Abstract
BACKGROUND AND PURPOSE
The cystic fibrosis transmembrane conductance regulator (CFTR) is a cAMP-dependent chloride channel in the plasma membrane of epithelia whose mutation is the cause of the genetic disease cystic fibrosis (CF). The most frequent CFTR mutation is deletion of Phe508 and this mutant protein (delF508CFTR) does not readily translocate to the plasma membrane and is rapidly degraded within the cell. We hypothesized that treating epithelial cells with resveratrol, a natural polyphenolic, phyto-ooestrogenic compound from grapes, could modulate both the expression and localization of CFTR.
EXPERIMENTAL APPROACH
Cells endogenously expressing CFTR (MDCK1 and CAPAN1 cells) or delF508CFTR (CFPAC1 and airway epithelial cells, deriving from human bronchial biopsies) were treated with resveratrol for 2 or 18 h. The effect of this treatment on CFTR and delF508CFTR expression and localization was evaluated using RT-PCR, Western blot and immunocytochemistry. Halide efflux was measured with a fluorescent dye and with halide-sensitive electrodes. Production of interleukin-8 by these cells was assayed by ELISA.
KEY RESULTS
Resveratrol treatment increased CFTR expression or maturation in immunoblotting experiments in MDCK1 cells or in CFPAC1 cells. Indirect immunofluorescence experiments showed a shift of delF508CFTR localization towards the (peri)-membrane area in CFPAC1 cells and in human airway epithelial cells. A cAMP-dependent increase in membrane permeability to halide was detected in resveratrol-treated CFPAC1 cells, and was inhibited by a selective inhibitor of CFTR.
CONCLUSION AND IMPLICATIONS
These results show that resveratrol modulated CFTR expression and localization and could rescue cAMP-dependent chloride transport in delF508CFTR cells.
Keywords: CFTR, cystic fibrosis, resveratrol, CFPAC1, human airways cell culture
Introduction
The autosomal and recessive genetic disease cystic fibrosis (CF) is related to mutations in CFTR, the gene encoding for the cystic fibrosis transmembrane conductance regulator (CFTR). This protein is a cAMP-dependent anionic channel, mediating chloride and bicarbonate secretion from the apex of epithelial cells. The deletion of Phe508 in the first nucleotide binding domain of CFTR (delF508CFTR) accounts for most of Caucasian CF genotypes (Bobadilla et al., 2002). The reduced chloride permeability in cells expressing delF508CFTR is related to the very few delF508CFTR that achieve final maturation and delivery to the plasma membrane. More than 95% of synthesized delF508CFTR proteins are targeted to degradation at the endoplasmic reticulum (Champigny et al., 1995; Wang et al., 2006). Moreover, delF508CFTR channels exhibit a reduced open probability and a lower phosphorylation-induced activation compared with the wild-type CFTR (Dalemans et al., 1991; Wang et al., 2000). In principle, cell permeability to chloride could be increased by improving transcription, stabilization, maturation, and/or folding of the protein (i.e. by using correctors) and/or by stimulating directly or indirectly channel activity (i.e. by using potentiators) (Kunzelmann and Mall, 2003). The acute stimulation of CFTR-mediated transport by low concentrations of polyphenolic compounds such as genistein has been observed for a long time (Kunzelmann and Mall, 2001). More recently, prolonged exposure (24 h) to genistein enhanced the maturation of CFTR and its localization to the cell surface of baby hamster kidney (BHK) cells, transfected with CFTR or delF508CFTR, albeit failing to restore delF508CFTR function (Schmidt et al., 2008). Curcumin, another polyphenol, was reported to increase CFTR synthesis, to stimulate channel activity and to rescue delF508CFTR in cellular and animal models (Egan et al., 2004; Lipecka et al., 2006; Wang et al., 2007). These reports raise the interesting possibility that some molecules may act as both correctors and potentiators, thus being promising candidates for an optimized CF therapy.
Until now, little attention has been paid to the effects on CFTR of resveratrol, a naturally occurring polyphenolic compound from grapes. This might be due to its weak acute stimulatory effect on CFTR in Calu-3 cells, compared with the effects of other phenolic compounds (Illek et al., 2000). Interestingly, curcumin, genistein and resveratrol may also act as phyto-oestrogens (Matsumura et al., 2005; Bachmeier et al., 2010). In a recent study, a functional rescue of delF508CFTR was achieved by treating a cell line with 17β-oestradiol (E2) because of the increased expression of the regulatory protein, Na-H exchanger regulatory factor 1 (NHERF-1; Fanelli et al., 2008). NHERF-1 is a positive regulator of CFTR membrane expression (Guerra et al., 2005; Cushing et al., 2008) and its gene possesses multiple oestrogen half-response elements that elicit a robust up-regulation of the protein expression (Ediger et al., 1999; Ediger et al., 2002). Also, oestrogens are known to increase CFTR mRNA and protein levels in tissues known to be E2 targets (Rowlands et al., 2001; Ajonuma et al., 2005). Besides a potential oestrogenic-like effect, resveratrol has potent anti-oxidant and anti-inflammatory properties (Bisht et al., 2010), but its effects in CF models remain to be investigated.
Our hypothesis that a prolonged exposure of cells to resveratrol would modulate CFTR expression has been supported by our present results obtained in a range of cell lines. In particular, resveratrol treatment of the cystic fibrosis pancreatic adenocarcinoma (CFPAC1) cells induced the mature form of CFTR and enhanced its localization to the cell membrane. Such a re-localization of delF508CFTR to the cell membrane was also observed in resveratrol-treated cultures of human airways epithelium cells from a CF patient. Also, in resveratrol-treated CFPAC1 cells, a cAMP-dependent anionic efflux, inhibited by the CFTR inhibitor CFTRinh-172, was demonstrated. These results show that pretreating cultured cells with resveratrol could rescue delF508CFTR function. Further experiments are needed to understand the mechanism(s) underlying the effect of resveratrol on CFTR expression, and to evaluate its potency in CF therapy.
Methods
Cell culture and treatment
Madin Darby canine kidney (MDCK) type 1 and type 2 cells were cultured in Dulbecco's Modified Eagle Medium +GlutaMAX™ (Gibco, Invitrogen, Cergy-Pontoise, France), and for immunofluorescence experiments, were cultured at an air-liquid interface. MDCK1 cells are derived from distal renal tubules and express endogenous CFTR at a low level whereas MDCK2 cells, derived from proximal tubules lack CFTR (Mohamed et al., 1997). CAPAN1 and CFPAC1 cells, obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA), were grown according to the manufacturer's recommendations. Both lines are derived from human pancreatic duct adenocarcinomas; the former expresses CFTR and retains characteristics of normal pancreatic duct function, whereas the latter expresses the mutant form, delF508CFTR (Wang et al., 2003). Human airway epithelial cells, differentiated in a polarized epithelium primary culture (MucilAir™) are derived from control and CF (homozygous for the delF508 mutation) bronchial biopsies and were provided by Epithelix Sàrl (http://www.epithelix.com/, Plan les Ouates, Switzerland). They were maintained at an air-liquid interface according to the manufacturer's instructions, using MucilAir™ culture medium (Wiszniewski et al., 2006).
Cells were deprived of fetal calf serum (FCS, 10% for MDCK1 and CFPAC1 cells, 20% for CAPAN1 cells), 24 h before adding resveratrol into the culture medium. Except when stated, experiments were carried out after 18 h of treatment. There was no difference in cell count number between treated and control cells.
