Capitalizing on the opportunities presented by the surface tension of aqueous liquids against air shows natural selection at its most creative. The paper in PNAS by Rico-Guevara and Rubega (1) describes how protruded hummingbird tongues curl lengthwise into a pair of tubes containing nectar. As a tongue contacts nectar on its lower surface or is withdrawn from full submersion, the surface tension of the residual air–nectar interface draws the sides of the tubes up into nearly complete cylinders. Together with the hydrophilic surface of the tongue, lengthwise stiffening rods ensure proper reshaping with no need for muscular activity. After retraction, subsequent protrusion through a tighter bill aperture strips the tongue of the fluid. Neither capillarity nor active pumping needs to take place, and therefore, the high viscosity from the concentrated sugar in the nectar does not present a problem. Viscosity may minimize lengthwise leakage from the cylinders, while viscosity rises steeply with increasing concentrations of sugar, and therefore, surface tension changes much less.
Making a simple model of the purely physical process of filling is trivially easy (Fig. 1). One just curls a small strip of flexible polyethylene sheet by rolling it around a nail. Empty, it has a wide U-shaped cross section; with a few drops of water, it curls into a nearly or fully cylindrical form, with the reduction in free surface doing a small amount of work against gravity and elasticity. A crude version does not even require a particularly hydrophilic surface.
Fig. 1.
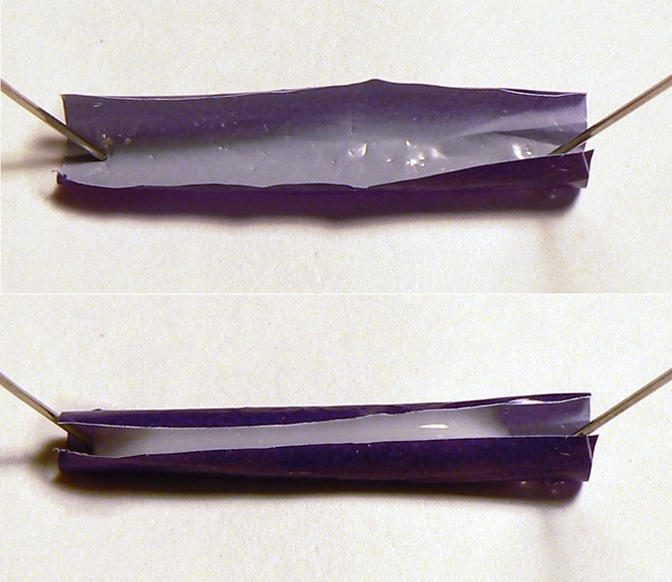
A piece of polyethylene, 1.3 × 3.5 cm, cut from a plastic bag. (Upper) Its initial curl. (Lower) The same piece after the addition of a few drops of skim milk.
Hummingbirds feed from a liquid using surface tension in a completely different manner from that of phalaropes, which was described by Rubega and Obst (2). In phalaropes, differences in radii of curvature between the top and bottom of a droplet drive its ascent between diverging top and bottom bills after a brief dip in water. Like the present mechanism, it almost certainly has been independently hit on in several lineages of birds (3). It can also be shown with a simple model. Hinge a vertically held pair of clean glass microscope slides at their upper ends with adhesive tape and introduce a drop of water between them; increasing the angle of divergence between the slides will cause the drop to move up.
The very mundane nature of these demonstrations illustrates our long-standing disregard for what can be accomplished by surface tension as well as what has been accomplished by a technology (nature) whose default operating scale is smaller than that of most of our own contrivances. Focusing merely on surface tension, other examples abound. The most startling must surely be the way some fungi takes advantage of the collapse of tiny aqueous drops (Buller's droplet, named after A. H. R. Buller, 1874–1944) to launch spores with initial accelerations in excess of 10,000 × g and speeds of over 1 m/s (4). The one that looms largest in our immediate world draws surprisingly little attention. Surface tension allows the tiny, curved air–water interfaces in the hydrophilic cellulosic walls of many of the interior cells of leaves, with radii of curvature of the order of one-tenth of a micrometer, to keep air out of the ascending conduits of plants, despite negative pressures of tens of atmospheres (5).
Less widespread, perhaps, but also convergently evolved in many lineages are the so-called plastrons of aquatic arthropods, which are coatings of air supported, much like tents on multiple tent poles, atop hydrophobic hairs. These coatings provide respiratory surfaces for these air breathers (6). Of particular interest recently has been the recognition that nature combines surface chemistry and microtexture to achieve unprecedented levels of hydrophobicity (superhydrophobicity), most notably enabling many leaves to shed water drops that, as they roll off, collect and carry contaminant particles (7). Water-walking insects seem to use superhydrophobic feet, and a biomimetic material (Lotusan) has recently been developed (8). These are not the only known applications of surface tension, and we have every reason to believe that others lurk unrecognized.
