for these practical applications to be realized, it will be important to answer the question: How does it work? We offer below, as a hypothesis to be tested, an initial biophysical explanation of the nectar-trapping mechanism. This conceptual model can serve to generate testable predictions. Some qualitative predictions can be addressed with observations from this study, but most will require a deeper mathematical treatment to generate quantitative predictions that are testable with measurements of the tongue action under a variety of conditions.
Biophysical Hypothesis.
We hypothesize that the dynamic nectar-trapping process we have observed results from the interplay among surface tension, Laplace pressure, and the elastic properties of the keratinous materials making up the tongue tip (Figs. 3 and 4).
We define the start of nectar feeding as the point at which the bird first approaches and inserts its beak into a flower, with the tongue inside the closed bill. The bird protrudes its tongue through a small aperture of the bill tips (cf. ref. 33), and past this point the tongue continues to be flattened (Fig. 4 A and B, frames on first column). We posit that at this point (past the compression point of the bill tips) the cohesive and adhesive forces of liquid previously trapped inside the tongue and Laplace pressure keep the lamellae, and hence the grooves, at the tongue tip furled and in a dorsoventrally flattened configuration (Fig. 4A, cross-section diagram).
In further support of the idea that physical forces (acting on the fluid trapped inside the tongue) are responsible for keeping the lamellae furled, we have observed in post mortem specimens that when the tongue is completely dry the lamellae open and the grooves lose their cylindrical shape. Thus lamellar furling stores potential energy by bending the flexible lamellae. We suggest that this elastic potential energy is then transformed into kinetic energy when the lamellae unfurl as the tongue enters the nectar. This occurs because as the lamellae are immersed (with liquid on both the outside and the inside of the tongue), opposing surface tension forces at the air–nectar interface cancel each other out (Fig. 3C), allowing the lamellae to open. Thus, inside the liquid, the tongue structures should be released from the forces acting on them outside the nectar pool (Fig. 4C). Two of our observations are consistent with our hypothesis of the forces acting on the lamellae. First, as each lamella crosses the air–nectar interface, it unfurls (Fig. 4B); second, as the tongue penetrates further, the tongue tips separate (Movie S2).
We have also observed (both in vivo and post mortem) that when the tongue is withdrawn from the liquid, each lamella refurls as it reaches the air–nectar interface, thereby trapping nectar. We hypothesize that surface tension at the tongue–fluid interface and Laplace pressure combine to refurl the structure using the supporting rod as a closing and rotational axis (Fig. 3 B and C and Movies S3 and S4). In this model, the surface energy acting on each lamella is expected to build up when the structure approaches the air–nectar interface and should decrease with the subsequent lamellar furling (Fig. 4 D and E). The combination of surface tension along the contact line (the change in meniscal width represented by the three-dimensionally inclined yellow arrows in Fig. 3A) and Laplace pressure should be sufficient to overcome the bending force opposing the lamellar closing (Figs. 3 B and C and 4E). The magnitude of the bending force involved will be quantifiable only through an understanding we currently lack of the physical properties of the keratinized tongue tissue.
Finally, we have observed that when the tongue is entirely free from the nectar pool, the forked tongue tips stick together again; we hypothesize that this results from the cohesive and adhesive forces of the liquid layer between them (Fig. 2A, 25 ms, and Fig. 4D, cross-section diagram).
Future Directions.
Now that we have shown how nectar is captured at the tongue tip, the next step is to document the mechanics and path of nectar transport along the portions of the tongue that remain outside the nectar and inside the beak. In order to complete the cycle and initiate the nectar-ingestion process, the bird must retract the tongue within the bill and offload the trapped nectar, using an as-yet undocumented process; thereafter the cycle can start again.
Our videos showing that the tongue is dorsoventrally compressed during protraction (cf. ref. 33, Movie S1), suggest that nectar offloading might be accomplished during the tongue protrusion phase by the beak tips “squeezing” nectar off the tongue and into the interior of the bill. It is worth noting that we expect this nectar offloading to clear fluid only from the distal-most portion of the tongue at the start of every tongue cycle. However, the portion of the tongue (and attendant grooves) that remains inside the bill would still be filled with nectar and would also need somehow to be offloaded. Furthermore, after the final lick and tongue retraction at a given flower, the whole tongue would still be loaded with nectar. This hypothesis, that hummingbirds are squeezing nectar from the tongue by protracting it through narrowly opened bill tips, is consistent with the common observation that wild hummingbirds continue cycling their tongues, with a much greater protraction distance than would be necessary inside a flower, even after the tongue has been withdrawn from it.
