Abstract
The mechanism by which ECM elasticity induces lineage specification of stem cells has not been clearly understood. Integrins are well-documented mechanosensors that are positioned at the beginning of the sensing pathway. By using an antibody specifically recognizing the active conformation of β1 integrin, we observed that β1 integrin activation in bone marrow mesenchymal stem cells (BMMSCs) was induced by soft substrate to a significantly greater degree than by stiff substrate. In contrast, however, the level of cell surface integrin on soft substrate was significantly lower than that on stiff substrate. Soft substrate markedly enhanced the internalization of integrin, and this internalization was mediated mainly through caveolae/raft-dependent endocytosis. The inhibition of integrin internalization blocked the neural lineage specification of BMMSCs on soft substrate. Furthermore, soft substrate also repressed the bone morphogenetic protein (BMP)/Smad pathway at least partially through integrin-regulated BMP receptor endocytosis. A theoretical analysis based on atomic force microscopy (AFM) data indicated that integrin–ligand complexes are more easily ruptured on soft substrate; this outcome may contribute to the enhancement of integrin internalization on soft substrate. Taken together, our results suggest that ECM elasticity affects integrin activity and trafficking to modulate integrin BMP receptor internalization, thus contributing to stem cell lineage specification.
Keywords: integrin trafficking, mesencymal stem cells, neurogenic lineage, traction force
Mechanical environment plays an important role in regulating cellular function and behavior, including proliferation, migration, apoptosis, and differentiation (1–3). It has been shown recently that the mechanical properties (e.g., elasticity) of adhesion substrates modulate stem cell fate in both 2D (4, 5) and 3D (6) cultures. However, the mechanism by which mechanical properties of ECM affect the chemical signaling processes has not been clearly understood.
Mechanical stimuli induce changes in focal adhesion (FA) protein activities and FA remodeling (7, 8). The growth and elongation of FAs vary with changes in substrate stiffness, indicating that ECM elasticity regulates FA assembly (4). FA complexes consist of many signaling molecules (including Src, Cas, vinculin, and integrins), which can undergo tension-dependent conformational changes to affect kinase activity, phosphorylation site availability, intracellular localization, and/or ligand affinity (9–12). Among these molecules, integrins are necessary for most mechanosensing processes and are positioned at the beginning of the sensing pathway (13).
We aimed to explore the mechanism by which stem cells sense ECM elasticity, especially the role of β1 integrin in bone marrow mesenchymal stem cells (BMMSCs) (14). Activation of β1 integrin in BMMSCs was significantly greater on softer than on stiffer substrate. Most importantly, the intracellular localization of β1 integrin varied with substrate elasticity, being present primarily in the cortical regions on stiff substrate, but mainly in the cytoplasm on soft substrate. The internalization of cell surface integrin via caveolae/raft-dependent endocytosis was markedly enhanced on the soft substrate, and this enhancement is shown to contribute to the neurogenic BMMSC fate on soft substrates. A theoretical analysis based on atomic force microscopy (AFM) experimental data (15) indicates that integrin–ligand complexes are more easily ruptured on soft substrate, which may contribute to the greater integrin internalization. Thus, our findings provide unique insights into the mechanism of differential MSC fate in response to substrate rigidity that is modulated by the dynamic behavior of integrins in terms of their activation, bond stability, and caveolae-mediated internalization.
Results
β1 Integrin Activation Is Significantly Enhanced in BMMSCs on Soft Substrate.
BMMSCs were seeded on collagen I-coated substrates of two levels of elasticity (elastic moduli: Esoft ∼ 0.1–1 kPa and Estiff ∼ 50–100 kPa). The levels of activated integrin were determined 2 h after seeding by Western blotting and immunocytochemical staining, with a β1 integrin antibody (clone HUTS-4; Chemicon) recognizing epitopes in the 355–425 region (hybrid domain), whose expression parallels the activity of β1 integrin (16, 17). The level of activated β1 integrin recognized by this antibody increased upon Mn2+ stimulation (Fig. S1 A and B). The activated β1 integrin level on soft substrate was dramatically higher than that on stiff substrate, whereas the total (including both active and inactive) β1 integrin levels were equal on the two substrates (Fig. 1). Similar results were obtained by using an activated β1 integrin antibody of the same clone from another company (LSBio) (Fig. S1 C, D, and G).
