Abstract
Fasciola hepatica releases excretory–secretory products (FhESP), and immunomodulatory properties have been described for the carbohydrates present in these parasite products. The interaction of FhESP with the innate immune cells, such as macrophages, is crucial in the early stage of infection. In this work we observed that peritoneal macrophages from naive BALB/c mice stimulated in vitro with FhESP presented: an increased arginase activity as well as Arginase I expression, and high levels of transforming growth factor-β and interleukin-10. A similar macrophage population was also observed in the peritoneum of infected mice. A partial inhibition of the immunomodulatory effects described above was observed when macrophages were pre-incubated with Mannan, anti-mannose receptor, Laminarin or anti-Dectin-1, and then stimulated with FhESP. In addition, we observed a partial inhibition of these effects in macrophages obtained from mice that were intraperitoneally injected with Mannan or Laminarin before being infected. Taken together, these results suggest the participation of at least two C-type lectin receptors, mannose receptor and Dectin-1, in the interaction of FhESP with macrophages, which allows this parasite to induce immunoregulatory effects on these important innate immune cells and may constitute a crucial event for extending its survival in the host.
Keywords: Dectin-1, Fasciola hepatica, macrophages, mannose receptor
Introduction
C-type lectin receptors (CLRs) are a large superfamily of soluble and membrane-bound proteins that share a common domain structure, the carbohydrate recognition domain, which is required for binding to specific carbohydrate structures of endogenous self-molecules as well as specific pathogens and pathogen-derived ligands.1–3 Interestingly, not all interactions between CLRs and pathogenic ligands have beneficial outcomes, with some pathogens appearing to have evolved immunoevasive activities through these receptors.4,5
Among the CLRs, the mannose receptor (MR) is mainly expressed in alveolar and peritoneal macrophages (MΦ), and derived from blood monocytes.6 It is able to bind branched sugars with terminal mannose, fucose or N-acetyl-glucosamine,7 and mediate both endocytosis and phagocytosis, hence facilitating the clearance of both particulate and soluble ligands. In addition, it binds numerous microorganisms and microbial products including Mycobacterium tuberculosis,8Trypanosoma cruzi,9Streptococcus pneumoniae, Klebsiella pneumonia,10 human immunodeficiency virus gp120,11 and helminths such as Trichinella spiralis12 and Trichuris muris.13 However, the importance of MR in pathogen recognition is unclear because mice lacking this receptor show normal immune responses to Candida albicans,14???Pneumocystis carinii15 and Leishmania sp.,16 despite all these pathogens possessing structures that bind to the receptor. Therefore, MR could be considered to be an important molecule of the innate immune system, which may be exploited to influence the activation of the acquired immune system during pathological conditions.
Dectin-1 is another CLR normally expressed on monocytes, MΦ, dendritic cells, neutrophils and a subset of splenic T cells,17 which recognizes particulate and soluble β-1,3-linked and β-1,6-linked glucans from fungi, bacteria and plants, and is the principal receptor for these carbohydrates on leucocytes.18,19 This receptor can also recognize the following ligands: an unidentified endogenous ligand on T cells, which interacts with Dectin-1 through a different binding site to β-glucans,20,21 and an unidentified molecule on mycobacteria, which does not express β-glucans.22–24 Recently it has also been implicated in the recognition and uptake of apoptotic cells and the cross-presentation of cellular antigens.25 Of all the known CLRs, only Dectin-1 has been conclusively demonstrated to function as a signalling pattern recognition receptor (PRR),26 by regulating the expression of innate response genes, including those encoding co-stimulatory molecules, and pro-inflammatory cytokines and chemokines.27–29 Paradoxically, the recognition of fungal β-glucans by Dectin-1 also triggers the production of non-protective cytokines, such as IL-23 and IL-10, and although the reasons for this are not fully understood, it is likely that Dectin-1 plays a central role in the immunomodulatory activities of these carbohydrates.27,29,30
Helminth parasites, on the other hand, are able to interfere in MΦ activation or induce the development of regulatory MΦ, which results in killing defects in cells, and enhanced survival and spread of parasites in the host.31Fasciola hepatica releases excretory–secretory products (FhESP) that have different immunomodulatory effects,32–40 with the interaction of FhESP with the innate immune cells, such as peritoneal macrophages (pMΦ), being crucial in the early stage of the infection for the establishment of this parasite in the host. In addition, different experimental models have demonstrated the induction of alternatively activated MΦ by the parasite-released products.41–45
The way in which helminth infections drive polarized T helper type 2 and anti-inflammatory responses is likely to be due in part to the nature and quantity of the parasite pathogen-associated molecular patterns (PAMPs) present on their surfaces or in the excretory/secretory products, among which glycans seem to modulate the anti-parasite immune response.46–49
Although little is known about the recognition of extracellular parasite PAMPs by antigen-presenting cells, such as MΦ, or about the subsequent immunoregulatory effects, numerous immunomodulatory properties have been described for helminth carbohydrates, which are well-known components of FhESP.32,43,50 Therefore, the aim of this study was to evaluate the participation of CLRs, particularly of MR and Dectin-1, in the interaction of FhESP with pMΦ, and in the immunomodulatory effects induced by these parasite products on these innate immune cells.
