Abstract
Reciprocal selective effects between coevolving species are often influenced by interactions with the broader ecological community. Community-level interactions may also influence macroevolutionary patterns of coevolution, such as cospeciation, but this hypothesis has received little attention. We studied two groups of ecologically similar feather lice (Phthiraptera: Ischnocera) that differ in their patterns of association with a single group of hosts. The two groups, “body lice” and “wing lice,” are both parasites of pigeons and doves (Columbiformes). Body lice are more host-specific and show greater population genetic structure than wing lice. The macroevolutionary history of body lice also parallels that of their columbiform hosts more closely than does the evolutionary history of wing lice. The closer association of body lice with hosts, compared with wing lice, can be explained if body lice are less capable of switching hosts than wing lice. Wing lice sometimes disperse phoretically on parasitic flies (Diptera: Hippoboscidae), but body lice seldom engage in this behavior. We tested the hypothesis that wing lice switch host species more often than body lice, and that the difference is governed by phoresis. Our results show that, where flies are present, wing lice switch to novel host species in sufficient numbers to establish viable populations on the new host. Body lice do not switch hosts, even where flies are present. Thus, differences in the coevolutionary history of wing and body lice can be explained by differences in host-switching, mediated by a member of the broader parasite community.
Keywords: coevolutionary biology, community ecology, phoresy, ectoparasites
Coevolving species do not live in isolation; they are part of a broader ecological community. Community-level interactions are known to have an important influence on the dynamics of reciprocal selection and other microevolutionary aspects of coevolution (1–6). Community-level interactions may also influence cospeciation and other macroevolutionary patterns of coevolution, yet this topic has received relatively little attention. Here we report the results of a study showing that interactions between unrelated groups of parasites govern host-switching, with fundamental implications for patterns of host–parasite coevolutionary history.
A well-documented example of the influence of community interactions on coevolutionary dynamics involves Red Crossbills (Loxia curvirostra) and Rocky Mountain lodgepole pines (Pinus contorta) in the western United States. In regions where crossbills are the dominant seed predator, they select for larger, thicker-scaled cones that protect pine seeds. Increased cone size exerts reciprocal selection on crossbills for increased beak size. However, in areas where red squirrels (Tamiasciurus hudsonicus) are the dominant seed predator and out-compete crossbills, the birds adapt to average cone size and have smaller beaks (7, 8). Because populations of crossbills with different beak sizes tend not to interbreed, crossbills in regions with and without squirrels have undergone speciation (9). Thus, squirrels influence the coevolutionary dynamics of crossbills and pines over both micro- and macroevolutionary time.
Another example of the influence of community interactions on coevolutionary patterns involves fungus-growing attini ants, the fungi they cultivate, and parasitic fungi of the cultivars. Phylogenies of these three groups are broadly congruent, reflecting 50 million years of close association (10). However, the phylogenies do not mirror one another perfectly because of periodic switching of fungi between unrelated host lineages. Although mechanisms governing host-switching are not well understood, parasitoid wasps or mites are thought to play a role in dispersing fungi between host lineages (11, 12). Transport of one species by another, known as phoresis (13), is another way in which members of a broad community can conceivably alter patterns of coevolutionary history (14, 15).
Another system in which phoresis may play a role in influencing coevolutionary history consists of birds and feather-feeding lice (Phthiraptera: Ischnocera). Lice are permanent ectoparasites that complete their entire life cycle on the body of the host. Feather lice are so specialized for life on feathers that they seldom, if ever, venture onto the host's skin, much less away from the host's body. Lice are usually transmitted to new hosts during periods of direct contact, such as that between parent birds and their offspring in the nest; however, some groups of lice are also capable of transmission by hitchhiking rides on more mobile parasitic flies (16). Lice feed on feathers and dead skin, which are metabolized with the aid of endosymbiotic bacteria (17). The feather damage they cause has thermoregulatory costs that lead to reductions in host fitness (18). Birds defend themselves against feather lice by destroying or removing the lice with their bill during frequent bouts of preening (19).
We concentrated on two groups of feather lice, “wing” and “body” lice, found on pigeons and doves (Columbiformes). Although distantly related within Ischnocera, wing and body lice have similar natural histories and are considered “ecological replicates” (20). Wing lice spend most of their time and lay their eggs on the large flight feathers of the wings and tail. They have a long, slender shape that facilitates hiding between the coarse barbs of the flight feathers where they are protected from preening (18). In contrast, body lice spend all of their time and lay their eggs on abdominal feathers. Their oval shape and short legs allow them to escape preening by burrowing into the downy regions of these feathers (18). Despite their different distributions on the host, wing and body lice both require the downy regions of abdominal feathers for food.