Quantitative real-time (q RT)-PCR experiments
Total RNAs were isolated from each well (9.6 cm2) using TRIzol® reagent following the manufacturer's recommendations (Invitrogen, Cergy-Pontoise, France). cDNA was synthesized from 5 µg of total RNA using a First-Strand cDNA Synthesis Kit (Amersham, GE Healthcare, Orsay, France) and gene expression was studied using the cycle threshold-based method and the LightCycler FastStart DNA Master PLUS SYBR Green I kit (Roche Applied Science, Meylan, France). Each reaction was performed in quadruplicate and the relative expression of CFTR was normalized to hypoxanthine phosphoribosyltransferase (HPRT) expression. The forward and reverse primers were respectively: ATGCCCTTCGGCGATGTTTT and TGATTCTTCCCAGTAAGAGAGGC for CFTR; TGGACAGGACTGAACGTCTTG and CCAGCAGGTCAGCAAAGAATTTA for HPRT.
Protein analysis
Total proteins were extracted from cells disrupted in ice-cold RIPA buffer, supplemented with a protease inhibitor cocktail (Roche Diagnostics GmdH, Germany); membrane extracts were prepared as previously described (Brouillard et al., 2001). Proteins were either directly immunoblotted or after the immuno-precipitation of CFTR. In this case, magnetic Dynabeads® protein G (Invitrogen Dynal AS, Oslo, Norway) were incubated overnight (4°C) with 1 µg of the mouse anti-CFTR COOH tail monoclonal MAB 25031 antibody (Ab), clone 24–1 (R&D Systems Minneapolis, MN, USA). The bead-Ab complexes were incubated (30 min at room temperature) with 500 µg of total protein extracts and washed three times with 0.1% phosphate buffered saline (PBS)-Tween. Protein elution was achieved using Laemmli buffer containing 2% β-mercaptoethanol (15 min, 37°C). Proteins were separated by SDS-polyacrylamide (6–8%) gel electrophoresis, and electro-blotted on nitrocellulose membranes (Bio-Rad, Marnes la Coquette, France) as previously reported (Brouillard et al., 2001; Lipecka et al., 2006). Preliminary experiments performed on membrane extracts confirmed that CFTR was in the membrane-bound fraction, not in the soluble, cytosolic fraction. The following primary antibodies were used: mouse anti-CFTR Ab MAB 25031 diluted to 1:5000, mouse anti-CFTR NH2 tail MM13-4 diluted to 1:1000 (Chemicon International, Temecula, CA, USA), rabbit polyclonal anti-NHERF-1 Ab diluted to 1:300 (Santa Cruz Biotechnology Inc., Heidelberg, Germany), rabbit monoclonal anti-keratin-18 Ab, diluted to 1:500 (DC10, Santa Cruz Biotechnology Inc. Heidelberg, Germany) and rabbit polyclonal anti-phosphorylated keratin-18 (phospho-Ser52) Ab diluted to 1:300 (Santa Cruz Biotechnology Inc. Heidelberg, Germany). The mouse secondary Ab and the goat anti-rabbit IgG HRP-conjugated secondary Ab (AbCys, Paris, France) were diluted to 1:5000 (Amersham Biosciences, UK) and to 1:1000 respectively. Protein loading control was checked using a mouse anti-tubulin Ab (Abcam, UK) diluted to 1:15 000. Stained proteins were detected by ECL plus Western Blotting detection system (GE Biotechnologies, Orsay, France). Films were recorded digitally and quantified using the NIH image J 1.42q software (National Institutes of Health, Bethesda, MD) (available at: http://rsb.info.nih.gov/).
Immunocytochemistry
The protocol for indirect immunofluorescence experiments has been detailed elsewhere (Lipecka et al., 2006; Wiszniewski et al., 2006). In brief, cells were fixed with cold acetone, re-hydrated using cold PBS and permeabilized by adding 0.25% Triton X-100. Nonspecific binding sites were blocked by incubation (1 h, room temperature) in PBS supplemented with 0.1% Triton X-100, 10% FCS and 3% BSA. Cells were incubated overnight at 4°C with the following antibodies: anti-CFTR MAB 25031 (diluted to 1:100 for MDCK1 cells, and to 1:50 for airway epithelial cell cultures), rabbit anti-CFTR MP-CT1 Ab (diluted to 1:100 for CFPAC1 cells) anti-keratin-18 Ab (DC10, diluted 1:100). After washing in 0.1% Triton X-100/PBS, cells were incubated (1 h, room temperature) with Alexa conjugated 594- or 488- goat anti-mouse IgG secondary antibodies (Molecular Probe Europe, Leiden, Netherlands) diluted to 1:1000 in 0.1% Triton X-100/PBS. Nuclei were stained with DAPI (Molecular Probe Europe, Leiden, Netherlands). Cells were observed under Zeiss LSM Pascal (Zeiss Jana, Germany) or Leica TCS SP5 AOBS (Heidelberg, Germany) confocal laser-scanning microscopes, using argon ion laser or helium neon laser for Alexa 488 or Alexa 594 fluorochromes respectively. Images were collected with Zeiss/Leica 63x oil objectives. MucilAir™ images were analysed using the Imaris 6.4.2 software (Bitplane AG, Zurich, Switzerland).
Interleukin-8 (IL-8) production
As an index of the inflammatory response, IL-8 was measured in supernatants of cell cultures, using an ELISA kit (Quantikine IL-8 ELISA kit, R&D Systems, Minneapolis, MN, USA). Supernatants were collected after resveratrol or DMSO treatment. Only assays having standard curves with a calculated regression line value >0.98 were accepted for analysis.
Functional assays of halide efflux
Two functional approaches were applied to assess halide efflux in CFPAC1 cells (with or without resveratrol (50 µM) for 18 h). The change in intracellular fluorescence intensity (ΔF) as a function of time (Δt) of the halide-quenched (I− > Br− > Cl−) dye 6-methoxy-N-(-sulphopropyl)quinolinium (SPQ, Molecular Probe Europe, Leiden, Netherland) was taken as an index of the change in intracellular chloride concentration. Fluorescence intensity was monitored using an inverted fluorescence microscope stage (Olympus 1X70, equipped with a x40 immersion objective) coupled to a charge device camera (Digital ICCD, Princetown Inst) and was recorded every 20 s using Metafluor software (Roper Scientific, Evry, France). Excitation and emission wavelengths were 360 and 456 nm respectively. Non-confluent living CFPAC1 cells grown on a glass coverslip were transferred to a perfusion chamber. SPQ loading was achieved by hypotonic shock (5 min at room temperature) in a fluorescence-quenching iodide solution (containing in mM: 135 NaI, 1 CaSO4, 1 MgSO4, 0.6 KH2PO4, 2.4 K2HPO4, 10 glucose, 10 HEPES, pH 7.4) diluted to 1:2 with water, and supplemented with 10 mM SPQ. After recovering in an isotonic iodide containing solution, cells were superfused with an ‘efflux’ solution that contained nitrate instead of iodide (equimolar replacement of NaI by NaNO3). This induced a loss of the quenching of SPQ fluorescence, as shown by a gradual increase in cell fluorescence at a rate that depends on the basal permeability of the cell to halide. To increase intracellular cyclic AMP (cAMPi), a stimulating mixture [forskolin, 25 µM + isobutylmethylxanthine (IBMX), 100 µM, +8-4-chlorophenylthio-adenosine 3′,5′-cyclic monophosphate sodium salt (pCPT-cAMP), 200 µM] was added to the efflux solution. In the presence of a cAMP-dependent halide pathway, an abrupt change in fluorescence occurred. At the end of the experiment, the minimal fluorescence intensity, F0, was obtained by totally quenching the cell fluorescence by superfusion of the iodide solution. Analysis of single-cell images was performed with Metafluor Offline, version 4.6r5 software (Univ Imaging Corp Downington, PA, USA). Fluorescence was normalized as F = (F − F0)/F0 × 100. The three successive experimental data points recorded after exposure to the stimulating mixture were fitted using linear regression analysis; the slope of this straight line was taken as an index of the cAMP-dependent cell permeability to halide.
