Abstract
CCR5 is a potent mediator of regulatory T cell (Treg) chemotaxis. In murine histoplasmosis, mice lacking CCR5 or endogenous CCL4 have a reduced number of Tregs in the lungs, which results in accelerated resolution of infection. In this study, we demonstrate that CCR5 controls the outcome of Histoplasma capsulatum infection by dictating thymic and lymph node egress of Tregs. Mice lacking CCR5 or treated with a mAb to CCL4 had more Tregs in the thymus prior to and during infection. Thymic accumulation was associated with diminished transcription of the sphingosine 1-phosphate 1 receptor and Krüppel-like factor 2, both of which regulate thymic and lymph node emigration of T cells. The significance of CCR5 in Treg egress was demonstrated by generating mixed bone marrow chimeras. Chimeric mice had an increased proportion of CCR5−/− Tregs in the thymus and lymph nodes and a decreased proportion of Tregs in the lungs prior to and during H. capsulatum infection. Hence, CCR5 signaling regulates pathogen persistence in murine histoplasmosis by regulating Tregs exiting from the thymus and lymph nodes and, consequently, their subsequent homing in the periphery.
The transcription factor Foxp3 is the master regulator for the development and function of regulatory T cells (Tregs) (1–3). Two general subsets of Tregs have been identified: natural Tregs, which mature in the thymus, and induced Tregs, which develop from CD4+ cells in the periphery in the presence of Ag and TGF-β. In several models of infection, the accumulation of Tregs, likely a combination of natural and induced, regulate cytokine production and protective immunity. Homing of Tregs to inflamed tissue is influenced by chemokines, a superfamily of chemotactic cytokines that signal through G protein-coupled receptors. Notably, CCR5 and CXCR3 have been identified as potent mediators of Treg chemotaxis. In murine histoplasmosis, as well as in dermal leishmaniasis and paracoccidioidomycosis, CCR5-dependent homing of Tregs regulates the magnitude of the proinflammatory response to favor pathogen persistence (4–6). Additionally, the proinflammatory cytokine TNF-α exerts an immunoregulatory function in Histoplasma capsulatum infection. TNF-α antagonism causes mice to succumb to infection as a result of the emergence of a population of Ag-specific suppressor CD4+ CD25+ T cells (7).
The factors that drive Tregs to exit the thymus remain unresolved. In this study, we reveal a role for CCR5 in thymic emigration under normal and inflammatory conditions. The paucity of Tregs in CCR5−/− and CCL4-neutralized lungs is associated with thymic and lymph node accumulation. Stimulation of CCR5 by CCL3, CCL4, or CCL5 regulated expression of the transcription factor Krüppel-like factor 2 (KLF2) and sphingosine 1-phosphate receptor 1 (S1P1) in vitro, and expression of both were diminished in thymocytes from CCR5−/− and CCL4-neutralized mice prior to and during infection. CCR5−/− Tregs homed to the lungs as efficiently as wild-type (WT) when transferred into RAG1−/− mice, suggesting CCR5 mediates thymic egress but is dispensable for Treg migration in the periphery. Collectively, this study demonstrates the importance of CCR5-regulated thymic egress in dictating the outcome of infection with the intracellular pathogen H. capsulatum.
Materials and Methods
Mice
C57BL/6 (WT), CCR5−/−, CXCR3−/−, RAG1−/−, and B6.PL-Thy1a/CyJ (Thy1.1 congenic C57BL/6) mice were purchased from The Jackson Laboratory and maintained by the Department of Laboratory Animal Medicine, University of Cincinnati, which is accredited by the American Association for Accreditation of Laboratory Animal Medicine. All animal experiments were done in accordance with the Animal Welfare Act guidelines of the National Institutes of Health.
Generation of mixed bone marrow chimeras
Bone marrow was harvested from femurs and tibias of B6.PL-Thy1a/CyJ and CCR5−/− mice and transferred i.v. into lethally irradiated RAG1−/− mice at a 1:1 ratio. Mice received a total of 8 × 106 cells. After 8–10 wk, mice were infected intranasally with 2 × 106 H. capsulatum.
In vivo neutralization of CCL4 and simvastatin treatment
For neutralization of CCL4, mice were treated with an mAb to CCL4 (R&D Systems, Minneapolis, MN). Mice received 100 μg anti-CCL4 or rat IgG three times a week. Mice were given 40 μg simvastatin or vehicle control i.p. daily for 2 wk.
H. capsulatum infection and organ culture
Mice 5 to 6 wk in age were intranasally inoculated with 30–50 μl 2 × 106 H. capsulatum yeasts (strain G217B) diluted in HBSS. To assess fungal burden, lungs were homogenized and serially diluted onto mycosel blood agar plates and incubated for 1 wk at 28°C. Fungal burden was expressed as the mean CFU per whole organ ± SEM. The limit of detection is 1 × 102 CFU.
Isolation of leukocytes
Thymi and lymph nodes were teased apart in HBSS using the ends of two frosted glass slides. Lungs were homogenized in HBSS using a gentleMACS Dissociator (Miltenyi Biotec, Auburn CA), and Lympholyte M (Cedarlane Laboratories, Hornby, ON, Canada) was used to isolate leukocytes. All cell solutions were filtered through 60-μm nylon mesh (Spectrum Laboratories, Rancho Dominguez, CA).
Flow cytometry
The following mAbs were purchased from BD Biosciences: PE-conjugated streptavidin and CD69, allophycocyanin-conjugated CD25, CD8α, and CD62L, peridin-chlorophyll protein-conjugated CD4, and FITC-conjugated CD3ε, CD24, and β7 integrin. Allophycocyanin- and biotin-conjugated CD90.1 (Thy1.1) and FITC-conjugated CD90.2 (Thy1.2) were purchased from eBioscience (San Diego, CA). For surface staining, cells were washed with 1% BSA in HBSS (pH 7.4) and were stained at 4°C for 15 min. To characterize Foxp3 expression, cells were incubated with Cytofix/Cytoperm (BD Biosciences, San Diego, CA), washed in Permeabilization buffer (BD Biosciences), and stained for 1 h with PE-conjugated Foxp3 (eBioscience). To characterize apoptosis in the thymus, the In Situ Cell Death Detection Kit (Roche Diagnostics, Indianapolis, IN) was used to characterize TUNEL+ cells as previously described (8). Cells were characterized using an FACSCalibur Flow Cytometer (BD Biosciences) and FCS Express Software.
BrdU labeling and detection
To measure Treg proliferation in the thymus, a FITC Flow kit was purchased from BD Biosciences. BrdU (1 mg/ml) was administered i.p. for two consecutive days before being sacrificed. Cells were stained following the manufacturer’s recommendations.