Wing and body lice differ substantially in their patterns of host use. Body lice are significantly more host-specific, and exhibit more population genetic structure than wing lice (21). The evolutionary history of body lice also parallels that of the host more closely than does the evolutionary history of wing lice. Despite equally thorough sampling and phylogenetic resolution, 67% of nodes in the North American body louse phylogeny are congruent with the host phylogeny (P < 0.0006); only 25% of nodes in the wing louse phylogeny are congruent with the host phylogeny, which is no more than expected by chance (P > 0.15) (22). These differences in specificity, population genetic structure, and phylogenetic congruence demonstrate that wing lice are less closely associated with particular host lineages than body lice, both over micro- and macroevolutionary time.
Recent studies have tried to explain these different patterns of host use. One hypothesis is that wing lice switch host species more often than body lice. This switching could result if wing lice are better than body lice at establishing viable populations on novel host species. To test this hypothesis, Bush and Clayton (23) experimentally transferred lice among several species of captive North American pigeons and doves and closely monitored the survival and reproductive success of the lice on each host. Their results showed that the two groups of lice are equally capable of establishing viable populations on novel hosts. In other words, differences in establishment ability could not explain different patterns of host use in wing and body lice.
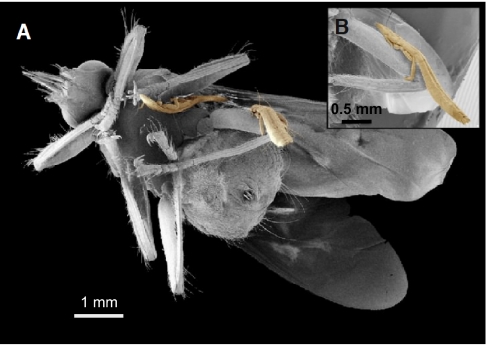
Another hypothesis to explain the different patterns of host use is that wing and body lice differ in their ability to disperse to novel host species in the first place. Neither group is capable of independent locomotion off the body of the host; however, wing lice are known to attach phoretically to hippoboscid flies (Fig. 1) (24–28). Harbison et al. (16) conducted experiments with captive Rock Pigeons (Columba livia), in which they showed that wing lice (Columbicola columbae and Columbicola tschulyschman) transmit horizontally between individual pigeons by hitchhiking on hippoboscid flies. Body lice (Campanulotes compar) were not capable of phoresis because they could not remain attached to flies, even when they were placed on them experimentally (16, 29). This functional constraint likely results from morphological adaptations of body lice for living in abdominal regions; they have short legs for burrowing in dense downy feathers, which limits their ability to remain attached to flies (29). In contrast, the long “outrigger” legs of wing lice provide a wide stance suited to the coarse surface of flight feathers. Using their long legs, wing lice are clearly able to hang onto hipposboscid flies (Fig. 1) (29).
Fig. 1.
(A) Pigeon fly (Pseudolynchia canariensis) with two wing lice (Columbicola columbae) attached. (B) Enlarged view showing how the louse uses its third pair of legs to grasp the fly's leg [scanning electron microscopy by E. H. Burtt, Jr. and J. Ichida (Ohio Wesleyan University)].
Many species of hippoboscid flies parasitize more than one species of host (25); therefore, it is conceivable that wing lice switch hosts via phoresis. Differences in phoretic ability could explain the differences in host specificity, population genetic structure, and cophylogenetic congruence of wing versus body lice. We conducted an experiment to test whether wing and body lice do, in fact, differ in their rates of host-switching, and whether phoresis plays a role. Our experiment involved Rock Pigeons, their wing and body lice, the pigeon fly Pseudolynchia canariensis, and a novel species of host: the Mourning Dove (Zenaida macroura).
Results
We tested for host-switching by lice from donor Rock Pigeons to Mourning Doves in experimental sheds with flies and control sheds without flies (Fig. 2). Flies were observed regularly on birds in the experimental sheds, but never on birds in the control sheds. Of 120 flies that were captured and examined, three (2.5%) had wing lice attached (Fig. 1); none of the 120 flies had body lice attached.
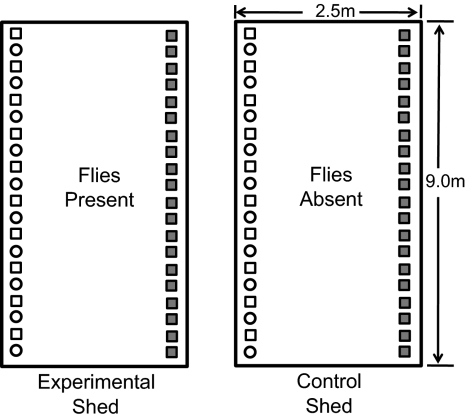
Fig. 2.