To actually consume the nectar, the bird must transport the offloaded nectar into the pharynx, where it can be swallowed. The mechanics of this crucial last step of nectar feeding is completely unknown, and the understanding of this process requires further study. Capillary transport of nectar in tongue grooves alone cannot account for transport of nectar from the tongue into the pharynx. In the absence of any additional forces, once the tongue grooves are fully loaded the system should reach equilibrium, and the nectar should cease to move any further. We suspect that a variety of mechanisms (such as suction, surface tension transport, and hydraulic pressure) are mediated by bill–tongue interactions actively controlled by the bird in order to move nectar to the pharynx and thence into the esophagus. Achieving an understanding of this intraoral transport system is likely to be challenging, because the process cannot be observed directly through the bill.
The conceptual hypothesis we offer here for the observed dynamic nectar trapping is in agreement with the empirical data available on hummingbird foraging preferences (21, 24, 26, 29–32). Because the force of gravity should be negligible in comparison to other forces during the lamellar closing process (Figs. 3 and 4), no variation in the extraction rate is expected when varying flower position [in contrast to the capillarity models in which gravity is a determinant (19, 20)] and in fact, none is consistently seen in experiments with living birds (21, 26). Similarly, given the Reynolds number (approximately 1–10) for the different interactions at the tongue–fluid boundary, any drag due to viscosity [also a determinant in the capillarity models (19, 20)] should be overcome by Laplace pressure and surface tension (Figs. 3 and 4). Higher nectar concentrations are not, therefore, expected to limit fluid intake rate [nectar volume uptake (μL/s)]. Hence, the optimal sugar concentration for a foraging hummingbird should not be limited by the loading portion of the lingual cycle. In contrast, the capillarity models predict that optimal sugar concentrations should be in the range of 20–40% (mass/mass) because those models assume that tongue loading is the rate-limiting step of uptake (19, 20). Instead, concentrations preferred by living birds [45–65% (21, 24, 30–32)] are more likely to be determined by mechanisms of intraoral transport yet to be investigated, or by physiological constraints on uptake and metabolism of the sugars in the nectar (52, 53).
Our work raises anew the question: How do hummingbirds feed? Much work remains before we can explain the whole nectar-feeding process in hummingbirds and other nectarivores. Achieving a fuller understanding of the mechanics of the nectar-feeding process may help eliminate the disparity between the theoretical predictions of how birds should act and empirical observations of what they actually do. We believe that investigations of the physical basis of dynamic nectar trapping can also lead to new tools for the development of engineering applications in microfluidics.
Methods
Morphological Survey of the Tongue Tips.
We examined the tongues of 20 species (three adults/sex/species, for a total of 120 specimens) representing the nine major clades of hummingbirds (Table S1) at magnifications up to 90×. We scrutinized the hummingbird tongues, focusing on their distal region and characterizing the three-dimensional arrangement of their different structures (grooves, supporting rods, lamellae). In our survey, we included morphologically extreme species (e.g., White-tipped Sicklebill Eutoxeres aquila, with a strongly decurved bill) as well as the species with the longest and shortest tongues (Sword-billed Hummingbird Ensifera ensifera, and Purple-backed Thornbill Ramphomicron microrhynchum, respectively). We used whole, alcohol-preserved specimens from: the Instituto de Ciencias Naturales, Universidad Nacional de Colombia; the Vertebrate Research Collection, University of Connecticut; the National Bird Collection, Smithsonian Institution; and the Department of Ornithology, American Museum of Natural History.
In Vivo Filming of the Tongue–Nectar Interactions.
We worked at three different elevations (1700, 2400, 2800 m above sea level) in the Andes mountains in Colombia, South America. We filmed free-living hummingbirds of 10 species (three individuals per species; Table S1) feeding at flat-sided (as opposed to tubular, to minimize image distortion) transparent feeders filled with artificial nectar (18.6% mass/mass sucrose concentration). We filmed the tongue–fluid interactions with high-speed cameras (Phantom Miro eX4, monochrome and color) with macro lenses (Nikon 105 mm f/2.8) running at 1,260 frames/s (Fig. 4 and Movies S1 and S2).
