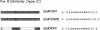Abstract
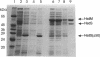
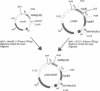
We have developed a complementation assay which allows us to distinguish between mutations affecting subunit assembly and mutations affecting DNA binding in the DNA recognition subunit (HsdS) of the multimeric restriction endonuclease EcoR1241. A number of random point mutations were constructed to test the validity of this assay. Two of the mutants produced were found to be truncated polypeptides that were still capable of complementation with the EcoR1241 Hsd subunits to give an active restriction enzyme of novel DNA specificity. The N-terminal variable domain (responsible for recognition of GAA from the EcoR1241 recognition sequence GAAnnnnnnRTCG) and the spacer region (central conserved region) is intact in both of these mutants. One of these mutant genes (hsdS(delta 50) has been cloned as an active Mtase. Purification of the Mtase proved to be difficult because the complex is weak. However, Mtase activity was obtained from a soluble cell extract, and this allowed us to determine the DNA recognition sequence of the Mtase to be GAAnnnnnnnTTC. This recognition sequence is an inverted repeat of 5'-end of the EcoR1241 recognition sequence. This suggests that the mutant Mtase is assembled from two inverted HsdS(D50) subunits, possibly held together by the HsdM subunits.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firman K., Price C., Glover S. W. The EcoR124 and EcoR124/3 restriction and modification systems: cloning the genes. Plasmid. 1985 Nov;14(3):224–234. doi: 10.1016/0147-619x(85)90006-x. [DOI] [PubMed] [Google Scholar]
- Fuller-Pace F. V., Cowan G. M., Murray N. E. EcoA and EcoE: alternatives to the EcoK family of type I restriction and modification systems of Escherichia coli. J Mol Biol. 1985 Nov 5;186(1):65–75. doi: 10.1016/0022-2836(85)90257-8. [DOI] [PubMed] [Google Scholar]
- Fuller-Pace F. V., Murray N. E. Two DNA recognition domains of the specificity polypeptides of a family of type I restriction enzymes. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9368–9372. doi: 10.1073/pnas.83.24.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gann A. A., Campbell A. J., Collins J. F., Coulson A. F., Murray N. E. Reassortment of DNA recognition domains and the evolution of new specificities. Mol Microbiol. 1987 Jul;1(1):13–22. doi: 10.1111/j.1365-2958.1987.tb00521.x. [DOI] [PubMed] [Google Scholar]
- Glover S. W., Colson C. Genetics of host-controlled restriction and modification in Escherichia coli. Genet Res. 1969 Apr;13(2):227–240. doi: 10.1017/s0016672300002901. [DOI] [PubMed] [Google Scholar]
- Gough J. A., Murray N. E. Sequence diversity among related genes for recognition of specific targets in DNA molecules. J Mol Biol. 1983 May 5;166(1):1–19. doi: 10.1016/s0022-2836(83)80047-3. [DOI] [PubMed] [Google Scholar]
- Gubler M., Bickle T. A. Increased protein flexibility leads to promiscuous protein--DNA interactions in type IC restriction-modification systems. EMBO J. 1991 Apr;10(4):951–957. doi: 10.1002/j.1460-2075.1991.tb08029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler M., Braguglia D., Meyer J., Piekarowicz A., Bickle T. A. Recombination of constant and variable modules alters DNA sequence recognition by type IC restriction-modification enzymes. EMBO J. 1992 Jan;11(1):233–240. doi: 10.1002/j.1460-2075.1992.tb05046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberman A., Heywood J., Meselson M. DNA modification methylase activity of Escherichia coli restriction endonucleases K and P. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3138–3141. doi: 10.1073/pnas.69.11.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubacek J., Glover S. W. Complementation analysis of temperature-sensitive host specificity mutations in Escherichia coli. J Mol Biol. 1970 May 28;50(1):111–127. doi: 10.1016/0022-2836(70)90108-7. [DOI] [PubMed] [Google Scholar]