Experimental design. Rectangles are identical wooden sheds. Shaded squares were cages with louse-infested “donor” Rock Pigeons. Empty squares or circles were cages with “recipient” Rock Pigeons and Mourning Doves, respectively. Recipient birds had no lice at the start of the experiment. Flies were introduced to the experimental shed, but not the control shed. Treatments were reversed between years.
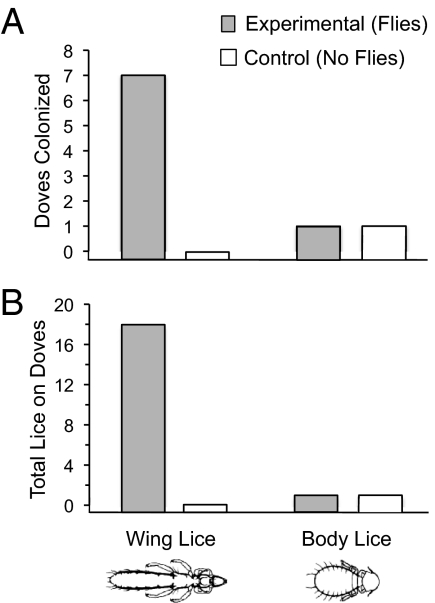
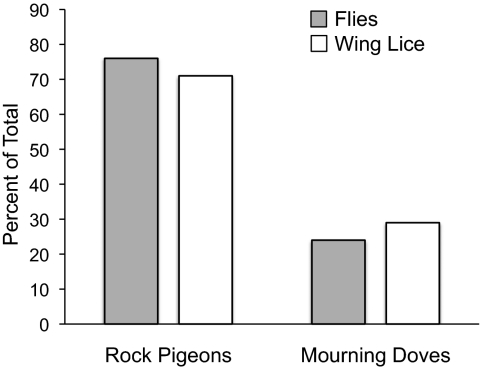
More doves had wing lice than body lice over the course of the experiment. Wing lice were observed on seven doves (21%) in experimental sheds, but none were observed on doves in control sheds (Fig. 3A) (Fisher's exact test, P = 0.03). Body lice were observed on one dove in experimental sheds, and one dove in control sheds (Fig. 3A) (Fisher's exact test, P = 1.0).
Fig. 3.
Host-switching results. (A) Seven doves were colonized by wing lice in the experimental sheds (flies present), but no doves were colonized by wing lice in the control sheds (flies absent). One dove was colonized by body lice in each type of shed. (B) A total of 18 wing lice colonized doves in the experimental sheds, but no wing lice colonized doves in the control sheds. One body louse colonized a dove in each type of shed.
The number of wing lice on doves was higher than that of body lice. Doves in experimental sheds had a total of 18 wing lice, but only one body louse (Fig. 3B) (Fisher's exact test, P = 0.0001). Doves in control sheds had no wing lice and only one body louse (Fig. 3B). Although immature lice were not included in calculating rates of phoresis (Materials and Methods), in the experimental sheds 32 immature wing lice were found on recipient pigeons, and seven immature wing lice were found on doves. In the control sheds no immature lice were found on either host species.
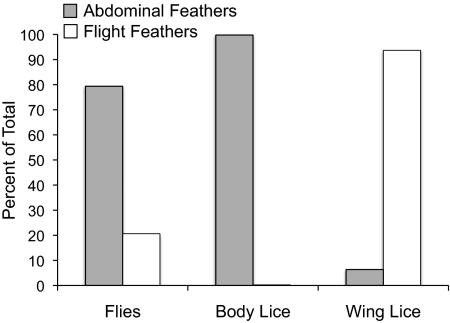
The differences in prevalence and abundance of wing versus body lice on doves were not artifacts of larger populations of wing lice on donor pigeons. Indeed, the number of body lice per donor bird was greater than the number of wing lice per donor in the experimental sheds (Table 1) (t = 2.4, df = 118, P = 0.02). Nor was the larger number of wing versus body lice an artifact of flies preferring the same body regions on the host as wing lice. Flies showed a preference for regions actually preferred by body lice (Fig. 4). Most body lice were observed on abdominal feathers, and most wing lice were observed on flight feathers (Table 1) (χ2 test, P < 0.0001). Like body lice, most flies were observed on abdominal feathers (Table 1) (χ2 test, P < 0.0001).