Fig. 1.
β1 Integrin activation in BMMSCs significantly increases on soft substrate. (A) Activated and total β1 integrin levels in BMMSCs 2 h after seeding on stiff or soft substrate analyzed by Western blotting. GAPDH was used to normalize for equal loading. Gels are representative of five experiments. (B and C) Statistical analysis of results in A. P values are for differences in β1 integrin levels between stiff and soft substrates (mean ± SEM; n = 5). (D) Immunocytochemical staining of activated and total β1 integrin levels in BMMSCs 2 h after seeding on stiff or soft substrate. In photos marked “activated integrin” and “total integrin,” solid arrowheads indicate β1 integrin on the cell surface and open triangles indicate β1 integrin in cytosol. Microphotographs are representative of six experiments. (Scale bar: 20 μm.) (E and F) Fluorescence intensity of β1 integrin quantified using ImageJ software. Statistical analysis of results in D is shown. P values are for differences in β1 integrin levels between stiff and soft substrates (mean ± SEM; n = 6).
β1 integrin displayed cell surface localization on stiff substrate, but a cytoplasmic distribution on soft substrate (Fig. S2). These results led us to quantify the surface distribution of active and total integrin in these cells.
Surface Distribution of β1 Integrin in BMMSCs Is Reduced on Soft Substrate.
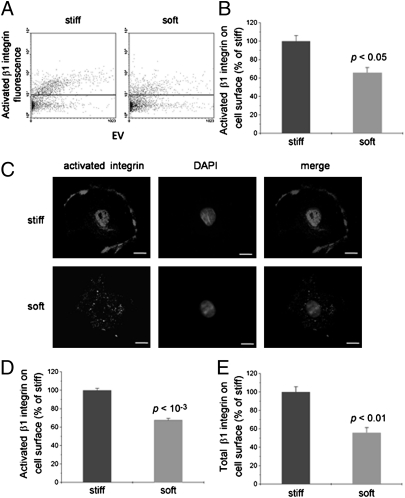
The levels of β1 integrin on the cell surface and in the whole cell were measured by using several techniques. Flow cytometry (Fig. 2 A and B) and immunocytochemistry (Fig. 2C) showed that activated β1 integrin on the cell surface was lower in BMMSCs cultured on the soft than the stiff substrate. Immunofluorescence staining showed that the ratio of activated integrin on the cell surface normalized to that of the whole cell (cell surface plus cytosol) was ∼85% on stiff substrate and 25% on soft substrate (Fig. S3). This difference in activated β1 integrin on the cell surface was further confirmed by using biotin-labeled cell surface proteins (Fig. 2D), which revealed that the activated β1 integrin level on the surface of BMMSCs on soft substrate was only 67% of that on stiff substrate. Both the total β1 integrin (including activated and inactivated) and activated β1 integrin on the cell surface of BMMSCs were lower on soft than stiff substrate (Fig. 2E). These results indicate that substrate stiffness affects the surface distribution of integrin. We next asked why soft substrate impacts the surface distribution of β1 integrin by examining integrin trafficking.
Fig. 2.
Substrate stiffness affects integrin subcellular localization in BMMSCs. (A) Flow cytometry dot plots of BMMSCs freshly harvested and analyzed for cell surface expression of activated β1 integrin. Data are representative of three experiments. (B) Statistical analysis of results in A. P values are for differences in activated β1 integrin levels between stiff and soft substrates (mean ± SEM; n = 3). (C) Typical immunocytochemical images of activated β1 integrin expression in BMMSCs cultured on stiff or soft substrate. (Scale bar: 10 μm.) (D and E) Activated β1 integrin (D) and total integrin (E) on the cell surface analyzed by biotin labeling and capture ELISA. P values are for differences in β1 integrin levels between stiff and soft substrates (mean ± SEM; n = 3).