Conversely, for a long time, we attributed several impressive tricks to surface tension for which careful analyses show it to have little or no relevance. Several lineages of lizards, basilisks in Central America and agamids in Southeast Asia, that are characterized by especially large hind legs and feet commonly run across the surfaces of streams and small ponds. Unlike water-walking insects, they take advantage of the density of water and its consequent inertial resistance to acceleration, performing what has been called slapping locomotion (9). Similarly dependent on acceleration resistance, felines lap liquids by rapidly raising their tongues from the surface while drawing liquid up on the tongues’ lower surfaces (10). Again, one suspects that other examples await discovery.
For that matter, tongues perform remarkably diverse tasks using impressively diverse mechanisms. Many use surface tension in a less counterintuitive way, taking advantage of wet adhesion to provide brief attachment for small edible items. What seems unusual about the present case is how it does not rely on any intrinsic muscular activity but limits muscular action to extrinsic protrusion and retraction. Tongues are almost by definition muscular, and muscle-centered investigations have focused on systems such as power amplification in the ballistic tongues of amphibians, which may accelerate at over 100 × g (11), the widely used device called a muscular hydrostat, by which tongues enable a contractile engine to power either forceful or rapid extension (12), and the scheme (and the counteracting tactics of the targets) with which bovid tongues enwrap grasses and pull bunches out of the ground (13).
Besides surface tension and tongues, the present investigation can be viewed in a third context. Biologists have most often looked to chemistry for reductionist explanations. Physical mechanisms, at least beyond those involved in locomotion, have been invoked less often. This traditional disinterest might be blamed on the poor exposure of biologists to the relevant basic variables and phenomena or the lack of exposure of physical scientists and engineers to the wondrous diversity of organisms and organism-level functional devices. With increasing cross-over between physical and biological domains, collaborations have become everyday occurrences; engineering, physics, and mathematics departments hire faculty with degrees in biology, and biology departments absorb physical scientists. The allure of biomimetics, whatever its basis or merits, supports the intrinsic goad of a particularly infectious form of intellectual curiosity. As a result, remarkable and unexpected mechanisms have been uncovered at an unprecedented rate during the past few decades.
Acknowledgments
I thank Howard Reisner for help in preparing Fig. 1.
Footnotes
The author declares no conflict of interest.
See companion article on page 9356.
References
- 1.Rico-Guevara A, Rubega MA. The hummingbird tongue is a fluid trip, not a capillary tube. Proc Natl Acad Sci USA. 2011;108:9356–9360. doi: 10.1073/pnas.1016944108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubega MA, Obst BS. Surface-tension feeding in phalaropes: Discovery of a novel feeding mechanism. Auk. 1993;110:169–178. [Google Scholar]
- 3.Prakash M, Quéré D, Bush JWM. Surface tension transport of prey by feeding shorebirds: The capillary ratchet. Science. 2008;320:931–934. doi: 10.1126/science.1156023. [DOI] [PubMed] [Google Scholar]
- 4.Pringle A, Patek SN, Fischer M, Stolze J, Money NP. The captured launch of a ballistospore. Mycologia. 2005;97:866–871. doi: 10.3852/mycologia.97.4.866. [DOI] [PubMed] [Google Scholar]
- 5.Nobel PS. Physicochemical and Environmental Plant Physiology. 3rd Ed. New York: Freeman; 2005. [Google Scholar]
- 6.Hinton HE. Plastron respiration in bugs and beetles. J Insect Physiol. 1976;22:1529–1550. [Google Scholar]
- 7.Neinhuis C, Barthlott W. Characterization and distribution of water-repellant, self-cleaning plant surfaces. Ann Bot. 1997;79:667–677. [Google Scholar]
- 8.Bush JWM, Hu DL. Walking on water: Biolocomotion at the interface. Annu Rev Fluid Mech. 2006;38:339–369. [Google Scholar]
- 9.Glasheen JW, McMahon TA. Size-dependence of water-running ability in basilisk lizards (Basiliscus basiliscus) J Exp Biol. 1996;199:2611–2618. doi: 10.1242/jeb.199.12.2611. [DOI] [PubMed] [Google Scholar]
- 10.Reis PM, Jung S, Aristoff JM, Stocker R. How cats lap: Water uptake by Felis catus. Science. 2010;330:1231–1234. doi: 10.1126/science.1195421. [DOI] [PubMed] [Google Scholar]
- 11.Lappin AK, et al. Storage and recovery of elastic potential energy powers ballistic prey capture in toads. J Exp Biol. 2006;209:2535–2553. doi: 10.1242/jeb.02276. [DOI] [PubMed] [Google Scholar]
- 12.Smith KK, Kier WM. Trunks, tongues and tentacles: Moving with skeletons of muscle. Am Sci. 1989;77:28–35. [Google Scholar]
- 13.Vincent JFV. Fracture properties of plants. Adv Bot Res. 1990;17:235–287. [Google Scholar]