Purification and transfer of CD4+CD25+ T cells
The T regulatory isolation kit from Miltenyi Biotec was used for magnetic separation of CD4+CD25− cells and CD4+CD25+ (Treg) cells from un-infected spleens. Purity of each population was >90%. RAG1−/− mice were reconstituted with 8 × 106 WT CD4+ cells and 5 × 105 WT or CCR5−/− CD4+CD25+ cells 1 d prior to infection.
Quantitative real-time PCR
TRIzol reagent (Invitrogen) was used to extract RNA from the thymus and mediastinal lymph nodes. A Reverse Transcription Systems kit (Promega, Madison, WI) was used to synthesize cDNA. KLF2 and S1P1 transcription was characterized by quantitative real-time PCR using TaqMan Fast Universal Master Mix and primer/probe sets from Applied Biosystems (Carlsbad, CA). Samples were normalized to hypoxanthine-guanine phosphoribosyltransferase (HPRT) (Applied Biosystems) and analyzed on an ABI Prism 7500 instrument (Applied Biosystems).
In vitro stimulation of thymocytes with chemokines
Thymocytes were plated at 1 × 106 cells/ml in RPMI 1640 supplemented with 5% FBS and treated with 1 ng/ml CCL3, CCL4, or CCL5 (PeproTech, Rocky Hill, NJ) for 24 h.
Measurement of Akt phosphorylation
To measure phosphorylation of Akt, WT and CCR5−/− thymocytes were plated in a 96-well plate and stimulated with media alone, 1, 10, or 100 ng/ml IL-2, or 10 ng/ml CCL3, CCL4, or CCL5 for 30 min (PeproTech). The Akt In-Cell ELISA kit from Thermo Scientific (Waltham, MA) was used to measure total Akt and phosphorylated Akt.
Statistical analysis
Student t test was used for the comparison of two groups, and ANOVA was used to compare multiple groups. Statistical significance was characterized by a p value ≤0.05.
Results
T cells accumulate in the thymus in the absence of CCR5
In the course of our studies, we unexpectedly observed that CCR5−/− mice had visually larger thymi than WT (Fig. 1A). Mice lacking CCR5 had a significant increase in the number of thymocytes compared with controls (0.77 ± 0.08 × 108 versus 1.57 ± 0.12 × 108; p < 0.001; Fig. 1B). We assessed the phenotype and absolute number of cells in the thymus. WT and CCR5−/− thymi had an equal proportion of CD4−CD8− double-negative (DN) (4.7 ± 0.2% versus 4.2 ± 0.2%), CD8+ single-positive (SP) (3.9 ± 0.3% versus 3.3 ± 0.2%), and CD4+CD8+ double-positive (DP) cells (82.9 ± 0.7% versus 82.7 ± 0.6%). A slight yet statistically significant increase in CD4+ SP thymocytes (6.3 ± 0.16% versus 7.7 ± 0.29%; p < 0.001) was observed in CCR5−/− thymi. Because CCR5−/− mice had 2-fold more thymocytes, the absolute number of each of these populations was increased (Fig. 1C).
FIGURE 1.
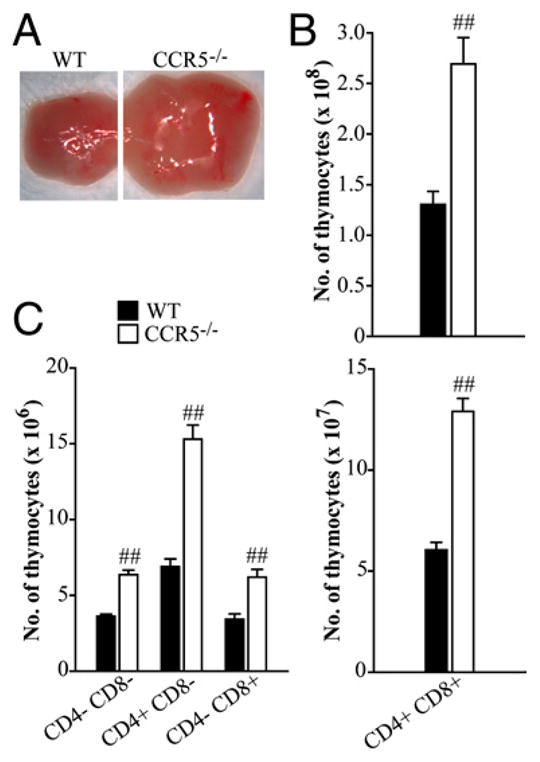
T cells accumulate in the thymus in the absence of CCR5. Thymi were harvested from WT and CCR5−/− mice 5 to 6 wk in age and visualized using a compound microscope (A). Total number of thymocytes was enumerated (B), and the absolute number of CD4−CD8− DN, CD4+ SP, CD8+ SP, and CD4+CD8+ DP cells (C) was characterized by flow cytometry. Data represent the mean ± SEM (n = 8–12) from two to three experiments. ##p < 0.001.
One explanation for the accumulation of cells within the thymi of CCR5−/− mice is that cells from these animals experience less apoptosis than those from WT. We assessed the proportion of apoptotic cells in the thymi of age-matched WT and CCR5−/− mice using TUNEL staining. The mean percentage (± SEM) of TUNEL+ thymocytes was similar (p > 0.05) in WT and CCR5−/− mice (0.63 ± 0.05% versus 0.51 ± 0.04%; n = 8–10).
We also considered that differences in cellularity might be accompanied by altered proliferation of thymocytes. To probe this possibility, we analyzed the proliferative activity of various thymic cell populations from WT and CCR5−/− mice using BrdU. In the thymi of the latter, a higher proportion of DP, CD8+ SP, and CD4+ Foxp3+ cells incorporated BrdU than in the respective populations from WT (Table I).
Table I.
Proliferation of thymic populations
| Phenotype | % Proliferating (BrdU+)
|
|
|---|---|---|
| WT | CCR5−/− | |
| DN (CD4−CD8−) | 32.0 ± 1.0 | 34.2 ± 0.9 |
| DP (CD4+CD8+) | 31.7 ± 3.0 | 37.1 ± 2.5a |
| CD4+ SP (CD4+CD8−) | 5.9 ± 0.2 | 6.6 ± 0.3 |
| CD8+ SP (CD4−CD8+) | 31.2 ± 1.1 | 37.4 ± 1.8a |
| CD4+CD25+ | 8.3 ± 1.2 | 7.7 ± 1.2 |
| CD4+Foxp3+ | 29.6 ± 2.4 | 42.3 ± 3.1a |
Data represent the mean ± SEM of two to three experiments (n = 6–12). Proliferation was measured by BrdU incorporation and expressed as percent of total thymic population.
p < 0.05 versus WT.