Integrin Internalization in BMMSCs Is Enhanced by Soft Substrate.
To elucidate the mechanism by which substrate elasticity affects the distribution of activated β1 integrin, we studied integrin endocytosis and recycling. Endocytosis of activated β1 integrin was analyzed by antibody internalization assay and confocal microscopy. Antibody internalization assay showed the presence of activated β1 integrin antibody in the characteristic vesicular structures in cytoplasm (Fig. S4A). In contrast, no intracellular antibodies were detected in control cells, which were incubated at 4 °C to prevent endocytosis. The soft substrate-enhanced internalization did not appear to be a general response of membrane proteins on soft substrate because CD71, as a control, failed to show such enhanced internalization. The soft substrate significantly increased the number of cytosolic vesicles containing internalized activated β1 integrin (Fig. S4 A and B).
Because antibody binding may cluster integrins on the cell surface and thus artificially promote their endocytosis, we also used a surface biotinylation assay to independently examine the steady-state internalization of β1 integrin. As shown in Fig. 3, the internalization rates of both active and total β1 integrin were higher for BMMSCs cultured on the soft than the stiff substrate.
Fig. 3.
β1 integrin internalization is enhanced on soft substrate. Internalization of total β1 integrin (A) and activated β1 integrin (B) in BMMSCs on stiff or soft substrate in the absence or presence of 0.6 μM PMQ (inhibitor of receptor recycling) was determined for the times indicated by biotin labeling and capture ELISA (mean ± SEM; n = 4).
Because the difference in internalization rate of β1 integrin between stiff and soft substrates may have resulted from different recycling rates, a surface biotinylation assay was performed in the presence of primaquine (PMQ), a well-established reversible inhibitor of receptor recycling (18, 19). PMQ did not affect the internalization of β1 integrin, indicating that recycling of integrin back to the membrane is not involved in the up-regulation of β1 integrin internalization on a soft substrate. Taken together, these results demonstrate that soft substrate enhances integrin internalization through endocytosis.
Soft Substrate Enhances Integrin Internalization via Caveolae/Raft-Dependent Endocytosis.
Confocal microscopy observation revealed that β1 integrin was mainly localized in the enriched vesicle-like structures in the BMMSCs cultured on soft substrate (Fig. 2D), suggesting that receptor-mediated endocytosis might be involved in soft substrate-induced integrin internalization.
Because receptor-mediated endocytosis is primarily clathrin or caveolin dependent, we analyzed whether soft substrate enhances integrin internalization via such endocytosis processes. On soft substrate, BMMSCs were double labeled using antibodies recognizing clathrin or caveolin-1, in addition to those for activated β1 integrin. As shown in Fig. 4A, activated β1 integrin colocalized with caveolin-1 in a vesicle-like structure, but not with clathrin. These findings suggest a role for caveolin-1, but not clathrin, in the integrin internalization on soft substrate. The possible roles of caveolin-1 and clathrin in soft substrate-induced integrin internalization were further examined by surface biotinylation assay in the presence of their respective inhibitors. The internalization of β1 integrin on soft substrate was not affected by the inhibitor for clathrin-mediated endocytosis (monodansylcadaverine, MDC) (20), but was dramatically repressed by the caveolae/raft inhibitor methyl-β-cyclodextrin (MBCD) (21) (Fig. 4 B and C). Moreover, the addition of MBCD blocked the decrease in cell surface β1 integrin on the soft substrate, in concert with the repression of integrin internalization (Fig. 4 D and E). We also used siRNA to down-regulate caveolin-1 expression. As shown in Fig. 4 F and G, caveolin-1 siRNA-transfected cells showed a significant reduction in β1 integrin endocytosis on the soft substrate.
Fig. 4.
