Abstract
Within the preoptic region, nitric oxide (NO) production varies during the ovarian cycle and has the ability to impact hypothalamic reproductive function. One mechanism for the regulation of NO release mediated by estrogens during the estrous cycle includes physical association of the calcium-activated neuronal NO synthase (nNOS) enzyme with the glutamate N-methyl-D-aspartate (NMDA) receptor channels via the postsynaptic density 95 (PSD 95) scaffolding protein. Here, we demonstrate that endogenous variations in estrogens levels during the estrous cycle also coincide with corresponding changes in the state of nNOS Ser1412 phosphorylation, the level of association of this isoform with the NMDA receptor/PSD-95 complex at the plasma membrane and the activity of NOS. Neuronal NOS Ser1412 phosphorylation is maximal on the afternoon of proestrus, when both the levels of estrogens and the physical association of nNOS with NMDA receptors are highest. Estradiol mimicked these effects in ovariectomized (OVX) rats. In addition, the catalytic activity of NOS in membrane protein extracts from the preoptic region, i.e., independent of any functional protein-protein interactions or cell-cell signaling, was significantly increased in estradioltreated OVX rats compared to OVX rats. Finally, λ phosphatase-mediated nNOS dephosphorylation dramatically impaired NOS activity in preoptic region protein extracts, thus demonstrating the important role of phosphorylation in the regulation of NO production in the preoptic region. Taken together, these results yield new insights into the regulation of neuron-derived NO production by gonadal steroids within the preoptic region and raise the possibility that changes in nNOS phosphorylation during fluctuating physiological conditions may be involved in the hypothalamic control of key neuroendocrine functions, such as reproduction.
Keywords: NOS1, sex steroids, LHRH, GnRH, preoptic area, brain
Keywords: Animals; Blotting, Western; Cell Membrane; metabolism; Estradiol; pharmacology; Estrogens; metabolism; Female; Hypothalamus; drug effects; metabolism; Immunoprecipitation; Menstrual Cycle; metabolism; physiology; Nitric Oxide; metabolism; Nitric Oxide Synthase Type I; metabolism; Ovariectomy; Phosphorylation; drug effects; Protein Binding; physiology; Rats; Rats, Sprague-Dawley; Receptors, N-Methyl-D-Aspartate; metabolism
Introduction
Neuronal nitric oxide synthase (nNOS) produces nitric oxide (NO) as a byproduct of the conversion of L-arginine to L-citruline. Nitric oxide has been implicated in the regulation of various cellular functions in the brain such as apoptosis, differentiation, development, synaptic plasticity and neurosecretion (for review see (1–5)). In the hypothalamus, NO is proposed to play a role in development (6) and is involved in the regulation of the biological clock (7), drinking behavior (8), food intake (9) and glucose homeostasis (10, 11). It was recently shown to play an important role in the stress response as well as in neural-immune interactions (12). Accumulating evidence also suggests that NO plays a key role in the control of reproduction (3, 13, 14). Nitric oxide influences the oxytocin system during pregnancy (15). Receptive behavior (lordosis) can also be induced with intracerebroventricular injection of NO donors, whereas NO synthesis inhibitors prevent progesterone-induced lordosis (16). In the hypothalamic preoptic region, GnRH-expressing neuronal cell bodies are surrounded by nNOS containing neurons (17, 18), and NO mediates GnRH release in response to norepinephrine (19, 20), progesterone (21), 17β-estradiol (22) and glutamate (23, 24). Furthermore, NO has been suggested to be a synchronizing agent for GnRH neuronal activity (25) and for pulsatile GnRH release (26) based on studies in brain slices and in the GT1-7 hypothalamic cell line, respectively. In mutant mice, targeted deletion of the catalytic domain of nNOS was shown to impair central hormonal regulation of reproductive function (27).
Neuronal NOS activity is primarily regulated by an increase in intracellular Ca++, which activates nNOS through calmodulin binding (28, 29). In neurons, nNOS activity is specifically coupled to calcium influx through N-methyl-D-aspartate (NMDA)-type glutamate receptors (29, 30), which are receptor channels tethered to calmodulin (31). The physical interaction of nNOS with NMDA receptors involves the post-synaptic density-95 (PSD-95) scaffolding protein and the assembly of a ternary complex (32). This complex formation is required to efficiently couple Ca++ influx, via NMDA receptors, to nNOS activity (33–35). In parallel, nNOS has also been shown to be subjected to posttranscriptional modifications (such as phoshorylation) that could impact its catalytic activity (36–39).
Estrogens have well-known effects on the hypothalamic–pituitary-gonadal axis (40, 41), but they also have profound effects on brain structure and physiology (42–49). For example, estrogens cause cyclic changes in neurogenesis (50), dendritic spine density and synaptogenesis (51) in the rat hippocampus during the estrous cycle. Likewise, estradiol treatment in ovariectomized female rats controls dynamic changes in spine density and/or the number of synapses in both the hippocampus (52) and hypothalamus (53–56). In addition to these structural effects on neuronal connectivity, recent studies showed that estrogens play a distinct role in controlling NO production within the hypothalamus (57–60) as well as in other brain areas (61).
Accumulating evidence suggests that one mechanism for the regulation of nNOS activity in the hypothalamus could reside in the differential coupling of nNOS to NMDA receptors at the plasma membrane in the context of changing physiological conditions. Indeed, recent data have demonstrated that assembly of an nNOS/PSD-95/NMDA receptor ternary complex is modulated by the natural fluctuations of estrogens across the estrous cycle in neurons of the preoptic region (58) and that these effects likely require ligand-dependent activation of estrogen receptors (62). Here, we investigated whether, in parallel to these estrogenmediated changes in protein-protein interactions, NO production is regulated by changes in nNOS phosphorylation during the estrous cycle. Our findings indicate that cyclic changes in estrogen levels are indeed associated with marked variations in nNOS phoshorylation. Furthermore, the physical association of the phosphorylation-activated nNOS isoform with NMDA receptors at the plasma membrane in neurons of the hypothalamic preoptic region displayed cyclic changes, which we previously shown to interact with the neuronal network involved in the central control of reproduction (25, 58).
Material and Methods
Animals
Sprague Dawley female rats (Janvier, Saint-Berthevin, France) weighing 200–220g were housed in a room with controlled photoperiods (14 h of light beginning at 0700h and 10 h of darkness) and temperature (21–23°C) with food and water ad libitum. Rats were fed a regular chow diet that contained trace amounts of phytoestrogen (2016; Harlan France, Gannat, France) (Odum et al., 2001). Vaginal smears were examined daily, and only rats that exhibited at least two consecutive 4-day estrous cycles were used for experiments. Diestrus I and II were defined by a predominance of leukocytes in the vaginal lavage. The day of proestrus was identified by a predominance of nucleated rounded epithelial cells. Finally, the day of estrous was defined by large numbers of clustered cornified squamous epithelial cells. All experiments were performed in accordance with guidelines on animal use specified by the European Communities Council Directive of November 24, 1986 (86/609/EEC) regarding mammalian research and were approved by the University of Lille 2 Animal Use Committee.
Antibodies
Antibodies used for coimmunoprecipitation and Western blot experiments
Rabbit polyclonal anti-nNOS (sc-8309; 1:500 dilution for immunoblotting) and goat polyclonal antibody antiactin (sc-1616; 1:1000 for immunoblotting) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal anti-PSD95 (MA1-045 and MA1-046 both used at 1 μg/750 μl for immunoprecipitation; MA1-046 only used for immunoblotting at 1:500) and the rabbit polyclonal anti-phospho-nNOS-Ser1412 (anti-P-nNOS) (PA1-032 at 2 μg/750 μl for immunoprecipitation and 1:1000 for immunodetection) were purchased from Affinity BioReagents (Golden, CO). Control experiments included incubation of size-fractioned proteins transferred onto polyvinylidene difluoride membrane in antisera that had been preabsorbed with the immunizing peptide (PEP-188; Affinity BioReagents). The specificity of both anti-PSD-95 was described by Kornau et al. (63). The polyclonal rabbit antibody anti- NR2B (71–8600; 1:250 for immunoblotting) was obtained from Zymed (San Francisco, CA) (32). Secondary antibodies used for Western blot detection (anti-mouse (1:8000), anti-rabbit (1:10,000) and anti-goat/sheep (1:10,000) all peroxidase (HRP)-conjugated) were purchased from Sigma (Saint-Quentin Fallavier, France).
Antibodies used for immunohistofluorescence experiments
Sheep polyclonal anti-nNOS (1:3000) was a generous gift from Dr. P. C. Emson (Medical Research Council, Laboratory for Molecular Biology, Cambridge, UK) (18) and anti-P-nNOS (71–8600; 1:500) were purchased from Affinity BioReagents (Golden, CO). The secondary donkey anti-rabbit conjugated Alexa Fluor 568 (A-21099; 1:500) used for nNOS and the conjugated Alexa Fluor 488 (S-11223; 1:500) used for P-nNOS detection were purchased from Invitrogen (Eugene, OR).
Protein extraction and coimmunoprecipitation
Female rats were decapitated at 4pm on the day of diestrus II (Di16h) or at 4 pm on the day of proestrus (Pro16h). After rapid removal of the brain, the meninges and optic chiasma were removed and the preoptic region was dissected under a binocular magnifying glass with Wecker’s scissors (Moria, France). The external limits for this dissection are: lateral, the external border of the Medial Preoptic Area (MPO); dorsal, the internal border of anterior commissures (aco); and antero-posterior limits are +0.95 to −0.51 mm from bregma, according to the Swanson Atlas (64). After dissection, each fragment was placed in a microcentrifuge tube, snap frozen in liquid nitrogen and stored at −80°C.