Table 1.
Relative abundance of flies and lice on donor birds in experimental sheds, and their relative distribution by host body region
| Mean (±SE) per bird | Total on abdominal feathers | Total on flight feathers | |
| Flies | 0.95 (±0.04) | 177 | 46 |
| Body Lice | 523 (±60) | 12,132 | 26 |
| Wing Lice | 370 (±24) | 798 | 11,802 |
Fig. 4.
Relative distribution of flies, body lice and wing lice on abdominal versus flight feathers (wings and tail) of donor birds in the experimental sheds; see Table 1 for data.
Over the 3 y of the experiment, 350 flies were observed on recipient Rock Pigeons (0.90 ± 0.09 per pigeon) and 108 flies were observed on Mourning Doves (0.27 ± 0.05 per dove). Flies showed a preference for the normal pigeon host, even when the number of flies on pigeons was reduced by two-thirds to account for the fact that Mourning Doves are one-third the size of Rock Pigeons (Wilcoxon Signed Rank, z = −3.96, P < 0.0001).
The number of wing lice observed on recipient pigeons versus doves was not significantly different from the relative distributions of flies on the two hosts (Fig. 5) (Fisher's exact test, P = 0.35), suggesting that wing lice left flies for a new bird host at the first opportunity.
Fig. 5.
Relative distribution of flies and wing lice on recipient pigeons versus doves in the experimental sheds; see Results for data.
Discussion
Our results show that wing lice switch hosts, and that switching is mediated by hippoboscid flies. Body lice did not switch hosts or engage in phoresis, despite the fact that they outnumbered wing lice on donor pigeons, and that flies spent more time in microhabitats favored by body lice. Only two body lice were ever found on Mourning Doves—one in an experimental shed and one in a control shed—suggesting accidental dispersal on our clothing.
The first step in phoresis-mediated host switching is that the louse must be capable of locating a fly to which it can attach. Our results are consistent with recent behavioral work showing that wing lice orient to hippoboscid flies, whereas body lice show no response to the same flies (29). Our results are also consistent with published accounts of Rock Pigeon wing lice found attached to wild flies (SI Appendix) (24–28). In contrast, the literature contains no accounts of Rock Pigeon body lice attached to wild flies (SI Appendix). Furthermore, published accounts of columbiform lice found on noncolumbiform birds mostly involve wing lice (30–33).
An underlying assumption of phoresis-mediated host-switching is that individual hippoboscid flies move between host species under natural conditions. To our knowledge, only one study has tested this assumption; Corbet (34) showed that about 7% of flies in a marked population dispersed between host species over the course of a week. Flies in our experiment were observed on both recipient pigeons and doves. Of 458 flies observed, 24% were on doves, despite the fact that P. canariensis is a parasite of Rock Pigeons. Thus, both data from the field and our experimental data from captive birds confirm that hippoboscid flies move between species of birds.
Another assumption of phoresis-mediated host switching is that lice attach to flies. Three of 120 (2.5%) flies in our study were observed with lice on them. Published studies of wild fly populations report lice on 8% to 43% of flies (25, 35–41). Although a single gravid female may be capable of founding a new population, dispersal of several lice to a new host undoubtedly improves the probability of establishing a viable population on that host. In our analysis of over 400 published records of lice attached to flies (SI Appendix), 60% of cases involved multiple lice on single flies, with up to 31 individual lice attached to a single fly (42). Recipient doves in our experiment had both adult and immature lice, suggesting that lice were capable of reproducing on doves after they dispersed to them. It is possible, however, that most, if not all, of the immature lice on doves were themselves phoretic. Published records of immature lice attached to flies do exist (42, 43).
Little is known about the frequency of lice dispersing between wild birds, but several records involve lice from distantly related hosts. For example, a fly removed from a woodpecker was carrying four Brueelia marginata, a species of louse that normally infests songbirds (37). Similarly, the literature contains two records of flies removed from swifts with ischnoceran songbird lice attached (25, 43). Swifts are normally host only to lice from the suborder Amblycera, which do not engage in phoresis because their mouthparts do not allow them to hang onto flies (44).
The final step in phoresis-mediated host-switching is that lice must disembark onto a novel host species. In our experiment the percentage of wing lice found on doves was not significantly different from the percentage of flies found on doves (Fig. 5), suggesting that lice leave flies at the first opportunity. It is also possible that flies sometimes knock lice off onto novel hosts. Harbison et al. (29) showed that, in vitro, flies use grooming to dislodge lice that have been placed on them experimentally. Although flies presumably cannot groom when they are flying, newly attached lice can be groomed off once the fly has landed on a new host.