Laboratory (Post Mortem) Filming of the Tongue–Nectar Interactions.
We used whole tongues of five recently deceased individuals (salvaged specimens) of four species (Archilochus colubris, Colibri coruscans, Eriocnemis vestita, and Metallura tyrianthina). We fixed each tongue in place and then slid a drop of artificial nectar (18.6% sucrose concentration) on a glass microscope slide onto and off of the tongue tip (Figs. 2A and 3A and Movies S3 and S4). We filmed the tongue–fluid interaction by coupling high-speed cameras (TroubleShooter HR and Phantom Miro eX4) running up to 2,400 frames/s to a dissecting microscope (Olympus SZX-12) at magnifications up to 50× (Movie S4). We also coupled a digital camera (Casio EX-FH20) to the dissecting microscope to take high-resolution (7 Megapixels) still pictures at 40 frames/s (Fig. 2A).
Animal Welfare Statement.
All hummingbird filming activities in this study were reviewed and authorized by the Institutional Animal Care and Use Committee at the University of Connecticut; Institutional Animal Care and Use Committee Exemption Number E09-010.
Supplementary Material
Acknowledgments.
We thank D. Sustaita for extensive feedback, R. Colwell, K. Schwenk, C. Elphick, T. Fan, and two anonymous reviewers for critical reading, K. Hurme for substantial comments, C. Clark and R. Prum for the loan of high-speed cameras, and T. Landberg and B. Ryerson for discussion. Many thanks to G. Stiles, and the staff of the ornithological collections at the Universidad Nacional de Colombia, the University of Connecticut, the Smithsonian Institution, and the American Museum of Natural History. This work was supported by a Multidisciplinary Environmental Research Award from the Center for Environmental Sciences and Engineering of the University of Connecticut to A.R.-G.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
See Commentary on page 9321.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016944108/-/DCSupplemental.
References
- 1.Vogel S. Life’s Devices. Princeton, NJ: Princeton Univ Press; 1988. pp. 82–129. [Google Scholar]
- 2.Denny MW. Air and Water. Princeton, NJ: Princeton Univ Press; 1993. [Google Scholar]
- 3.Tributsch H, Nadeždina N, Čermák J. Infrared images of heat fields around a linear heater in tree trunks: What can be learned about sap flow measurement? Ann For Sci. 2006;63:1–8. [Google Scholar]
- 4.Tyree MT. Plant hydraulics: The ascent of water. Nature. 2003;423:923. doi: 10.1038/423923a. [DOI] [PubMed] [Google Scholar]
- 5.Suter RB, Rosenberg RB, Loeb S, Wildman H, Long JH. Locomotion on the water surface: Propulsive mechanisms of the fisher spider Dolomedes triton. J Exp Biol. 1997;200:2523–2538. doi: 10.1242/jeb.200.19.2523. [DOI] [PubMed] [Google Scholar]
- 6.Suter RB, Gruenwald J. Predator avoidance on the water surface? Kinematics and efficacy of vertical jumping by Dolomedes (Araneae: Pisauridae) J Arachnol. 2000;28:201–210. [Google Scholar]
- 7.Hu DL, Bush JWM. Meniscus-climbing insects. Nature. 2005;437:733–736. doi: 10.1038/nature03995. [DOI] [PubMed] [Google Scholar]
- 8.Hu DL, Bush JWM. The hydrodynamics of water-walking arthropods. J Fluid Mech. 2010;644:5–33. [Google Scholar]
- 9.Rubega MA, Obst BS. Surface-tension feeding in phalaropes: Discovery of a novel feeding mechanism. Auk. 1993;110:169–178. [Google Scholar]
- 10.Rubega MA. Surface tension prey transport in shorebirds: How widespread is it? Ibis. 1997;139:488–493. [Google Scholar]
- 11.Blossey R. Self-cleaning surfaces: Virtual realities. Nat Mater. 2003;2:301–306. doi: 10.1038/nmat856. [DOI] [PubMed] [Google Scholar]
- 12.Song W, Veiga DD, Custódio CA, Mano JF. Bioinspired degradable substrates with extreme wettability properties. Adv Mater. 2009;21:1830–1834. [Google Scholar]