Protein extracts of four preoptic region samples were prepared in 750 μl of lysis buffer (pH 7.4, 25 mM Tris, 50 mM β-glycerophosphate, 1.5 mM EGTA, 0.5 mM EDTA, 1 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 10 μg/ml leupeptin and pepstatin, 10 μg/ml aprotinin, 100 μg/ml PMSF, and 1% triton X-100) by homogenization of the fragments through 22 and 26 gauge needles in succession. The tissue lysates were cleared by centrifugation at 12,000 × g for 15 min and 30 μl of protein A–Sepharose beads (1:1 slurry in lysis buffer; P3391, Sigma) was added to the supernatants to remove endogenous G-type immunoglobulins. After 30 min of gentle rocking at 4 °C, beads were centrifuged (15 sec, 12,000 × g), and supernatants were collected. Protein content was determined using the Bradford method (Bio-Rad, Hercules, CA). An equal amount of protein (2.2 mg; four rats were required to obtain this quantity of protein) in a total volume of 750 μl of lysis buffer was incubated with gentle rocking at 4°C for 2 h with 2 μg of the antibodies used for immunoprecipitation in this study (anti-PSD-95 MA1-045, anti-PSD-95 MA1-046, or antiphospho- nNOS-Ser1412). Thereafter, 60 μl of protein A–sepharose beads in lysis buffer (1:1 blend) was added to each sample and incubated for one additional hour with gentle rocking at 4 °C. The Sepharose beads were pelleted by brief centrifugation, the supernatant was collected and 4× sample buffer (Invitrogen) was added for analysis of nonimmunoprecipitated proteins. The beads were then washed three times with ice-cold lysis buffer and boiled for 5 min in 50 μl of 2× sample buffer. When necessary, the samples were stored at −80 °C until use.
Plasma membrane extraction and Western blotting analysis
Tissues were homogenized in Tris buffer (25 mM, pH 7.4) containing 50 mM βglycerophosphate, 1.5 mM EGTA, 0.5 mM EDTA, 1 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 10 μg/ml leupeptin and pepstatin, 10 μg/ml aprotinin and 100 μg/ml PMSF and subsequently cleared by centrifugation at 3500 x g for 10 min. The supernatant was then collected and the volume was equalized with homogenization buffer and ultracentrifuged at 49,000 rpm in Beckmann Eppendorff tubes for 1 hour at 4 °C. After ultracentrifugation, the pellet was resuspended in 50μl of lysis buffer with Triton X-100 and sonicated for 10 seconds. Protein content was determined using the Bradford method (Bio- Rad, Hercules, CA). We added 4× sample buffer (Invitrogen) to the samples, which were boiled for 5 min before electrophoresis at 150 V for 75 min in 3–8% Tris-acetate precast SDS-polyacrylamide gels according to the protocol supplied with the NuPAGE system (Invitrogen). After size fractionation, the proteins were transferred onto nitrocellulose membrane (0.2 μm pore-size membranes; LC2002; Invitrogen) in the blot module of the NuPAGE system (Invitrogen) for 75 min at room temperature (RT). Blots were blocked for 1 h in TBS with 0.05% Tween 20 (TBST) and 5% nonfat milk at RT, incubated overnight at 4 °C with their respective primary antibody, and washed four times with TBST before being exposed to HRP-conjugated secondary antibodies diluted in 5% nonfat milk TBST for 1 h at RT. The immunoreactions were detected with enhanced chemiluminescence (NEL101; PerkinElmer, Boston, MA).
Neuronal NOS activity assay
Nitric oxide synthase activity was determined in plasma membrane-extracted protein by measuring the formation of nitrite and nitrate produced in samples using the Calbiochem nitric oxide synthase assay kit, calorimetric (Cat. No 482702; Calbiochem, International, Merck KGaA, Darmstadt, Germany). The total quantity of NO corresponds to the sum of both nitrate and nitrite products. Experiments were performed with plasma membrane extracts with equal amounts of protein obtained from preoptic region homogenates and processed according to the manufacturer’s instructions. Spectrophotometric quantification of nitrite and nitrate was performed at 540 nm using Greiss reagents. Nitrate and nitrite sample concentrations were deduced from a nitrate standard curve at 540nm.
λ-phosphatase treatment
An equal amount of protein was incubated 1 h at 37 °C with the presence or the absence of 800 U λ-phospatase (P0753S; Biolabs Ltd. New England, UK) in 1X λ-phospatase buffer, supplemented with 2 mM MgCl2, in a total volume of 50 μl. This reaction mixture was then used for western blot and NOS activity assays, as detailed above.
Effects of ovariectomy and estradiol treatment on nNOS phosphorylation and activity
Cycling female rats (n = 24) were bilaterally ovariectomized (OVX, day 0) under anesthesia by intraperitoneal injection of 10 mg/kg xylazine (Rompun 2% Bayer) and 60 mg/kg ketamine (Ketalar Parke-Davis). Animals were divided into two groups: 12 animals that were killed at 2 pm 17 days after the ovariectomy without receiving any treatment, and 12 animals that received a single subcutaneous injection of estradiol benzoate (E2, 30 μg/rat) at 10 am on day 15 and were killed at 2 pm 17 days after the ovariectomy. Control and E2-treated OVX rats were used for Western blots and nNOS assay experiments performed on membrane extract protein of the hypothalamic preoptic region. We observed that uterus was full of fluid in E2-treated rats (12/12) compared to OVX rats, in which the uteri were empty (12/12). In addition, there was a significant difference in the weight of the uteri between OVX (n = 12, 99.4 ± 2.68 mg) and E2-treated OVX rats (n= 11, 272.81±11.568 mg).
Immunohistofluorescence
Animals were anesthetized with chloral hydrate (400 mg/kg, i.p.) and perfused transcardially with 5–10 ml of saline, followed by 500 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH7.4. Brains were removed and immersed in the same fixative for 2 h at 4°C and stored in PB until slicing. Free floating coronal sections (40 μm thick) containing the preoptic region were cut on a Vibratome (VT1000S; Leica, Wetzlar, Germany), collected in ice-cold PB and blocked in PBS with 5% normal donkey serum (D9663; Sigma) and 0.3% Triton X-100 (Sigma) for 1 h at RT before incubation with sheep anti-nNOS (1:1000) and rabbit anti P-nNOS (1:1000) in the same blocking solution for 48 hours at 4°C. Sections were washed extensively in PBS and exposed to secondary antibodies (Alexa Fluor 568- conjugated anti-sheep (1:500) and anti rabbit Alexa Fluor 488 (1:500)) for 1 h in 5% normal donkey serum. After washes, slices were incubated for 2 min with 0.02% Hoechst 33258 bisbenzimide (H3569; Invitrogen) in PBS for nuclear fluorescent staining, and mounted on glass slides and coverslipped in Permafluor medium (434990; Immunon, Pittsburgh, PA). Control sections were incubated in the absence of a primary antibody. Sections were analyzed using an Axio Imager.Z1 ApoTome microscope (Zeiss, Germany), equipped with a motorized stage and an AxioCam MRm camera (Zeiss, Germany). Specific filter cubes were used for the visualization of green (EX: 475/40 nm, DM: 500 nm, BA: 530/50 nm) and red (EX: 550/25 nm, DM: 570 nm, BA: 605/70 nm). The P-nNOS-immunoreactive cells displayed a green fluorescence from Alexa 488, and nNOS-immunoreactive neurons displayed a red fluorescence from Alexa 568.
For illustration purposes, photomontages of the preoptic region were prepared with the help of Photoshop CS2 (Adobe Systems, San Jose, CA) using two to four digitalized images acquired with a 10× and 20× (numerical apertures: 0.3 and 0.8, respectively) objective. Analysis was undertaken as described previously (58). Briefly, the numbers of single immunoreactive cells and dual-labeled neurons in the upper focal plan of each section. The distribution and number of dual labeled cells were counted in the forebrain represented by plates 16–18 of the Swanson brain atlas (64) and more precisely, in the nucleus of the diagonal band (NDB), the median preoptic nucleus (MEPO) at the vascular organ of the lamina terminalis (OV) and the anteroventral preoptic nucleus (AVP), three forebrain regions where nNOS neurons are abundant and known to interact both morphologicaly (18, 25) and functionally (25) with GnRH neurons.
Statistics
The differences between several groups were analyzed using one-way ANOVA, followed by the Student–Newman–Keuls multiple comparison test for unequal replication. The comparison between two groups was subjected to an unpaired t test. The level of significance was set at p < 0.05.
