Abstract
The pregnane X receptor (PXR, NR1I2) is a ligand activated transcription factor that belongs to the nuclear hormone receptor (NR) superfamily. PXR is highly expressed in the liver and intestine, but low levels of expression have also been found in many other tissues. PXR plays an integral role in xenobiotic and endobiotic metabolism by regulating the expression of drug metabolizing enzymes and transporters, as well as genes implicated in the metabolism of endobiotics. PXR exerts its transcriptional regulation by binding to its DNA response elements as a heterodimer with the retinoid X receptor (RXR) and recruitment of a host of coactivators. The biological and physiological implications of PXR activation are broad, ranging from drug metabolism and drug-drug interactions to the homeostasis of numerous endobiotics, such as glucose, lipids, steroids, bile acids, bilirubin, retinoic acid, and bone minerals. The purpose of this article is to provide an overview on the transcriptional circuits and metabolic relevance controlled by PXR.
Keywords: Nuclear receptor, gene regulation, xenobiotic receptor, xenobiotics, endobiotics
1. Introduction
1.1. Discovery of PXR
The pregnane X receptor (PXR, NR1I2) belongs to the nuclear hormone receptor (NR) superfamily of ligand activated transcription factors [1]. PXR has been shown to play an essential role in xenobiotic metabolism in humans, mice, rats, and rabbits [2–5]. Subsequent studies have strongly suggested that PXR also plays an important role in endobiotic metabolism in humans, mice, and rats [6–16]. The mouse PXR (mPXR) was first discovered and cloned in 1998 based on sequence homology with other NRs, and was found to be activated by a variety of compounds, including natural and synthetic glucocorticoids, steroids, pregnane derivatives, antiglucocorticoids, macrocyclic antibiotics, antifungals, and herbal extracts [1, 3, 17–21]. The human PXR (hPXR) ortholog was subsequently reported as the steroid and xenobiotic receptor (SXR) and pregnane activated receptor (PAR), both exhibiting structural features and activation patterns similar to mPXR [19, 20]. SXR/PAR was later confirmed to be orthologous to mPXR by Xie and colleagues via the gene replacement experiment with the PXR knockout mice [22]. PXR has since been cloned from a wide array of species, including mammals, birds, and fish [3, 18–21, 23–25].
The structural organization of PXR follows that of a typical NR which includes an NH2 - terminal ligand independent activation function domain (AF-1, A/B region), a highly conserved DNA binding domain (DBD, C region), a less conserved hinge domain (D region), followed by a C-terminal ligand binding domain (LBD, E region) and an activation function 2 domain (AF-2, F region) [26–30].
1.2. PXR’s mode of action
When bound to and activated by ligands, PXR translocates from the cytoplasm to the nucleus of the cells [31]. PXR then binds to its DNA response elements as a heterodimer with the retinoid X receptor (RXR). PXR is also capable of recruiting a host of coactivators which includes members of the p160 family of coactivators such as steroid receptor coactivators 1 (SRC-1), TIF/GRIP (SRC-2), and peroxisome proliferator activated receptor gamma coactivator 1a (PGC-1a) [32–34]. The DBD of PXR facilitates DNA binding specificity via two highly conserved zinc finger motifs as well as a P-Box motif and D-Box motif which allow the receptor to target and bind its xenobiotic response elements (XREs) located in the 5′ promoter region of PXR target genes [35]. PXR can bind to a variety of DNA response elements containing two copies of the half site consensus sequence AG(G/T)TCA with various spacing, which includes direct repeats DR-3, DR-4, and DR-5, and everted repeats ER-6 and ER-8 [28].
2. PXR in Xenobiotic Metabolism
2.1. Regulation of Phase I enzymes
Functional characterization of PXR has shown that this receptor acts as a xenosensor, playing a major role in protecting organisms from exogenous chemical insults. PXR is highly expressed in the liver, intestine, and kidneys, but low levels have also been found in the peripheral blood monocytes, blood brain barrier, uterus, ovary, placenta, breast, osteoclasts, heart, adrenal glands, bone marrow, and specific brain regions of various species [23, 36–40]. Given such a broad range of expression pattern, PXR is well suited to accommodate its metabolic role through the induction of metabolizing/detoxifying enzymes and transporters.
The metabolism of exogenous and endogenous compounds is quintessential for normal physiological functioning of any living organism. PXR is capable of modulating this process through induction of the major Phase I cytochromes P450 enzymes (CYPs). CYPs are a superfamily of heme-dependent monooxygenases, which catalyze the first step of detoxification of aliphatic or lipophillic compounds [41, 42]. Highly expressed in the liver and intestine [41], CYPS use hydroxylation and/or oxidation reactions to convert target compounds into more soluble derivatives that are easier to excrete from the body [42]. Activation of PXR has been shown to lead to the transcription of a host of CYP genes in humans and rodents, including CYP3A4, CYP3A23, CYP3a11, CYP2B6, Cyp2b9, Cyp2c55, CYP2C8, CYP2C9, CYP2C19, and CYP1A [18, 37, 43–47].
It is apparent that since PXR controls the transcription of an array of CYPs, this receptor must be activated by a commensurate number of xenobiotic compounds. This is in fact the case: hPXR has been shown to be activated by a plethora of pharmaceutical drugs that include rifampicin (RIF), rifaximin [48], clotrimazole [3], dexamethasone [18], lovastatin [18] and metyrapone [49] to name a few. PXR is also activated by a variety of environmental pollutants such as 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane (DDT), di-n-butyl phthalate (DBP), chlordane, dieldrin, and endosulfan [50–52]. Finally, PXR can be activated by a variety of medicinal compounds derived from herbal sources including Schisandra chinensis (anti-perspiration), Piper methysticum (chloraseptic), and Agauria salicifolia (arrhythmia) [53].
The ligand dependent PXR activation has been shown to be species specific at times. For example, in humans and rabbits the antibiotic RIF is a potent PXR activator. However, the same drug has little effect on the mouse or rat PXR. In contrast, the synthetic anti-glucocorticoid pregnenolone-16a-carbonitrile (PCN) can activate the mouse and rat PXR but has no effect on hPXR. These species-species differences represent a challenge for pharmaceutical companies attempting to select appropriate animal models to evaluate candidate drugs. The same notion has also led to the initial creation and characterization of the hPXR humanized mice [22].
2.2. Regulation of Phase II enzymes
PXR also can regulate the expression of Phase II drug metabolizing enzymes, including UDP-glucuronosyl transferase (UGT), sulfotransferase (SULT) and glutathione S-transferase (GST) enzymes [54]. Phase II metabolic transformations are often, but do not have to be, preceded by Phase I oxidation reactions which expose or add sites that are ideal for Phase II conjugates. The Phase II metabolic enzymes add polar molecules onto xenobiotics and endobiotics, producing water soluble, non-toxic metabolites amenable to biliary and/or urinary excretion [55]. Indeed, a major consequence of PXR mediated Phase II metabolic enzyme regulation is the metabolism and detoxification of bile acids, estrogens, thyroxin, xenobiotics, and carcinogens [56].
UGTs are central Phase II metabolic enzymes which often have distinct as well as overlapping substrates [57]. In humans, 19 enzymes exist which contribute extensively to metabolism by catalyzing the addition of a UDP-glucuronic acid to endo- and xenobiotics, enhancing their water solubility and elimination [57, 58]. PXR activation by carbamazepin, RIF, dexamethasone and phenytoin have been linked to the transcriptional activation of several UGTs, including UGT1A1, UGT1A6, UGT1A3 and UGT1A4 [56, 57, 59, 60]. These UGT isoforms are also responsible for the metabolism of a plethora of other drugs such as lamotrigine, olanzapine, retigabine, irinotecan/SN38, acetaminophen, cyproheptadine, nicotine and imipramine, as well as carcinogens such as 4-nitrophenol and 4-OH-PhIP, benzo[a]pyrene [56, 57, 61, 62].
SULT enzyme activities represent another important Phase II pathway of metabolism. SULTs facilitate xenobiotic metabolism by catalyzing the addition of sulfate conjugates on drug molecules leading to more water soluble compounds [63]. In mice, PXR activation by PCN has been shown to up-regulate the transcription of several SULT isoforms including Sult1a1, Sult2a1, and Sult5a1 [64]. The role of PXR in human regulation of SULTs in response to xenobiotics is loosely established. Treatment with dexamethasone has been shown to up-regulate SULT2A1 in human liver cells, but rifampicin treatment has been shown to have both inductive and suppressive effects [65–67].