In a separate series of experiments, iodide efflux from CFPAC1 cells was measured according to the experimental procedure detailed by Long and Walsh (1997). In brief, CFPAC1 cells were iodide-loaded (1 h room temperature incubation) in a buffer containing 136 mM NaI, 3 mM KNO3, 2 mM Ca(NO3)2, 20 mM HEPES, 11 mM glucose, pH 7,4) then washed and bathed in an ‘efflux’ solution (equimolar replacement of NaI by NaNO3), in the presence or absence of CFTRinh-172, 20 µM. The amount of iodide released by the cells during exposure to the stimulating mixture was measured (at room temperature) using an iodide-selective electrode (ISE251, Radiometer Analytical, Lyon, France) connected to a pH/voltmeter (PHM250, Ion Analyzer, Radiometer Analytical SAS, France).
Statistics
Results are expressed as means ± SEM, with n = number of experiments, or N = number of cells. When appropriate, unpaired Student's t-test was applied using Sigma Plot software version 9.0. (Systat Software Inc, San Jose, CA, USA). Differences were considered significant when P < 0.05.
Materials
The selective CFTR blocker CFTRinh-172 (Taddei et al., 2004) was obtained from Calbiochem (LaJolla, CA, USA). Along with other reagents, resveratrol (trans-3,4′,5-trihydroxystilbene) was purchased from Sigma-Aldrich (Saint Quentin Fallavier, France). The stock solution of resveratrol (50 mM dissolved in DMSO) was kept in the dark at −20°C for less than 3 weeks. Immediately prior to use, the final required concentration (5 to 100 µM) was prepared by appropriate dilution of this stock solution with growth medium. An equivalent amount of DMSO was added to the medium (final concentration from 0.01% to 0.2%) of control cells. It is known that DMSO may increase CFTR expression by acting as a chemical chaperone (Tamarappoo and Verkman, 1998).
Results
Effect of resveratrol on CFTR expression in MDCK1 cells
First, we investigated the effect of resveratrol in MDCK1 cells that endogenously express CFTR (Mohamed et al., 1997). Cells were treated for 18 h with increasing concentrations of resveratrol (up to 100 µM). Western-blot analysis showed a concentration-dependent increase in CFTR expression up to 50 µM resveratrol (Figure 1). Further increase in resveratrol concentrations led to variable results compared with that observed using 50 µM, which was the concentration selected for further experiments. In the MDCK2 cell line, that does not express CFTR (Mohamed et al., 1997), CFTR was undetectable in both resveratrol-treated and untreated cells (data not shown), showing that the MAB 25031 antibody had specifically detected CFTR in the MDCK1 cell line. Quantitative RT-PCR revealed a significant increase in CFTR mRNA in resveratrol-treated cells, compared with control cells (Figure 1), showing that at least part of the effects of resveratrol on CFTR expression in MDCK1 cells was mediated by a transcriptional effect. NHERF-1 was undetectable at both the protein and mRNA level (the primers used for q RT-PCR experiments were shown to successfully amplify NHERF-1 mRNA in MDCK2 cells, Bossard et al., 2007). The resveratrol-induced increase in CFTR protein expression was also associated with a concomitant increase in CFTR cell surface expression, as shown by indirect immunofluorescence experiments: Figure 2 shows a widespread increase in CFTR staining associated with increased labelling in the (peri)membrane area of resveratrol-treated cells, compared with DMSO-treated cells. Taken together, these results are consistent with a resveratrol-induced increase of both total and membrane-localized expression of CFTR in MDCK1 cells.
Figure 1.
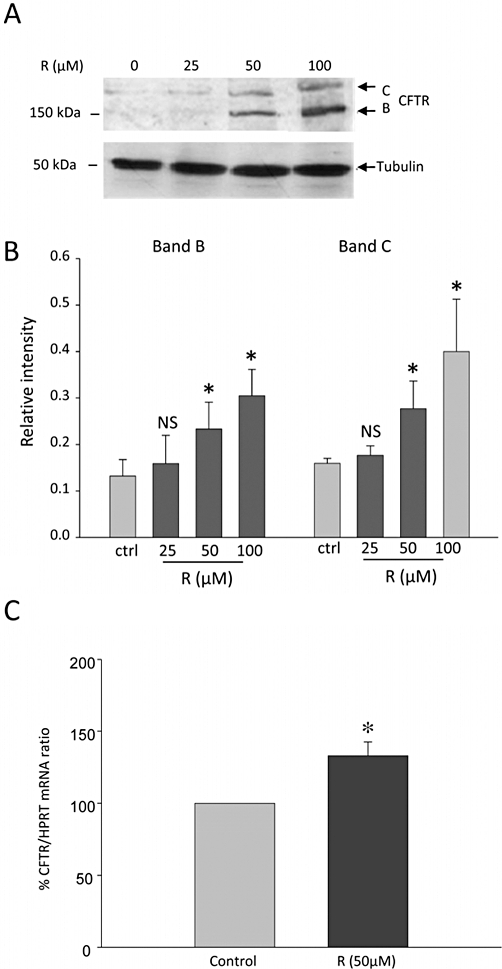
Effect of resveratrol on CFTR protein expression and CFTR mRNA in MDCK1 cells. (A) Representative Western blot analysis of CFTR and tubulin (used as loading control) protein expression from total cell lysates. Cells were pretreated for 18 h with increasing concentrations of resveratrol (R, as indicated above the lines) or with vehicle alone (control cells). The arrows indicate the positions of the mature (band C) and of the immature (band B) form of CFTR, at 175 and 150 kDa respectively. Samples from four separate experiments were analysed in duplicate and gave similar results on CFTR expression up to the 50 µM concentration of resveratrol. (B) Quantification of the staining intensity of CFTR band B and band C (over the staining intensity of tubulin) from cells pretreated with increasing concentrations of resveratrol (18 h), as indicated below the graph. Columns represent means ± SEM from four separate experiments, each Western blot analysis being performed in duplicate. *P < 0.05. (C) CFTR mRNA expression was analysed using (q RT)-PCR in MDCK1 cells pretreated for 18 h with 50 µM resveratrol (R) or with vehicle alone (control cells). The relative expression of CFTR was normalized to hypoxanthine phosphoribosyl transferase (HPRT) mRNA expression. Columns represent means ± SEM from three different experiments, each reaction being performed in quadruplicate. *P < 0.05. CFTR, cystic fibrosis transmembrane conductance regulator; MDCK, Madin Darby canine kidney.
Figure 2.
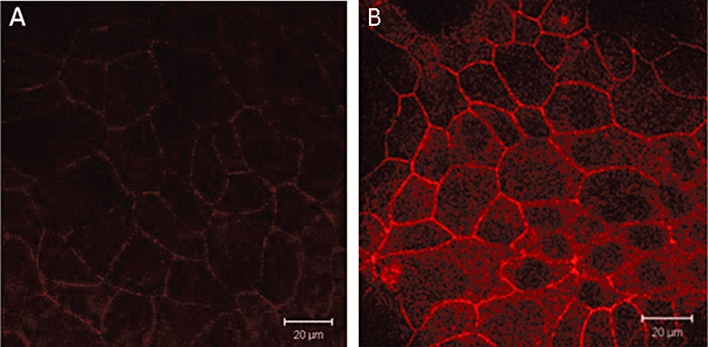
Effect of resveratrol pretreatment on CFTR expression and localization in MDCK1 cells. Confocal fluorescence immunocytochemical representative images of CFTR distribution in (A) control (DMSO-treated) and (B) resveratrol-treated (50 µM, 18 h) MDCK1 cells. Bar: 20 µm. Five independent experiments were performed, each in duplicate. Note the increased staining at the (peri)-membrane in resveratrol-treated cells. CFTR, cystic fibrosis transmembrane conductance regulator; MDCK, Madin Darby canine kidney.