Results
The estrous cycle promotes changes in nNOS protein phosphorylation in the hypothalamic preoptic region
To determine whether posttranslational modifications play a regulatory role in nNOS signaling under physiologically fluctuating conditions, we examined for putative changes in nNOS phosphorylation during the estrous cycle by using a phospho-specific antibody directed against Ser1412-phosphorylated nNOS (P-nNOS). Immunoblot analysis revealed that P-nNOS expression was easily detected in protein extracts from the rat preoptic region where the P-nNOS antiserum recognized a single band of 165 kDa (Figure 1A). Importantly, no reaction was present when the antibody was preabsorbed with the blocking peptide (Figure 1A). Figures 1B and 1C show that the estrous cycle significantly impacts nNOS Ser1412 phosphorylation, while (in agreement with previous studies (58)) nNOS protein levels remain steady (nNOS/actin signal ratio, n = 4 rats per estrous cycle stage, one-way ANOVA, p > 0.05). Quantification clearly showed that P-nNOS levels were maximal on the afternoon of proestrus (Figure 1C; n = 4 per estrous cycle stage, one-way ANOVA, p < 0.03 proestrus vs. all other groups). We next performed immunohistofluorescent studies on freefloating coronal sections of the preoptic region from rats in diestrus and proestrus using the same P-nNOS antiserum. Fluorescent microscopic analysis showed that P-nNOSimmunoreactivity was exclusively visualized in nNOS neurons, even though high power images revealed that the nNOS and P-nNOS immunoreactivities were sometimes distributed in different subcellular compartments (Figure 2). The intensity of the immunoreactive signal for P-nNOS showed strong variations between diestrus and proestrus. As shown in Figure 3 and quantified in Table 1, the number of nNOS neurons that exhibited an immunoreactive signal for P-NOS was significantly higher at the onset of the preovulatory surge (Pro 16h) than on the day of diestrus. These changes occurred in all three forebrain region that were analyzed, i.e. the nucleus of the diagonal band (NDB, Figure 3A), the median preoptic nucleus (MEPO) at the vascular organ of the lamina terminalis (Figure 3B), and in the anteroventral preoptic nucleus (AVP; Figure 3C). A large number of NOergic neurons were visualized with nNOS staining in the forebrain, and this number did not vary during the estrous cycle as previously described by us (58) and others (18). Altogether, these Western blot and immunofluorescent data clearly show that nNOS phosphorylation at Ser1412 fluctuates during the estrous cycle and is maximal at the onset of the preovulatory surge on the day of proestrus. To determine whether these changes in nNOS phosphorylation impact the activity of the enzyme, we measured NOS catalytic activity in protein extracts from the preoptic region, i.e., independently of any functional protein-protein interactions and/or cellcell signaling. The results showed that NOS intrinsic activity was significantly higher at the onset of the preovulatory surge at proestrus than in basal-stage diestrous rats (Figure 4A; n = 4 rats per stage, t-test, p < 0.01).
Figure 1.
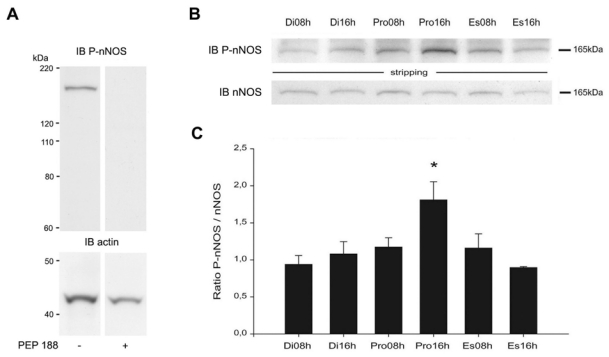
Estrous-cycle effects on nNOS Ser1412 phosphorylation. A. Immunoblotting of hypothalamic preoptic region extracts revealed high levels of nNOS phosphorylation at the Ser1412 site. All reaction disappeared in extracts when the antibody was preabsorbed with blocking peptide (PEP 188). B. Western blot analyses of the Ser1412-phosphorylated nNOS isoform (P-nNOS; upper panel) and the non-phosphorylated isoform (nNOS; lower panel) at six different stages of the estrous cycle. A total of 25 μg of protein per lane was electrophoresed and immunoblotted with an antibody specific to P-nNOS. The membranes were then stripped and incubated with an antibody against nNOS. A representative blot from four independent experiments is shown. C. The protein levels are expressed in arbitrary densitometric units as the ratio between the P-nNOS signal and the signal obtained with nNOS in each sample. Error bars indicate SEM. * p < 0.03 vs. all other groups, n = 4 per stage of the estrous cycle.
Figure 2.
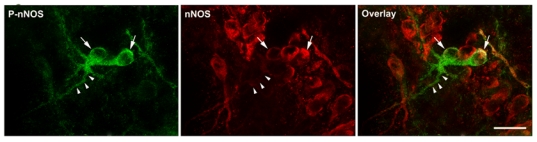
Localization of P-nNOS immunoreactivity in nNOS expressing neurons by fluorescent microscopy in the preoptic region. Note the presence of P-nNOS immunoreactive signal (green) in nNOS-immunonegative dendrites (arrowheads) of nNOS-expressing neurons (red, arrows). Scale bars: 20 μm.
Figure 3.
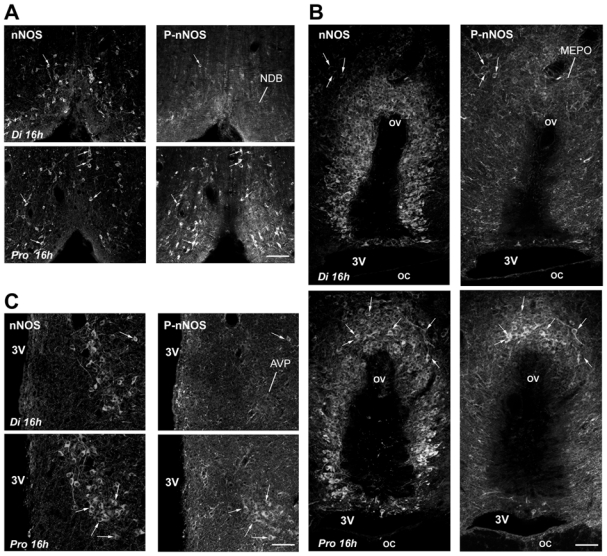
Microphotographs showing nNOS and P-nNOS immunoreactivities in coronal sections of the forebrain. Neuronal NOS and Ser1412 phosphorylated-nNOS positive neurons were detected by fluorescent immunocytochemistry on the same coronal brain sections passing through the nucleus of the diagonal band (NDB, A), the medial preoptic nucleus (MEPO) at the vascular organ of the lamina terminalis (ov) (B) and the anteroventral preoptic nucleus (AVP, C) from cycling female rats in diestrus (Di 16h) and proestrus (Pro 16h). Arrows show double-labeled cells. 3V, third ventricle; oc, optic chiasm. Scale bars: 120 μm in A; 60 μm in B and C.
Table 1. Percent number of nNOS neurons immunoreactive for P-nNOS in diestrus (Di) and proestrus (Pro) rats.
| NDB | MEPO/ov | AVP | |
|---|---|---|---|
| Di 16h | 24.1 ± 5.2 | 25.3 ± 3.7 | 38.4 ± 9.9 |
| Pro 16h | 58.4 ± 8.6** | 46.1 ± 3.2** | 77.7 ± 12.1* |
Four animals were used for counting in both stages of the estrous cycle; per animal, an average of 54 ± 6 nNOS-immunoreactive neurons were considered in the nucleus of the diagonal band (NDB), 88 ± 8 in the organ of the median preoptic nucleus (MEPO) at the vascular organ of the lamina terminalis (ov), 30 ± 3 in the anteroventral preoptic nucleus (AVP).
p < 0.01,
p < 0.05, t-test, Pro 16h vs. Di 16h.
Figure 4.
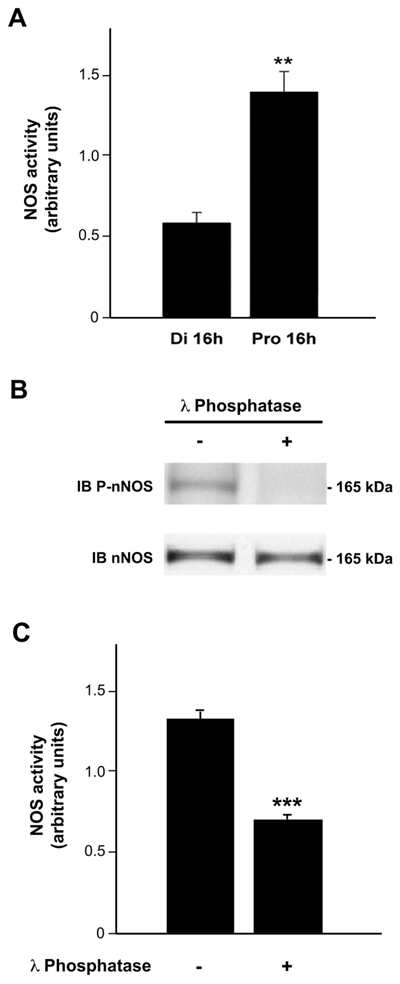
λ-phosphatase-mediated dephosphorylation of nNOS at Ser1412 impairs NOS activity in protein extracts from the preoptic region of the hypothalamus. A. Representative bargraph of NOS activity in homogenized preoptic region fragments from diestrus (n= 4) or proestrus (n= 5) female rats. NOS activity was determined by measuring the formation of nitrite produced in samples. Nitrite assays indicated that preoptic explants had a significantly increased NOS activity on proestrus compared to diestrus. B. Proteins extracts of the preoptic region from female rats in proestrus were first dephosphorylated or not (+ or -) by using λ-phosphatase. Samples were then submitted to electrophoresis and Western blotting using three different antibodies (anti-P-nNOS, anti-nNOS and anti-PSD95). Dephosphorylation of proteins with λ-phosphatase resulted in a complete abolishment of PnNOS signal, whereas no effect on nNOS expression was observed. C. The same experiments were repeated to determine whether dephosphorylation of nNOS affects NOS activity (n = 4 per group). The graph illustrates the significant decrease in NOS activity after dephosphorylation. Error bars indicate SEM. ** p < 0.01; *** p < 0.001.