We next investigated the expression of several cell-surface markers throughout the course of T cell development. Of particular interest were CD69 and CD24, which are both downregulated as medullary thymocytes mature (9, 10). Total CD69 expression was comparable among CD4+ and CD8+ SP thymocytes in WT and CCR5−/− mice. However, although variable, we observed differences in the proportion of CD69hi and CD69int thymocytes between the two groups. In several experiments, CCR5−/− mice manifested a reduced proportion of CD69hi cells and increased proportion of CD69int compared with controls (data not shown). CD24 was also differentially expressed on WT and CCR5−/− thymocytes. Notably, CD24 was not as efficiently downregulated on mature CCR5−/− CD69−CD8+ SP thymocytes. CD69−CD24hi constituted 10.1 ± 1% of the CD8+ SP population in WT and 17.5 ± 1.1% in CCR5−/− (p < 0.01).
CCR5 and its ligands influence S1P1 and KLF2 expression in the thymus
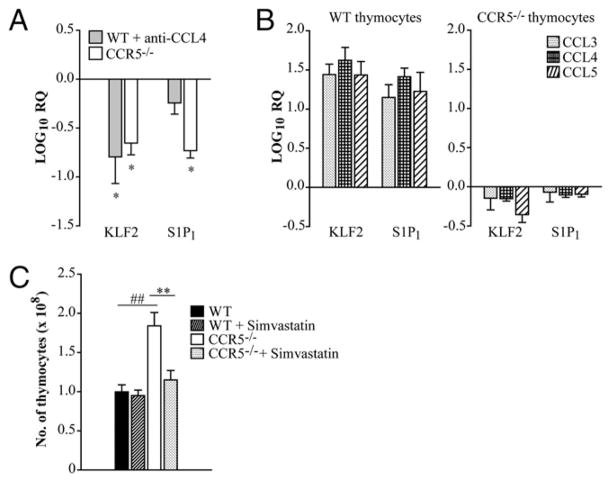
The receptor S1P1 is pivotal for T cell egress from the thymus and peripheral lymph nodes (11, 12). S1P1 expression is regulated by the zinc-finger transcription factor KLF2, and the absence of KLF2 results in defective peripheral migration of T cells as a result of unrestrained expression of chemokine receptors such as CCR3 and CCR5 (13). The factors that control KLF2 expression in T cells are unknown. Thus, one possible explanation for the accumulation of T cells in the thymus is that signaling through CCR5 controls the expression of KLF2 and. consequently, S1P1. Thymi were isolated from WT and CCR5−/− mice, and expression of KLF2 and S1P1 was measured by quantitative real-time PCR. KLF2 and S1P1 transcript was reduced by a log in thymocytes from CCR5−/− mice when normalized to age-matched WT controls (Fig. 2A). A similar decrement in KLF2 and S1P1 transcript was observed in thymi harvested from mice given an mAb to CCL4 (Fig. 2A).
FIGURE 2.
Thymic accumulation is associated with diminished expression of S1P1 and KLF2 in CCR5−/− and CCL4-neutralized mice. Thymi were isolated from WT, CCL4-neutralized, and CCR5−/− mice. Mice were given rat IgG or anti-CCL4 daily for 1 wk days prior to being sacrificed. RNA was extracted from thymi, and expression of KLF2 and S1P1 was measured by quantitative real-time PCR. HPRT was used as an endogenous control, and values represent log decrease compared with WT day 0 thymi (A). WT and CCR5−/− thymocytes were treated with 1 ng/ml CCL3, CCL4, or CCL5 overnight, and KLF2 and S1P1 expression was measured by quantitative real-time PCR. Values represent log change relative to the vehicle control (B). Mice were given 40 μg simvastatin or vehicle control i.p. daily. After 2 wk of treatment, the total number of thymocytes was enumerated (C). Data represent the mean ± SEM (n = 8–12) from two to three experiments. *p < 0.05, **p < 0.01, ##p < 0.001. RQ, relative quantification.
To determine if signaling through CCR5 directly regulated expression of these molecules, thymocytes were treated with CCL3, CCL4, or CCL5, and KLF2 and S1P1 transcription was quantified. Relative expression of KLF2 and S1P1 in thymocytes was elevated upon addition of any CCR5 ligand when normalized to the vehicle control (Fig. 2B). These results provide evidence that CCR5 signaling and the expression of KLF2 and S1P1 are linked and together likely influence thymic exiting.
Treatment with statins induces KLF2 expression in T cells (14). To determine if thymic accumulation was caused by diminished KLF2 expression in CCR5−/− mice, WT and CCR5−/− mice were administered simvastatin or vehicle control daily to induce KLF2 expression. After 2 wk, the number of thymocytes in CCR5−/− mice was reduced and comparable to controls (Fig. 2C).
Enhanced activation of Akt in CCR5−/− thymocytes
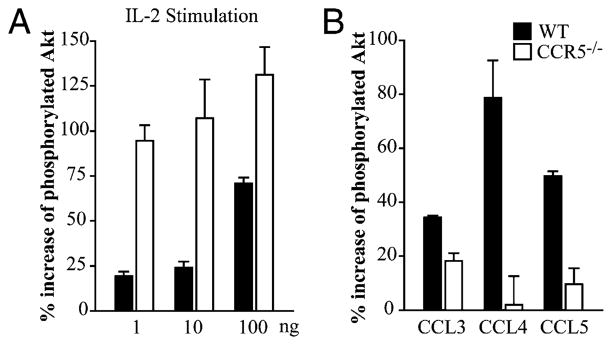
PI3K influences thymic egress (15). Constitutive activation of PI3K, measured by Akt activity, in the thymus results in accumulation of mature SP T cells in the thymus and delayed appearance in the periphery. We next characterized Akt activity in WT and CCR5−/− thymocytes isolated from uninfected mice. Thymocytes were stimulated with various concentrations of IL-2 to measure phosphorylation of Akt. When treated with media alone, Akt phosphorylation was comparable in WT and CCR5−/− thymocytes (56.3 ± 5.0% versus 62.3 ± 8.1%). Addition of IL-2 induced phosphorylation of Akt in WT and, to a greater extent, CCR5−/− thymocytes (Fig. 3A). To determine if CCR5 signaling directly activated Akt, WT and CCR5−/− thymocytes were stimulated with CCL3, CCL4, or CCL5. Each CCR5 ligand induced phosphorylation of Akt in WT, but not CCR5−/− thymocytes (Fig. 3B). These data suggest that CCR5 influences activation of this signaling pathway.
FIGURE 3.
Akt is hyperphosphorylated in CCR5−/− thymocytes. WT and CCR5−/− thymocytes were isolated to measure phosphorylation of Akt. Thymocytes were treated with media alone, 1, 10, or 100 ng/ml IL-2 (A) or 10 ng/ml CCL3, CCL4, or CCL5 (B) for 30 min. Graphs represent the percent increase of total Akt that was phosphorylated after stimulation. Data are representative of two individual experiments.