Our data indicate that differences in the coevolutionary history of wing and body lice can be explained by differences in host-switching. Nonphoretic body lice track host lineages more closely than phoretic wing lice, leading to greater congruence between the phylogenies of body lice and their hosts, compared with wing lice and the same hosts (22). Because body and wing lice are equally capable of establishing viable populations on novel hosts once they are reached (23), it is this difference in dispersal ability that best explains the different patterns of association. Future work comparing the gene flow of phoretic and nonphoretic lice within and between host species could further clarify the impact of phoresis on processes such as local adaptation, specialization, and patterns of host–parasite cospeciation.
Columbiform wing and body lice are a powerful comparative system in which to study ecological factors influencing macroevolutionary differences in host association. It is more difficult to assess the relative influence of factors, such as ongoing phoresis, in bird–louse associations that lack this “ecological replicate” framework. However, it is worth noting that our results are consistent with patterns of host use in another well-studied louse genus, Brueelia. Over one-third of all phoretic events found in our literature survey involved Brueelia, indicating that phoretic dispersal occurs frequently in this genus. Phoresis may govern differences in the specificity of Brueelia from brood parasitic indigobirds (Vidua spp.), versus nonphoretic lice in the genus Myrsidea (15). Phoretic dispersal may also explain how a single species of Brueelia is able to infest every North American migrant thrush species (Catharus spp.), and maintain gene flow among these species (45). Not surprisingly, the phylogeny for Brueelia shows no congruence with host phylogeny. This lack of congruence has been attributed to frequent host-switching mediated by phoresis (14, 46).
Linking community interactions to coevolutionary dynamics is a stated goal of evolutionary ecologists (2). Phoresis provides just one example of how broad community interactions can influence coevolutionary dynamics over both micro- and macroevolutionary time. Our results highlight the importance of adopting a broad, community approach in studies of coevolution. Our results also demonstrate the potential of host–parasite systems for unraveling connections between community ecology and coevolutionary biology.
Materials and Methods
We conducted an experiment using wild-caught birds to test for host-switching from Rock Pigeons to Mourning Doves by wing and body lice. The long-term experiment was conducted over 3 y using identical wooden animal sheds (Fig. 2). Birds were individually housed in adjacent wire mesh cages (30 × 30 × 56 cm) along the walls of each shed. Plexiglas dividers prevented lice from moving between the feather tips of birds in adjacent cages. The sheds were kept on a 12:12 light/dark cycle, and birds were provided ad libitum food, water, and grit. All procedures followed guidelines of the Institutional Animal Care and Use Committee of the University of Utah.
One wall of each shed was lined with 20 cages containing “donor” Rock Pigeons that were naturally infested with wing lice and body lice. The opposite wall of each shed was lined with 20 cages containing 10 louse-free pigeons, interspersed with 10 louse-free doves (Fig. 2). Before the start of the experiment, the louse-free birds were “dried” in low humidity (< 40% rh) animal rooms for at least 10 wk. The drying procedure kills 100% of lice and their eggs by desiccation (16). After drying, all birds were carefully examined to confirm that they were, in fact, free of lice.
During the experiment, all birds (donors and recipients) were fitted with C-shaped plastic bits, which create a 1- to 3-mm gap between the mandibles that impairs efficient preening; bits have no side effects and bitted birds are able to feed normally (18). Bits were used to prevent lice from being preened off by recipient pigeons or doves before we could observe them.
At the start of each experimental period, pigeon flies were added to the experimental shed from a culture of wild-caught flies. Additional flies were periodically added to maintain fly levels at one to two flies per bird. Fly abundance on wild pigeons ranges from zero to eight flies per bird (47–50). The flies could move freely between donor and recipient birds in each experimental shed. No flies were introduced to the control sheds. Years 1 and 2 included one experimental and one control shed (reversed between years). Year 3 had one experimental shed. Data collection each year lasted 6 mo, from October through March in 2003 to 2004, 2004 to 2005, and 2006 to 2007, to avoid the protracted molting period of pigeons and doves.
Host-switching was measured in two stages. First, every 2 wk we did a careful 15-min visual examination of each “recipient” bird. The visual examination method detects wing lice and body lice with comparable accuracy (51). When lice were seen on a recipient bird, that bird, and the corresponding bird in the control shed, were killed, placed in separate plastic bags, and frozen. The birds were immediately replaced with new louse-free birds of the same species. A reciprocal procedure was followed if lice were detected on recipient birds in the control shed. Louse loads on the killed birds were determined using a washing technique that accounts for 99% of the lice on a bird (51).