λ phosphatase-mediated nNOS dephosphorylation dramatically impairs NOS activity
Having shown that phosphorylation of the Ser1412 residue is paralleled by an increase of the activity of NOS in the preoptic region during the estrous cycle, we next investigated the effect of presence or absence of λ-phosphatase treatment on both nNOS phosphorylation and NO production. Interestingly, the results showed that the incubation of protein extracts from the preoptic region with λ-phosphatase resulted in a complete abolishment of the P-nNOS labeling intensity (Figure 4B), indicating that Ser1412 was dephosphorylated after phosphatase treatment. Immunoblotting with nNOS antibody displayed the same signal intensity with and without any phosphatase treatment, indicating that the dephosphorylation treatment did not affect the protein itself (Figure 4B). We then assayed NOS activity in preoptic region protein extracts treated or not treated with phosphatase. Figure 4C illustrates that NOS dephosphorylation resulted in a major decrease in NO production (n = 4 independent experiments, t-test, p < 0.001). These data clearly indicate that nNOS catalytic activity is related to its phosphorylation at Ser1412. Thus, NO secretory regulation may be attributable, at least in part, to changes in nNOS phosphorylation during the estrous cycle.
Serine 1412-phosphorylated nNOS has a high degree of physical association with the NMDA receptor NR2B subunit and PSD-95 at the plasma membrane on the day of proestrus
Because it was shown in ectopic expression systems that nNOS Ser1412 phosphorylation requires physical association with the plasma membrane (65) in a manner analogous to that observed with eNOS (65), and because we have recently shown that the estrous cycle modulates coupling of nNOS to NMDA receptors via the scaffolding protein PSD-95 (58), we investigated whether the increase in nNOS anchoring to the NR2B/PSD-95 complex reflected its phosphorylation state. Coimmunoprecipitation assays performed on protein extracts from the preoptic region demonstrated that immunoprecipitation of p-nNOS resulted in increased coprecipitation of the NMDA receptor NR2B subunit on the afternoon of proestrus compared to diestrus (Figure 5A). Similar assays to determine whether PSD-95 was in the complex with phosphorylated nNOS showed higher levels of coprecipitation on the afternoon of proestrus (Figure 5B). These results show that, within preoptic neurons, the physical association of P-nNOS with NR2B/PSD95 varies during the estrous cycle and is highest at proestrus. To test whether these changes in protein-protein interaction actually occur at the plasma membrane, we studied nNOS and its phosphorylation state in membrane protein extracts. In line with our previous results (58), the physical association of nNOS with the plasma membrane increased significantly in proestrus when compared to diestrus (Figure 5C,E; n = 4 animals per stage, t-test, p < 0.001), and this increase was noticeably associated with an augmented P-nNOS immunoreactivity (Figure 5C,D; n = 4 animals per stage, t-test, p < 0.001). Altogether, our results suggest that on the afternoon of proestrus, most NO-producing enzyme molecules are directly coupled to NMDA receptors at the plasma membrane, which constitutes the primary stimulatory calcium influx pathway for this enzyme (29, 30), and may correspond to increasing amounts of phosphorylationactivated nNOS.
Figure 5.
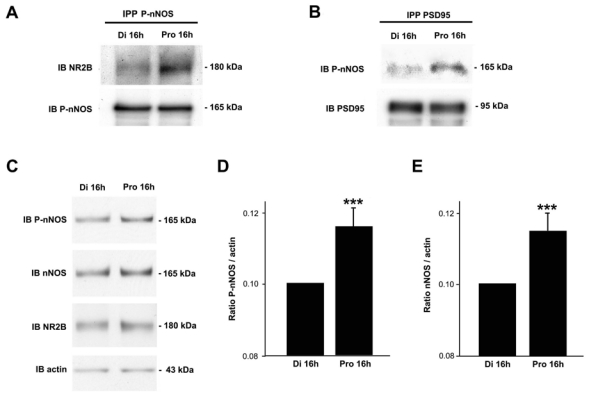
Estrous-cycle effects on phosphorylation-activated nNOS anchoring to the PSD95/NR2B complex in the adult preoptic region. A. Immunoprecipitation of proteins collected from the preoptic region with antibodies against P-nNOS results in the coprecipitation of NR2B. B. Immunoprecipitation of tissue extract with antibodies against PSD-95 results in the coprecipitation of P-nNOS. A–B. Each observation derives from preoptic region tissue pooled from four rats. A representative blot from two independent experiments is shown. C. Membrane extracts from adult rat preoptic region in diestrus (Di 16h) and proestrus (Pro 16h) were subjected to western blot analyses using P-nNOS, nNOS and NR2B antibodies. D. and E. Bar graph showing the quantitative analysis of the differential p-nNOS or nNOS association with the plasma membrane among stages of the estrous cycle, respectively. The protein levels are expressed in arbitrary densitometric units as the ratio between the P-nNOS (D) or nNOS (E) signal and the signal obtained with actin in each sample (n = 4 independent experiments, *** p < 0.001 Pro 16h vs. Di 16h). Error bars indicate SEM.
Estradiol promotes both nNOS phosphorylation and physical association with the plasma membrane in ovariectomized rats
To explore the possible role of estrogens in triggering nNOS phosphorylation and its physical linkage with the plasma membrane in preoptic neurons, we next performed experiments in OVX rats. Mimicking the preovulatory increase in plasma estrogen that occurs at proestrus (66) in OVX rats via subcutaneous injection of 17β-estradiol-3 benzoate-containing sesame oil (EB) (67, 68), resulted in a significant increase of nNOS Ser1412 phosphorylation two days later (Figure 6A,B n = 4 animals per condition, t-test, p < 0.05). Moreover, there was an increase in nNOS/P-nNOS physical association with the plasma membrane (Figure 6C-E; n = 4 animals per condition, t-test, p < 0.001) where the NMDA receptor NR2B subunit is located, thus reproducing the increase in nNOS phosphorylation and its physical association with the plasma membrane during a normal reproductive cycle on the afternoon of proestrus (Figure 5). Consistent with these data, measurements of NOS activity in plasma membrane protein extracts showed that NO production was greater in OVX+EB animals than in OVX animals (Figure 6F).
Figure 6.
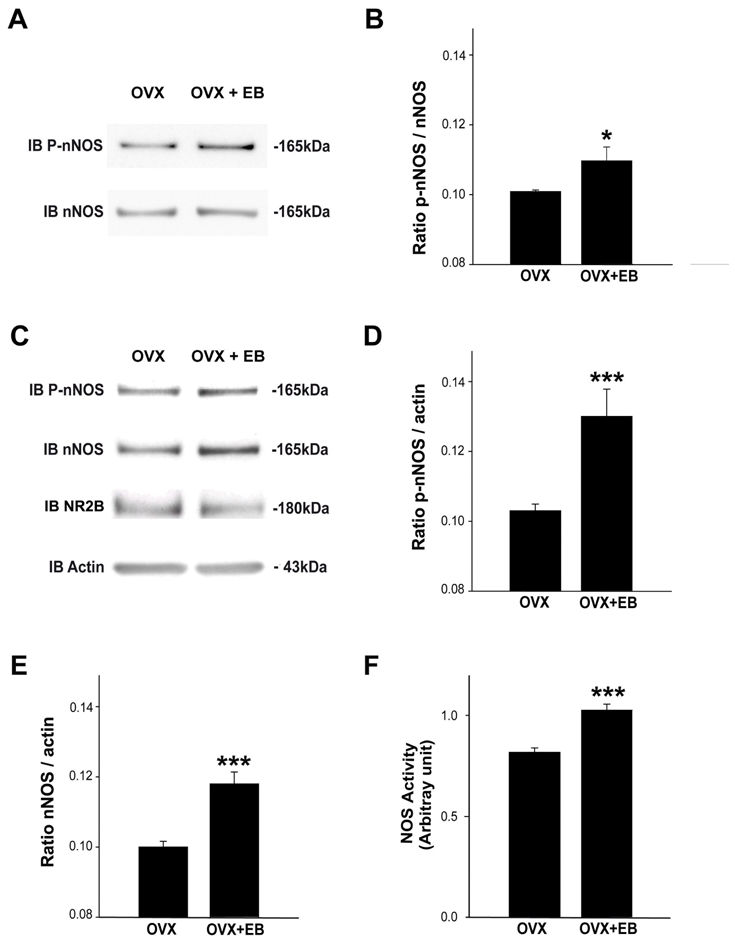
Effects of estradiol on nNOS phosphorylation and physical approximation to the plasma membrane in ovariectomized female rats. A. Western blot analyses of the Ser 1412- phosphorylated nNOS isoform (P-nNOS; upper panel) and the non-phosphorylated isoform (nNOS; lower panel) in preoptic region protein extracts from ovariectomized rats treated (OVX+E2) or not (OVX) with estradiol. A representative blot from three independent experiments is shown. B. The protein levels are expressed in arbitrary densitometric units as the ratio between the P-nNOS signal and the signal obtained with nNOS in each sample (* p < 0.05, n = 4 independent experiments per group). C. Preoptic region membrane extracts from OVX and OVX+E2 rats were subjected to electrophoresis and Western-blot. The antibodies used are anti-P-nNOS, anti-nNOS, anti-NR2B and anti-actin. Signal intensities of both P-nNOS (D) and nNOS (E) were quantified and reported in bar graphs (n = 4 independent experiments, ***, p < 0.001). F. Representative bargraph of NOS activity in membrane extracts from preoptic region fragments from OVX (n= 4) or OVX+E2 (n= 4) female rats (***, p < 0.001).