CCR5 influences thymic and lymph node egress during H. capsulatum infection
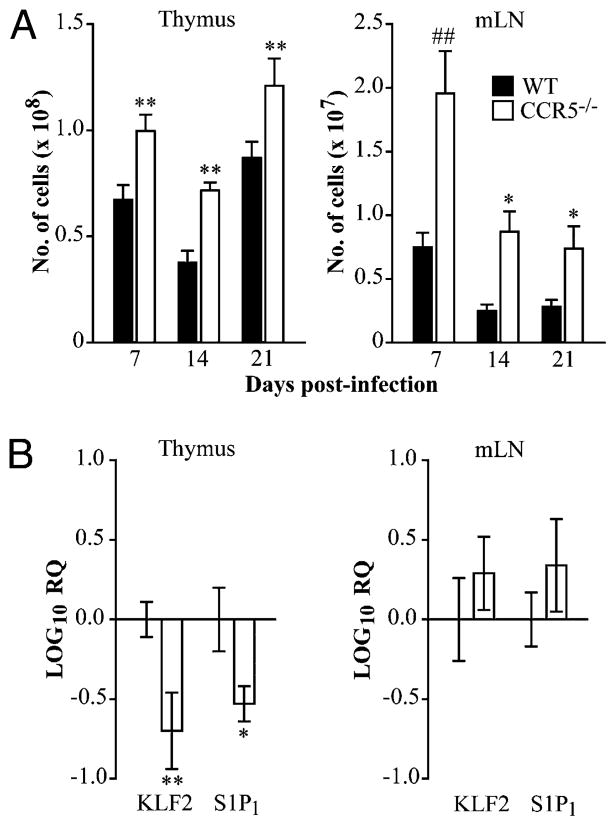
Infection with H. capsulatum results in thymic involution (16). We sought to determine if CCR5 was involved in this process. WT and CCR5−/− mice were infected, and thymic cellularity was monitored at various time points postinfection (Fig. 4A). Thymi from CCR5−/− mice were visually larger than controls throughout the course of infection. However, the difference in the number of thymocytes in WT and CCR5−/− mice narrowed upon infection. Consistent with thymi from uninfected CCR5−/− mice, KLF2 and S1P1 transcription was diminished compared with WT at day 14 postinfection (Fig. 4B).
FIGURE 4.
CCR5 influences thymic and lymph node egress during H. capsulatum infection. WT and CCR5−/− mice were infected intranasally with 2 × 106 H. capsulatum and sacrificed at days 7, 14, and 21 post-infection to determine the absolute number of leukocytes in the thymus and mediastinal lymph nodes (A). RNA was extracted from the thymus and mediastinal lymph nodes at day 14 postinfection to measure KLF2 and S1P1 transcription by quantitative real-time PCR. HPRT was used as an endogenous control, and values represent log decrease normalized to WT controls (B). Data represent the mean ± SEM (n = 8–12) from two to three experiments. *p < 0.05, **p < 0.01, ##p < 0.001.
In addition to the thymus, leukocytes also accumulated in the mediastinal lymph nodes of CCR5−/− mice during H. capsulatum infection (Fig. 4A). At day 14 postinfection, CD4+ T cells accounted for 16.4 ± 1.1% and 24.5 ± 0.8% of cells in WT and CCR5−/− lymph nodes, respectively (p < 0.001). The proportion of CD8+ T cells was comparable in both groups. Because CCR5−/− mice have 2-fold more cells than controls (0.44 ± 0.13 × 107 versus 1.0 ± 0.19 × 107; p < 0.01), the absolute number of both CD4+ and CD8+ T cells in the mediastinal lymph nodes was elevated. In contrast to the thymus, KLF2 and S1P1 expression was comparable in leukocytes isolated from WT and CCR5−/− mediastinal lymph nodes at day 14 postinfection (Fig. 4B).
The paucity of Tregs in the lungs correlates with accumulation in the thymus and lymph nodes during H. capsulatum infection
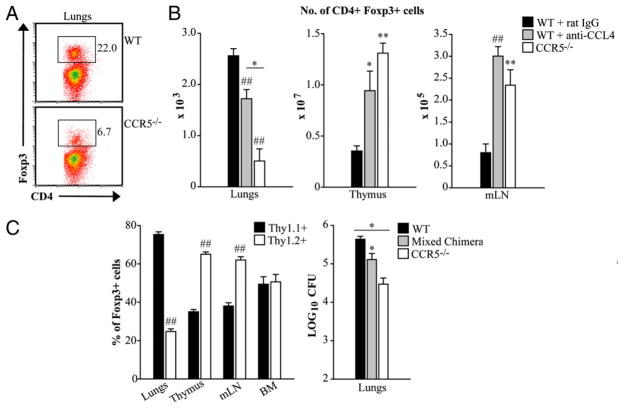
Natural Tregs mature in the thymus and are characterized by stable expression of Foxp3 (17). The signals required for thymic emigration of Tregs remain unclear. The receptor S1P1 is expressed on Tregs and was recently shown to influence Treg development, maintenance, and suppressor function (18, 19). CCR5 is highly expressed on Tregs and has been shown to dictate Treg trafficking in several models of infection and in tumor immunity (4, 5, 20). Mice lacking CCR5 or endogenous CCL4 resolve H. capsulatum infection more efficiently than controls due to a decrement in the proportion (Fig. 5A) and number of Tregs in the lungs (6). Hence, we hypothesized that the reduction of Tregs in infected lungs was a result of failed thymic egress in the absence of CCR5 activity.
FIGURE 5.
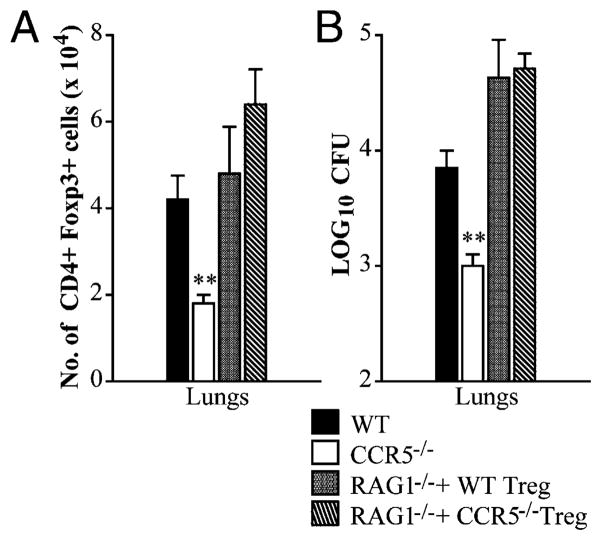
The paucity of Tregs in the lungs correlates with accumulation in the thymus and lymph nodes during H. capsulatum infection. Mice were infected with 2 × 106 yeasts intranasally. For neutralization of CCL4, mice were i.p. given an mAb to CCL4 at the time of infection and every other day thereafter. Tregs were characterized as CD4+ Foxp3+ cells. The proportion of Tregs in the lungs (A) and absolute number of Tregs in the lungs, thymus, and mediastinal lymph nodes were determined at day 14 postinfection (B). For the generation of mixed bone marrow chimeras, RAG1−/− mice were lethally irradiated and transplanted with a 1:1 ratio of Thy1.1+ (WT) and Thy1.2+ (CCR5−/−) bone marrow cells. After 8–10 wk, mice were infected with 2 × 106 H. capsulatum intranasally. Cells isolated from infected lungs, thymi, mediastinal lymph nodes (mLN), and bone marrow (BM) were stained and analyzed by flow cytometry. The graph represents the proportion of CD4+ Foxp3+ cells that were Thy1.1+ or Thy1.2+ at day 14 postinfection. WT and CCR5−/− mice were infected in parallel with mixed bone marrow chimeras to quantify fungal burden in the lungs at day 14 postinfection (C). Data represent the mean ± SEM (n = 6–12) from two to three experiments. *p < 0.05, **p < 0.01, ##p < 0.001.