GSTs are also major enzymes in Phase II metabolism, as well as many other cytoprotective pathways. GSTs protect cells, organelles, and macromolecules from chemical and oxidative stress, and electrophiles. GSTs catalyze nucleophilic attack via reduced glutathione (GSH) on non-polar compounds containing an electrophilic carbon, rendering them less reactive and more hydrophilic [68, 69]. In mice, PXR activation by spironolactone, dexamethasone, and PCN has been shown to induce several GSTs including Gsta3, Gstm1, Gstm2, Gstm3, Gstm4, and MGst1 [69]. The effect of genetic activation of PXR on GST expression in transgenic mice has been shown to be GST isoform-, gender-, and tissue-specific. Human PXR has not been extensively shown to induce GSTs, however a recent report by Naspinski and colleaguescorrelated PXR activation by benzo[a]pyrene with subsequent up-regulation of several GSTs including GSTA1, GSTA2 and GSTM1 [70].
2.3. Regulation of drug transporters
Drug disposition and metabolism is also regulated by an array of cellular uptake and efflux transporters that control intestinal and hepatic absorption, renal re-absorption, and biliary/urinary elimination. These transporters work in concert with Phase I and II enzymes. The major xenobiotic transporters subject to PXR regulation include the ATP binding cassette family (ABC) proteins expressed in hepatocytes, enterocytes, kidney, and blood brain barrier that regulate cellular export of drugs. Examples of PXR target ABC transporters include the multidrug resistance 1 or P-glycoprotein (MDR1/P-gp), multidrug resistance associated proteins (MRP2, MRP3, MRP4, and MRP5), and breast cancer resistance protein (BCRP) [71–74]. The organic anion transporting polypeptide family (SLC/OATP), which regulates drug and endobiotic influx/uptake into the liver, is also regulated by PXR [75]. The known PXR target SLC/OATP genes include SLCO1A2/OATP1A2, SLCO1B1/OATP1B1, and SLCO1B3/OATP1B3. Finally, the organic ion transporter family, particularly the organic cation transporter SLC22A5/OCTN2, are proposed to have moderate PXR related regulation [76].
2.4. Implication of PXR in drug-drug interactions (DDIs)
As previously discussed, CYPs play an integral role in Phase I metabolism. Among CYP isoforms, the CYP3A subfamily is the most abundant in the liver and also conveys broad substrate specificity [77]. In fact, CYP3As have been shown to be responsible for the metabolism of over 50% of pharmaceuticals on the market today [77]. PXR has been shown to be a major transcriptional regulator of CYP3As, and because of this, it became increasingly apparent that the PXR-mediated regulation of drug-metabolizing enzymes could be involved in clinical DDIs. Such interactions occur when one drug accelerates the metabolism of a second, potentially leading to adverse consequences [78]. In addition, DDIs have been shown to cause decreased or absent bioavailability for orally administered drugs and increased hepatic clearance or accelerated formation of reactive metabolites, which can lead to local or systemic toxicity [78]. The human CYP3A4 is induced by PXR and has been shown clinically to be involved in possible drug-drug interactions. An example is the effect of RIF, a hPXR agonist, on the metabolism of the antihypertensive drug Verapamil [78]. Long-term treatment with RIF caused increased hepatic and gastrointestinal levels of CYP3A4, which led to the reduced oral bioavailability of (S)-verapamil by 96%, and abolished the anti-hypertensive effect in patients [78]. Another example was reported by Maglich and colleagues when they demonstrated that the anxiolytic herb St. John’s Wort could induce CYP3A4 at low concentrations. This discovery explained prevalent clinical data which showed increased metabolism and reduced efficacy of oral contraceptives, cyclosporin, and indinavir when taken with St. John’s Wort [79]. Unfortunately, these are not anecdotal occurrences, as they have been demonstrated clinically in an array of situations [80–85]. In the future, it is imperative to continue to use relevant animal models, such as the hPXR humanized mice [86], in order to fully understand the pharmacokinetic profile of PXR ligands and CYP substrates.
3. PXR in Endobiotic Metabolism
Although PXR was originally characterized as a xenobiotic receptor, it has been demonstrated that PXR has equal importance as an endobiotic receptor. Many studies have revealed essential roles of PXR in glucose and lipid metabolism, steroid hormone homeostasis, bile acid and bilirubin detoxification, and bone mineral homeostasis. As a result, PXR activation has great implications in many patho-physiological conditions.
3.1. PXR in glucose metabolism
Blood glucose is tightly controlled by insulin and glucagon through gluconeogenesis, glycogenolysis, and glycogenesis. Glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase1 (PEPCK1) are rate-limiting enzymes in gluconeogenesis and glycogenolysis [87, 88]. These genes are up-regulated by glucagon and glucocorticoids. Glucagon increases the formation of intracellular cAMP, which activates protein kinase A (PKA) to stimulate cAMP-response element binding protein (CREB) that binds to and regulates the transcription of PEPCK1 and G6Pase [89, 90]. Similarly, glucocorticoids induce PEPCK1 expression through a glucocorticoid response element [91]. On the other hand, insulin suppresses gluconeogenesis by down-regulating the transcription of G6Pase and PEPCK1 [92]. The forkhead transcription factor 1 (FoxO1) functions as an activator of G6Pase and PEPCK1 in the absence of insulin [92, 93]. Upon binding to an insulin response sequence (IRS), insulin excludes FoxO1 from the nucleus through phosphatidylinositol3-kinase (PI3K)-Akt pathway [94], resulting in a repressed expression of G6Pase and PEPCK1 and decreased glucose production.
PXR plays a role in hepatic gluconeogenesis. The expression of PEPCK and G6Pase was reduced in VP-hPXR transgenic mice in which the expression of an activated PXR (VP-PXR) was directed to the liver [95]. The PXR agonist PCN down-regulated G6Pase gene expression in wild type but not PXR−/ − mice [93]. Studies by Kodama and colleagues [93] suggested cross-talk between PXR and CREB and FoxO1 in regulating gluconeogenesis. They used a gel shift assay to show that PXR directly interacted with CREB and prevented its binding to the G6Pase gene promoter. Next, through a chromatin immunoprecipitation (ChIP) assay they showed that treatment with PCN decreased CREB binding to the promoter of G6Pase only in wild-type, but not in PXR−/− mice. Thus, by forming a complex with phosphorylated CREB, ligand activated PXR repressed CREB-mediated gene transcription and gluconeogenesis. Finally, PXR inhibited the binding of FoxO1 to IRS by direct interaction with FoxO1, as demonstrated by both gel shift and GST pull-down assays. A reporter gene assay also showed that FoxO1-IRS activity was repressed by PXR activation [96]. Therefore, it seems that ligand activated PXR directly interacts with FoxO1, which prevents FoxO1 from binding to IRS, leading to the suppression of G6Pase and PEPCK1 gene expression and gluconeogenesis [97]. The hepatocyte nuclear factor 4α (HNF4α) also positively regulates gluconeogenesis with the nuclear receptor coactivator PGC-1α. Bhalla and colleagues showed that PXR could compete with HNF4α for PGC-1α and thus suppress gluconeogenesis [9].
3.2. PXR in lipid metabolism
Triglycerides and fatty acids are also vital metabolic fuels. Lipid homeostasis centers on balancing lipid uptake and synthesis with lipid catabolism and secretion. When glucose and fatty acids exceed the body’s energy needs, they are converted to triglycerides in the liver for storage. During fasting or exercise, fatty acid β-oxidation and ketogenesis are increased in adipocytes to enhance ketone body synthesis and to provide energy. The sterol regulatory element-binding protein 1c (SREBP-1c) is a master regulator of lipogenesis. Several nuclear receptors, such as LXR [98], HNF-4 [99] and LRH-1 [100] orchestratelipid homeostasis through regulating the transcriptional activity of SREBP.
Interestingly, Zhou and colleagues showed that PXR induced lipogenesis in an SREBP-independent manner [95]. VP-PXR transgenic mice showed increased triglyceride accumulation in the liver, which was linked to up-regulation of fatty acid translocase CD36 and several other accessory lipogenic enzymes, including SCD-1 and long-chain free fatty acid elongase. CD36 is a scavenger receptor with broad ligand specificity. Activation of CD36 facilitates free fatty acid uptake from the circulation [101] and might contribute to hepatic steatosis. Correlation between CD36 levels and triglyceride storage and secretion suggests the causative role of CD36 in hepatic steatosis [102]. In fact, PXR is both necessary and sufficient for activation ofCD36 transcription. Further studies established CD36as a direct PXR transcriptional target [95]. CD36 expression can also be positively regulated by LXR and PPARγ. Therefore, CD36 appears to be a shared transcriptional target of LXR, PXR and PPARγ in their regulation of lipid homeostasis [103].