Soft substrate enhances integrin internalization via caveolae/raft-dependent endocytosis. (A) BMMSCs on soft substrate were stained with anti-activated β1 integrin and either anti-clathrin or anti-caveolin-1 antibodies, followed by FITC-conjugated anti-mouse and TRITC-conjugated anti-rabbit and anti-goat secondary antibodies. Data are representative of 12 experiments. (Scale bar: 10 μm.) BMMSCs on stiff or soft substrate were pretreated with 200 μM MDC, 10 mM MBCD, or medium alone for 1 h, and internalization of total β1 integrin (B) and activated β1 integrin (C) for 15 min was determined by biotin labeling and capture ELISA. P values are for differences in internalized β1 integrin levels between stiff and soft substrates in each group (mean ± SEM; n = 4). BMMSCs on stiff or soft substrate were pretreated with 10 mM MBCD or medium alone for 1 h, and total β1 integrin (D) and activated β1 integrin (E) on the cell surface were analyzed by biotin labeling and capture ELISA. P values are for differences in β1 integrin levels on the cell surface between stiff and soft substrates in each group or MBCD+ vs. MBCD− on soft substrate (mean ± SEM; n = 3). BMMSCs transfected with CAV-1 siRNA or control RNA were cultured on stiff or soft substrate, and internalization of total β1 integrin (F) and activated β1 integrin (G) for 15 min was determined by biotin labeling and capture ELISA. P values are for differences in internalized β1 integrin levels on the cell surface between stiff and soft substrates in each group (mean ± SEM; n = 3).
These results suggest that soft substrate enhances β1 integrin internalization through caveolin-1–dependent endocytosis. To further confirm this finding, we investigated whether the internalized β1 integrin can be found in caveolin-enriched compartments. Caveolae were immunoaffinity isolated from BMMSCs on stiff or soft substrate as described in previous studies (22). The amount of β1 integrin in the caveolae derived from cells on soft substrate was significantly higher than that on stiff substrate (Fig. S4 C and D), further confirming that the soft substrate-enhanced integrin internalization occurs via caveolae/raft-dependent endocytosis.
Caveolin-1–Mediated β1 Integrin Internalization Contributes to BMMSC Fate Determination via Substrate Stiffness.
We investigated the role of soft substrate-enhanced integrin internalization in the neurogenic lineage specification of BMMSCs. Inhibition of caveolin-1–mediated integrin internalization by MBCD significantly blocked the soft substrate-induced differentiation of BMMSCs into neural cells (Fig. 5). Neither enhanced cell death nor commitment to other lineages was observed under the MBCD treatment (Fig. S5). More specifically, siRNA-induced caveolin-1 knock-down substantially inhibited the neural specification of BMMSCs on soft substrate (Fig. S6). A functional blocking anti-β1 integrin antibody, which markedly inhibited β1 integrin internalization (Fig. S7B), abrogated BMMSCs neurogenic differentiation on the soft substrate (Fig. S7 A and C–E). These results suggest that enhanced β1 integrin internalization is involved in the neurogenic lineage specification of BMMSCs on soft substrate. The molecular mechanisms were further investigated as described below.
Fig. 5.
Caveolin-1–mediated internalization may contribute to BMMSC fate determination via substrate stiffness. (A) BMMSCs were cultured on a plastic six-well plate or soft substrate for 7 d in the presence or absence of 10 mM MBCD, followed by determinations of Nestin, MAP2, and NFL expression by immunocytochemical staining. Data are representative of four experiments. (Scale bar: 30 μm.) (B–D) Fluorescence intensities of Nestin, MAP2, and NFL were quantified using ImageJ software. Statistical analysis of results in A is shown. P values are for differences between MBCD+ vs. MBCD− on soft substrate (mean ± SEM; n = 4).