The proportion of CD4+ SP cells expressing Foxp3 was comparable in WT and CCR5−/− mice prior to (1.3 ± 0.11% versus 1.4 ± 0.12%; p > 0.05) and at day 14 postinfection (5.8 ± 1.2% versus 4.6 ± 0.7%; p > 0.05). However, the absolute number of CD4+Foxp3+ cells in CCR5−/− thymi was elevated compared with controls at both time points (data not shown and Fig. 5B). Although Treg proliferation was augmented in the thymus of uninfected CCR5−/− mice (Table I), the percent of CD4+ Foxp3+ cells that incorporated BrdU in control and mutant animals was similar at day 14 postinfection (46.5 ± 5.5% versus 42.3 ± 4.7%; p > 0.05). Thus, during infection, more Tregs was more likely due to impaired thymic emigration rather than augmented expansion.
CCL4 is a potent chemotactic mediator for Tregs (21) as well as human thymocytes (22). Neutralization of CCL4 causes a similar reduction in Tregs in CCR5−/− lungs in infection (6). This prompted us to investigate the role of the CCL4–CCR5 signaling axis in Treg egress from the thymus. Thymocytes were isolated from mice treated with rat IgG or a mAb to CCL4 at days 0 and 14 postinfection. Similar to CCR5−/− mice, the absence of endogenous CCL4 was associated with more cells in the thymus. Although the proportion of CD4+ SP cells expressing Foxp3 was comparable, the absolute of Tregs was significantly elevated compared with IgG controls (p < 0.01; Fig. 5B).
Tregs also accumulated in the mediastinal lymph nodes of CCL4-neutralized and CCR5−/− mice during H. capsulatum infection. Although Foxp3+ cells constituted 8% of the CD4+ T cells in all groups, the absolute number was significantly higher in CCL4-neutralized and CCR5−/− lymph nodes days 7 and 14 postinfection (p < 0.001) (data not shown and Fig. 5B).
Generation of mixed bone marrow chimeras
To confirm that CCR5 influenced emigration of Tregs from the thymus and lymph nodes, lethally irradiated RAG1−/− mice were transplanted with a 1:1 ratio of WT (Thy1.1+) and CCR5−/− (Thy1.2+) bone marrow cells. At 8–10 wk posttransplant, chimeric mice were infected with H. capsulatum to determine the proportion of Thy1.1+ and Thy1.2+ Tregs in the thymus, mediastinal lymph nodes, lungs, and bone marrow. An increased proportion of Tregs in the thymus and lymph nodes of chimeric mice were Thy1.2+. In contrast, lungs from chimeric mice manifested an increased proportion of Thy1.1+Foxp3+ cells. An equal proportion of Thy1.1+ and Thy1.2+ Tregs were detected in the bone marrow (Fig. 5C). Mixed bone marrow chimeras were also infected in parallel with WT and CCR5−/− mice to characterize fungal burden. At day 14, the number of CFUs in the lungs of chimeric mice was intermediate to that of WT and CCR5−/− mice (Fig. 5C).
CCR5 is dispensable for Treg migration once in the periphery
The importance of CCR5 in thymic egress of Tregs was extended by adoptive transfer experiments. RAG1−/− mice were reconstituted with WT CD4+ T effector cells and WT or CCR5−/− CD4+CD25+ cells 1 d prior to infection. At day 14 postinfection, mice had a similar number of Foxp3+ T cells and fungal burden in the lungs whether given WT or CCR5−/− CD4+CD25+ cells (Fig. 6). Both groups contained an equal percent of CD4+ Foxp3+ cells in the spleen (0.39 ± 0.06% versus 0.56 ± 0.12%) and lymph nodes (2.3 ± 0.3% versus 2.1 ± 0.4%). These results demonstrate that WT and CCR5−/− Tregs manifest the same migratory capacity as WT once in circulation.
FIGURE 6.
CCR5 is dispensable for Treg migration once in the periphery. RAG1−/− mice were reconstituted with WT CD4+ T cells and WT or CCR5−/− CD4+ CD25+ cells purified from the spleen and infected with 2 × 106 yeasts 1 d later. Number of CD4+ CD25+ Foxp3+ cells (A) and fungal burden (B) in the lungs of RAG1−/− mice reconstituted with WT or CCR5−/− CD4+CD25+ cells at day 14 postinfection. Fungal burden is presented as the mean number of CFUs. Data represent the mean ± SEM (n = 8–12) from two to three experiments. **p < 0.01.