Two key enzymes involved in β-oxidation and ketogenesis are carnitine palmitoyltransferase 1A (CPT1A) and mitochondrial 3-hydroxy-3-methyl-glutarate-CoA synthase 2 (HMGCS2) [104, 105]. In the absence of insulin, a winged-helix/forkhead transcription factor FoxA2 stimulates the transcription of CPT1A and HMGCS2 [106]. Insulin induces the phosphorylation and nuclear exclusion of FoxA2, resulting in inactivation of FoxA2 and suppression of CPT1A and HMGCS2 transcription [107]. Nakamura and colleagues showed that PCN decreased transcription of Cpt1a and Hmgcs2 in wild-type, but not in PXR knockout mice. The underlying mechanism seemed to be direct binding of PXR to FoxA2 and suppression of Cpt1a and Hmgcs2 gene activation [108].
Cholesterol is essential to form cell membranes, bile acids and steroid hormones. On the other hand, oxidized cholesterol metabolites are cytotoxic and represent risk factors for atherosclerosis [109]. Therefore, cholesterol detoxification is crucial to protect the body from excess cholesterol. The mitochondrial sterol 27-hydroxylase (CYP27A1) is required for the cleavage and hydroxylation of cholesterol in most tissues [110, 111]. Li et al [112] showed that PXR activates CYP27A1, as well as cholesterol efflux transporters ABCA1 and ABCG1 in intestinal cells. The ‘good cholesterol’ HDL and its major constituent apolipoprotein A-I (ApoA-I) are involved in reverse cholesterol transport and have been associated with a reduced risk of atherosclerosis. ApoA-I and HDL cholesterol levels were elevated by PXR agonist in wild-type, but not in PXR−/ −mice [10]. Cholic acid mediated down- regulation of HDL cholesterol and plasma ApoA-1 was abolished in human PXR transgenic mice [113].
On the other hand, there were also studies supporting the pro-atherogenic role of PXR. Activation of PXR decreased the expression of ABCA1 in hepatocytes [114]. Clinically used PXR activating drugs caused hyperlipidemia in some patient populations [11, 115, 116]. Future studies are necessary to further define the role of PXR in the pathogenesis of hyperlipidemia.
3.3. PXR in glucocorticoid and mineralocorticoid homeostasis
Studies by Zhai et al [117] showed the importance of PXR in adrenal steroidhomeostasis. Both genetic and pharmacological activation of PXR increased plasma levels of corticosterone and aldosterone. Thisincrease was accompanied by activation of adrenal steroidogenic enzymes, such asCYP11a1, CYP11b1, CYP11b2, and 3β-hydroxysteroiddehydrogenase (3β-HSD). Interestingly, the PXR transgenic mice exhibited normal ACTH secretion in pituitary, and intact suppression of dexamethasone by corticosterone, indicating a functionalhypothalamus-pituitary-adrenal axis in spite of severely disrupted adrenal steroidhomeostasis. Consistent with these observations, several clinical studies reported that rifampicin increased steroid secretion in urine and may have resulted in misdiagnosis of Cushing’s syndrome [118, 119]. Therefore, PXR has a potential to disrupt endocrine homeostasis, and it may be broadly implicated in drug-hormone interactions.
3.4. PXR in androgen metabolism
The androgen-androgen receptor signaling pathway plays an important role in the initiation and progression of prostate cancer. Accordingly, androgen deprivation has been the most effective endocrine therapy for hormone-dependent prostate cancer. There are at least two major PXR target genes, CYP3A and SULT2A1, which are known to play a role in the metabolic deactivation of androgens. CYP3A is a key enzyme in catalyzing hydroxylation of testosterone and progesterone, leading to inactive hormones [120]. Dehydroepiandrosterone (DHEA) sulfotransferase (SULT2A1) is the primary SULT isoform responsible for androgen sulfonation [121]. Based on these notions, Zhang and colleagues recently reported a novel PXR-mediated and metabolism-based androgen deprivation [122]. In this study, the authors showed that activation of PXR lowered androgenic activity and inhibited androgen-dependent prostate regeneration in castrated male mice that received daily injections of testosterone by inducing the expression of CYP3Asand SULT2A1. In human prostate cancer cells, treatment with the PXR agonist RIF inhibited androgen-dependent proliferation of LAPC-4 cells, but had little effect on the growth of the androgen-independent isogenic LA99 cells. Down-regulation of PXR or SULT2A1 in LAPC-4 cells by shRNA or siRNA abolished the RIF effect, indicating that the inhibitory effect of RIF on androgens wasPXR- and SULT2A1-dependent. PXR may represent a novel therapeutic target to lower androgen activity and may aid in the treatment and prevention of hormone-dependent prostate cancer.
3.5. PXR in bile acid and bilirubin detoxification
Bile acids, synthesized in the liver, are end products of cholesterol catabolism and represent the primary pathway for cholesterol elimination from our bodies [123]. When excreted into the intestine, bile acids promote the absorption of cholesterol and fat-soluble vitamins. However, excess bile acids are cytotoxic and can lead to pathological cholestasis [124]. Therefore, bile acid levels need to be tightly regulated to protect the human body from their toxic effects.
PXR plays a critical role in bile acid detoxification [12, 14]. PXR agonist PCN reduced lithocholic acid (LCA) induced toxicity in wild-type, but not in PXR knockout mice. PXR transgenic mice were also resistant to LCA toxicity [12]. The protective effect of PXR can be explained by its regulation of genes involved in bile acid metabolism. PXR induced the expression of CYP3A that is essential for bile acid hydroxylation and excretion [14]. The Phase II enzyme SULT2A is also a PXR target gene that contributes to bile acid detoxification [47]. In addition to bile acid synthesis and metabolism, PXR also regulates the expression of bile acid transporters, such as MRP2 [125] and OATP2 [14].
There were also reports suggesting that PXR may play a role in the regulation of CYP7A1. It was reported that ligand activated PXR reduced the expression of CYP7A1without affecting SHP expression in mice [14]. However, hPXR can regulate SHP expression directly in HepG2 cells [126]. PXR has also been reported as a FXR target gene [127]. Together, these results suggested a close evolutionary relationship between PXR and FXR in protecting the human body from bile acid toxicity.
Bilirubin is the breakdown product of heme proteins. Conjugation of bilirubin by UGT converts the neurotoxic unconjugated bilirubin to nontoxic bilirubinglucuronide. It has been reported that activation of PXR prevented experimental hyperbilirubinemia in mice [56]. PXR activates the transcription of UGT1A1 [13] and several other genes critically involved in bilirubin detoxification, such as OATP2 [14] and MRP2 [125]. OATP2 mediates bilirubin uptake from blood into liver, whereas MRP2 facilitates the excretion of conjugated bilirubin to bile canaliculus. As a result, PXR ligands may represent potential therapeutic agents in treating hyperbilirubinemia.
3.6. PXR in vitamin metabolism and bone mineral homeostasis
Vitamin K2 is critical for bone formation and has been clinically used to treat osteoporosis. It has been reported that Vitamin K2 can activate PXR and stimulate PXR target gene expression. Treatment of osteosarcoma cells with Vitamin K2 increased the mRNA expression of osteoblast markers bone alkaline phosphatase, osteoprotegerin, osteopontin, and matrix Gla protein [128]. Vitamin K2 induced the expression of bone markers in primary osteocytes from wild-type, but not PXR−/ −mice [128]. Ichikawa et al [129] identified several PXR target genes with bone related functions in osteoblastic cells. Igarashi et al [130] showed that activation of PXR by Vitamin K2 induced the expression of osteoblastogenic transcription factor Msh homeobox 2, which is involved in osteoblast differentiation.
Calcium is a major component in bone development and maintenance. Calcium absorption and excretion are regulated by vitamin D, whose active metabolite 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) binds to the vitamin D receptor (VDR) [131]. VDR activates 25-hydroxyvitamin D(3)-24-hydroxylase (CYP24)-mediated 24-hydroxylation, which is critical in 1,25(OH)2D3 metabolism. Pascussi et al [132] reported that activation of PXR up-regulated CYP24 gene expression. However, Zhou et al [133] showed that PXR activation inhibited CYP24 gene expression. Although controversial, these results suggested a potential role of PXR in bone homeostasis, which warrants further investigation. Ligand activated PXR also suppresses the transcription of CYP2D25, an important hydroxylase in 1, 25(OH) 2D3 biosynthesis. It has been known for decades that prolonged treatment with anticonvulsant drugs may result in Vitamin D deficiency or osteomalacia in patients [134, 135]. Since many anticonvulsant drugs, such as carbamazepine and phenobarbital, are PXR ligands, these results urge caution in preventing drug-induced osteomalacia in patients.