At the end of each experiment, all remaining birds were killed, bagged, frozen, and washed to determine louse loads. Because doves were examined every 2 wk, we were able to distinguish phoretic adult lice from any offspring of those lice born after dispersal. Lice develop from egg to adult in ∼24 d (24); thus, adult lice observed during the 2-wk period must have come from donor birds, and immature lice could have come from donor birds, or hatched from eggs laid by newly arrived adult lice on recipient birds. Only adult lice were included when calculating rates of phoresis.
The number of wing and body lice on donor pigeons was also estimated each month using a 5-min visual examination (51). Fly abundance and the location of flies on birds were recorded during visual examinations of both donor and recipient birds. When possible, flies were caught, examined for phoretic lice, and then released. At the start of the experiment, the number of flies observed was 21% of the total number released in the shed. We used this percentage to extrapolate to the total number of flies present over the course of each year. Data from the sheds were combined over the 3 y of the experiment to compare rates of dispersal by wing versus body lice from donor pigeons to recipient pigeons and doves.
Supplementary Material
Acknowledgments
We thank Joe Atkin, Craig Benkman, Kurtis Birch, Stuart Howe, Kevin Johnson, Marcy Lloyd, Jael Malenke, Doug Neilson, David Slager, and Kevin Wilding for help with data collection and for comments on previous versions of the manuscript; Sarah Bush for comments and assistance with the figures; and E. H. Burtt, Jr. and J. Ichida for the scanning electron microscopy used in Fig. 1. This work was supported by National Science Foundation awards DEB-0118794, 0614565, and 0816877 (to D.H.C.) and National Science Foundation Graduate Fellowship DGE-0338340 (to C.W.H.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102129108/-/DCSupplemental.
References
- 1.Thompson JN. The Geographic Mosaic of Coevolution. Chicago: University of Chicago Press; 2005. [Google Scholar]
- 2.Johnson MTJ, Stinchcombe JR. An emerging synthesis between community ecology and evolutionary biology. Trends Ecol Evol. 2007;22:250–257. doi: 10.1016/j.tree.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Strauss SY, Sahli H, Conner JK. Toward a more trait-centered approach to diffuse (co)evolution. New Phytol. 2005;165(1):81–89. doi: 10.1111/j.1469-8137.2004.01228.x. [DOI] [PubMed] [Google Scholar]
- 4.Haloin JR, Strauss SY. Interplay between ecological communities and evolution: Review of feedbacks from microevolutionary to macroevolutionary scales. Ann N Y Acad Sci. 2008;1133:87–125. doi: 10.1196/annals.1438.003. [DOI] [PubMed] [Google Scholar]
- 5.Urban MC, et al. The evolutionary ecology of metacommunities. Trends Ecol Evol. 2008;23:311–317. doi: 10.1016/j.tree.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Dercole F, Ferriere R, Rinaldi S. Chaotic Red Queen coevolution in three-species food chains. Proc Biol Sci. 2010;277:2321–2330. doi: 10.1098/rspb.2010.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benkman CW, Holimon WC, Smith JW. The influence of a competitor on the geographic mosaic of coevolution between crossbills and lodgepole pine. Evolution. 2001;55:282–294. doi: 10.1111/j.0014-3820.2001.tb01293.x. [DOI] [PubMed] [Google Scholar]
- 8.Benkman CW, Parchman TL, Favis A, Siepielski AM. Reciprocal selection causes a coevolutionary arms race between crossbills and lodgepole pine. Am Nat. 2003;162:182–194. doi: 10.1086/376580. [DOI] [PubMed] [Google Scholar]
- 9.Smith JW, Benkman CW. A coevolutionary arms race causes ecological speciation in crossbills. Am Nat. 2007;169:455–465. doi: 10.1086/511961. [DOI] [PubMed] [Google Scholar]
- 10.Currie CR, et al. Ancient tripartite coevolution in the attine ant-microbe symbiosis. Science. 2003;299:386–388. doi: 10.1126/science.1078155. [DOI] [PubMed] [Google Scholar]