Chemokine-driven thymic egress is specific to CCR5
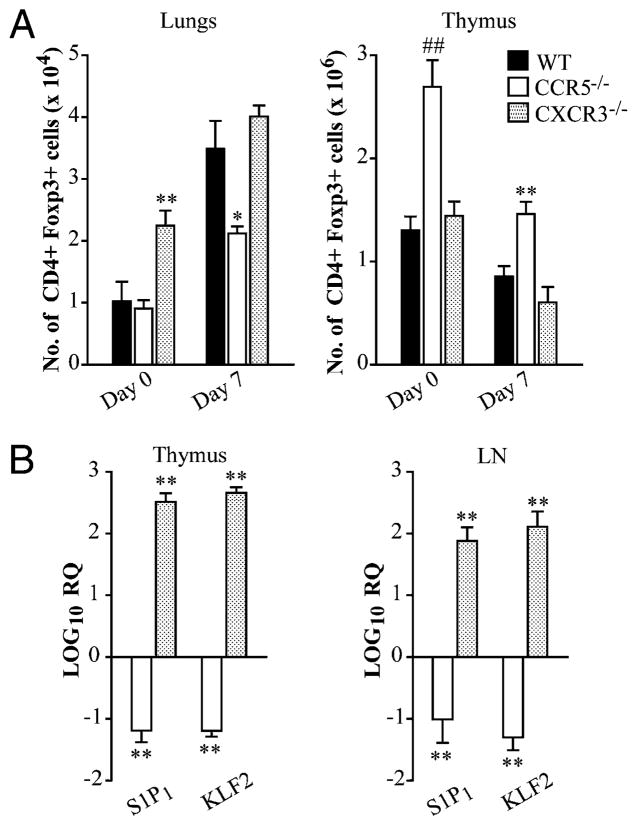
Other chemokines including CXCR3 and CCR4 also influence Treg migration. CXCR3 is upregulated in a unique population of Tregs that coexpress the Th1-specifying transcription T-bet and Foxp3 to facilitate Treg homing in Mycobacterium tuberculosis infection (23). To establish if impaired thymic egress and subsequent homing of Tregs was specific to a deficiency in CCR5 signaling, mice lacking CXCR3 were infected because this chemokine receptor has been implicated in Treg trafficking during Th1 responses (23). At days 0 and 7 postinfection, CXCR3−/− mice had a similar number of Foxp3+ cells in the thymus as WT. Furthermore, Tregs lacking CXCR3 homed to the lungs as efficiently as WT both prior to and during infection (Fig. 7A). Interestingly, S1P1 and KLF2 were dramatically upregulated in thymi and lymph nodes harvested from uninfected CXCR3−/− mice (Fig. 7B).
FIGURE 7.
Chemokine-driven thymic egress is specific to CCR5. WT and CXCR3−/− mice were sacrificed at days 0 and 7 postinfection to quantify the number of CD4+ Foxp3+ cells in the lungs, thymus, and mediastinal lymph nodes (A). RNA was extracted from the thymus and lymph nodes of uninfected mice to measure expression of KLF2 and S1P1 by quantitative real-time PCR. HPRT was used as an endogenous control, and values represent log increase compared with WT controls (B). Data represent the mean ± SEM (n = 6–8) from two to three experiments. *p < 0.05, **p < 0.01, ##p < 0.001.
Discussion
CCR5 is expressed on Tregs and has been shown to dictate Treg trafficking in models of infection and tumor immunity (4, 5, 20). H. capsulatum infection in CCR5−/− mice manifests an accelerated clearance of the fungus beginning on day 14 coincident with the activation of the cellular immune response. The heightened elimination of H. capsulatum was associated with a marked reduction in the number of Tregs in lungs and an amplified Th17 response. The latter was critically important for enhanced fungal clearance (6). In this study, we discovered that the paucity of Tregs in CCR5−/− lungs was coincident with an accumulation of Tregs in the thymus and mediastinal lymph nodes. The involvement of CCR5 in thymic and lymph node emigration was demonstrated by the generation of mixed bone marrow chimeras. Chimeric mice exhibited a greater proportion of CCR5−/− Tregs in the thymus and mediastinal lymph nodes and a reduced proportion in the lungs. Thus, by influencing Treg homing to the lungs, CCR5 regulates the outcome of H. capsulatum infection.
The exiting of T cells from the thymus is dependent on S1P1 (12, 24). Expression of this receptor is regulated by the transcription factor KLF2 (25). Chemokine receptors have also been shown to influence thymic egress. CCR7 and CXCR4 direct thymic egress and lymph node accumulation of T cells in homeostatic states (26, 27). In contrast, CCR5 influences S1P1-dependent thymic egress (28). In vivo activation of S1P1 with the sphingosine analog FTY720 reduces the number of thymocytes in CCR5−/−, but not WT mice. Consistent with these observations, we found a correlation between thymocyte number and S1P1 expression in CCR5−/− mice. The absence of CCR5 was accompanied by diminished transcription of KLF2 and S1P1. However, this finding was not merely correlative because in vitro stimulation of thymocytes from WT mice with CCL3, CCL4, or CCL5 induced expression of KLF2 and S1P1. These results argue for a direct relationship between CCR5 signaling and expression of these molecules.
The cholesterol-lowering drug simvastatin has been shown to upregulate KLF2 and, therefore, S1P1 expression in T cells (14). Simvastatin-induced upregulation of KLF2 reduced the total number of thymocytes in CCR5−/− mice, suggesting that CCR5-driven exiting occurs in a KLF2- and S1P1-dependent manner. However, because simvastatin has been shown to have multiple anti-inflammatory effects (29–31), we are unable to exclude the possibility that the reduction of thymocytes in CCR5−/− mice upon administration of simvastatin is solely dependent on upregulation of KLF2. Furthermore, because T cell migration is largely influenced by chemokine receptor expression, the inability to sense a chemokine gradient in the absence of CCR5 is also plausible.
Maturation of medullary thymocytes is associated with upregulation of molecules that facilitate T cell entry into the periphery such as CD62L and β7 integrin (9, 10, 32, 33). Similar to S1P1, CD62L expression is regulated by KLF2 and has been implicated in thymic emigration (34). For unknown reason, CD62L expression like CD69 was highly variable on CCR5−/− SP thymocytes. In multiple, but not all, experiments, the proportion of CD4+ and CD8+ SP thymocytes that were CD62Lhi was diminished in the absence of CCR5. Thus, diminished KLF2 in CCR5−/− mice may hinder upregulation of CD62L and, consequently, thymic exiting. Additionally, expression of β7 integrin was altered in the absence of CCR5. The expression of β7 integrin was diminished on CCR5−/− CD8+ SP thymocytes compared with WT controls (48.5 ± 1.4% versus 36.1 ± 0.9%; p < 0.001).
PI3K/Akt appears to contribute to thymic emigration (15). Constitutive activation of this pathway is associated with more mature T cells in the thymus and delayed appearance in the periphery. Stimulation of thymocytes with IL-2 enhanced phosphorylation of Akt in CCR5−/− thymocytes compared with the control. Thus, hyperactivation of Akt in the absence of CCR5 may influence thymic accumulation. Additionally, treatment of WT thymocytes with CCL3, CCL4, or CCL5 resulted in phosphorylation of Akt, which was significantly diminished in the absence of CCR5. These results demonstrate that CCR5 signaling directly activates Akt. Because the Akt–mammalian target of rapamycin pathway regulates Foxp3 expression and Treg development in the thymus (35, 36), studies are planned to explore the role of CCR5 in this signaling pathway.