Vitamin E is often taken as a dietary supplement for antioxidation purpose. Vitamin E is metabolized by CYPs mediated -oxidation [136], followed by β-oxidation, conjugation including glucuronidation and sulfation [137, 138], and then excretion [139, 140]. These processes are facilitated by an array of enzymes and transporters that happen to be PXR target genes. It was reported that Vitamin E can activate PXR [141, 142], and therefore may regulate xenobiotic detoxifying genes involved in its own metabolism. The study by Landes et al [141] showed that PXR can be activated by several forms of Vitamin E in a reporter gene assay. The most potent PXR activating -tocotrienol can induce the expression of endogenous CYP3A4 as efficaciousas rifampicin. Urinary Vitamin E metabolite was significantly decreased upon PCN treatment in wild type but not PXR−/− mice, which was suggested due to PXR mediated down regulation of hepatic sterol carrier protein 2 and attenuated β-oxidation[8]. These findings raise the concern of potential drug-drug interactions between Vitamin E and PXR regulators, which requires further studies.
3.7. PXR in retinoic acid metabolism
Retinoic acid (RA) is the metabolite of Vitamin A that binds to and activates the retinoic acid receptor (RAR). RAR forms a heterodimer with RXR and activates the transcription of genes associated with cell differentiation [143] and apoptosis [144], leading to inhibition of cell growth. Therefore, RAs have been used or tested as anti-cancer agents in several human cancer types [145]. However, RA resistance represents a major limit to its clinical use, which might be explained at least in part by the co-administration of a PXR agonist. Ligand activated PXR can induce expression of CYP3A and transporters such as MDR1A, MRP3 and OATP2, which accelerate RA metabolism [146]. It has been suggested that PXR antagonists might be useful in preventing RA resistance.
3.8. PXR in inflammation
A negative correlation between infectious disease/inflammation and drug metabolism capacity has been long suggested [147]. In understanding the molecular mechanisms behind this correlation, Teng et al showed that treatment of wild type mice with IL-6 caused a marked decrease in PXR protein level, as well the mRNA expression of PXR and its target genes Mrp2, Bsep, and Cyp3a11. This reduction was not seen in PXR−/− mice subjected to the same treatment, suggesting that this reduction was mediated by PXR. The same group showed that IL-6 could attenuate the upregulation of PXR and its target genes after PCN treatment in wild type mice [148]. Additionally, Beigneux et al showed that induction of a specific type of inflammation called acute phase response by lipopolysaccharide (LPS) caused a marked decrease in the mRNA expression of PXR and its target genes in the liver of wild type mice [149].
Reciprocally, treatment of human patients with rifampicin is known to activate PXR, leading to increased expression of PXR target genes. It is also known that patients treated with rifampicin showed marked immunosuppression. Zhou et al showed in vivo an in vitro that activation of PXR via rifampicin can attenuate NF-κb proteins, which are important in facilitating immune response and inflammation. Moreover, activation of NF-κb was shown to inhibit PXR, and inhibition of NF-κb enhanced PXR activity. The same group also showed that PXR−/− mice had increased small bowel inflammation and expression of NF-κb target genes [150]. Taken together, inhibition of PXR may be one mechanism of inflammation responsive repression of drug metabolism, whereas activation of PXR may be one mechanism for the drug responsive immunosuppression.
4. Conclusions and Perspectives
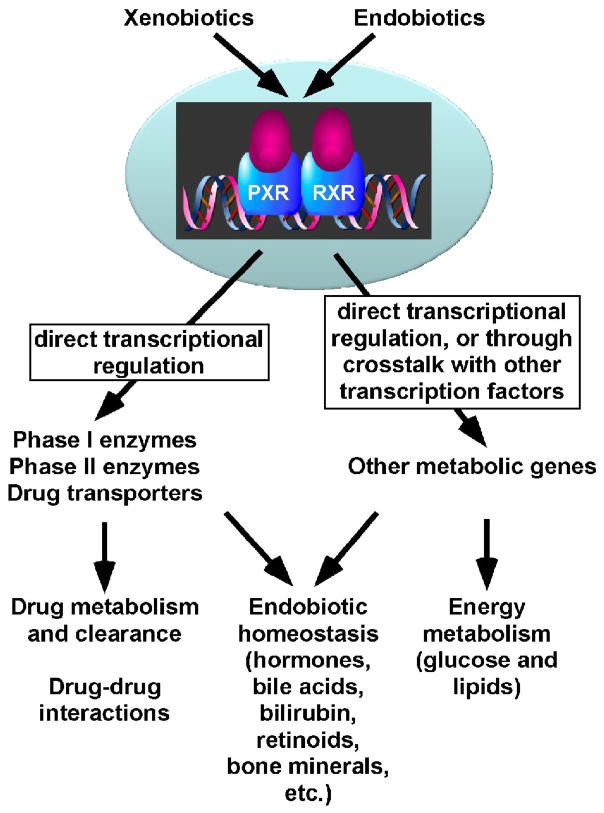
As summarized in Figure 1, many xenobiotics and endobiotics, as well as their metabolites can activate PXR. Subsequently, the activated PXR regulates the transcription of key enzymes involved in the metabolism of xenobiotics and endobiotics. Although the endobiotic functions of PXR have been appreciated, identification of physiologically relevant endogenous ligands for PXR will be beneficial in understanding the role of PXR as an endobiotic sensor. As we learn more about the roles of PXR in xenobiotic and endobiotic gene regulation, it remains to be determined whether the regulatory functions of PXR can be taken advantage of in preventing and treating human diseases.
Figure 1. Summary of the transcriptional circuits and metabolic relevance controlled by PXR.
PXR, pregnane X receptor; RXR, retinoid X receptor.
The role of PXR in xenobiotic metabolism is vast, nuanced, and extremely important. With the burgeoning number of pharmaceuticals on the market that are PXR ligands, more research must be done toward investigating receptor-mediated drug-drug interactions. The development of humanized mice offers a promise in using the in-vivo model to predict PXR-mediated drug-drug interactions.
In addition, polymorphisms of PXR and its major transcriptional targets such as CYP3A4 and MDR1 can affect xenobiotic and endobiotic metabolism. Considering the broad and complex transcription network controlled by PXR, the genetic variation of PXR might have broad implications in physiology and diseases [30]. To date, many splice variants of PXR have been identified and even more single nucleotide polymorphisms (SNPs) have been reported [151]. These polymorphisms exist in the coding or non-coding regions of the PXR gene, and several polymorphisms are associated with functional changes of PXR [30]. Future studies are necessary to assess metabolic differences in populations of different PXR genotypes [151].
Today’s medicine and biomedical research are at the interface of the old standardized approach and the new pharmacogenetically personalized future. In order for scientists and doctors to make headway into the future, it will be important to characterize genetics-based metabolic differences. Since its inception, the importance of PXR in xenobiotic and endobiotic metabolism has been repeatedly redefined. We have discussed many of the metabolic circuits that can be controlled by PXR, and described how alterations in these pathways could have substantial physiological consequences. As we move into the future, a full genetic assessment of the metabolic characteristics of PXR is imperative in order to further research that may lead to development of novel drugs or improvements on current drug therapies.