- JACOB F., WOLLMAN E. L. Etude génétique d'un bactériophage tempéré d'Escherichia coli. l. Le système genétique du bactériophage. Ann Inst Pasteur (Paris) 1954 Dec;87(6):653–673. [PubMed] [Google Scholar]
- Kannan P., Cowan G. M., Daniel A. S., Gann A. A., Murray N. E. Conservation of organization in the specificity polypeptides of two families of type I restriction enzymes. J Mol Biol. 1989 Oct 5;209(3):335–344. doi: 10.1016/0022-2836(89)90001-6. [DOI] [PubMed] [Google Scholar]
- Lehtovaara P. M., Koivula A. K., Bamford J., Knowles J. K. A new method for random mutagenesis of complete genes: enzymatic generation of mutant libraries in vitro. Protein Eng. 1988 Apr;2(1):63–68. doi: 10.1093/protein/2.1.63. [DOI] [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- Meselson M., Yuan R., Heywood J. Restriction and modification of DNA. Annu Rev Biochem. 1972;41:447–466. doi: 10.1146/annurev.bi.41.070172.002311. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Gough J. A., Suri B., Bickle T. A. Structural homologies among type I restriction-modification systems. EMBO J. 1982;1(5):535–539. doi: 10.1002/j.1460-2075.1982.tb01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraja V., Shepherd J. C., Bickle T. A. A hybrid recognition sequence in a recombinant restriction enzyme and the evolution of DNA sequence specificity. Nature. 1985 Jul 25;316(6026):371–372. doi: 10.1038/316371a0. [DOI] [PubMed] [Google Scholar]
- Nagaraja V., Shepherd J. C., Pripfl T., Bickle T. A. Two type I restriction enzymes from Salmonella species. Purification and DNA recognition sequences. J Mol Biol. 1985 Apr 20;182(4):579–587. doi: 10.1016/0022-2836(85)90243-8. [DOI] [PubMed] [Google Scholar]
- Patel J., Taylor I., Dutta C. F., Kneale G., Firman K. High-level expression of the cloned genes encoding the subunits of and intact DNA methyltransferase, M.EcoR124. Gene. 1992 Mar 1;112(1):21–27. doi: 10.1016/0378-1119(92)90298-4. [DOI] [PubMed] [Google Scholar]
- Price C., Lingner J., Bickle T. A., Firman K., Glover S. W. Basis for changes in DNA recognition by the EcoR124 and EcoR124/3 type I DNA restriction and modification enzymes. J Mol Biol. 1989 Jan 5;205(1):115–125. doi: 10.1016/0022-2836(89)90369-0. [DOI] [PubMed] [Google Scholar]
- Price C., Pripfl T., Bickle T. A. EcoR124 and EcoR124/3: the first members of a new family of type I restriction and modification systems. Eur J Biochem. 1987 Aug 17;167(1):111–115. doi: 10.1111/j.1432-1033.1987.tb13310.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzypek E., Piekarowicz A. The EcoDXX1 restriction and modification system: cloning the genes and homology to type I restriction and modification systems. Plasmid. 1989 May;21(3):195–204. doi: 10.1016/0147-619x(89)90043-7. [DOI] [PubMed] [Google Scholar]
- Taylor I., Patel J., Firman K., Kneale G. Purification and biochemical characterisation of the EcoR124 type I modification methylase. Nucleic Acids Res. 1992 Jan 25;20(2):179–186. doi: 10.1093/nar/20.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Yuan R., Heywood J., Meselson M. ATP hydrolysis by restriction endonuclease from E. coli K. Nat New Biol. 1972 Nov 8;240(97):42–43. doi: 10.1038/newbio240042a0. [DOI] [PubMed] [Google Scholar]
- Yuan R., Meselson M. A specific complex between a restriction endonuclease and its DNA substrate. Proc Natl Acad Sci U S A. 1970 Feb;65(2):357–362. doi: 10.1073/pnas.65.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkevich V. E., Weiserová M., Kryukov V. M., Hubácek J. A mutation that converts serine340 of the HsdSK polypeptide to phenylalanine and its effects on restriction and modification in Escherichia coli K-12. Gene. 1990 May 31;90(1):125–128. doi: 10.1016/0378-1119(90)90447-y. [DOI] [PubMed] [Google Scholar]
- Zinkevich V., Heslop P., Glover S. W., Weiserova M., Hubácek J., Firman K. Mutation in the specificity polypeptide of the type I restriction endonuclease R.EcoK that affects subunit assembly. J Mol Biol. 1992 Oct 5;227(3):597–601. doi: 10.1016/0022-2836(92)90210-b. [DOI] [PubMed] [Google Scholar]