Discussion
We show that natural fluctuations of estrogens across the ovarian cycle in adult female rats regulate the state of activation of nNOS through changes in nNOS stimulatory phosphorylation levels in the preoptic region. Our results also demonstrate that association of this phosphorylation-activated nNOS isoform with NMDA receptor/PSD-95 complexes at the plasma membrane varies with cyclic changes in estrogen levels. At proestrus, when circulating estrogen levels are at their highest, increased nNOS phosphorylation is associated with parallel increases in NOS catalytic activity. Dephosphorylation experiments suggested that these two events are causally linked. Concomitantly, physical approximation of phosphorylation-activated nNOS with NMDA receptor/PSD-95 complexes at the plasma membrane is maximal. Previous studies have demonstrated that nNOS/PSD-95/NMDA receptor ternary complex formation in the hypothalamic preoptic region is required for NO production in vivo, it parallels cyclic changes in NO release during the ovarian cycle in situ (58) and that estrogen receptors can mediate these effects (62). Our results show that most NO-producing enzyme molecules are directly coupled to Ca++ stimulatory influxes via NMDA receptor channels (29, 30) on the afternoon of proestrus, and this corresponds to increasing amounts of phosphorylation-activated nNOS. These data suggest that, at the onset of the preovulatory surge, estrogens provide optimal conditions to favor maximal production of NO within the hypothalamus (Figure 7). This pathway may be used to alternate coupling and uncoupling of glutamatergic fluxes for NO production during the ovarian cycle, thereby regulating NOergic neurotransmission and the properties of synaptic transmission (69, 70). Because NO is a short-acting, rapidly diffusible mediator of volume transmission (71), estrogen-promoted glutamate-dependent NO release (62) could coordinate neuronal activity in functional microdomains of the hypothalamus (25, 72–74).
Figure 7.
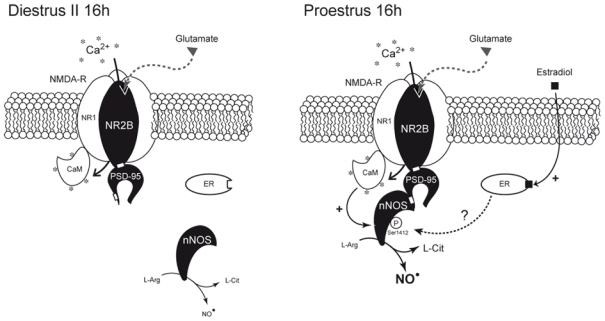
Schematic representation of the possible estradiol-mediated changes in proteinprotein interactions involved in the control of nNOS activity in the preoptic region of the hypothalamus during the ovarian cycle. Neuronal NOS activity is primarily regulated by increases in the local intracellular [Ca2+] (*), which activates nNOS through calmodulin (CaM) binding (29). The physical interaction of nNOS with NMDA receptors involves the postsynaptic density-95 (PSD-95) scaffolding protein and the assembly of a ternary complex (32). Only Ca2+ influx through the NMDA receptor promotes an efficient NO production (29, 30). In parallel, nNOS is also subjected to posttranscriptional modifications (such as phoshorylation) that modulates its catalytic activity (36–39). Natural fluctuations of estrogen levels across the ovarian cycle, i.e., low in diestrus (Di 16h) and high in proestrus (Pro 16h), regulate the activation state of nNOS by modulating its coupling with NR2B-containing NMDA receptors by the PSD-95 scaffolding protein. In turn, this regulation, that could be mediated by estrogen-dependent estrogen receptor (ER) activation (62), results in the phosphorylation of nNOS, an effect known to increase nNOS enzymatic activity (37, 65).
Hormonal signals (such as circulating estrogens) may influence glutamatergic neurotransmission in the brain not only by regulating gene transcription (46, 75, 76) and signaling pathway activation (42, 46), but also by regulating protein complex assembly and the organization of the postsynaptic density (77), resulting in the elaboration of synaptic glutamate responses with different properties (78). In the preoptic region, most NMDA receptor-expressing neurons also contain estrogen receptor α (79), which is localized to cell nuclei, perykarial cytoplasm and dendrites (80, 81). Interestingly, virtually all preoptic nNOS neurons, which are also known to express estrogen receptor α (82, 83), were recently shown to express NMDA receptors (58).
While some previous studies have suggested that estrogens could modulate hypothalamic nNOS gene expression (59, 84–87), our results show that nNOS protein expression levels do not vary during the estrous cycle in the preoptic region. This is consistent with previous data from our own (58) and other laboratories (18). In addition, and in accordance with previous studies (58, 59, 88), our present results clearly show that NOS activity significantly varies between diestrus (when estrogen levels are low) and proestrus (when estrogen levels are highest). Thus, NO secretory regulation during the estrous cycle may not be directly linked to changes in nNOS protein synthesis but rather involve posttranslational modifications and/or a differential association of the enzyme with stimulatory or inhibitory proteins.
Posttranslational modifications known to modulate nNOS catalytic activity comprise both stimulatory and repressive sets of serine phosphorylations. The Ser1412 site is analogous to the established Akt site found in endothelial NOS (65, 89). While this site causes nNOS activation (37, 65), phosphorylation at the Ser847 site by calcium-calmodulin-dependent kinase II (CAMKII) results in nNOS autoinhibition (37, 90, 91). Our in vivo studies convincingly show that estrogens promote nNOS Ser1412 phosphorylation during the ovarian cycle. These results are consistent with previous in vitro data obtained in primary cultures of hypothalamic neurons showing that estradiol induces phosphorylation of nNOS at Ser1412 (92). In this model system, estradiol (10 nM) has acute effects that vanish as soon as 15 min after initiation of the treatment. Unfortunately, in the present study, we were unable to address the question as to whether nNOS is differentially phosphorylated at its Ser847 site during the ovarian cycle as, in our hands, commercially available anti-Ser847-phosphorylated nNOS failed to detect any signal in protein extracts from the preoptic region, the hippocampus or the cortex (data not shown). Notwithstanding the putative involvement of Ser847 phosphorylation in the regulation of nNOS activity across the estrous cycle, our results demonstrating that λ-phosphatase-mediated dephosphorylation of nNOS dramatically impairs NOS activity in protein extracts from the preoptic region suggest that phosphorylation-mediated posttranslational modifications of nNOS in the preoptic region are primarily stimulatory. However, because hypothalamic endothelial cells are able to synthesize NO (93) and express endothelial NOS (93, 94), which is also known to be subjected to regulatory phosphorylation (65, 89), the possible participation of nonneuronal sources of NOS activity in our protein extracts cannot be excluded.
In addition to linking nNOS activity to phosphorylation of Ser1412 in preoptic neurons during the estrous cycle, our results show that in proestrus, when estrogen levels are highest, the activated Ser 1412-phosphorylated nNOS is physically associated with the NMDA receptor/PSD-95 signaling system in preoptic neurons. It remains unclear the mechanism by which estrogen receptors promote nNOS anchoring to the PSD-95-NMDA receptor complex at the cell membrane (62). Perhaps the nNOS association with PSD-95 is favored at synaptic sites in response to dynamic events at the post-synaptic density. One potential mechanism for this could be that, upon activation by sex steroids, the estrogen receptor mediates the coalescence of cytoskeleton-tethered nNOS (95) to PSD-95 through spine formation. This would require remodeling of the actin cytoskeleton (96), a phenomenon known to occur in neurons of the preoptic region (97). Interestingly, estrogen-dependent regulation of dendritic spine growth in the preoptic region has also been functionally associated with increased PI3 kinase-promoted phosphorylation of Akt (56), which is one of the serine/threonine protein kinases shown to phosphorylate nNOS at Ser1412 when the enzyme interacts with plasma membranes (37, 65). We may therefore speculate that circulating levels of estrogen regulate the activation state of nNOS by modulating its coupling with NMDA receptors. In turn, this regulation results in the serine phosphorylation of nNOS, an effect known to increase nNOS enzymatic activity (37, 65). These changes, among others, would be well suited to account for variations in the level of activation of NO production in hypothalamic neurons, which are largely dependent on fluctuations in circulating estrogen levels.
Growing evidence indicates that hypothalamic production of NO is required for the onset of the preovulatory GnRH/LH surge (19, 27, 58, 60, 93, 98, 99) that depends on the coordinated and timely activation of GnRH neurons, which represent the final common pathway for the neural control of ovulation (40, 41). The increase of GnRH release, signaling the onset of the preovulatory GnRH surge, requires changes in transsynaptic communication within the neuronal network associated with GnRH neurons in the preoptic region (100–102). These changes involve, at least in part, interaction between estrogens, NMDA receptordependent glutamatergic transmission (103, 104) and NO signaling (21, 58). We have recently shown that NO directly modulates GnRH neuronal activity (25) and that estrogens promote NMDA receptor-nNOS complex assembly in hypothalamic neurons (58, 62), thus potentiating NO secretion by coupling nNOS to its main calcium influx pathway (29, 30). These findings, in addition to our present data demonstrating that estrogens further activate NMDA receptor-associated nNOS catalytic activity via phosphorylation, strengthen the hypothesis that nNOS neurons may be key targets for gonadal steroids during their positive feedback on the reproductive brain.