- 11.Currie CR. Prevalence and impact of a virulent parasite on tripartite mutualism. Oecologia. 2001;128(1):99–106. doi: 10.1007/s004420100630. [DOI] [PubMed] [Google Scholar]
- 12.Caldera EJ, Poulsen M, Suen G, Currie CR. Insect symbioses: A case study of past, present, and future fungus-growing ant research. Environ Entomol. 2009;38(1):78–92. doi: 10.1603/022.038.0110. [DOI] [PubMed] [Google Scholar]
- 13.Houck MA, O'Conner BM. Ecological and evolutionary significance of phoresy in the Astigmata. Annu Rev Entomol. 1991;36:611–636. [Google Scholar]
- 14.Clayton DH, Bush SE, Johnson KP. Ecology of congruence: Past meets present. Syst Biol. 2004;53(1):165–173. doi: 10.1080/10635150490265102. [DOI] [PubMed] [Google Scholar]
- 15.Balakrishnan CN, Sorenson MD. Dispersal ecology versus host specialization as determinants of ectoparasite distribution in brood parasitic indigobirds and their estrildid finch hosts. Mol Ecol. 2007;16:217–229. doi: 10.1111/j.1365-294X.2006.03142.x. [DOI] [PubMed] [Google Scholar]
- 16.Harbison CW, Bush SE, Malenke JR, Clayton DH. Comparative transmission dynamics of competing parasite species. Ecology. 2008;89:3186–3194. doi: 10.1890/07-1745.1. [DOI] [PubMed] [Google Scholar]
- 17.Fukatsu T, et al. Bacterial endosymbiont of the slender pigeon louse, Columbicola columbae, allied to endosymbionts of grain weevils and tsetse flies. Appl Environ Microbiol. 2007;73:6660–6668. doi: 10.1128/AEM.01131-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clayton DH, Lee PLM, Tompkins DM, Brodie ED., III Reciprocal natural selection on host-parasite phenotypes. Am Nat. 1999;154:261–270. doi: 10.1086/303237. [DOI] [PubMed] [Google Scholar]
- 19.Clayton DH, et al. Adaptive significance of avian beak morphology for ectoparasite control. Proc Biol Sci. 2005;272:811–817. doi: 10.1098/rspb.2004.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson KP, Clayton DH. Coevolutionary history of ecological replicates: Comparing phylogenies of wing and body lice to Columbiform hosts. In: Page RDM, editor. Tangled Trees: Phylogenies, Cospeciation, and Coevolution. Chicago: The University of Chicago Press; 2003. pp. 262–285. [Google Scholar]
- 21.Johnson KP, Williams BL, Drown DM, Adams RJ, Clayton DH. The population genetics of host specificity: Genetic differentiation in dove lice (Insecta: Phthiraptera) Mol Ecol. 2002;11(1):25–38. doi: 10.1046/j.0962-1083.2001.01412.x. [DOI] [PubMed] [Google Scholar]
- 22.Clayton DH, Johnson KP. Linking coevolutionary history to ecological process: Doves and lice. Evolution. 2003;57:2335–2341. doi: 10.1111/j.0014-3820.2003.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 23.Bush SE, Clayton DH. The role of body size in host specificity: Reciprocal transfer experiments with feather lice. Evolution. 2006;60:2158–2167. [PubMed] [Google Scholar]
- 24.Martin M. Life history and habits of the pigeon louse (Columbicola columbae [Linnaeus]) Can Entomol. 1934;66(1):6–16. [Google Scholar]
- 25.Bequaert J. The Hippoboscidae or louse-flies (Diptera) of mammals and birds. Part I. Structure, physiology and natural history. Entomol Am. 1953;32:1–209. [Google Scholar]
- 26.Ward RA. Additional record of phoresy of Mallophaga on Hippoboscidae. Bull Brooklyn Entomol Soc. 1953;48:128. [Google Scholar]
- 27.Iannacone JA. Registering a case of phoresis: Columbicola columbae (L) (Phthiraptera: Insecta) on Psuedolynchia canariensis (Diptera: Insecta) in the area of Lima, Peru. (Translated from Spanish) Boletin de Lima. 1992;84:17–18. [Google Scholar]
- 28.Macchioni F, Magi M, Mancianti F, Perrucci S. Phoretic association of mites and mallophaga with the pigeon fly Pseudolynchia canariensis. Parasite. 2005;12:277–279. doi: 10.1051/parasite/2005123277. [DOI] [PubMed] [Google Scholar]