Effects of resveratrol on (delF508)CFTR expression in human pancreatic cell lines
Next, we investigated the effects of resveratrol in the pancreatic cell lines CAPAN1 and CFPAC1. In control conditions, Western blot analysis showed that in CAPAN1 cells, CFTR was stained as two bands (B and C), whereas in CFPAC1 cells only the band B was detected. Pretreating CAPAN1 and CFPAC cells with resveratrol (50 µM, 18 h) had only a barely detectable effect on (delF508)CFTR total expression, consistent with (qRT)-PCR experiments that did not show a resveratrol-induced increase in CFTR mRNA level (n = 2). In resveratrol-treated CFPAC1 cells however, a CFTR band C was detectable (see Figure 3) in four out of nine immunoblots, consistent with an increased maturation of the protein. To allow a better visualization of immunostained bands, CFTR was immuno-precipitated in a separate series of experiments (n = 5, Figure 3): band C was detected in all experiments, confirming that pretreating CFPAC1 cells with resveratrol induced the expression of a mature CFTR. Moreover, CFTR immunoprecipitation allowed the detection of CFTR band C in CFPAC1 cells that were pretreated with resveratrol, 5 µM (data not shown). Further experiments on CFPAC1 cells were performed using the 50 µM concentration. Indirect immunofluorescence experiments showed clear differences in delF508CFTR pattern between control and resveratrol-treated CFPAC1 cells (Figure 4). First, CFTR labelling was stronger in resveratrol-treated than in control CFPAC1 cells. Second, delF508CFTR appeared to be localized near the plasma membrane in resveratrol-treated cells, whereas it had a widespread cytoplasmic localization in control (DMSO-treated) cells. These results indicate an increased membrane expression of CFTR in CFPAC1 cells after resveratrol treatment.
Figure 3.
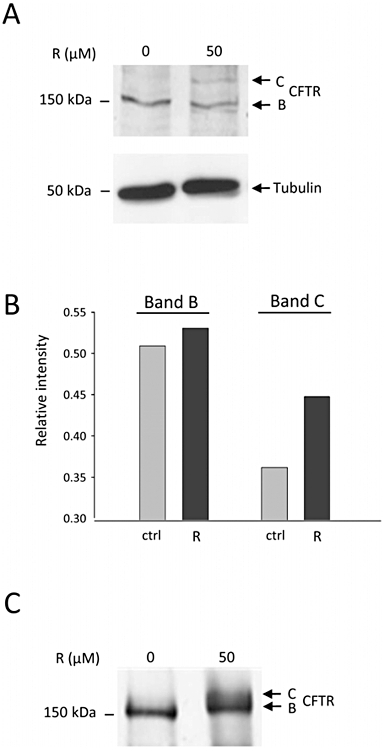
CFTR protein expression in CFPAC1 cells pretreated with resveratrol. (A) CFTR expression was analysed by Western blot from equal amounts of protein from total cell lysates. CFPAC1 cells were pretreated for 18 h with resveratrol (R) 50 µM, or with vehicle alone (DMSO). Tubulin expression was used as loading control. The arrows indicate the position of CFTR band B and C (at 150 and 175 kDa respectively). Note the faint staining of band C, detectable only in resveratrol-treated cells. (B) Quantification of the Western blot analysis presented in (A), showing the staining intensity of CFTR band B and C, relative to that of tubulin. (C) CFTR was immunoprecipitated from 500 µg of protein from control (DMSO-treated), or from resveratrol-treated CFPAC1 cells (50 µM, 18 h), using anti-CFTR 24–1 antibody. Immunoprecipitated proteins were separated by SDS-PAGE, transferred to a nitrocellulose membrane and probed with anti-CFTR NH2 tail MM13-4 antibody. Five separate experiments analysed in duplicate gave similar results. CFTR, cystic fibrosis transmembrane conductance regulator; CFPAC1, cystic fibrosis pancreatic adenocarcinoma.
Figure 4.
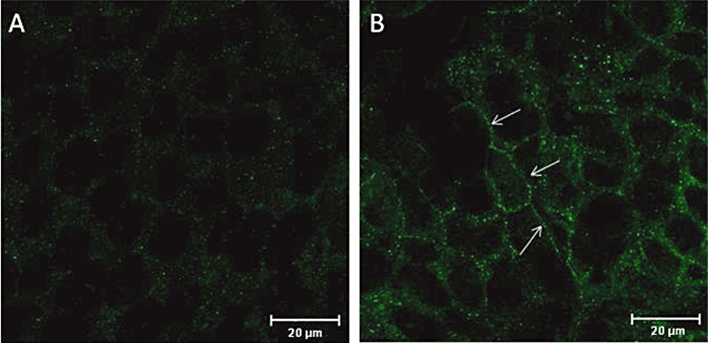
Effect of resveratrol pretreatment on CFTR expression and localization in CFPAC1 cells. Confocal fluorescence immunocytochemical representative images (from five experiments, each performed in duplicate) of CFTR distribution in (A) control (DMSO-treated) and (B) resveratrol-treated (50 µM, 18 h) CFPAC1 cells. Bar: 20 µm. Note the increased staining at the (peri)-membrane in resveratrol-treated cells (indicated by arrows). CFTR, cystic fibrosis transmembrane conductance regulator; CFPAC1, cystic fibrosis pancreatic adenocarcinoma.
Concentrations of IL-8 in CFPAC1 supernatants were significantly reduced after resveratrol treatment (50 µM, 18 h), compared with control values, after DMSO (7359 ± 776, vs. 10 652 ± 803 pg·mL−1, n = 9 for both conditions, P < 0.05), suggesting that resveratrol treatment blunted the inflammatory response in these cells. As shown in the Supplementary data, treating CFPAC1 cells also induced a reorganization of the keratin-18 network that was associated with increased keratin-18 phosphorylation and an increase in NHERF-1 expression.
Functional rescue of a cAMP-dependent anionic efflux in resveratrol-treated CFPAC1 cells
The above results suggest that resveratrol may have induced Golgi-processing of CFTR and translocation to peri-membrane sites in CFPAC1 cells, raising the question of a functional rescue of chloride transport. To explore this possibility, the change in cell membrane halide permeability induced by stimulation of protein kinase A (PKA) was monitored in SPQ-loaded CFPAC1 cells. Superfusion of the PKA-activating mixture did not change the rate of fluorescence increase in control cells. However, it induced an abrupt change in ΔF/Δt in resveratrol-treated cells (Figure 5). This cAMP-dependent increase in halide permeability of resveratrol-treated CFPAC1 cells is consistent with the rescue of a cAMP-triggered chloride efflux. The inhibition of this response in the presence of CFTRinh-172 could not be tested, because in our hands the compound's fluorescence interfered with the measurements. To address this issue, we used an iodide-selective electrode to measure the release of iodide from iodide-loaded CFPAC1 cells, with or without the CFTRinh-172 (20 µM). After adding the stimulating mixture in the efflux solution, iodide efflux, measured at the ‘plateau’, was 53 ± 5% higher in resveratrol-treated cells than in control cells (n = 3, P < 0.05), a response that was totally abolished in the presence of CFTRinh-172 (Figure 5). These results indicate the presence of a cAMP-dependent, CFTRinh-172-inhibited, chloride flux in resveratrol-treated CFPAC1 cells.
Figure 5.