Together, our results showing that association of the phosphorylation-activated nNOS isoform with NMDA receptor/PSD-95 complexes at the plasma membrane varies with cyclic changes in levels of estrogens levels provide novel insights into the steroid-mediated molecular mechanism that enables the adult hypothalamus to control the activity of a major brain neuronal signaling pathway in an ever-changing physiological context. Because NO cannot be stored in synaptic vesicles, as with other neurotransmitters, unraveling mechanisms that regulate its synthesis with respect to time and space is crucial, as precise regulation of nNOS activity during fluctuating conditions is key to major hypothalamic functions (7–11) and in particular, to the central control of reproduction (15, 16, 27, 58).
Acknowledgments
This research was supported by the Institut National de la Santé et de la Recherche Médicale (Inserm, France) grant U837, the Fondation pour le Recherche Médicale (Equipe FRM), l’Agence Nationale de la Recherche (ANR), the Indo-French Centre for the Promotion of Advanced Research (IFCPAR), the Université Lille 2 and the imaging Core of IFR114. JP was a postdoctoral fellow supported by IFCPAR. XdAdT and CC were students supported by a fellowship from the Inserm and the Région Nord Pas de Calais. We thank Dr. PC Emson (Medical Research Council, Laboratory for Molecular Research, Cambridge, UK) for his generous supply of antibodies against nNOS.
Footnotes
Disclosure statement: Authors have nothing to disclose
References
- 1.Boehning D, Snyder SH. Novel neural modulators. AnnuRevNeurosci. 2003;26:105–131. doi: 10.1146/annurev.neuro.26.041002.131047. [DOI] [PubMed] [Google Scholar]
- 2.Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci. 2008;27:2783–2802. doi: 10.1111/j.1460-9568.2008.06285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCann SM, Haens G, Mastronardi C, Walczewska A, Karanth S, Rettori V, Yu WH. The role of nitric oxide (NO) in control of LHRH release that mediates gonadotropin release and sexual behavior. Curr Pharm Des. 2003;9:381–390. doi: 10.2174/1381612033391766. [DOI] [PubMed] [Google Scholar]
- 4.Prast H, Philippu A. Nitric oxide as modulator of neuronal function. ProgNeurobiol. 2001;64:51–68. doi: 10.1016/s0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- 5.Prevot V, Dehouck B, Poulain P, Beauvillain JC, Buee-Scherrer V, Bouret S. Neuronal-glial-endothelial interactions and cell plasticity in the postnatal hypothalamus: implications for the neuroendocrine control of reproduction. Psychoneuroendocrinology. 2007;32:S46–S51. doi: 10.1016/j.psyneuen.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Edelmann M, Wolfe C, Scordalakes EM, Rissman EF, Tobet S. Neuronal nitric oxide synthase and calbindin delineate sex differences in the developing hypothalamus and preoptic area. Dev Neurobiol. 2007;67:1371–1381. doi: 10.1002/dneu.20507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding JM, Chen D, Weber ET, Faiman LE, Rea MA, Gillette MU. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science. 1994;266:1713–1717. doi: 10.1126/science.7527589. [DOI] [PubMed] [Google Scholar]
- 8.Calapai G, Squadrito F, Altavilla D, Zingarelli B, Campo GM, Cilia M, Caputi AP. Evidence that nitric oxide modulates drinking behaviour. Neuropharmacology. 1992;31:761–764. doi: 10.1016/0028-3908(92)90038-q. [DOI] [PubMed] [Google Scholar]
- 9.Morley JE, Flood JF. Evidence that nitric oxide modulates food intake in mice. Life Sci. 1991;49:707–711. doi: 10.1016/0024-3205(91)90102-h. [DOI] [PubMed] [Google Scholar]
- 10.Canabal DD, Potian JG, Duran RG, McArdle JJ, Routh VH. Hyperglycemia impairs glucose and insulin regulation of nitric oxide production in glucose-inhibited neurons in the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2007;293:R592–600. doi: 10.1152/ajpregu.00207.2007. [DOI] [PubMed] [Google Scholar]
- 11.Murphy BA, Fakira KA, Song Z, Beuve A, Routh VH. AMP-activated Protein Kinase (AMPK) and Nitric Oxide (NO) regulate the glucose sensitivity of ventromedial hypothalamic (VMH) glucose-inhibited (GI) neurons. Am J Physiol Cell Physiol. 2009 doi: 10.1152/ajpcell.00127.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di S, Maxson MM, Franco A, Tasker JG. Glucocorticoids regulate glutamate and GABA synapse-specific retrograde transmission via divergent nongenomic signaling pathways. J Neurosci. 2009;29:393–401. doi: 10.1523/JNEUROSCI.4546-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hull EM, Dominguez JM. Getting his act together: roles of glutamate, nitric oxide, and dopamine in the medial preoptic area. Brain Res. 2006;1126:66–75. doi: 10.1016/j.brainres.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 14.Panzica GC, Viglietti-Panzica C, Sica M, Gotti S, Martini M, Pinos H, Carrillo B, Collado P. Effects of gonadal hormones on central nitric oxide producing systems. Neuroscience. 2006;138:987–995. doi: 10.1016/j.neuroscience.2005.07.052. [DOI] [PubMed] [Google Scholar]
- 15.Srisawat R, Ludwig M, Bull PM, Douglas AJ, Russell JA, Leng G. Nitric oxide and the oxytocin system in pregnancy. J Neurosci. 2000;20:6721–6727. doi: 10.1523/JNEUROSCI.20-17-06721.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mani SK, Allen JM, Rettori V, McCann SM, O’Malley BW, Clark JH. Nitric oxide mediates sexual behavior in female rats. Proc Natl Acad Sci U S A. 1994;91:6468–6472. doi: 10.1073/pnas.91.14.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhat GK, Mahesh VB, Lamar CA, Ping L, Aguan K, Brann DW. Histochemical localization of nitric oxide neurons in the hypothalamus: association with gonadotropin-releasing hormone neurons and co-localization with N-methyl-Daspartate receptors. Neuroendocrinology. 1995;62:187–197. doi: 10.1159/000127004. [DOI] [PubMed] [Google Scholar]
- 18.Herbison AE, Simonian SX, Norris PJ, Emson PC. Relationship of neuronal nitric oxide synthase immunoreactivity to GnRH neurons in the ovariectomized and intact female rat. J Neuroendocrinol. 1996;8:73–82. doi: 10.1111/j.1365-2826.1996.tb00688.x. [DOI] [PubMed] [Google Scholar]
- 19.Rettori V, Belova N, Dees WL, Nyberg CL, Gimeno M, McCann SM. Role of nitric oxide in the control of luteinizing hormone-releasing hormone release in vivo and in vitro. Proc Natl Acad Sci USA. 1993;90:10130–10134. doi: 10.1073/pnas.90.21.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rettori V, Gimeno M, Lyson K, McCann SM. Nitric oxide mediates norepinephrine-induced prostaglandin E2 release from the hypothalamus. Proc Natl Acad Sci USA. 1992;89:11543–11546. doi: 10.1073/pnas.89.23.11543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonavera JJ, Sahu A, Kalra PS, Kalra SP. Evidence that nitric oxide may mediate the ovarian steroid-induced luteinizing hormone surge: involvement of excitatory amino acids. Endocrinology. 1993;133:2481–2487. doi: 10.1210/endo.133.6.8243268. [DOI] [PubMed] [Google Scholar]
- 22.Prevot V, Croix D, Rialas CM, Poulain P, Fricchione GL, Stefano GB, Beauvillain JC. Estradiol coupling to endothelial nitric oxide stimulates gonadotropin-releasing hormone release from rat median eminence via a membrane receptor. Endocrinology. 1999;140:652–659. doi: 10.1210/endo.140.2.6484. [DOI] [PubMed] [Google Scholar]
- 23.Bhat GK, Mahesh VB, Ping L, Chorich L, Wiedmeier VT, Brann DW. Opioid-glutamate-nitric oxide connection in the regulation of luteinizing hormone secretion in the rat. Endocrinology. 1998;139:955–960. doi: 10.1210/endo.139.3.5844. [DOI] [PubMed] [Google Scholar]
- 24.Mahachoklertwattana P, Black SM, Kaplan SL, Bristow JD, Grumbach MM. Nitric oxide synthesized by gonadotropin-releasing hormone neurons is a mediator of N-methyl-D-aspartate (NMDA)-induced GnRH secretion. Endocrinology. 1994;135:1709–1712. doi: 10.1210/endo.135.4.7523101. [DOI] [PubMed] [Google Scholar]
- 25.Clasadonte J, Poulain P, Beauvillain JC, Prevot V. Activation of neuronal nitric oxide release inhibits spontaneous firing in adult gonadotropin-releasing hormone neurons: a possible local synchronizing signal. Endocrinology. 2008;149:587–596. doi: 10.1210/en.2007-1260. [DOI] [PubMed] [Google Scholar]
- 26.Lopez FJ, Moretto M, Merchenthaler I, Negro-Vilar A. Nitric oxide is involved in the genesis of pulsatile LHRH secretion from immortalized LHRH neurons. J Neuroendocrinol. 1997;9:647–654. doi: 10.1046/j.1365-2826.1997.t01-1-00618.x. [DOI] [PubMed] [Google Scholar]
- 27.Gyurko R, Leupen S, Huang PL. Deletion of exon 6 of the neuronal nitric oxide synthase gene in mice results in hypogonadism and infertility. Endocrinology. 2002;143:2767–2774. doi: 10.1210/endo.143.7.8921. [DOI] [PubMed] [Google Scholar]