Abbreviations
- CYP
cytochrome P450
- DDI
drug-drug interaction
- G6Pase
Glucose-6-phosphatase
- GST
glutathione S-transferase
- NR
nuclear receptor
- PCN
prenenolone-16a-carbonitrile
- PEPCK
phosphoenolpyruvate carboxykinase
- PXR
pregnane X receptor
- RIF
rifampicin
- SULT
sulfotransferase
- UGT
UDP-glucuronosyl transferase
- XRE
xenobiotic response element
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kliewer SA, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92(1):73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 2.Francis GA, et al. Nuclear receptors and the control of metabolism. Annu Rev Physiol. 2003;65:261–311. doi: 10.1146/annurev.physiol.65.092101.142528. [DOI] [PubMed] [Google Scholar]
- 3.Jones SA, et al. The pregnane X receptor: a promiscuous xenobiotic receptor that has diverged during evolution. Mol Endocrinol. 2000;14(1):27–39. doi: 10.1210/mend.14.1.0409. [DOI] [PubMed] [Google Scholar]
- 4.Tolson AH, Wang H. Regulation of drug-metabolizing enzymes by xenobiotic receptors: PXR and CAR. Adv Drug Deliv Rev. 2010;62(13):1238–49. doi: 10.1016/j.addr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chirulli V, et al. CAR and PXR expression and inducibility of CYP2B and CYP3A activities in rat and rabbit lungs. Life Sci. 2005;76(22):2535–46. doi: 10.1016/j.lfs.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 6.Zollner G, et al. Role of nuclear receptors in the adaptive response to bile acids and cholestasis: pathogenetic and therapeutic considerations. Mol Pharm. 2006;3(3):231–51. doi: 10.1021/mp060010s. [DOI] [PubMed] [Google Scholar]
- 7.Iyer M, Reschly EJ, Krasowski MD. Functional evolution of the pregnane X receptor. Expert Opin Drug Metab Toxicol. 2006;2(3):381–97. doi: 10.1517/17425255.2.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho JY, et al. Metabolomics reveals a novel vitamin E metabolite and attenuated vitamin E metabolism upon PXR activation. J Lipid Res. 2009;50(5):924–37. doi: 10.1194/jlr.M800647-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhalla S, et al. Ligand-activated pregnane X receptor interferes with HNF-4 signaling by targeting a common coactivator PGC-1alpha. Functional implications in hepatic cholesterol and glucose metabolism. J Biol Chem. 2004;279(43):45139–47. doi: 10.1074/jbc.M405423200. [DOI] [PubMed] [Google Scholar]
- 10.Bachmann K, et al. PXR and the regulation of apoA1 and HDL-cholesterol in rodents. Pharmacol Res. 2004;50(3):237–46. doi: 10.1016/j.phrs.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Khogali AM, et al. Hyperlipidaemia as a complication of rifampicin treatment. Tubercle. 1974;55(3):231–3. doi: 10.1016/0041-3879(74)90050-6. [DOI] [PubMed] [Google Scholar]
- 12.Xie W, et al. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci U S A. 2001;98(6):3375–80. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugatani J, et al. Transcriptional regulation of human UGT1A1 gene expression: activated glucocorticoid receptor enhances constitutive androstane receptor/pregnane X receptor-mediated UDP-glucuronosyltransferase 1A1 regulation with glucocorticoid receptor-interacting protein 1. Mol Pharmacol. 2005;67(3):845–55. doi: 10.1124/mol.104.007161. [DOI] [PubMed] [Google Scholar]
- 14.Staudinger JL, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A. 2001;98(6):3369–74. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geier A, et al. Principles of hepatic organic anion transporter regulation during cholestasis, inflammation and liver regeneration. Biochim Biophys Acta. 2007;1773(3):283–308. doi: 10.1016/j.bbamcr.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Matic M, et al. Pregnane X receptor: promiscuous regulator of detoxification pathways. Int J Biochem Cell Biol. 2007;39(3):478–83. doi: 10.1016/j.biocel.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Kliewer SA, et al. The PPARs and PXRs: nuclear xenobiotic receptors that define novel hormone signaling pathways. Recent Prog Horm Res. 1999;54:345–67. discussion 367–8. [PubMed] [Google Scholar]
- 18.Lehmann JM, et al. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest. 1998;102(5):1016–23. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertilsson G, et al. Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc Natl Acad Sci U S A. 1998;95(21):12208–13. doi: 10.1073/pnas.95.21.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blumberg B, et al. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12(20):3195–205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore LB, et al. Pregnane X receptor (PXR), constitutive androstane receptor (CAR), and benzoate X receptor (BXR) define three pharmacologically distinct classes of nuclear receptors. Mol Endocrinol. 2002;16(5):977–86. doi: 10.1210/mend.16.5.0828. [DOI] [PubMed] [Google Scholar]
- 22.Xie W, et al. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature. 2000;406(6794):435–9. doi: 10.1038/35019116. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, et al. Rat pregnane X receptor: molecular cloning, tissue distribution, and xenobiotic regulation. Arch Biochem Biophys. 1999;368(1):14–22. doi: 10.1006/abbi.1999.1307. [DOI] [PubMed] [Google Scholar]
- 24.Savas U, et al. Rabbit pregnane X receptor is activated by rifampicin. Drug Metab Dispos. 2000;28(5):529–37. [PubMed] [Google Scholar]
- 25.Handschin C, Podvinec M, Meyer UA. CXR, a chicken xenobiotic-sensing orphan nuclear receptor, is related to both mammalian pregnane X receptor (PXR) and constitutive androstane receptor (CAR) Proc Natl Acad Sci U S A. 2000;97(20):10769–74. doi: 10.1073/pnas.97.20.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chawla A, et al. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294(5548):1866–70. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 27.Klarlund JK, et al. Signaling complexes of the FERM domain-containing protein GRSP1 bound to ARF exchange factor GRP1. J Biol Chem. 2001;276(43):40065–70. doi: 10.1074/jbc.M105260200. [DOI] [PubMed] [Google Scholar]
- 28.Orans J, Teotico DG, Redinbo MR. The nuclear xenobiotic receptor pregnane X receptor: recent insights and new challenges. Mol Endocrinol. 2005;19(12):2891–900. doi: 10.1210/me.2005-0156. [DOI] [PubMed] [Google Scholar]
- 29.Robinson-Rechavi M, Escriva Garcia H, Laudet V. The nuclear receptor superfamily. J Cell Sci. 2003;116(Pt 4):585–6. doi: 10.1242/jcs.00247. [DOI] [PubMed] [Google Scholar]
- 30.Zhang B, Xie W, Krasowski MD. PXR: a xenobiotic receptor of diverse function implicated in pharmacogenetics. Pharmacogenomics. 2008;9(11):1695–709. doi: 10.2217/14622416.9.11.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Squires EJ, Sueyoshi T, Negishi M. Cytoplasmic localization of pregnane X receptor and ligand-dependent nuclear translocation in mouse liver. J Biol Chem. 2004;279(47):49307–14. doi: 10.1074/jbc.M407281200. [DOI] [PubMed] [Google Scholar]
- 32.Li T, Chiang JY. Mechanism of rifampicin and pregnane X receptor inhibition of human cholesterol 7 alpha-hydroxylase gene transcription. Am J Physiol Gastrointest Liver Physiol. 2005;288(1):G74–84. doi: 10.1152/ajpgi.00258.2004. [DOI] [PubMed] [Google Scholar]
- 33.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83(6):841–50. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 34.McKenna NJ, Lanz RB, O’Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20(3):321–44. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 35.Umesono K, Evans RM. Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell. 1989;57(7):1139–46. doi: 10.1016/0092-8674(89)90051-2. [DOI] [PubMed] [Google Scholar]
- 36.Pollock CB, Rogatcheva MB, Schook LB. Comparative genomics of xenobiotic metabolism: a porcine-human PXR gene comparison. Mamm Genome. 2007;18(3):210–9. doi: 10.1007/s00335-007-9007-7. [DOI] [PubMed] [Google Scholar]
- 37.Miki Y, et al. Steroid and xenobiotic receptor (SXR), cytochrome P450 3A4 and multidrug resistance gene 1 in human adult and fetal tissues. Mol Cell Endocrinol. 2005;231(1–2):75–85. doi: 10.1016/j.mce.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Lamba V, et al. PXR (NR1I2): splice variants in human tissues, including brain, and identification of neurosteroids and nicotine as PXR activators. Toxicol Appl Pharmacol. 2004;199(3):251–65. doi: 10.1016/j.taap.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 39.Bauer B, et al. Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol Pharmacol. 2004;66(3):413–9. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 40.Lamba J, Lamba V, Schuetz E. Genetic variants of PXR (NR1I2) and CAR (NR1I3) and their implications in drug metabolism and pharmacogenetics. Curr Drug Metab. 2005;6(4):369–83. doi: 10.2174/1389200054633880. [DOI] [PubMed] [Google Scholar]