- 29.Harbison CW, Jacobsen MV, Clayton DH. A hitchhiker's guide to parasite transmission: The phoretic behaviour of feather lice. Int J Parasitol. 2009;39:569–575. doi: 10.1016/j.ijpara.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Teel PD, Fleetwood SC, Hopkins SW, Cruz D. Ectoparasites of Eastern and Western meadowlarks from the Rio Grande plains of south Texas. J Med Entomol. 1988;25(1):32–38. doi: 10.1093/jmedent/25.1.32. [DOI] [PubMed] [Google Scholar]
- 31.Aguirre ULA, Lozoya SA. Mallophaga of domestic birds in southeastern Coahuila, Mexico. Folia Entomol Mexicana. 1991;82:93–105. [Google Scholar]
- 32.Pavlovic I, et al. Significance of Arthropoda in health problems of pheasants which are bred artificially. Veterinarski Glasnik. 1995;49:745–749. [Google Scholar]
- 33.Whiteman NK, Santiago-Alarcon D, Johnson KP, Parker PG. Differences in straggling rates between two genera of dove lice (Insecta: Phthiraptera) reinforce population genetic and cophylogenetic patterns. Int J Parasitol. 2004;34:1113–1119. doi: 10.1016/j.ijpara.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Corbet GB. The life-history and host-relations of a hippoboscid fly Ornithomyia fringillina Curtis. J Anim Ecol. 1956;25:403–420. [Google Scholar]
- 35.Markov GS. The presence of phoresy in Mallophaga. Zool Zh. 1938;17:634–636. [Google Scholar]
- 36.Thompson GB. Association of Hippoboscidae and Mallophaga: Further notes and records. Ent Mon Mag. 1947;83:212–214. [Google Scholar]
- 37.Ash J. Records of Hippoboscidae (Dipt.) from Berkshire and Co., Durham, in 1950, with notes on their bionomics. Entomol Mon Mag. 1952;88:25–30. [Google Scholar]
- 38.Edwards R. Flatflies taken in the laboratory during 1951. Fair Isle Bird Obs Bull. 1952;6:37–38. [Google Scholar]
- 39.Corbet GB. The phoresy of Mallophaga on a population of Ornithomyia fringillina Curtis (Dipt., Hippoboscidae) Entomol Mon Mag. 1956;92:207–211. [Google Scholar]
- 40.Bennett GF. On three species of Hippoboscidae (Diptera) on birds in Ontario. Can J Zool. 1961;39:379–406. [Google Scholar]
- 41.Baum VH. Biology and ecology of the feather lice of blackbirds. (Translated from German) Angew Parasitol. 1968;9:129–176. [Google Scholar]
- 42.Peters HS. Mallophaga carried by hippoboscids. Ann Carnegie Mus. 1935;24:57–58. [Google Scholar]
- 43.Walter VG. Phoresy and hyperparasitism in Ornithomyia (Diptera, Hippoboscidae) in the Federal Republic of Germany. (Translated from German) Angew Parasitol. 1989;30(1):43–46. [PubMed] [Google Scholar]
- 44.Price RD, Hellenthal RA, Palma RL, Johnson KP, Clayton DH. The Chewing Lice World Checklist and Biological Overview. Special Publication: Illinois Natural History Survey; 2003. [Google Scholar]
- 45.Bueter C, Weckstein J, Johnson KP, Bates JM, Gordon CE. Comparative phylogenetic histories of two louse genera found on Catharus thrushes and other birds. J Parasitol. 2009;95:295–307. doi: 10.1645/GE-1642.1. [DOI] [PubMed] [Google Scholar]
- 46.Johnson KP, Adams RJ, Clayton DH. The phylogeny of the louse genus Brueelia does not reflect host phylogeny. Biol J Linn Soc Lond. 2002;77:233–247. [Google Scholar]
- 47.Brown NS. A survey of the Arthropod parasites of Pigeons (Columba livia) in Boston. J Parasitol. 1971;6:1379–1380. [PubMed] [Google Scholar]
- 48.Klei TR, DeGiusti DL. Seasonal occurrence of Haemoproteus columbae Kruse and its vector Pseudolynchia canariensis Bequaert. J Wildl Dis. 1975;11:130–135. doi: 10.7589/0090-3558-11.1.130. [DOI] [PubMed] [Google Scholar]
- 49.Dranzoa C, Ocaido M, Katete P. The ecto-, gastro-intestinal and haemo-parasites of live pigeons (Columba livia) in Kampala, Uganda. Avian Pathol. 1999;28:119–124. doi: 10.1080/03079459994830. [DOI] [PubMed] [Google Scholar]
- 50.Sol D, Jovani R, Torres J. Geographical variation in blood parasites in feral pigeons: The role of vectors. Ecography. 2000;23:307–314. [Google Scholar]
- 51.Clayton DH, Drown DM. Critical evaluation of five methods for quantifying chewing lice (Insecta: Phthiraptera) J Parasitol. 2001;87:1291–1300. doi: 10.1645/0022-3395(2001)087[1291:CEOFMF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.