Engler et al. (4) showed that the Runx2 (CBFα) and Smad1, -5 expression levels in cells on soft substrate are suppressed compared with those on stiff substrate. In our experiment, we found that the phosphorylated Smad1, -5, -8 levels on soft substrate were also significantly reduced in comparison with those on stiff substrate (Fig. 6 B and C). Smad1, -5, -8 are phosphorylated by the transmembrane serine/threonine kinase bone morphogenetic protein (BMP) type-I receptor (i.e., BMPRIA or BMPRIB) (23, 24). In concert with a previous report that BMPRIA forms a complex with β1 integrin (25), we found that BMPRIA and β1 integrin colocalized in the vesicle-like structures on soft substrate (Fig. 6A). By biotin labeling of cell membrane proteins, we found that the BMPRIA level on cell membrane was substantially decreased (Fig. 6D) and that its internalization was enhanced on soft substrate (Fig. 6E). MBCD significantly reduced the internalization of BMPRIA on soft substrate (Fig. 6E), indicating that the soft substrate-induced BMPRIA endocytosis is mediated by caveolae. Furthermore, down-regulation of β1 integrin by siRNA transfection also decreased the internalization of BMPRIA (Fig. 6F). Treatment with β1 integrin blocking antibody, which reduced β1 integrin internalization (Fig. S7B), also inhibited BMPRIA internalization (Fig. 6G) and the decreases in phosphorylated Smad1, -5, -8 levels on soft substrate (Fig. 6 H and I). These results suggested that β1 integrin internalization plays a crucial role in BMPRIA membrane localization and subsequent signaling. Furthermore, knock-down of BMPRIA by siRNA interference significantly induced neurogenic differentiation of BMMSCs on stiff substrate and synergistically promoted the neural lineage specification on soft substrate (Fig. S8), implying an essential role of BMPRIA in substrate elasticity-induced BMMSC neural differentiation. Taken together, our findings indicate that the caveolae-mediated β1 integrin internalization in BMMSCs may contribute to the soft substrate-triggered neurogenic fate determination through inhibition of the BMP/Smad signal pathway.
Fig. 6.
Soft substrate represses the BMP–Smad pathway probably through caveolin-dependent endocytosis of BMPRIA. (A) BMMSCs on soft substrate were stained with anti-activated β1 integrin and anti-BMPRIA antibodies, followed by FITC-conjugated anti-mouse and TRITC-conjugated anti-rabbit secondary antibodies. Data are representative of 8 experiments. (Scale bar: 10 μm.) (B) Phosphorylated and total Smad1, -5, -8 levels in BMMSCs 2 h after seeding on stiff or soft substrate were analyzed by Western blotting. Gels are representative of 6 experiments. (C) Statistical analysis of results in B. P values are for differences in ratios of phosphorylated/total Smad1, -5, -8 between stiff and soft substrates (mean ± SEM; n = 6). (D) BMPRIA on the cell surface was analyzed by biotin labeling and capture ELISA. P values are for differences in BMPRIA levels between stiff and soft substrates (mean ± SEM; n = 3). (E) BMMSCs on stiff or soft substrate were pretreated with 10 mM MBCD or medium alone for 1 h, and internalization of BMPRIA for 15 min was determined by biotin labeling and capture ELISA. P values are for differences in internalized BMPRIA levels between stiff and soft substrates in each group (mean ± SEM; n = 4). (F) BMMSCs transfected with β1 integrin siRNA or control RNA were cultured on soft substrate, and internalization of BMPRIA for 15 min was determined by biotin labeling and capture ELISA. P values are for differences in internalized BMPRIA levels on the cell surface between β1 integrin siRNA- and control RNA-transfected cells (mean ± SEM; n = 3). (G) After biotin labeling of cell surface proteins in BMMSCs on the soft substrate, 25 μg/mL of β1 integrin blocking antibody or isotype control IgG was added, and internalization of BMPRIA for 15 min was determined by biotin labeling and capture ELISA. P values are for differences in internalized BMPRIA levels between β1 integrin blocking antibody and isotype control IgG-treated cells (mean ± SEM; n = 3). (H) Phosphorylated and total Smad1, -5, -8 levels in BMMSCs 2 h after seeding on stiff or soft substrate in the presence of 25 μg/mL β1 integrin blocking antibody or isotype control IgG were analyzed by Western blotting. Gels are representative of 3 experiments. (I) Statistical analysis of results in B. P values are for differences in the ratios of phosphorylated/total Smad1, -5, -8 between stiff and soft substrates in each group (mean ± SEM; n = 3).