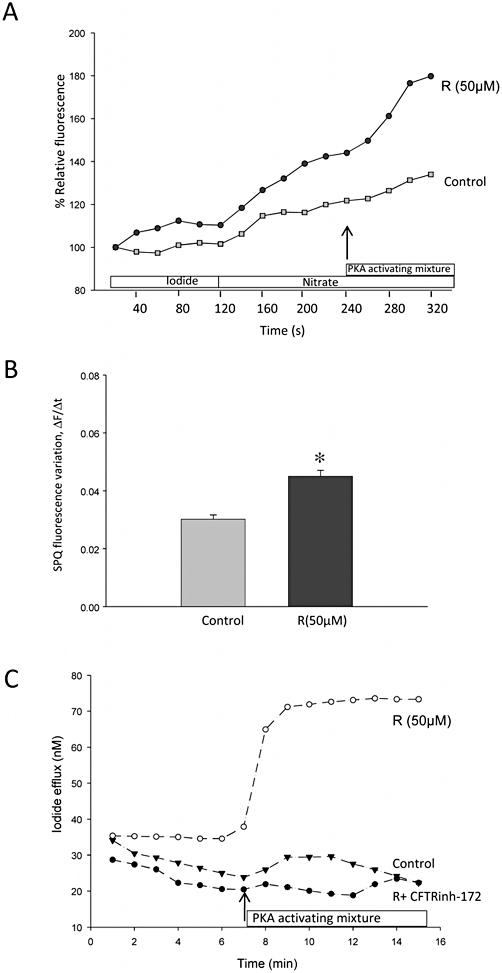
Activation of a cAMP-dependent halide efflux in resveratrol-treated CFPAC1 cells. (A) Representative changes in SPQ relative fluorescence as a function of time monitored in a resveratrol pretreated cell (R, 50 µM, 18 h, circles) or a control CFPAC1 cell (squares). Changing the superfusate from an iodide- to a nitrate-containing solution (as indicated by the bars) induced in both cells a slight increase in fluorescence intensity, denoting a basal finite anionic permeability. In the presence of a PKA activating mixture (indicated by arrow), an abrupt change in fluorescence intensity was observed in the resveratrol-treated cell, consistent with the activation of a cAMP-dependent halide efflux. (B) The change in fluorescence intensity as a function of time (ΔF/Δt) following superfusion of the PKA-stimulating mixture (forskolin, IBMX and pCPT-cAMP) in control (DMSO-treated, n = 58 cells) or resveratrol-treated cells (R, n = 66 cells) from three independent experiments. Results are given as means ± SEM The significance of difference was analysed using unpaired Student's t-test. *P < 0.05. (C) Representative changes in iodide efflux as a function of time measured by an iodide-selective electrode. As in (A), the addition of the stimulating mixture is indicated by arrow. Data shown are from control cells, cells treated with resveratrol (50 µM, 18 h) and cells treated with resveratrol (50 µM, 18 h) in the presence of CFTRinh-172 (20 µM). CFPAC1, cystic fibrosis pancreatic adenocarcinoma; IBMX, isobutyl methylxanthine; PKA, protein kinase A; SPQ, 6-methoxy-N-(-sulphopropyl)quinolinium.
Effects of resveratrol on CFTR localization in human airway epithelial cells
We performed immunofluorescence experiments on Mucilair™ cultures, which provide a model close to an ex vivo preparation. These primary cell cultures were either a normal epithelium (deriving from bronchial biopsies from a healthy subject, wild type for CFTR), or a CF epithelium (deriving from a patient homozygous for the delF508 mutation). Resveratrol treatment was applied for 2 or 18 h. Treatment had no effect on CFTR staining in the normal epithelium, whereas a clear shift of delF508CFTR towards the apical membrane was observed in the CF epithelium. The effect of resveratrol was more striking with the 2 h than with the 18 h treatment. The effect of the 2 h treatment is shown in Figure 6. We also measured IL-8 in the cell supernatants (collected from the resveratrol- or DMSO-treated CF cells, before using them for immunofluorescence experiments) and found an almost twofold decrease in IL-8 concentration in the supernatant of the cells treated for 2 h with resveratrol, compared with the control.
Figure 6.
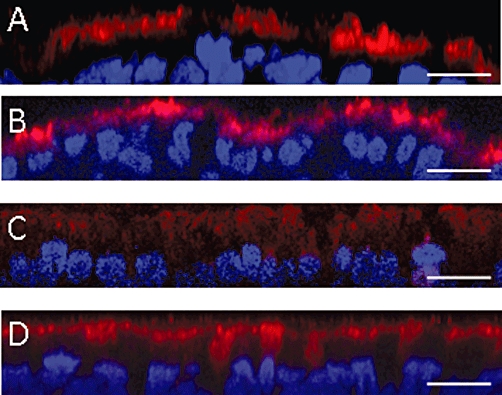
Effect of resveratrol pretreatment on CFTR expression and localization in human airway epithelial cells. Fluorescent x-z confocal immunocytochemical images of wild-type or mutant CFTR distribution in MucilAir™ cells, differentiated from a healthy subject (A and B) or from a CF (homozygous for delF508CFTR) patient (C and D). (A) and (C) show control cultures (DMSO-treated). (B) and (D) show resveratrol-treated (50 µM, 2 h) cultures. Nuclei are stained in blue, the CFTR proteins are stained in red. Bar: 10 µm. CFTR, cystic fibrosis transmembrane conductance regulator.
Discussion
Dysfunction and defects in CFTR leads to altered fluid and electrolyte secretion. The defective epithelial chloride permeability of delF508CFTR cells is primarily due to the inability of delF508CFTR to reach the plasma membrane. Moreover, whereas normal CFTR exhibits a very efficient endocytosis-recycling process, delF508CFTR is subjected to a rapid degradation after its retrieval from the plasma membrane (Lukacs et al., 1993; Swiatecka-Urban et al., 2002; Farmen et al., 2005). The basolateral membrane acts as a limiting barrier for transepithelial chloride net flux so that the presence of even a low amount of delF508CFTR at the apical membrane would be enough to add to the defective chloride transport (Farmen et al., 2005). Thus, a major goal of CF therapy is to increase the expression/stabilization of delF508CFTR at the plasma membrane.
Recent reports shed light on the potentiality of some phyto-oestrogenic, polyphenolic molecules, such as curcumin and genistein, to act as both correctors and activators of CFTR (Wang et al., 2007; Schmidt et al., 2008). Here, we investigated the long-term effect of another polyphenolic compound, resveratrol. Our results show that the long-term exposure (18 h) of cultured cells to this natural compound increased the expression of either the normal CFTR or the mutant, delF508CFTR, at the cell membrane. Moreover, in the CF pancreatic cell line, CFPAC1, resveratrol induced the functional rescue of cAMP-dependent anionic transport. Other effects of resveratrol were also shown in the present study – re-organization of the keratin-18 network, diminished IL-8 secretion and enhanced NHERF-1 expression – although their direct or indirect relationship with delF508CFTR rescue remains to be clarified.
The similarities between the structure of resveratrol (trans-3,4′,5-trihydroxystilbene) and that of the synthetic oestrogen diethylstilbestrol (4, 4′-dihydroxy-trans-α,β-diethylstilbene, DES) suggest that resveratrol can behave as a phyto-oestrogen. By acting as weak oestrogen agonists, phyto-oestrogens may up-regulate the expression of oestrogen-sensitive proteins, such as NHERF-1. However, it is not yet known if the CFTR gene possesses oestrogen response elements consensus palindromic sequences, and if so, if they may lead to a biological effect of E2 on its gene transcription. Our computational analyses indicate that two putative binding sites for the oestrogen-α receptor (ER-α) are found in the CFTR gene promoter, in a region about 2 kb upstream of the major transcription initiation start site and that they are separated by a relatively long distance (around 1 kb) (data not shown). This might suggest that a direct oestrogenic effect can affect CFTR transcriptional activity, but the biological relevance of such a hypothesis requires further study. Also, how other transcription factors collaborate with the ER-α to regulate targeted gene promoters (putatively CFTR) in a tissue/cell-specific manner or drug-concentration dependency needs to be specified (Levy et al., 2007). Depending on the cell lines we used, resveratrol effects on NHERF-1 and CFTR expression were different. NHERF-1 protein was increased in CFPAC1, but not found in MDCK1 cells; CFTR was increased at mRNA and protein levels in MDCK1, but not in CFPAC1 cells. The band B is the endoplasmic reticulum-associated core glycosylated form of CFTR, and band C represents the mature, Golgi-processed glycoform of CFTR (Wang et al., 2006). Thus, the consistent effect of resveratrol seems to be an increased maturation and trafficking of wild-type and mutant forms of CFTR to the membrane, rather than an increased total expression.