- 41.Poulos TL. Structural and functional diversity in heme monooxygenases. Drug Metab Dispos. 2005;33(1):10–8. doi: 10.1124/dmd.104.002071. [DOI] [PubMed] [Google Scholar]
- 42.Poulos TL. Cytochrome P450: molecular architecture, mechanism, and prospects for rational inhibitor design. Pharm Res. 1988;5(2):67–75. doi: 10.1023/a:1015920931701. [DOI] [PubMed] [Google Scholar]
- 43.Kishida T, et al. Strain differences in hepatic cytochrome P450 1A and 3A expression between Sprague-Dawley and Wistar rats. J Toxicol Sci. 2008;33(4):447–57. doi: 10.2131/jts.33.447. [DOI] [PubMed] [Google Scholar]
- 44.Drocourt L, et al. Calcium channel modulators of the dihydropyridine family are human pregnane X receptor activators and inducers of CYP3A, CYP2B, and CYP2C in human hepatocytes. Drug Metab Dispos. 2001;29(10):1325–31. [PubMed] [Google Scholar]
- 45.Ferguson SS, et al. Human CYP2C8 is transcriptionally regulated by the nuclear receptors constitutive androstane receptor, pregnane X receptor, glucocorticoid receptor, and hepatic nuclear factor 4alpha. Mol Pharmacol. 2005;68(3):747–57. doi: 10.1124/mol.105.013169. [DOI] [PubMed] [Google Scholar]
- 46.Mueller SO, et al. Species-specific activation of nuclear receptors correlates with the response of liver drug metabolizing enzymes to EMD 392949 in vitro. Toxicol Lett. 2010;193(1):120–3. doi: 10.1016/j.toxlet.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 47.Sonoda J, et al. Regulation of a xenobiotic sulfonation cascade by nuclear pregnane X receptor (PXR) Proc Natl Acad Sci U S A. 2002;99(21):13801–6. doi: 10.1073/pnas.212494599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma X, et al. Rifaximin is a gut-specific human pregnane X receptor activator. J Pharmacol Exp Ther. 2007;322(1):391–8. doi: 10.1124/jpet.107.121913. [DOI] [PubMed] [Google Scholar]
- 49.Goodwin B, Hodgson E, Liddle C. The orphan human pregnane X receptor mediates the transcriptional activation of CYP3A4 by rifampicin through a distal enhancer module. Mol Pharmacol. 1999;56(6):1329–39. doi: 10.1124/mol.56.6.1329. [DOI] [PubMed] [Google Scholar]
- 50.Coumoul X, Diry M, Barouki R. PXR-dependent induction of human CYP3A4 gene expression by organochlorine pesticides. Biochem Pharmacol. 2002;64(10):1513–9. doi: 10.1016/s0006-2952(02)01298-4. [DOI] [PubMed] [Google Scholar]
- 51.Wyde ME, et al. The environmental pollutant 1,1-dichloro-2,2-bis (p-chlorophenyl)ethylene induces rat hepatic cytochrome P450 2B and 3A expression through the constitutive androstane receptor and pregnane X receptor. Mol Pharmacol. 2003;64(2):474–81. doi: 10.1124/mol.64.2.474. [DOI] [PubMed] [Google Scholar]
- 52.Wyde ME, et al. Di-n-butyl phthalate activates constitutive androstane receptor and pregnane X receptor and enhances the expression of steroid-metabolizing enzymes in the liver of rat fetuses. Toxicol Sci. 2005;86(2):281–90. doi: 10.1093/toxsci/kfi204. [DOI] [PubMed] [Google Scholar]
- 53.Chang TK. Activation of pregnane X receptor (PXR) and constitutive androstane receptor (CAR) by herbal medicines. AAPS J. 2009;11(3):590–601. doi: 10.1208/s12248-009-9135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu C, Li CY, Kong AN. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res. 2005;28(3):249–68. doi: 10.1007/BF02977789. [DOI] [PubMed] [Google Scholar]
- 55.Wang H, LeCluyse EL. Role of orphan nuclear receptors in the regulation of drug-metabolising enzymes. Clin Pharmacokinet. 2003;42(15):1331–57. doi: 10.2165/00003088-200342150-00003. [DOI] [PubMed] [Google Scholar]
- 56.Xie W, et al. Control of steroid, heme, and carcinogen metabolism by nuclear pregnane X receptor and constitutive androstane receptor. Proc Natl Acad Sci U S A. 2003;100(7):4150–5. doi: 10.1073/pnas.0438010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bock KW. Functions and transcriptional regulation of adult human hepatic UDP-glucuronosyl-transferases (UGTs): Mechanisms responsible for interindividual variation of UGT levels. Biochem Pharmacol. 2010 doi: 10.1016/j.bcp.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 58.Buckley DB, Klaassen CD. Induction of mouse UDP-glucuronosyltransferase mRNA expression in liver and intestine by activators of aryl-hydrocarbon receptor, constitutive androstane receptor, pregnane X receptor, peroxisome proliferator-activated receptor alpha, and nuclear factor erythroid 2-related factor 2. Drug Metab Dispos. 2009;37(4):847–56. doi: 10.1124/dmd.108.024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trottier J, et al. Coordinate regulation of hepatic bile acid oxidation and conjugation by nuclear receptors. Mol Pharm. 2006;3(3):212–22. doi: 10.1021/mp060020t. [DOI] [PubMed] [Google Scholar]
- 60.Gardner-Stephen D, et al. Human PXR variants and their differential effects on the regulation of human UDP-glucuronosyltransferase gene expression. Drug Metab Dispos. 2004;32(3):340–7. doi: 10.1124/dmd.32.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borlak J, et al. N-Glucuronidation of the antiepileptic drug retigabine: results from studies with human volunteers, heterologously expressed human UGTs, human liver, kidney, and liver microsomal membranes of Crigler-Najjar type II. Metabolism. 2006;55(6):711–21. doi: 10.1016/j.metabol.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 62.Linnet K. Glucuronidation of olanzapine by cDNA-expressed human UDP-glucuronosyltransferases and human liver microsomes. Hum Psychopharmacol. 2002;17(5):233–8. doi: 10.1002/hup.403. [DOI] [PubMed] [Google Scholar]
- 63.Bian HS, et al. Induction of human sulfotransferase 1A3 (SULT1A3) by glucocorticoids. Life Sci. 2007;81(25–26):1659–67. doi: 10.1016/j.lfs.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 64.Alnouti Y, Klaassen CD. Regulation of sulfotransferase enzymes by prototypical microsomal enzyme inducers in mice. J Pharmacol Exp Ther. 2008;324(2):612–21. doi: 10.1124/jpet.107.129650. [DOI] [PubMed] [Google Scholar]
- 65.Duanmu Z, et al. Effects of dexamethasone on aryl (SULT1A1)- and hydroxysteroid (SULT2A1)-sulfotransferase gene expression in primary cultured human hepatocytes. Drug Metab Dispos. 2002;30(9):997–1004. doi: 10.1124/dmd.30.9.997. [DOI] [PubMed] [Google Scholar]
- 66.Fang HL, et al. Positive and negative regulation of human hepatic hydroxysteroid sulfotransferase (SULT2A1) gene transcription by rifampicin: roles of hepatocyte nuclear factor 4alpha and pregnane X receptor. J Pharmacol Exp Ther. 2007;323(2):586–98. doi: 10.1124/jpet.107.124610. [DOI] [PubMed] [Google Scholar]
- 67.Fang HL, et al. Regulation of human hepatic hydroxysteroid sulfotransferase gene expression by the peroxisome proliferator-activated receptor alpha transcription factor. Mol Pharmacol. 2005;67(4):1257–67. doi: 10.1124/mol.104.005389. [DOI] [PubMed] [Google Scholar]
- 68.Cui JY, et al. Genetic and epigenetic regulation and expression signatures of glutathione S-transferases in developing mouse liver. Toxicol Sci. 2010;116(1):32–43. doi: 10.1093/toxsci/kfq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Knight TR, Choudhuri S, Klaassen CD. Induction of hepatic glutathione S-transferases in male mice by prototypes of various classes of microsomal enzyme inducers. Toxicol Sci. 2008;106(2):329–38. doi: 10.1093/toxsci/kfn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Naspinski C, et al. Pregnane X receptor protects HepG2 cells from BaP-induced DNA damage. Toxicol Sci. 2008;104(1):67–73. doi: 10.1093/toxsci/kfn058. [DOI] [PubMed] [Google Scholar]
- 71.Mills JB, et al. Induction of drug metabolism enzymes and MDR1 using a novel human hepatocyte cell line. J Pharmacol Exp Ther. 2004;309(1):303–9. doi: 10.1124/jpet.103.061713. [DOI] [PubMed] [Google Scholar]
- 72.Schrenk D, et al. Up-regulation of transporters of the MRP family by drugs and toxins. Toxicol Lett. 2001;120(1–3):51–7. doi: 10.1016/s0378-4274(01)00306-x. [DOI] [PubMed] [Google Scholar]