Theoretical Analysis Revealed Unstable Integrin–Ligand Binding on Soft Substrate.
del Pozo et al. reported that the disruption of integrin–ECM binding may trigger raft endocytosis (26, 27). Consistent with their reports, our results showed that soft substrate induced more caveolae in the cytoplasm than stiff substrate (Fig. S8G). The enhanced integrin internalization on soft substrate implies a weak integrin-mediated adhesion, which may be readily disrupted. We introduced a theoretical model to investigate whether integrin detachment from the ECM is affected by substrate stiffness. Binding of integrin and ECM proteins forges a link between the ECM and the actin cytoskeleton that is tensioned by myosin II motors. In cells, integrin–ECM protein complexes are ruptured by tension applied by actin bundles. According to the Bell model and AFM analysis of integrin–ECM protein bonds dissociation by Trache et al. (15), the most probable rupture force on soft or stiff substrate is
 |
where K0 represents the dissociation rate constant in the absence of applied force, KB the Boltzmann constant, T the absolute temperature, and γ the distance from the energy minimum to the barrier. r is the loading rate, defined as a differential of applied force with respect to time.
In our cellular experiments, the elastic moduli of soft and stiff substrates are ∼500 Pa and 105 Pa, respectively. The most probable rupture forces on soft and stiff substrates were calculated to be 37 and 95 pN, respectively (SI Appendix).
Thus, our theoretical analysis showed that the integrin–ECM protein bond is more easily dissociated on soft than stiff substrate; this result may shed light on the mechanism of integrin internalization on soft substrate. However, whether integrin detachment actually occurs in cells on soft substrate is worthy of further experimental studies.
Discussion
There is a need to elucidate the mechanism for the regulation of cell behavior by mechanical stimuli. In particular, it remains to be determined how cells sense exogenous mechanical stimuli and what are the pathways that transduce these mechanical stimuli into intracellular biochemical signals. Recently, it has been demonstrated that ECM elasticity, which differs in different tissues, is crucial to multiple cellular behaviors (1, 4). It is believed that in vivo, ECM elasticity may contribute to organismal development and function. However, the molecular mechanism underlying the regulation of cell behavior by ECM elasticity remains to be established.
In the present study, we found that the activation and internalization of integrin was significantly enhanced by a soft substrate in comparison with a stiff substrate. We found that the internalization of cell surface integrin on soft substrate is mainly mediated through caveolae/raft-dependent endocytosis. This internalization is shown to contribute to BMMSC neural lineage specification on soft substrate by inhibiting the BMP–Smad pathway. Thus, the regulation of integrin internalization may provide a unique mechanism for the induction of stem cell lineage specification by ECM elasticity.
Being an important membrane receptor for ECM proteins, integrin directly affects FA assembly, cytoskeletal organization, and signal transduction via its activation (28). The antibody we used to analyze the activation of integrin is well documented (16, 17) to recognize the active conformation of β1 integrin, whether it is assembled in FAs or endocytic vesicles. Endocytic vesicles may contain internalized integrins and ECM proteins detached from the substrate. These detached integrins may remain active (29, 30) and can trigger endocytosis in a cholesterol-enriched membrane microdomain (raft) (26, 27). For the mechanism underlying the enhanced integrin detachment/internalization on a soft gel, our analysis with a theoretical model indicates that the integrin–ligand complex is unstable on soft substrate because of a low rupture force. For an adherent cell, integrin–ECM protein complexes or FAs undergo a dynamic turnover process of assembly–disassembly–reassembly during the cell life cycle. At the beginning stage of each integrin–ECM binding site formation, no tension is put on the integrin–ECM protein complex because the tension-generating elements, actin and myosin II bundles, can be triggered into formation only after integrin binding to ECM. As more myosin II motors are assembled, the tension in the actin bundles gradually increases. Our theoretical analysis indicates that, in comparison with stiff substrate, the rupture on a soft substrate tends to occur at a relatively lower stress level during tension increase. Taken together, we hypothesize that the high level of activated integrin that we observed on the soft substrate resulted from the enhanced rupture of integrin–ECM protein complexes, which in turn induced integrin internalization.