If CCR5 was absolutely required for thymic egress, we would expect accumulation of mature SP thymocytes due to failed egress and, consequently, a reduced proportion of DN and DP cells. Although CCR5−/− mice displayed a slight yet statistically significant increase in CD4+ SP thymocytes (6.3 ± 0.16% versus 7.7 ± 0.29%; p < 0.001), its biological relevance is unresolved. Thus, other additional factors likely contribute to CCR5−/− mice having a more capacious thymus. Microarray analysis has revealed several families of genes that were differentially expressed in CCR5−/− thymi. Notably, >40 genes associated with the cell cycle process were upregulated. A large proportion of these cell cycle genes were specifically linked to M phase. Thus, dysregulation of the cell cycle may contribute to thymus enlargement in CCR5−/− mice. Genes associated with apoptosis and abnormal T cell development and function were also upregulated in CCR5−/− thymi. Downregulated genes included those associated with TGF-β signaling, arachidonic acid metabolism, regulation of the inflammatory response, biological adhesion, and epithelial cell development (data not shown).
The influence of CCR5 signaling on intrathymic trafficking has not been explored. CCR7 and CCR9 are involved in thymic entry of bone marrow progenitor cells (37). CCR7 has been recognized has the predominant chemokine receptor in intrathymic trafficking of thymocytes (38–40). However, it was recently demonstrated that CCR7 directs migration of CD4+ SP cells toward the medulla, but is dispensable for entry and accumulation. Treatment of CD4+ SP cells with pertussis toxin inhibits directional trafficking of CD4+ SP cells from the cortex to the medulla, as well as accumulation in the medulla (41). These results suggest that other chemokine receptors likely influence the entry of CD4+ SP cells into the medulla. CCL4 has been shown to potently stimulate migration and activation of CD4+ SP, CD8+ SP, and CD4+CD8+ DP human thymocytes (22). CCL3, CCL4, and CCL5 are highly expressed by CD90− cells in thymus (data not shown). Thus, it is possible that CCR5 influences intrathymic trafficking of thymocytes in addition to thymic emigration.
Although our findings support the assertion that CCR5 influences thymic exiting, CCR5−/− mice exhibit an equal proportion of CD8+ and an elevated proportion of CD4+ T cells in peripheral organs (6). The increased proportion of CD4+ cells in H. capsulatum infection is not a result of augmented proliferation in the periphery. CCR5 is required for optimal IL-2 production and CD4+ T cell proliferation. In fact, T cells isolated from CCR5−/− mice or CCR5Δ32 homozygotes display a diminished proliferative capacity (6, 42). A deficiency in Tregs and accumulation of effector T cells has been described in mice and humans that have mutations in the genes encoding IL-2 or its receptor (43). The development of severe autoimmune disease in IL-2−/− mice is associated with reduced proportion of Tregs and an increased proportion and number of Th1 and Th17 cells in multiple organs (44). Furthermore, in experimental tuberculosis, Ag-specific Tregs prevent accumulation of effector CD4+ T cells in the lungs (45). Based on these findings, a decrement in Tregs in CCR5−/− lungs could allow accumulation of effector CD4+ T cells in histoplasmosis. Unlike the scenario in experimental tuberculosis, a portion of the effector CD4+ T cells in H. capsulatum-infected mice differentiate into Th17, which are central in the accelerated clearance.
In summary, we demonstrate that CCR5 signaling influences thymic egress, likely in a KLF2- and S1P1-dependent manner. Further studies focusing on how the CCR5, KLF2, and S1P1 signaling pathways overlap will likely clarify the mechanism in which T cells exit the thymus. Of considerable importance is the influence of the thymus in coordinating the protective immune response during infection, as CCR5-dependent trafficking of Tregs dictates the outcome to the fungal pathogen H. capsulatum.
Acknowledgments
This work was supported by a Merit Review from the Veterans Affairs Hospital and National Institutes of Health Grants AI-07337 and AI-083313.
Abbreviations used in this article
- DN
double-negative
- DP
double-positive
- HPRT
hypoxanthine-guanine phosphoribosyltransferase
- KLF2
Krüppel-like factor 2
- S1P1
sphingosine 1-phosphate receptor 1
- SP
single-positive
- Treg
regulatory T cell
- WT
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 2.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 3.Hori S, Sakaguchi S. Foxp3: a critical regulator of the development and function of regulatory T cells. Microbes Infect. 2004;6:745–751. doi: 10.1016/j.micinf.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Moreira AP, Cavassani KA, Massafera Tristão FS, Campanelli AP, Martinez R, Rossi MA, Silva JS. CCR5-dependent regulatory T cell migration mediates fungal survival and severe immunosuppression. J Immunol. 2008;180:3049–3056. doi: 10.4049/jimmunol.180.5.3049. [DOI] [PubMed] [Google Scholar]
- 5.Yurchenko E, Tritt M, Hay V, Shevach EM, Belkaid Y, Piccirillo CA. CCR5-dependent homing of naturally occurring CD4+ regulatory T cells to sites of Leishmania major infection favors pathogen persistence. J Exp Med. 2006;203:2451–2460. doi: 10.1084/jem.20060956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kroetz DN, Deepe GS., Jr CCR5 dictates the equilibrium of proinflammatory IL-17+ and regulatory Foxp3+ T cells in fungal infection. J Immunol. 2010;184:5224–5231. doi: 10.4049/jimmunol.1000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deepe GS, Jr, Gibbons RS. TNF-α antagonism generates a population of antigen-specific CD4+CD25+ T cells that inhibit protective immunity in murine histoplasmosis. J Immunol. 2008;180:1088–1097. doi: 10.4049/jimmunol.180.2.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen HL, Deepe GS., Jr Apoptosis modulates protective immunity to the pathogenic fungus. Histoplasma capsulatum. J Clin Invest. 2005;115:2875–2885. doi: 10.1172/JCI25365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge Q, Chen WF. Phenotypic identification of the subgroups of murine T-cell receptor alphabeta+ CD4+ CD8− thymocytes and its implication in the late stage of thymocyte development. Immunology. 1999;97:665–671. doi: 10.1046/j.1365-2567.1999.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian T, Zhang J, Gao L, Qian XP, Chen WF. Heterogeneity within medullary-type TCRalphabeta(+)CD3(+)CD4(−)CD8(+) thymocytes in normal mouse thymus. Int Immunol. 2001;13:313–320. doi: 10.1093/intimm/13.3.313. [DOI] [PubMed] [Google Scholar]