- 73.Jigorel E, et al. Differential regulation of sinusoidal and canalicular hepatic drug transporter expression by xenobiotics activating drug-sensing receptors in primary human hepatocytes. Drug Metab Dispos. 2006;34(10):1756–63. doi: 10.1124/dmd.106.010033. [DOI] [PubMed] [Google Scholar]
- 74.Assem M, et al. Interactions between hepatic Mrp4 and Sult2a as revealed by the constitutive androstane receptor and Mrp4 knockout mice. J Biol Chem. 2004;279(21):22250–7. doi: 10.1074/jbc.M314111200. [DOI] [PubMed] [Google Scholar]
- 75.Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447(5):653–65. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- 76.Rae JM, et al. Rifampin is a selective, pleiotropic inducer of drug metabolism genes in human hepatocytes: studies with cDNA and oligonucleotide expression arrays. J Pharmacol Exp Ther. 2001;299(3):849–57. [PubMed] [Google Scholar]
- 77.Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23(5):687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- 78.Fuhr U. Induction of drug metabolising enzymes: pharmacokinetic and toxicological consequences in humans. Clin Pharmacokinet. 2000;38(6):493–504. doi: 10.2165/00003088-200038060-00003. [DOI] [PubMed] [Google Scholar]
- 79.Moore LB, et al. St. John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci U S A. 2000;97(13):7500–2. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mai I, et al. Hyperforin content determines the magnitude of the St John’s wort-cyclosporine drug interaction. Clin Pharmacol Ther. 2004;76(4):330–40. doi: 10.1016/j.clpt.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 81.Niemi M, et al. Pharmacokinetic interactions with rifampicin : clinical relevance. Clin Pharmacokinet. 2003;42(9):819–50. doi: 10.2165/00003088-200342090-00003. [DOI] [PubMed] [Google Scholar]
- 82.Reimers D, Jezek A. The simultaneous use of rifampicin and other antitubercular agents with oral contraceptives. Prax Pneumol. 1971;25(5):255–62. [PubMed] [Google Scholar]
- 83.Backman JT, Olkkola KT, Neuvonen PJ. Rifampin drastically reduces plasma concentrations and effects of oral midazolam. Clin Pharmacol Ther. 1996;59(1):7–13. doi: 10.1016/S0009-9236(96)90018-1. [DOI] [PubMed] [Google Scholar]
- 84.Polk RE, et al. Pharmacokinetic Interaction between amprenavir and rifabutin or rifampin in healthy males. Antimicrob Agents Chemother. 2001;45(2):502–8. doi: 10.1128/AAC.45.2.502-508.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grub S, et al. The interaction of saquinavir (soft gelatin capsule) with ketoconazole, erythromycin and rifampicin: comparison of the effect in healthy volunteers and in HIV-infected patients. Eur J Clin Pharmacol. 2001;57(2):115–21. doi: 10.1007/s002280100277. [DOI] [PubMed] [Google Scholar]
- 86.Moore JT, Kliewer SA. Use of the nuclear receptor PXR to predict drug interactions. Toxicology. 2000;153(1–3):1–10. doi: 10.1016/s0300-483x(00)00300-0. [DOI] [PubMed] [Google Scholar]
- 87.Foster JD, Nordlie RC. The biochemistry and molecular biology of the glucose-6-phosphatase system. Exp Biol Med (Maywood) 2002;227(8):601–8. doi: 10.1177/153537020222700807. [DOI] [PubMed] [Google Scholar]
- 88.Quinn PG, Yeagley D. Insulin regulation of PEPCK gene expression: a model for rapid and reversible modulation. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5(4):423–37. doi: 10.2174/156800805774912962. [DOI] [PubMed] [Google Scholar]
- 89.Herzig S, et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413(6852):179–83. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 90.Kurukulasuriya R, et al. Potential drug targets and progress towards pharmacologic inhibition of hepatic glucose production. Curr Med Chem. 2003;10(2):123–53. doi: 10.2174/0929867033368556. [DOI] [PubMed] [Google Scholar]
- 91.Imai E, et al. Characterization of a complex glucocorticoid response unit in the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol. 1990;10(9):4712–9. doi: 10.1128/mcb.10.9.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nakae J, et al. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest. 2001;108(9):1359–67. doi: 10.1172/JCI12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kodama S, et al. Human nuclear pregnane X receptor cross-talk with CREB to repress cAMP activation of the glucose-6-phosphatase gene. Biochem J. 2007;407(3):373–81. doi: 10.1042/BJ20070481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matsuzaki H, et al. Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc Natl Acad Sci U S A. 2003;100(20):11285–90. doi: 10.1073/pnas.1934283100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou J, et al. A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J Biol Chem. 2006;281(21):15013–20. doi: 10.1074/jbc.M511116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kodama S, et al. Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol Cell Biol. 2004;24(18):7931–40. doi: 10.1128/MCB.24.18.7931-7940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Konno Y, Negishi M, Kodama S. The roles of nuclear receptors CAR and PXR in hepatic energy metabolism. Drug Metab Pharmacokinet. 2008;23(1):8–13. doi: 10.2133/dmpk.23.8. [DOI] [PubMed] [Google Scholar]
- 98.Yoshikawa T, et al. Identification of liver X receptor-retinoid X receptor as an activator of the sterol regulatory element-binding protein 1c gene promoter. Mol Cell Biol. 2001;21(9):2991–3000. doi: 10.1128/MCB.21.9.2991-3000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Misawa K, et al. Sterol regulatory element-binding protein-2 interacts with hepatocyte nuclear factor-4 to enhance sterol isomerase gene expression in hepatocytes. J Biol Chem. 2003;278(38):36176–82. doi: 10.1074/jbc.M302387200. [DOI] [PubMed] [Google Scholar]
- 100.Kanayama T, et al. Interaction between sterol regulatory element-binding proteins and liver receptor homolog-1 reciprocally suppresses their transcriptional activities. J Biol Chem. 2007;282(14):10290–8. doi: 10.1074/jbc.M700270200. [DOI] [PubMed] [Google Scholar]
- 101.Febbraio M, Silverstein RL. CD36: implications in cardiovascular disease. Int J Biochem Cell Biol. 2007;39(11):2012–30. doi: 10.1016/j.biocel.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Koonen DP, et al. Increased hepatic CD36 expression contributes to dyslipidemia associated with diet-induced obesity. Diabetes. 2007;56(12):2863–71. doi: 10.2337/db07-0907. [DOI] [PubMed] [Google Scholar]
- 103.Zhou J, et al. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology. 2008;134(2):556–67. doi: 10.1053/j.gastro.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 104.Hegardt FG. Mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase: a control enzyme in ketogenesis. Biochem J. 1999;338(Pt 3):569–82. [PMC free article] [PubMed] [Google Scholar]
- 105.Louet JF, et al. Regulation of liver carnitine palmitoyltransferase I gene expression by hormones and fatty acids. Biochem Soc Trans. 2001;29(Pt 2):310–6. doi: 10.1042/0300-5127:0290310. [DOI] [PubMed] [Google Scholar]
- 106.Wolfrum C, et al. Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature. 2004;432(7020):1027–32. doi: 10.1038/nature03047. [DOI] [PubMed] [Google Scholar]
- 107.Wolfrum C, et al. Insulin regulates the activity of forkhead transcription factor Hnf-3beta/Foxa-2 by Akt-mediated phosphorylation and nuclear/cytosolic localization. Proc Natl Acad Sci U S A. 2003;100(20):11624–9. doi: 10.1073/pnas.1931483100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakamura K, et al. Nuclear pregnane X receptor cross-talk with FoxA2 to mediate drug-induced regulation of lipid metabolism in fasting mouse liver. J Biol Chem. 2007;282(13):9768–76. doi: 10.1074/jbc.M610072200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Leonarduzzi G, Sottero B, Poli G. Oxidized products of cholesterol: dietary and metabolic origin, and proatherosclerotic effects (review) J Nutr Biochem. 2002;13(12):700–710. doi: 10.1016/s0955-2863(02)00222-x. [DOI] [PubMed] [Google Scholar]
- 110.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–74. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 111.Chiang JY. Regulation of bile acid synthesis. Front Biosci. 1998;3:d176–93. doi: 10.2741/a273. [DOI] [PubMed] [Google Scholar]
- 112.Li T, Chen W, Chiang JY. PXR induces CYP27A1 and regulates cholesterol metabolism in the intestine. J Lipid Res. 2007;48(2):373–84. doi: 10.1194/jlr.M600282-JLR200. [DOI] [PubMed] [Google Scholar]