- 13.Parker AR, Lawrence CR. Water capture by a desert beetle. Nature. 2001;414:33–34. doi: 10.1038/35102108. [DOI] [PubMed] [Google Scholar]
- 14.Zheng Y, et al. Directional water collection on wetted spider silk. Nature. 2010;463:640–643. doi: 10.1038/nature08729. [DOI] [PubMed] [Google Scholar]
- 15.Schwenk K, Rubega MA. Diversity of vertebrate feeding systems. In: Starck JM, Wang T, editors. Physiological and Ecological Adaptations to Feeding in Vertebrates. Enfield, NH: Science Publishers; 2005. pp. 1–41. [Google Scholar]
- 16.Stiles FG. Geographical aspects of bird-flower coevolution, with particular reference to Central America. Ann Mo Bot Gard. 1981;68:323–351. [Google Scholar]
- 17.Fleming TH, Muchhala N. Nectar-feeding bird and bat niches in two worlds: Pantropical comparisons of vertebrate pollination systems. J Biogeogr. 2008;35:764–780. [Google Scholar]
- 18.Martin WCL. In: The Naturalist’s Library: A General History of Humming-Birds or the Trochilidae. Jardine W, editor. Vol 41. London: H.G. Bohn; 1833. pp. 65–68. [Google Scholar]
- 19.Kingsolver JG, Daniel TL. Mechanical determinants of nectar feeding strategy in hummingbirds: Energetics, tongue morphology, and licking behavior. Oecologia. 1983;60:214–226. doi: 10.1007/BF00379523. [DOI] [PubMed] [Google Scholar]
- 20.Heyneman AJ. Optimal sugar concentrations of floral nectars: Dependence on sugar intake efficiency and foraging costs. Oecologia. 1983;60:198–213. doi: 10.1007/BF00379522. [DOI] [PubMed] [Google Scholar]
- 21.Tamm S, Gass CL. Energy intake rates and nectar concentration preferences by hummingbirds. Oecologia. 1986;70:20–23. doi: 10.1007/BF00377107. [DOI] [PubMed] [Google Scholar]
- 22.Stromberg MR, Johnsen PB. Hummingbird sweetness preferences: Taste or viscosity? Condor. 1990;92:606–612. [Google Scholar]
- 23.Gass CL, Roberts WM. The problem of temporal scale in optimization: Three contrasting views of hummingbird visits to flowers. Am Nat. 1992;140:829–853. doi: 10.1086/285443. [DOI] [PubMed] [Google Scholar]
- 24.Roberts WM. Hummingbirds’ nectar concentration preferences at low volume: The importance of time scale. Anim Behav. 1996;52:361–370. [Google Scholar]
- 25.Bateson M, Healy SD, Hurly TA. Context-dependent foraging decisions in rufous hummingbirds. Proc Biol Sci. 2003;270:1271–1276. doi: 10.1098/rspb.2003.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins BG. Nectar intake and foraging efficiency: Responses of honeyeaters and hummingbirds to variations in floral environments. Auk. 2008;125:574–587. [Google Scholar]
- 27.Renvoisé P, Bush JWM, Prakash M, Quéré D. Drop propulsion in tapered tubes. Europhys Lett. 2009;86:64003. [Google Scholar]
- 28.Köhler A, Leseigneur CDC, Verburgt L, Nicolson SW. Dilute bird nectars: Viscosity constrains food intake by licking in a sunbird. Am J Physiol Regul Integr Comp Physiol. 2010;299:1068–1074. doi: 10.1152/ajpregu.00208.2010. [DOI] [PubMed] [Google Scholar]
- 29.Montgomerie RD. Nectar extraction by hummingbirds: Response to different floral characters. Oecologia. 1984;63:229–236. doi: 10.1007/BF00379882. [DOI] [PubMed] [Google Scholar]
- 30.Stiles FG. Taste preferences, color preferences, and flower choice in hummingbirds. Condor. 1976;78:10–26. [Google Scholar]
- 31.Van Riper W. Hummingbird feeding preferences. Auk. 1958;75:100–101. [Google Scholar]
- 32.Pyke GH, Waser NM. The production of dilute nectars by hummingbird and honeyeater flowers. Biotropica. 1981;13:260–270. [Google Scholar]
- 33.Ewald PW, Williams WA. Function of the bill and tongue in nectar uptake by hummingbirds. Auk. 1982;99:573–576. [Google Scholar]