An enhanced trafficking to the cell membrane was shown by the immunofluorescence images. The reasons why results from Western blot analysis appear less impressive than results from immunochemistry are unknown; one possibility is that they could be due to the technique-dependent sensitivity and reactivity of the antibodies. In both MDCK1 and CFPAC1 cell lines, a striking enhancement in membrane localization of fluorescent labelling of both wild-type and mutant CFTR was detected in resveratrol-treated cells. Importantly, in a CF human airway epithelial cell culture, a shift of delF508CFTR to the apical membrane was also clearly detected (see Figure 6), indicating that results from CFPAC1 cells can be extended to models closer to ex vivo models.
Most of our results were obtained using resveratrol at 50 µM, a concentration that is commonly used in cell cultures but is unrealistic for human trials (for a review on the bioavailability and pharmakocinetics of resveratrol and of its metabolites depending on models, see Rocha-Gonzàlez et al., 2008 and Bisht et al., 2010). However, in our study, a 10-fold lower concentration of resveratrol (i.e. twofold higher than the resveratrol plasma concentration in clinical trials, Boocock et al., 2007) also induced band C in CFPAC1 cells. Moreover, it is likely that in the very near future, drug-encapsulated nanoparticules will greatly improve the delivery of molecules to their targets. Obviously, further studies are necessary to define the optimal parameters for resveratrol use, as well as investigating its possible deleterious effects. For instance, we observed that at concentrations above 50 µM, exposure to resveratrol for 18 h may reduce CFTR expression. At high concentrations (>50 µM), resveratrol may induce an endoplasmic reticulum stress in dopaminergic cells (Chinta et al., 2009); also it was reported that a reduction in CFTR expression may follow endoplasmic reticulum stress (Bartoszewski et al., 2008). Thus, it was emphasized that the effects of molecules on CFTR expression should be investigated in models that endogenously express wild-type and mutant CFTR rather than in cells transfected with the wild-type and mutant proteins, in order to be able to detect the reduction in CFTR expression, consequent on a drug-induced endoplasmic reticulum stress (Bartoszewski et al., 2008).
In the present study, only cells that endogenously express wild-type and mutant CFTR were used. Most experiments were performed on two cell lines (MDCK1 cells and CFPAC1 cells) that are commonly used to study the expression and function of wild-type and delF508CFTR (Li et al., 2004; Lipecka et al., 2006; Bartoszewski et al., 2008; Caohuy et al., 2009). In these cells, resveratrol improved CFTR expression, maturation and/or folding, and induced a shift and/or an increased staining of immuno-labelled wild-type and mutant CFTR towards the plasma membrane. Functional experiments performed in CFPAC1 cells eliminated the possibility that the protein, despite being more mature, remains trapped, thus devoid of function: the cAMP-dependent, CFTRinh-172-sensitive, anionic flux in resveratrol-treated cells were compatible with rescue of delF508CFTR. This functional rescue was associated with an increased phosphorylation of keratin-18, an event that regulates the structural network of keratin-18. Proteomic, biochemical and functional studies have shown that these intermediate filaments in epithelial cells directly interact with CFTR and influence delivery of both wild-type and mutant proteins to the plasma membrane (Davezac et al., 2004; Lipecka et al., 2006).
From the present study, one can only speculate about the mechanism that leads to post-translational keratin-18 modification. Resveratrol, like other polyphenols, targets many enzymes and proteins, including the serine/threonine kinases (Stevenson and Hurst, 2007). It is thus possible that a resveratrol-induced activation of AMP-activated protein kinase or of protein kinase C δ contributed to the increased phosphorylation of keratin-18 (Bastianetto et al., 2009; Vijayaraj et al., 2009). The functional rescue of delF508CFTR in CFPAC1 cells was also associated with a decrease in the inflammatory cytokine response. Of note, resveratrol, known to increase histone deacetylase activities, also reduces the oxidative stress that induces IL-8 promoter hyperacetylation in CF models (Bartling and Drumm, 2009). Our observations agree with numerous other studies emphasizing the beneficial pleiotropic effects of resveratrol, but to our knowledge these are the first observations dealing with CF cells. Potent anti-inflammatory and anti-oxidant properties of resveratrol have been proposed to play major roles in its cytoprotective effects, as resveratrol inhibits the transcription factors NF-κB and activator-protein 1 (AP1), and also prevents lipid peroxidation (Bisht et al., 2010). Because inflammatory and oxidative processes complicate CF, resveratrol may also have beneficial effects in CF cells and is worth further evaluating as a potential candidate for CF therapy. Further investigations are needed to unravel the mechanism(s) underlying the increase in CFTR membrane expression and the rescue of a cAMP-dependent halide transport in CFPAC1 cells, as well as evaluating the effects of resveratrol in other CF models.
Acknowledgments
This study was supported by grants from Vaincre la Mucoviscidose, Chancellerie des Universités (Legs Poix), Paris Descartes University and Inserm. NH, MK, SM, DLV, JC were supported by Vaincre la Mucoviscidose. The authors thank Dr Robert Dormer (Cardiff Univ, UK) for the generous gift of rabbit anti-CFTR MP-CT1 Ab, and Dr Ludovic Wiszniewski (Epithelix Sàrl, Plan les Ouates, Switzerland; http://www.epithelix.com/) for providing Mucilair™. MDCK1 and MDCK2 cells were provided by Drs Dina Kremsdorf (Inserm U 845, Paris, France) and Corinne Antignac (Inserm U 983, Paris, France) respectively. They acknowledge Meriem Garfa-Traore and Fatna-Léa Makaci for skilful assistance. They thank Drs Naziha Bakouh and Maurice Bichara for advice and Dr Charlotte Sumida for correcting the manuscript. GP is indebted to Dr Daniel Hurvy for his valuable comments.
Glossary
Abbreviations
- AP-1
activator protein 1
- BHK
baby hamster kidney
- CF
cystic fibrosis
- CFPAC1
cystic fibrosis pancreatic adenocarcinoma
- CFTR
cystic fibrosis transmembrane conductance regulator
- CFTRinh-172
CFTR channel inhibitor
- delF508CFTR
CFTR with deletion of Phe508
- DES
4, 4′-dihydroxy-trans-α, β-diethylstilbene
- E2
17β-oestradiol
- ER-α
oestrogen α receptor
- ERE
oestrogen response element
- FCS
fetal calf serum
- HPRT
hypoxanthine phosphoribosyltransferase
- IBMX
isobutyl methylxanthine
- IL-8
interleukin 8
- MDCK
Madin Darby canine kidney
- NF-κB
nuclear factor-κB
- NHERF-1
Na+/H+ exchanger regulatory factor 1
- pCPT-cAMP
8-4-chlorophenylthio-adenosine 3′,5′-cyclic monophosphate sodium salt
- PKA
protein kinase A
- SPQ
6-methoxy-N-(-sulphopropyl)quinolinium
Conflicts of interest
The authors declare no conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Effect of resveratrol pretreatment on keratin 18 (K18) network in CFPAC1 cells. Confocal fluorescence immunocytochemical images of K18 network of control DMSO-treated CFPAC1 cells (A and C) and resveratrol-treated (50 µM, 18 h) CFPAC1 cells (B and D). Bar: 20 µm (A and B) and 10 µm (C and D). Images are representative of two independent experiments, each in triplicate. Note that in resveratrol treated cells, K18 network was scattered whereas it in untreated cells, it was concentrated around the nucleus.