- 113.Masson D, et al. Expression of the pregnane X receptor in mice antagonizes the cholic acid-mediated changes in plasma lipoprotein profile. Arterioscler Thromb Vasc Biol. 2005;25(10):2164–9. doi: 10.1161/01.ATV.0000183674.88817.fb. [DOI] [PubMed] [Google Scholar]
- 114.Sporstol M, et al. Pregnane X receptor-agonists down-regulate hepatic ATP-binding cassette transporter A1 and scavenger receptor class B type I. Biochem Biophys Res Commun. 2005;331(4):1533–41. doi: 10.1016/j.bbrc.2005.04.071. [DOI] [PubMed] [Google Scholar]
- 115.Carr A, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12(7):F51–8. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 116.Eiris JM, et al. Effects of long-term treatment with antiepileptic drugs on serum lipid levels in children with epilepsy. Neurology. 1995;45(6):1155–7. doi: 10.1212/wnl.45.6.1155. [DOI] [PubMed] [Google Scholar]
- 117.Zhai Y, et al. Activation of pregnane X receptor disrupts glucocorticoid and mineralocorticoid homeostasis. Mol Endocrinol. 2007;21(1):138–47. doi: 10.1210/me.2006-0291. [DOI] [PubMed] [Google Scholar]
- 118.Zawawi TH, al-Hadramy MS, Abdelwahab SM. The effects of therapy with rifampicin and isoniazid on basic investigations for Cushing’s syndrome. Ir J Med Sci. 1996;165(4):300–2. doi: 10.1007/BF02943098. [DOI] [PubMed] [Google Scholar]
- 119.Terzolo M, et al. Misdiagnosis of Cushing’s syndrome in a patient receiving rifampicin therapy for tuberculosis. Horm Metab Res. 1995;27(3):148–50. doi: 10.1055/s-2007-979927. [DOI] [PubMed] [Google Scholar]
- 120.Niwa T, et al. Contribution of human hepatic cytochrome P450 isoforms to regioselective hydroxylation of steroid hormones. Xenobiotica. 1998;28(6):539–47. doi: 10.1080/004982598239290. [DOI] [PubMed] [Google Scholar]
- 121.Strott CA. Sulfonation and molecular action. Endocr Rev. 2002;23(5):703–32. doi: 10.1210/er.2001-0040. [DOI] [PubMed] [Google Scholar]
- 122.Zhang B, et al. Pregnane X receptor as a therapeutic target to inhibit androgen activity. Endocrinology. 2010;151(12):5721–9. doi: 10.1210/en.2010-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fiorucci S, et al. Targeting farnesoid X receptor for liver and metabolic disorders. Trends Mol Med. 2007;13(7):298–309. doi: 10.1016/j.molmed.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 124.Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med. 1999;159(22):2647–58. doi: 10.1001/archinte.159.22.2647. [DOI] [PubMed] [Google Scholar]
- 125.Kast HR, et al. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem. 2002;277(4):2908–15. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- 126.Frank C, et al. Identification of pregnane X receptor binding sites in the regulatory regions of genes involved in bile acid homeostasis. J Mol Biol. 2005;346(2):505–19. doi: 10.1016/j.jmb.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 127.Jung D, Mangelsdorf DJ, Meyer UA. Pregnane X receptor is a target of farnesoid X receptor. J Biol Chem. 2006;281(28):19081–91. doi: 10.1074/jbc.M600116200. [DOI] [PubMed] [Google Scholar]
- 128.Tabb MM, et al. Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J Biol Chem. 2003;278(45):43919–27. doi: 10.1074/jbc.M303136200. [DOI] [PubMed] [Google Scholar]
- 129.Ichikawa T, et al. Steroid and xenobiotic receptor SXR mediates vitamin K2-activated transcription of extracellular matrix-related genes and collagen accumulation in osteoblastic cells. J Biol Chem. 2006;281(25):16927–34. doi: 10.1074/jbc.M600896200. [DOI] [PubMed] [Google Scholar]
- 130.Igarashi M, et al. Vitamin K induces osteoblast differentiation through pregnane X receptor-mediated transcriptional control of the Msx2 gene. Mol Cell Biol. 2007;27(22):7947–54. doi: 10.1128/MCB.00813-07. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 131.Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78(4):1193–231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 132.Pascussi JM, et al. Possible involvement of pregnane X receptor-enhanced CYP24 expression in drug-induced osteomalacia. J Clin Invest. 2005;115(1):177–86. doi: 10.1172/JCI21867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhou C, et al. Steroid and xenobiotic receptor and vitamin D receptor crosstalk mediates CYP24 expression and drug-induced osteomalacia. J Clin Invest. 2006;116(6):1703–12. doi: 10.1172/JCI27793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dent CE, et al. Osteomalacia with long-term anticonvulsant therapy in epilepsy. Br Med J. 1970;4(5727):69–72. doi: 10.1136/bmj.4.5727.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shah SC, et al. Rifampicin induced osteomalacia. Tubercle. 1981;62(3):207–9. doi: 10.1016/0041-3879(81)90008-8. [DOI] [PubMed] [Google Scholar]
- 136.Birringer M, Drogan D, Brigelius-Flohe R. Tocopherols are metabolized in HepG2 cells by side chain omega-oxidation and consecutive beta-oxidation. Free Radic Biol Med. 2001;31(2):226–32. doi: 10.1016/s0891-5849(01)00574-3. [DOI] [PubMed] [Google Scholar]
- 137.Swanson JE, et al. Urinary excretion of 2,7, 8-trimethyl-2-(beta-carboxyethyl)-6-hydroxychroman is a major route of elimination of gamma-tocopherol in humans. J Lipid Res. 1999;40(4):665–71. [PubMed] [Google Scholar]
- 138.Stahl W, et al. Quantification of the alpha- and gamma-tocopherol metabolites 2,5,7, 8-tetramethyl-2-(2′-carboxyethyl)-6-hydroxychroman and 2,7, 8-trimethyl-2-(2′-carboxyethyl)-6-hydroxychroman in human serum. Anal Biochem. 1999;275(2):254–9. doi: 10.1006/abio.1999.4312. [DOI] [PubMed] [Google Scholar]
- 139.Brigelius-Flohe R, Traber MG. Vitamin E: function and metabolism. FASEB J. 1999;13(10):1145–55. [PubMed] [Google Scholar]
- 140.Kiyose C, et al. Alpha-tocopherol affects the urinary and biliary excretion of 2,7,8-trimethyl-2 (2′-carboxyethyl)-6-hydroxychroman, gamma-tocopherol metabolite, in rats. Lipids. 2001;36(5):467–72. doi: 10.1007/s11745-001-0744-2. [DOI] [PubMed] [Google Scholar]
- 141.Landes N, et al. Vitamin E activates gene expression via the pregnane X receptor. Biochem Pharmacol. 2003;65(2):269–73. doi: 10.1016/s0006-2952(02)01520-4. [DOI] [PubMed] [Google Scholar]
- 142.Brigelius-Flohe R. Vitamin E and drug metabolism. Biochem Biophys Res Commun. 2003;305(3):737–40. doi: 10.1016/s0006-291x(03)00811-8. [DOI] [PubMed] [Google Scholar]
- 143.Park DJ, et al. CCAAT/enhancer binding protein epsilon is a potential retinoid target gene in acute promyelocytic leukemia treatment. J Clin Invest. 1999;103(10):1399–408. doi: 10.1172/JCI2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Altucci L, et al. Retinoic acid-induced apoptosis in leukemia cells is mediated by paracrine action of tumor-selective death ligand TRAIL. Nat Med. 2001;7(6):680–6. doi: 10.1038/89050. [DOI] [PubMed] [Google Scholar]
- 145.Soprano DR, Qin P, Soprano KJ. Retinoic acid receptors and cancers. Annu Rev Nutr. 2004;24:201–21. doi: 10.1146/annurev.nutr.24.012003.132407. [DOI] [PubMed] [Google Scholar]
- 146.Wang T, et al. Role of pregnane X receptor in control of all-trans retinoic acid (ATRA) metabolism and its potential contribution to ATRA resistance. J Pharmacol Exp Ther. 2008;324(2):674–84. doi: 10.1124/jpet.107.131045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gu X, et al. Role of NF-kappaB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J Biol Chem. 2006;281(26):17882–9. doi: 10.1074/jbc.M601302200. [DOI] [PubMed] [Google Scholar]
- 148.Teng S, Piquette-Miller M. The involvement of the pregnane X receptor in hepatic gene regulation during inflammation in mice. J Pharmacol Exp Ther. 2005;312(2):841–8. doi: 10.1124/jpet.104.076141. [DOI] [PubMed] [Google Scholar]
- 149.Beigneux AP, et al. Reduction in cytochrome P-450 enzyme expression is associated with repression of CAR (constitutive androstane receptor) and PXR (pregnane X receptor) in mouse liver during the acute phase response. Biochem Biophys Res Commun. 2002;293(1):145–9. doi: 10.1016/S0006-291X(02)00196-1. [DOI] [PubMed] [Google Scholar]
- 150.Zhou C, et al. Mutual repression between steroid and xenobiotic receptor and NF-kappaB signaling pathways links xenobiotic metabolism and inflammation. J Clin Invest. 2006;116(8):2280–2289. doi: 10.1172/JCI26283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.He P, et al. Human pregnane X receptor: genetic polymorphisms, alternative mRNA splice variants, and cytochrome P450 3A metabolic activity. J Clin Pharmacol. 2006;46(11):1356–69. doi: 10.1177/0091270006292125. [DOI] [PubMed] [Google Scholar]