- 11.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 12.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 13.Sebzda E, Zou Z, Lee JS, Wang T, Kahn ML. Transcription factor KLF2 regulates the migration of naive T cells by restricting chemokine receptor expression patterns. Nat Immunol. 2008;9:292–300. doi: 10.1038/ni1565. [DOI] [PubMed] [Google Scholar]
- 14.Bu DX, Tarrio M, Grabie N, Zhang Y, Yamazaki H, Stavrakis G, Maganto-Garcia E, Pepper-Cunningham Z, Jarolim P, Aikawa M, et al. Statin-induced Krüppel-like factor 2 expression in human and mouse T cells reduces inflammatory and pathogenic responses. J Clin Invest. 2010;120:1961–1970. doi: 10.1172/JCI41384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbee SD, Alberola-Ila J. Phosphatidylinositol 3-kinase regulates thymic exit. J Immunol. 2005;174:1230–1238. doi: 10.4049/jimmunol.174.3.1230. [DOI] [PubMed] [Google Scholar]
- 16.Watson SR, Bullock WE. Immunoregulation in disseminated histoplasmosis: characterization of the surface phenotype of splenic suppressor T lymphocytes. Infect Immun. 1982;37:940–945. doi: 10.1128/iai.37.3.940-945.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 18.Sawicka E, Dubois G, Jarai G, Edwards M, Thomas M, Nicholls A, Albert R, Newson C, Brinkmann V, Walker C. The sphingosine 1-phosphate receptor agonist FTY720 differentially affects the sequestration of CD4+/CD25+ T-regulatory cells and enhances their functional activity. J Immunol. 2005;175:7973–7980. doi: 10.4049/jimmunol.175.12.7973. [DOI] [PubMed] [Google Scholar]
- 19.Liu G, Burns S, Huang G, Boyd K, Proia RL, Flavell RA, Chi H. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat Immunol. 2009;10:769–777. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan MC, Goedegebuure PS, Belt BA, Flaherty B, Sankpal N, Gillanders WE, Eberlein TJ, Hsieh CS, Linehan DC. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J Immunol. 2009;182:1746–1755. doi: 10.4049/jimmunol.182.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG. B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol. 2001;2:1126–1132. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- 22.Dairaghi DJ, Franz-Bacon K, Callas E, Cupp J, Schall TJ, Tamraz SA, Boehme SA, Taylor N, Bacon KB. Macrophage inflammatory protein-1β induces migration and activation of human thymocytes. Blood. 1998;91:2905–2913. [PubMed] [Google Scholar]
- 23.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem. 2004;279:15396–15401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- 25.Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, Wani MA, Lingrel JB, Hogquist KA, Jameson SC. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- 26.Zaitseva MB, Lee S, Rabin RL, Tiffany HL, Farber JM, Peden KW, Murphy PM, Golding H. CXCR4 and CCR5 on human thymocytes: biological function and role in HIV-1 infection. J Immunol. 1998;161:3103–3113. [PubMed] [Google Scholar]
- 27.Ueno T, Hara K, Willis MS, Malin MA, Höpken UE, Gray DH, Matsushima K, Lipp M, Springer TA, Boyd RL, et al. Role for CCR7 ligands in the emigration of newly generated T lymphocytes from the neonatal thymus. Immunity. 2002;16:205–218. doi: 10.1016/s1074-7613(02)00267-4. [DOI] [PubMed] [Google Scholar]
- 28.Yopp AC, Fu S, Honig SM, Randolph GJ, Ding Y, Krieger NR, Bromberg JS. FTY720-enhanced T cell homing is dependent on CCR2, CCR5, CCR7, and CXCR4: evidence for distinct chemokine compartments. J Immunol. 2004;173:855–865. doi: 10.4049/jimmunol.173.2.855. [DOI] [PubMed] [Google Scholar]
- 29.Youssef S, Stüve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, Bravo M, Mitchell DJ, Sobel RA, Steinman L, Zamvil SS. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 30.Hakamada-Taguchi R, Uehara Y, Kuribayashi K, Numabe A, Saito K, Negoro H, Fujita T, Toyo-oka T, Kato T. Inhibition of hydroxymethylglutaryl-coenzyme a reductase reduces Th1 development and promotes Th2 development. Circ Res. 2003;93:948–956. doi: 10.1161/01.RES.0000101298.76864.14. [DOI] [PubMed] [Google Scholar]
- 31.Mausner-Fainberg K, Luboshits G, Mor A, Maysel-Auslender S, Rubinstein A, Keren G, George J. The effect of HMG-CoA reductase inhibitors on naturally occurring CD4+CD25+ T cells. Atherosclerosis. 2008;197:829–839. doi: 10.1016/j.atherosclerosis.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 32.Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- 33.Pribila JT, Quale AC, Mueller KL, Shimizu Y. Integrins and T cell-mediated immunity. Annu Rev Immunol. 2004;22:157–180. doi: 10.1146/annurev.immunol.22.012703.104649. [DOI] [PubMed] [Google Scholar]
- 34.Bai A, Hu H, Yeung M, Chen J. Kruppel-like factor 2 controls T cell trafficking by activating L-selectin (CD62L) and sphingosine-1-phosphate receptor 1 transcription. J Immunol. 2007;178:7632–7639. doi: 10.4049/jimmunol.178.12.7632. [DOI] [PubMed] [Google Scholar]
- 35.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O’Connor E, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zlotoff DA, Sambandam A, Logan TD, Bell JJ, Schwarz BA, Bhandoola A. CCR7 and CCR9 together recruit hematopoietic progenitors to the adult thymus. Blood. 2010;115:1897–1905. doi: 10.1182/blood-2009-08-237784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ueno T, Saito F, Gray DH, Kuse S, Hieshima K, Nakano H, Kakiuchi T, Lipp M, Boyd RL, Takahama Y. CCR7 signals are essential for cortex-medulla migration of developing thymocytes. J Exp Med. 2004;200:493–505. doi: 10.1084/jem.20040643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwan J, Killeen N. CCR7 directs the migration of thymocytes into the thymic medulla. J Immunol. 2004;172:3999–4007. doi: 10.4049/jimmunol.172.7.3999. [DOI] [PubMed] [Google Scholar]
- 40.Kurobe H, Liu C, Ueno T, Saito F, Ohigashi I, Seach N, Arakaki R, Hayashi Y, Kitagawa T, Lipp M, et al. CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity. 2006;24:165–177. doi: 10.1016/j.immuni.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 41.Ehrlich LI, Oh DY, Weissman IL, Lewis RS. Differential contribution of chemotaxis and substrate restriction to segregation of immature and mature thymocytes. Immunity. 2009;31:986–998. doi: 10.1016/j.immuni.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Camargo JF, Quinones MP, Mummidi S, Srinivas S, Gaitan AA, Begum K, Jimenez F, VanCompernolle S, Unutmaz D, Ahuja SS, Ahuja SK. CCR5 expression levels influence NFAT translocation, IL-2 production, and subsequent signaling events during T lymphocyte activation. J Immunol. 2009;182:171–182. doi: 10.4049/jimmunol.182.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 44.Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, Hirahara K, Sun HW, Wei L, Vahedi G, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shafiani S, Tucker-Heard G, Kariyone A, Takatsu K, Urdahl KB. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J Exp Med. 2010;207:1409–1420. doi: 10.1084/jem.20091885. [DOI] [PMC free article] [PubMed] [Google Scholar]