The traction force of the cells has been measured from the displacement fields that were mapped from embedded beads within a soft substratum (4, 31, 32). The traction stress measured by this method reflects an average value of an indicated region, in which the FA density and stress fibers density are affected by the substrate elasticity. Our analysis shares the same trend with the these measurements; however, it is rather difficult to compare the theoretical analysis of a single integrin–ECM binding site to the measured traction stress over a larger area.
The detachment of integrin-associated adhesion has been reported to trigger caveolae/raft endocytosis, which can affect signal transduction from the cell surface. In this paper, the enhanced internalization of integrin via caveolae on soft substrate is shown to affect BMPRIA membrane location that is essential for the BMPs signaling. Notably, integrin also modulates Ras/Erk, Rac, and PI3-kinase/Akt signaling by controlling endocytosis and recycling lipid rafts (26, 27). Our initial studies showed that inhibiting caveolae/raft endocytosis by MBCD also repressed osteogenic and myogenic lineages. Hence, elucidating the role of the localization of integrin-modulated signal molecules in the regulation of stem cell differentiation by ECM elasticity is an important goal for future work.
In summary, our results indicate that ECM elasticity affects integrin activity and trafficking to modulate the internalization of signaling molecules involved in cell fate and function.
Materials and Methods
Cultured primary BMMSC was derived from Sprague–Dawley rats. Detailed information on methods is described in SI Materials and Methods. These include cell culture, substrate preparation, and immunochemical analysis.
Supplementary Material
Acknowledgments
We thank Profs. Cheng Zhu and Adam J. Engler for their valuable suggestions on the experiments. We also thank Prof. Yanping Cao for his help in the theoretical analysis. This work was financially supported by the National Basic Research Program (973 Project) of China (2009CB522108 and 2010CB631005); the National Natural Science Foundation of China (10732050, 31000418, and 30870602); the opening foundation of the State Key Laboratory of Space Medicine Fundamentals and Application, Chinese Astronaut Research and Training Center (SMFA09K06); Tsinghua University (2009THZ02122); and National Heart, Lung, and Blood Institute research Grant HL 104402 from the National Institutes of Health of the US Public Health Service.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1106467108/-/DCSupplemental.
References
- 1.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 2.Zajac AL, Discher DE. Cell differentiation through tissue elasticity-coupled, myosin-driven remodeling. Curr Opin Cell Biol. 2008;20:609–615. doi: 10.1016/j.ceb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 5.Chowdhury F, et al. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat Mater. 2010;9:82–88. doi: 10.1038/nmat2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huebsch N, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okuda M, et al. Shear stress stimulation of p130(cas) tyrosine phosphorylation requires calcium-dependent c-Src activation. J Biol Chem. 1999;274:26803–26809. doi: 10.1074/jbc.274.38.26803. [DOI] [PubMed] [Google Scholar]
- 8.Tzima E, et al. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J. 2002;21:6791–6800. doi: 10.1093/emboj/cdf688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown AE, Discher DE. Conformational changes and signaling in cell and matrix physics. Curr Biol. 2009;19:R781–R789. doi: 10.1016/j.cub.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riveline D, et al. Focal contacts as mechanosensors: Externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawada Y, et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Na S, et al. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc Natl Acad Sci USA. 2008;105:6626–6631. doi: 10.1073/pnas.0711704105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls alpha5beta1 function. Science. 2009;323:642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- 14.Ip JE, et al. Mesenchymal stem cells use integrin beta1 not CXC chemokine receptor 4 for myocardial migration and engraftment. Mol Biol Cell. 2007;18:2873–2882. doi: 10.1091/mbc.E07-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trache A, Trzeciakowski JP, Meininger GA. Mg2+ modulates integrin-extracellular matrix interaction in vascular smooth muscle cells studied by atomic force microscopy. J Mol Recognit. 2010;23:316–321. doi: 10.1002/jmr.985. [DOI] [PubMed] [Google Scholar]