Figure S2 Effect of resveratrol on Ser 52 phosphorylated keratin 18 (p K18 Ser52) and on NHERF-1 expression in CFPAC1 cells. (A) Equal amounts of protein from total cell lysates were analysed by Western blot, using anti-K18 antibodies. Cells were pretreated with resveratrol (R, 50 µM for 18 h) or with vehicle alone (control cells). An increased amount of p K18 Ser52 is detected in resveratrol-treated CFPAC1 cells, whereas the total amount of K18 is unchanged (B). This latter was obtained from dehybridation of the membrane previously probed with an anti-phosphorylated Ser52 K18 antibody, and also represented the loading control. Similar results were obtained in duplicate in two separate experiments. (B) Quantification of the Western blot analysis presented in (A) showing the staining intensity of p K18 Ser52 over the staining intensity of total K18 in resveratrol-treated cells. (C) Equal amounts from total cell lysates were analysed by Western blot, using an anti-NHERF-1 antibody. CFPAC1 cells were pretreated for 18 h with resveratrol 50 µM, or with vehicle alone (DMSO). The arrow indicates the position of NHERF-1 protein, 50 kDa. Tubulin expression was used as loading control. (D) Quantification of the staining intensity of NHERF-1 (over the staining intensity of tubulin) from CFPAC1 cells pretreated with resveratrol, R (50 µM, 18 h) or with vehicle (control cells, C). Columns represent means ± SEM from six separate experiments, each Western blot analysis being performed in duplicate. *P < 0.05.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Ajonuma LC, Tsang LL, Zhang GH, Wong CH, Lau MC, Ho LS, et al. Estrogen-induced abnormally high cystic fibrosis transmembrane conductance regulator expression results in ovarian hyperstimulation syndrome. Mol Endocrinol. 2005;19:3038–3044. doi: 10.1210/me.2005-0114. [DOI] [PubMed] [Google Scholar]
- Bachmeier BE, Mirisola V, Romeo F, Generoso L, Esposito A, Dell'eva R, et al. Reference profile correlation reveals estrogen-like trancriptional activity of curcumin. Cell Physiol Biochem. 2010;26:471–482. doi: 10.1159/000320570. [DOI] [PubMed] [Google Scholar]
- Bartling TR, Drumm ML. Oxidative stress causes IL8 promoter hyperacetylation in cystic fibrosis airway cell models. Am J Respir Cell Mol Biol. 2009;40:58–65. doi: 10.1165/rcmb.2007-0464OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoszewski R, Rab A, Twitty G, Stevenson L, Fortenberry J, Piotrowski A, et al. The mechanism of cystic fibrosis transmembrane conductance regulator transcriptional repression during the unfolded protein response. J Biol Chem. 2008;283:12154–12165. doi: 10.1074/jbc.M707610200. [DOI] [PubMed] [Google Scholar]
- Bastianetto S, Dumont Y, Han Y, Quirion R. Comparative neuroprotective properties of stilbene and catechin analogs: action via a plasma membranereceptor site? CNS Neurosci Ther. 2009;15:76–83. doi: 10.1111/j.1755-5949.2008.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht K, Wagner KH, Bulmer AC. Curcumin, resveratrol and flavonoids as anti-inflammatory, cyto- and DNA-protective dietary compounds. Toxicology. 2010;278:88–100. doi: 10.1016/j.tox.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Bobadilla JL, Macek M, Jr, Fine JP, Farrell PM. Cystic fibrosis: a worldwide analysis of CFTR mutations – correlation with incidence data and application to screening. Hum Mutat. 2002;19:575–606. doi: 10.1002/humu.10041. [DOI] [PubMed] [Google Scholar]
- Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- Bossard F, Robay A, Toumaniantz G, Dahimene S, Becq F, Merot J, et al. NHERF1 protein rescues DeltaF508-CFTR function. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1085–L1094. doi: 10.1152/ajplung.00445.2005. [DOI] [PubMed] [Google Scholar]
- Brouillard F, Tondelier D, Edelman A, Baudouin-Legros M. Drug résistance induced by ouabain via the stimulation of MDR1 gene expression in humancarcinomatous pulmonary cells. Cancer Res. 2001;61:1693–1698. [PubMed] [Google Scholar]
- Caohuy H, Jozwik C, Pollard HB. Rescue of DeltaF508-CFTR by the SGK1/Nedd4-2 signaling pathway. J Biol Chem. 2009;284:25241–25253. doi: 10.1074/jbc.M109.035345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champigny G, Imler JL, Puchelle E, Dalemans W, Gribkoff V, Hinnrasky J, et al. A change in gating mode leading to increased intrinsic Cl− channel activity compensates for defective processing in a cystic fibrosis mutant corresponding to a mild form of the disease. EMBO J. 1995;14:2417–2423. doi: 10.1002/j.1460-2075.1995.tb07239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinta SJ, Poksay KS, Kaundinya G, Hart M, Bredesen DE, Andersen JK, et al. Endoplasmic reticulum stress-induced cell death in dopaminergic cells: effect of resveratrol. J Mol Neurosci. 2009;39:157–168. doi: 10.1007/s12031-008-9170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing PR, Fellows A, Villone D, Boisguérin P, Madden DR. The relative binding affinities of PDZ partners for CFTR: a biochemical basis for efficient endocytic recycling. Biochemistry. 2008;47:10084–10098. doi: 10.1021/bi8003928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalemans W, Barbry P, Champigny G, Jallat S, Dott K, Dreyer D, et al. Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature. 1991;354:526–528. doi: 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- Davezac N, Tondelier D, Lipecka J, Fanen P, Demaugre F, Debski J, et al. Global proteomic approach unmasks involvement of keratins 8 and 18 in the delivery of cystic fibrosis transmembrane conductance regulator (CFTR)/deltaF508-CFTR to the plasma membrane. Proteomics. 2004;4:3833–3844. doi: 10.1002/pmic.200400850. [DOI] [PubMed] [Google Scholar]
- Ediger TR, Kraus WL, Weinman EJ, Katzenellenbogen BS. Estrogen receptor regulation of the Na+/H+ exchange regulatory factor. Endocrinology. 1999;140:2976–2982. doi: 10.1210/endo.140.7.6885. [DOI] [PubMed] [Google Scholar]
- Ediger TR, Park SE, Katzenellenbogen BS. Estrogen receptor inducibility of the human Na+/H+ exchanger regulatory factor/ezrin-radixin-moesin binding protein 50 (NHE-RF/EBP50) gene involving multiple half-estrogen response elements. Mol Endocrinol. 2002;16:1828–1839. doi: 10.1210/me.2001-0290. [DOI] [PubMed] [Google Scholar]
- Egan ME, Pearson M, Weiner SA, Rajendran V, Rubin D, Glöckner-Pagel J, et al. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science. 2004;304:600–602. doi: 10.1126/science.1093941. [DOI] [PubMed] [Google Scholar]
- Fanelli T, Cardone RA, Favia M, Guerra L, Zaccolo M, Monterisi S, et al. Beta-oestradiol rescues DeltaF508CFTR functional expression in human cystic fibrosis airway CFBE41o- cells through the up-regulation of NHERF1. Biol Cell. 2008;100:399–412. doi: 10.1042/BC20070095. [DOI] [PubMed] [Google Scholar]