- 34.Guo X, et al. Two- and three-dimensional folding of thin film single-crystalline silicon for photovoltaic power applications. Proc Natl Acad Sci USA. 2009;106:20149–20154. doi: 10.1073/pnas.0907390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu JL, Nie ZX, Jiang WG. Deformation field of the soft substrate induced by capillary force. Phys B Condensed Matter. 2009;404:1195–1199. [Google Scholar]
- 36.Vestad T, Marr DWM, Oakey J. Flow control for capillary-pumped microfluidic systems. J Micromech Microeng. 2004;14:1503–1506. [Google Scholar]
- 37.Luo JK, et al. Moving-part-free microfluidic systems for lab-on-a-chip. J Micromech Microeng. 2009;19:054001. [Google Scholar]
- 38.Bico J, Roman B, Moulin L, Boudaoud A. Elastocapillary coalescence in wet hair. Nature. 2004;432:690. doi: 10.1038/432690a. [DOI] [PubMed] [Google Scholar]
- 39.Py C, et al. Capillary origami: Spontaneous wrapping of a droplet with an elastic sheet. Phys Rev Lett. 2007;98:156103. doi: 10.1103/PhysRevLett.98.156103. [DOI] [PubMed] [Google Scholar]
- 40.Weymouth RD, Lasiewski RC, Berger AJ. The tongue apparatus in hummingbirds. Acta Anat. 1964;58:252–270. doi: 10.1159/000142586. [DOI] [PubMed] [Google Scholar]
- 41.Scharncke H. Contribution to the morphology and developmental evolution of the tongue of the Trochilidae, Meliphagidae and Picidae (Beiträge zur Morphologie und Entwicklungsgeschichte der Zunge der Trochilidae, Meliphagidae und Picidae) J Ornithol. 1931;79:425–491. (in German) [Google Scholar]
- 42.Hainsworth FR. On the tongue of a hummingbird: Its role in the rate and energetics of feeding. Comp Biochem Physiol. 1973;46:64–78. doi: 10.1016/0300-9629(73)90559-8. [DOI] [PubMed] [Google Scholar]
- 43.Stiles FG. Seasonal patterns and coevolution in the hummingbird-flower community of a Costa Rican subtropical forest. Ornithol Monogr. 1985;36:757–787. [Google Scholar]
- 44.Weathers WW, Stiles FG. Energetics and water balance in free-living tropical hummingbirds. Condor. 1989;91:324–331. [Google Scholar]
- 45.Baum KA, Grant WE. Hummingbird foraging behavior in different patch types: Simulation of alternative strategies. Ecol Modell. 2001;137:201–209. [Google Scholar]
- 46.Berns CM, Adams DC. Bill shape and sexual shape dimorphism between two species of temperate hummingbirds: Black-Chinned hummingbird (Archilochus alexandri) and Ruby-Throated hummingbird (A. colubris) Auk. 2010;127:626–635. [Google Scholar]
- 47.Temeles EJ, Roberts WM. Effect of sexual dimorphism in bill length on foraging behavior: An experimental analysis of hummingbirds. Oecologia. 1993;94:87–94. doi: 10.1007/BF00317307. [DOI] [PubMed] [Google Scholar]
- 48.McGuire JA, Witt CC, Remsen JV, Jr, Dudley R, Altshuler DL. A higher-level taxonomy for hummingbirds. J Ornithol. 2009;150:155–165. [Google Scholar]
- 49.Paton DC, Collins BG. Bills and tongues of nectar-feeding birds: A review of morphology, function and performance, with intercontinental comparisons. Austral Ecol. 1989;14:473–506. [Google Scholar]
- 50.Downs CT. Some preliminary results of studies on the bill and tongue morphology of Gurney’s Sugarbird and some southern African sunbirds. Ostrich. 2004;75:169–175. [Google Scholar]
- 51.Sohn YS, et al. A microbead array chemical sensor using capillary-based sample introduction: Toward the development of an “electronic tongue”. Biosens Bioelectron. 2005;21:303–312. doi: 10.1016/j.bios.2004.08.050. [DOI] [PubMed] [Google Scholar]
- 52.Diamond JM, Karasov WH, Phan D, Carpenter FL. Digestive physiology is a determinant of foraging bout frequency in hummingbirds. Nature. 1986;320:62–63. doi: 10.1038/320062a0. [DOI] [PubMed] [Google Scholar]
- 53.McWhorter TJ, Martínez del Rio C. Does gut function limit hummingbird food intake? Physiol Biochem Zool. 2000;73:313–324. doi: 10.1086/316753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.