- 16.Luque A, et al. Activated conformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region (355-425) of the common beta 1 chain. J Biol Chem. 1996;271:11067–11075. doi: 10.1074/jbc.271.19.11067. [DOI] [PubMed] [Google Scholar]
- 17.Berger BW, et al. Consensus motif for integrin transmembrane helix association. Proc Natl Acad Sci USA. 2010;107:703–708. doi: 10.1073/pnas.0910873107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reid PA, Watts C. Cycling of cell-surface MHC glycoproteins through primaquine-sensitive intracellular compartments. Nature. 1990;346:655–657. doi: 10.1038/346655a0. [DOI] [PubMed] [Google Scholar]
- 19.Roberts M, Barry S, Woods A, van der Sluijs P, Norman J. PDGF-regulated rab4-dependent recycling of alphavbeta3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr Biol. 2001;11:1392–1402. doi: 10.1016/s0960-9822(01)00442-0. [DOI] [PubMed] [Google Scholar]
- 20.Schütze S, et al. Inhibition of receptor internalization by monodansylcadaverine selectively blocks p55 tumor necrosis factor receptor death domain signaling. J Biol Chem. 1999;274:10203–10212. doi: 10.1074/jbc.274.15.10203. [DOI] [PubMed] [Google Scholar]
- 21.Yancey PG, et al. Cellular cholesterol efflux mediated by cyclodextrins. Demonstration of kinetic pools and mechanism of efflux. J Biol Chem. 1996;271:16026–16034. doi: 10.1074/jbc.271.27.16026. [DOI] [PubMed] [Google Scholar]
- 22.Oh P, Schnitzer JE. Immunoisolation of caveolae with high affinity antibody binding to the oligomeric caveolin cage. Toward understanding the basis of purification. J Biol Chem. 1999;274:23144–23154. doi: 10.1074/jbc.274.33.23144. [DOI] [PubMed] [Google Scholar]
- 23.Hoodless PA, et al. MADR1, a MAD-related protein that functions in BMP2 signaling pathways. Cell. 1996;85:489–500. doi: 10.1016/s0092-8674(00)81250-7. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Bhushan A, Vale W. Smad8 mediates the signaling of the ALK-2 [corrected] receptor serine kinase. Proc Natl Acad Sci USA. 1997;94:12938–12943. doi: 10.1073/pnas.94.24.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai CF, Cheng SL. Alphavbeta integrins play an essential role in BMP-2 induction of osteoblast differentiation. J Bone Miner Res. 2005;20:330–340. doi: 10.1359/JBMR.041013. [DOI] [PubMed] [Google Scholar]
- 26.del Pozo MA, et al. Integrins regulate Rac targeting by internalization of membrane domains. Science. 2004;303:839–842. doi: 10.1126/science.1092571. [DOI] [PubMed] [Google Scholar]
- 27.del Pozo MA, et al. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol. 2005;7:901–908. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miranti CK, Brugge JS. Sensing the environment: A historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4:E83–E90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- 29.Shi F, Sottile J. Caveolin-1-dependent beta1 integrin endocytosis is a critical regulator of fibronectin turnover. J Cell Sci. 2008;121:2360–2371. doi: 10.1242/jcs.014977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng T, et al. PKCalpha regulates beta1 integrin-dependent cell motility through association and control of integrin traffic. EMBO J. 1999;18:3909–3923. doi: 10.1093/emboj/18.14.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang HB, Dembo M, Wang YL. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am J Physiol Cell Physiol. 2000;279:C1345–C1350. doi: 10.1152/ajpcell.2000.279.5.C1345. [DOI] [PubMed] [Google Scholar]
- 32.Hur SS, Zhao Y, Li YS, Botvinick E, Chien S. Live cells exert 3-dimensional traction forces on their substrata. Cell Mol Bioeng. 2009;2:425–436. doi: 10.1007/s12195-009-0082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.