- Farmen SL, Karp PH, Ng P, Palmer DJ, Koehler DR, Hu J, et al. Gene transfer of CFTR to airway epithelia: low levels of expression are sufficient to correct Cl− transport and overexpression can generate basolateral CFTR. Am J Physiol Lung Cell Mol Physiol. 2005;289:L1123–L1130. doi: 10.1152/ajplung.00049.2005. [DOI] [PubMed] [Google Scholar]
- Guerra L, Fanelli T, Favia M, Riccardi SM, Busco G, Cardone RA, et al. Na+/H+ exchanger regulatory factor isoform 1 overexpression modulates cystic fibrosis transmembrane conductance regulator (CFTR) expression and activity in human airway 16HBE14o- cells and rescues DeltaF508 CFTR functional expression in cystic fibrosis cells. J Biol Chem. 2005;280:40925–40933. doi: 10.1074/jbc.M505103200. [DOI] [PubMed] [Google Scholar]
- Illek B, Lizarzaburu ME, Lee V, Nantz MH, Kurth MJ, Fischer H. Structural determinants for activation and block of CFTR-mediated chloride currents by apigenin. Am J Physiol Cell Physiol. 2000;279:C1838–C1846. doi: 10.1152/ajpcell.2000.279.6.C1838. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Mall M. Pharmacotherapy of the ion transport defect in cystic fibrosis. Clin Exp Pharmacol Physiol. 2001;28:857–867. doi: 10.1046/j.1440-1681.2001.03541.x. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Mall M. Pharmacotherapy of the ion transport defect in cystic fibrosis: role of purinergic receptor agonists and other potential therapeutics. Am J Respir Med. 2003;2:299–309. doi: 10.1007/BF03256658. [DOI] [PubMed] [Google Scholar]
- Levy N, Zhao X, Tang H, Jaffe RB, Speed TP, Leitman DC. Multiple transcription factor elements collaborate with estrogen receptor alpha to activate an inducible estrogen response element in the NKG2E gene. Endocrinology. 2007;148:3449–3458. doi: 10.1210/en.2006-1632. [DOI] [PubMed] [Google Scholar]
- Li H, Findlay IA, Sheppard DN. The relationship between cell proliferation, Cl− secretion, and renal cyst growth: a study using CFTR inhibitors. Kidney Int. 2004;66:1926–1938. doi: 10.1111/j.1523-1755.2004.00967.x. [DOI] [PubMed] [Google Scholar]
- Lipecka J, Norez C, Bensalem N, Baudouin-Legros M, Planelles G, Becq F, et al. Rescue of DeltaF508-CFTR (cystic fibrosis transmembrane conductance regulator) by curcumin: involvement of the keratin 18 network. J Pharmacol Exp Ther. 2006;317:500–505. doi: 10.1124/jpet.105.097667. [DOI] [PubMed] [Google Scholar]
- Long KJ, Walsh KB. Iodide efflux measurements with iodide-selective electrode: a non-radioactive procedure for monitoring cellular chloride transport. Methods Cell Sci. 1997;19:207–212. [Google Scholar]
- Lukacs GL, Chang XB, Bear C, Kartner N, Mohamed A, Riordan JR, et al. The delta F508 mutation decreases the stability of cystic fibrosis transmembrane conductance regulator in the plasma membrane. Determination of functional half-lives on transfected cells. J Biol Chem. 1993;268:21592–21598. [PubMed] [Google Scholar]
- Matsumura A, Ghosh A, Pope GS, Darbre PD. Comparative study of oestrogenic properties of eight phytoestrogens in MCF7 human breast cancer cells. J Steroid Biochem Mol Biol. 2005;94:431–443. doi: 10.1016/j.jsbmb.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Mohamed A, Ferguson D, Seibert FS, Cai HM, Kartner N, Grinstein S, et al. Functional expression and apical localization of the cystic fibrosis transmembrane conductance regulator in MDCK I cells. Biochem J. 1997;322:259–265. doi: 10.1042/bj3220259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Gonzàlez HI, Ambriz-Tututi M, Granados-Soto V. Resveratrol: a natural compound with pharmacological potential in neurodegenerative diseases. CNS Neurosci Ther. 2008;14:234–247. doi: 10.1111/j.1755-5949.2008.00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands DK, Tsang LL, Cui YG, Chung YW, Chan LN, Liu CQ, et al. Upregulation of cystic fibrosis transmembrane conductance regulator expression by oestrogen and Bak Foong Pill in mouse uteri. Cell Biol Int. 2001;25:1033–1035. doi: 10.1006/cbir.2001.0746. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Hughes LK, Cai Z, Mendes F, Li H, Sheppard DN, et al. Prolonged treatment of cells with genistein modulates the expression and function of the cystic fibrosis transmembrane conductance regulator. Br J Pharmacol. 2008;153:1311–1323. doi: 10.1038/sj.bjp.0707663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson DE, Hurst RD. Polyphenolic phytochemicals – just antioxidants or much more? Cell Mol Life Sci. 2007;64:2900–2916. doi: 10.1007/s00018-007-7237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiatecka-Urban A, Duhaime M, Coutermarsh B, Karlson KH, Collawn J, Milewski M. PDZ domain interaction controls the endocytic recycling of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2002;277:40099–40105. doi: 10.1074/jbc.M206964200. [DOI] [PubMed] [Google Scholar]
- Taddei A, Folli C, Zegarra-Moran O, Fanen P, Verkman AS, Galietta LJ. Altered channel gating mechanism for CFTR inhibition by a high-affinity thiazolidinone blocker. FEBS Lett. 2004;558:52–56. doi: 10.1016/S0014-5793(04)00011-0. [DOI] [PubMed] [Google Scholar]
- Tamarappoo BK, Verkman AS. Defective aquaporin-2 trafficking in nephrogenic diabetes insipidus and correction by chemical chaperones. J Clin Invest. 1998;101:2257–2267. doi: 10.1172/JCI2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayaraj P, Kröger C, Reuter U, Windoffer R, Leube RE, Magin TM. Keratins regulate protein biosynthesis through localization of GLUT1 and -3 upstream of AMP kinase and Raptor. J Cell Biol. 2009;187:175–184. doi: 10.1083/jcb.200906094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zeltwanger S, Hu S, Hwang TC. Deletion of phenylalanine 508 causes attenuated phosphorylation-dependent activation of CFTR chloride channels. J Physiol. 2000;524:637–648. doi: 10.1111/j.1469-7793.2000.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XF, Zhou CX, Shi QX, Yuan YY, Yu MK, Ajonuma LC, et al. Involvement of CFTR inuterine bicarbonate secretion and the fertilizing capacity of sperm. Nat Cell Biol. 2003;5:902–906. doi: 10.1038/ncb1047. [DOI] [PubMed] [Google Scholar]
- Wang X, Venable J, LaPointe P, Hutt DM, Koulov AV, Coppinger J. Hsp90 cochaperone Aha1 downregulation rescues misfolding of CFTR in cystic fibrosis. Cell. 2006;127:803–816. doi: 10.1016/j.cell.2006.09.043. [DOI] [PubMed] [Google Scholar]
- Wang W, Bernard K, Li G, Kirk KL. Curcumin opens cystic fibrosis transmembrane conductance regulator channels by a novel mechanism that requires neither ATP binding nor dimerization of the nucleotide-binding domains. J Biol Chem. 2007;282:4533–4544. doi: 10.1074/jbc.M609942200. [DOI] [PubMed] [Google Scholar]
- Wiszniewski L, Jornot L, Dudez T, Pagano A, Rochat T, Lacroix JS, et al. Long-term cultures of polarized airway epithelial cells from patients with cystic fibrosis. Am J Respir Cell Mol Biol. 2006;34:39–48. doi: 10.1165/rcmb.2005-0161OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