- 28.Abu-Soud HM, Yoho LL, Stuehr DJ. Calmodulin controls neuronal nitricoxide synthase by a dual mechanism. Activation of intra- and interdomain electron transfer. J Biol Chem. 1994;269:32047–32050. [PubMed] [Google Scholar]
- 29.Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulinrequiring enzyme. Proc Natl Acad Sci USA. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garthwaite J, Charles SL, Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988;336:385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- 31.Hisatsune C, Umemori H, Inoue T, Michikawa T, Kohda K, Mikoshiba K, Yamamoto T. Phosphorylation-dependent regulation of N-methyl-D-aspartate receptors by calmodulin. J Biol Chem. 1997;272:20805–20810. doi: 10.1074/jbc.272.33.20805. [DOI] [PubMed] [Google Scholar]
- 32.Christopherson KS, Hillier BJ, Lim WA, Bredt DS. PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem. 1999;274:27467–27473. doi: 10.1074/jbc.274.39.27467. [DOI] [PubMed] [Google Scholar]
- 33.Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science. 2002;298:846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- 34.Ishii H, Shibuya K, Ohta Y, Mukai H, Uchino S, Takata N, Rose JA, Kawato S. Enhancement of nitric oxide production by association of nitric oxide synthase with N-methyl-D-aspartate receptors via postsynaptic density 95 in genetically engineered Chinese hamster ovary cells: real-time fluorescence imaging using nitric oxide sensitive dye. J Neurochem. 2006;96:1531–1539. doi: 10.1111/j.1471-4159.2006.03656.x. [DOI] [PubMed] [Google Scholar]
- 35.Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD- 95 protein. Science. 1999;284:1845–1848. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- 36.Komeima K, Hayashi Y, Naito Y, Watanabe Y. Inhibition of neuronal nitricoxide synthase by calcium/calmodulin-dependent protein kinase IIalpha through Ser847 phosphorylation in NG108-15 neuronal cells. J Biol Chem. 2000;275:28139–28143. doi: 10.1074/jbc.M003198200. [DOI] [PubMed] [Google Scholar]
- 37.Rameau GA, Tukey DS, Garcin-Hosfield ED, Titcombe RF, Misra C, Khatri L, Getzoff ED, Ziff EB. Biphasic coupling of neuronal nitric oxide synthase phosphorylation to the NMDA receptor regulates AMPA receptor trafficking and neuronal cell death. J Neurosci. 2007;27:3445–3455. doi: 10.1523/JNEUROSCI.4799-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song T, Hatano N, Horii M, Tokumitsu H, Yamaguchi F, Tokuda M, Watanabe Y. Calcium/calmodulin-dependent protein kinase I inhibits neuronal nitric-oxide synthase activity through serine 741 phosphorylation. FEBS Lett. 2004;570:133–137. doi: 10.1016/j.febslet.2004.05.083. [DOI] [PubMed] [Google Scholar]
- 39.Adak S, Santolini J, Tikunova S, Wang Q, Johnson JD, Stuehr DJ. Neuronal nitric-oxide synthase mutant (Ser-1412 --> Asp) demonstrates surprising connections between heme reduction, NO complex formation, and catalysis. J Biol Chem. 2001;276:1244–1252. doi: 10.1074/jbc.M006857200. [DOI] [PubMed] [Google Scholar]
- 40.Herbison A. Physiology of the gonadotropin-releasing hormone neuronal network. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. 3. Elsevier; 2006. pp. 1415–1482. [Google Scholar]
- 41.Ojeda SR, Terasawa E, Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R. Neuroendocrine regulation of puberty. New York: Elsevier; 2002. pp. 589–659. [Google Scholar]
- 42.Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- 43.Simerly RB. Wired on hormones: endocrine regulation of hypothalamic development. Curr Opin Neurobiol. 2005;15:81–85. doi: 10.1016/j.conb.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 44.Maggi A, Ciana P, Belcredito S, Vegeto E. Estrogens in the nervous system: mechanisms and nonreproductive functions. Annu Rev Physiol. 2004;66:291–313. doi: 10.1146/annurev.physiol.66.032802.154945. [DOI] [PubMed] [Google Scholar]
- 45.Lightman SL, Windle RJ, Wood SA, Kershaw YM, Shanks N, Ingram CD. Peripartum plasticity within the hypothalamo-pituitary-adrenal axis. Prog Brain Res. 2001;133:111–129. doi: 10.1016/s0079-6123(01)33009-1. [DOI] [PubMed] [Google Scholar]
- 46.Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J Neurosci. 2006;26:10332–10348. doi: 10.1523/JNEUROSCI.3369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bodo C, Rissman EF. New roles for estrogen receptor beta in behavior and neuroendocrinology. Front Neuroendocrinol. 2006;27:217–232. doi: 10.1016/j.yfrne.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 48.Curtis KS. Estrogen and the central control of body fluid balance. Physiol Behav. 2009;97:180–192. doi: 10.1016/j.physbeh.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 49.McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Calizo LH, Flanagan-Cato LM. Estrogen selectively regulates spine density within the dendritic arbor of rat ventromedial hypothalamic neurons. J Neurosci. 2000;20:1589–1596. doi: 10.1523/JNEUROSCI.20-04-01589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao QMG, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CARJ, Gao XB, Mobbs C, Shulman GI, Diano S, Horvath TL. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med. 2007;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- 55.Garcia-Segura LM, Chowen JA, Duenas M, Torres-Aleman I, Naftolin F. Gonadal steroids as promoters of neuro-glial plasticity. Psychoneuroendocrinology. 1994;19:445–453. doi: 10.1016/0306-4530(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 56.Schwarz JM, Liang SL, Thompson SM, McCarthy MM. Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: a mechanism for organizational sex differences. Neuron. 2008;58:584–598. doi: 10.1016/j.neuron.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cherney A, Edgell H, Krukoff TL. NO mediates effects of estrogen on central regulation of blood pressure in restrained, ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R842–849. doi: 10.1152/ajpregu.00035.2003. [DOI] [PubMed] [Google Scholar]
- 58.d’Anglemont de Tassigny X, Campagne C, Dehouck B, Leroy D, Holstein GR, Beauvillain JC, Buee-Scherrer V, Prevot V. Coupling of neuronal nitric oxide synthase to NMDA receptors via postsynaptic density-95 depends on estrogen and contributes to the central control of adult female reproduction. JNeurosci. 2007;27:6103–6114. doi: 10.1523/JNEUROSCI.5595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lamar CA, Bhat GK, Mahesh VB, Brann DW. Evidence that neuronal nitric oxide synthase but not heme oxygenase increases in the hypothalamus on proestrus afternoon. Neuroendocrinology. 1999;70:360–367. doi: 10.1159/000054497. [DOI] [PubMed] [Google Scholar]
- 60.Knauf C, Prevot V, Stefano GB, Mortreux G, Beauvillain JC, Croix D. Evidence for a spontaneous nitric oxide release from the rat median eminence: influence on gonadotropin-releasing hormone release. Endocrinology. 2001;142:2343–2350. doi: 10.1210/endo.142.6.8073. [DOI] [PubMed] [Google Scholar]
- 61.Weiner CP, Lizasoain I, Baylis SA, Knowles RG, Charles IG, Moncada S. Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc Natl Acad Sci USA. 1994;91:5212–5216. doi: 10.1073/pnas.91.11.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.d’Anglemont de Tassigny X, Campagne C, Steculorum S, Prevot V. Estradiol induces physical association of neuronal nitric oxide synthase with NMDA receptor and promotes nitric oxide formation via estrogen receptor activation in primary neuronal cultures. J Neurochem. 2009;109:214–224. doi: 10.1111/j.1471-4159.2009.05949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- 64.Swanson LW. Structure of the rat brain. Amsterdam: Elsevier Science Publishers; 1996. [Google Scholar]
- 65.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- 67.Bouret S, Prevot V, Takumi T, Beauvillain JC, Mitchell V. Regulation by gonadal steroids of the mRNA encoding for a type I receptor for TGF-beta in the female rat hypothalamus. Neuroendocrinology. 2002;76:1–7. doi: 10.1159/000063678. [DOI] [PubMed] [Google Scholar]
- 68.Kalra P, McCann SM. Involvement of catecholamines in feedback mechanisms. Prog Brain Res. 1973;39:185–198. doi: 10.1016/s0079-6123(08)64076-5. [DOI] [PubMed] [Google Scholar]
- 69.Savchenko A, Barnes S, Kramer RH. Cyclic-nucleotide-gated channels mediate synaptic feedback by nitric oxide. Nature. 1997;390:694–698. doi: 10.1038/37803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang HG, Lu FM, Jin I, Udo H, Kandel ER, de Vente J, Walter U, Lohmann SM, Hawkins RD, Antonova I. Presynaptic and postsynaptic roles of NO, cGK, and RhoA in long-lasting potentiation and aggregation of synaptic proteins. Neuron. 2005;45:389–403. doi: 10.1016/j.neuron.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 71.Agnati LF, Zoli M, Stromberg I, Fuxe K. Intercellular communication in the brain: wiring versus volume transmission. Neuroscience. 1995;69:711–726. doi: 10.1016/0306-4522(95)00308-6. [DOI] [PubMed] [Google Scholar]
- 72.Li DP, Chen SR, Pan HL. Nitric oxide inhibits spinally projecting paraventricular neurons through potentiation of presynaptic GABA release. J Neurophysiol. 2002;88:2664–2674. doi: 10.1152/jn.00540.2002. [DOI] [PubMed] [Google Scholar]
- 73.Stern JE, Zhang W. Cellular sources, targets and actions of constitutive nitric oxide in the magnocellular neurosecretory system of the rat. JPhysiol. 2005;562:725–744. doi: 10.1113/jphysiol.2004.077735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang QZ, Hatton GI. Nitric oxide via cGMP-dependent mechanisms increases dye coupling and excitability of rat supraoptic nucleus neurons. JNeurosci. 1999;19:4270–4279. doi: 10.1523/JNEUROSCI.19-11-04270.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gu G, Varoqueaux F, Simerly RB. Hormonal regulation of glutamate receptor gene expression in the anteroventral periventricular nucleus of the hypothalamus. J Neurosci. 1999;19:3213–3222. doi: 10.1523/JNEUROSCI.19-08-03213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morissette M, Le Saux M, D’Astous M, Jourdain S, Al Sweidi S, Morin N, Estrada-Camarena E, Mendez P, Garcia-Segura LM, Di Paolo T. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J Steroid Biochem Mol Biol. 2008;108:327–338. doi: 10.1016/j.jsbmb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 77.Akama KT, McEwen BS. Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. J Neurosci. 2003;23:2333–2339. doi: 10.1523/JNEUROSCI.23-06-02333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McEwen B, Akama K, Alves S, Brake WG, Bulloch K, Lee S, Li C, Yuen G, Milner TA. Tracking the estrogen receptor in neurons: implications for estrogen-induced synapse formation. Proc Natl Acad Sci USA. 2001;98:7093–7100. doi: 10.1073/pnas.121146898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chakraborty TR, Ng L, Gore AC. Colocalization and hormone regulation of estrogen receptor alpha and N-methyl-D-aspartate receptor in the hypothalamus of female rats. Endocrinology. 2003;144:299–305. doi: 10.1210/en.2002-220749. [DOI] [PubMed] [Google Scholar]
- 80.Blaustein JD. Cytoplasmic estrogen receptors in rat brain: immunocytochemical evidence using three antibodies with distinct epitopes. Endocrinology. 1992;131:1336–1342. doi: 10.1210/endo.131.3.1380440. [DOI] [PubMed] [Google Scholar]
- 81.Blaustein JD, Lehman MN, Turcotte JC, Greene G. Estrogen receptors in dendrites and axon terminals in the guinea pig hypothalamus. Endocrinology. 1992;131:281–290. doi: 10.1210/endo.131.1.1612006. [DOI] [PubMed] [Google Scholar]
- 82.Sato S, Braham CS, Putnam SK, Hull EM. Neuronal nitric oxide synthase and gonadal steroid interaction in the MPOA of male rats: co-localization and testosterone-induced restoration of copulation and nNOS-immunoreactivity. Brain Res. 2005;1043:205–213. doi: 10.1016/j.brainres.2005.02.074. [DOI] [PubMed] [Google Scholar]
- 83.Scordalakes EM, Shetty SJ, Rissman EF. Roles of estrogen receptor alpha and androgen receptor in the regulation of neuronal nitric oxide synthase. JComp Neurol. 2002;453:336–344. doi: 10.1002/cne.10413. [DOI] [PubMed] [Google Scholar]
- 84.Okamura H, Yokosuka M, Hayashi S. Estrogenic induction of NADPH-diaphorase activity in the preoptic neurons containing estrogen receptor immunoreactivity in the female rat. J Neuroendocrinol. 1994;6:597–601. doi: 10.1111/j.1365-2826.1994.tb00624.x. [DOI] [PubMed] [Google Scholar]
- 85.Pu S, Kalra PS, Kalra SP. Ovarian steroid-independent diurnal rhythm in cyclic GMP/nitric oxide efflux in the medial preoptic area: possible role in preovulatory and ovarian steroid-induced LH surge. J Neuroendocrinol. 1998;10:617–625. doi: 10.1046/j.1365-2826.1998.00245.x. [DOI] [PubMed] [Google Scholar]
- 86.Rachman IM, Unnerstall JR, Pfaff DW, Cohen RS. Regulation of neuronal nitric oxide synthase mRNA in lordosis-relevant neurons of the ventromedial hypothalamus following short-term estrogen treatment. Brain Res Mol Brain Res. 1998;59:105–108. doi: 10.1016/s0169-328x(98)00131-4. [DOI] [PubMed] [Google Scholar]
- 87.Sica M, Martini M, Viglietti-Panzica C, Panzica G. Estrous cycle influences the expression of neuronal nitric oxide synthase in the hypothalamus and limbic system of female mice. BMC Neurosci. 2009;10:78. doi: 10.1186/1471-2202-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pu S, Xu B, Kalra SP, Kalra PS. Evidence that gonadal steroids modulate nitric oxide efflux in the medial preoptic area: effects of N-methyl-D-aspartate and correlation with luteinizing hormone secretion. Endocrinology. 1996;137:1949–1955. doi: 10.1210/endo.137.5.8612535. [DOI] [PubMed] [Google Scholar]
- 89.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 90.Hayashi Y, Nishio M, Naito Y, Yokokura H, Nimura Y, Hidaka H, Watanabe Y. Regulation of neuronal nitric-oxide synthase by calmodulin kinases. J Biol Chem. 1999;274:20597–20602. doi: 10.1074/jbc.274.29.20597. [DOI] [PubMed] [Google Scholar]
- 91.Rameau GA, Chiu LY, Ziff EB. Bidirectional regulation of neuronal nitricoxide synthase phosphorylation at serine 847 by the N-methyl-D-aspartate receptor. J Biol Chem. 2004;279:14307–14314. doi: 10.1074/jbc.M311103200. [DOI] [PubMed] [Google Scholar]
- 92.Gingerich S, Krukoff TL. Activation of ERbeta increases levels of phosphorylated nNOS and NO production through a Src/PI3K/Akt-dependent pathway in hypothalamic neurons. Neuropharmacology. 2008;55:878–885. doi: 10.1016/j.neuropharm.2008.06.058. [DOI] [PubMed] [Google Scholar]
- 93.De Seranno S, Estrella C, Loyens A, Cornea A, Ojeda SR, Beauvillain JC, Prevot V. Vascular endothelial cells promote acute plasticity in ependymoglial cells of the neuroendocrine brain. J Neurosci. 2004;24:10353–10363. doi: 10.1523/JNEUROSCI.3228-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gingerich S, Krukoff TL. Estrogen modulates endothelial and neuronal nitric oxide synthase expression via an estrogen receptor beta-dependent mechanism in hypothalamic slice cultures. Endocrinology. 2005;146:2933–2941. doi: 10.1210/en.2004-1375. [DOI] [PubMed] [Google Scholar]
- 95.Haraguchi K, Satoh K, Yanai H, Hamada F, Kawabuchi M, Akiyama T. The hDLG-associated protein DAP interacts with dynein light chain and neuronal nitric oxide synthase. Genes Cells. 2000;5:905–911. doi: 10.1046/j.1365-2443.2000.00374.x. [DOI] [PubMed] [Google Scholar]
- 96.Hering H, Sheng M. Dendritic spines: structure, dynamics and regulation. Nat Rev Neurosci. 2001;2:880–888. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- 97.Amateau SK, McCarthy MM. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostaglandin-E2. J Neurosci. 2002;22:8586–8596. doi: 10.1523/JNEUROSCI.22-19-08586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aguan K, Mahesh VB, Ping L, Bhat G, Brann DW. Evidence for a physiological role for nitric oxide in the regulation of the LH surge: effect of central administration of antisense oligonucleotides to nitric oxide synthase. Neuroendocrinology. 1996;64:449–455. doi: 10.1159/000127151. [DOI] [PubMed] [Google Scholar]
- 99.Bonavera JJ, Sahu A, Kalra PS, Kalra SP. Evidence that nitric oxide may mediate the ovarian steroid-induced luteinizing hormone surge: involvement of excitatory amino acids. Endocrinology. 1993;133:2481–2487. doi: 10.1210/endo.133.6.8243268. [DOI] [PubMed] [Google Scholar]
- 100.Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: The case for the rostral periventricular area of the third ventricle (RP3V) Brain ResRev. 2007 doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ojeda SR, Prevot V, Heger S. Regulation of puberty. Curr Opin Endocrinol Diabetes. 2001;8:154–160. [Google Scholar]
- 102.Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- 103.Brann DW, Mahesh VB. Endogenous excitatory amino acid involvement in the preovulatory and steroid-induced surge of gonadotropins in the female rat. Endocrinology. 1991;128:1541–1547. doi: 10.1210/endo-128-3-1541. [DOI] [PubMed] [Google Scholar]
- 104.Urbanski HF, Ojeda SR. A role for N-methyl-D-aspartate (NMDA) receptors in the control of LH secretion and initiation of female puberty. Endocrinology. 1990;126:1774–1776. doi: 10.1210/endo-126-3-1774. [DOI] [PubMed] [Google Scholar]