Materials and methods
Reagents
For cell cultures, RPMI-1640 supplemented with 10% fetal bovine serum (FBS), 2 mm glutamine and 50 μg/ml gentamycin (Sigma-Aldrich Co., St Louis, MO) were used. Mannan, Laminarin, Polymixin B, anti-mouse IgG horseradish peroxidase and lipopolysaccharide (LPS) were also acquired from Sigma-Aldrich. The anti-mouse Dectin-1 antibody and the Syk inhibitor were bought from R&D Systems (Minneapolis, MN) and Calbiochem (Merck, Darmstadt, Germany), respectively. Anti-mouse MR (CD206), anti-mouse arginase I, anti-mouse β-actin, anti-mouse α-tubulin and goat-anti-rabbit IgG horseradish peroxidase were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Phycoerythrin-conjugated anti-mouse F4/80 was bought from Invitrogen (Carlsbad, CA). Transforming growth factor-β (TGF-β) Cytoset and the IL-10 kit were obtained from Biosource (Camarillo, CA) and e-Bioscience (San Diego, CA), respectively.
FhESP preparation
The FhESP were prepared according to a procedure described by Diaz et al.51 with some variation. Briefly, live adult worms of F. hepatica were obtained from the bile ducts of bovine livers and then washed with PBS, pH 7·4, before being incubated (1 worm/2 ml PBS) for 3 hr at 37°. Then, the supernatant was centrifuged (16 000 g, 30 min, 4°) before being concentrated using a high-flow YM 10 membrane filter (Millipore-Amicon Corp., Billeria, MA), and stored at −20° until used. The protein concentration (500 μg/ml) was measured using a Bradford protein assay (Bio-Rad, Hercules, CA), and the quantity of contaminating LPS present in FhESP was determined using the Limulus amoebocyte lysate test (Endosafe Times; Charles River Laboratories, Wilmington, DE), resulting in endotoxin levels < 90 units/ml of FhESP, and < 3·6 endotoxin units/ml (or 0·36 ng/ml) in the final culture conditions. Also, cultures were carried out by adding 10 μg/ml polymixin B (Sigma-Aldrich), and as the effects exerted by FhESP on pMΦ were the same (data not shown), this made it unlikely that the results attributed to FhESP stimulation were actually the result of LPS contamination.
Mice and purification of macrophages from PECs
Six- to 8-week-old female BALB/c mice were purchased from the Ezeiza Atomic Centre (CNEA, Buenos Aires, Argentina), and housed and cared for in the animal resource facilities of the Department of Clinical Biochemistry, Faculty of Chemical Sciences, National University of Cordoba, following institutional guidelines. All experimental protocols were approved by the Animal Experimentation Ethics Committee, Faculty of Chemical Sciences, National University of Cordoba. To obtain pMΦ, the peritoneal cavity was washed with ice-cold PBS containing 0·1% FBS and 5 mm EDTA. After determination of viability by trypan blue exclusion (cell viability was > 95%), peritoneal exudate cells were re-suspended in RPMI supplemented with 10% FBS, 2 mm glutamine and 50 μg/ml gentamycin, and adjusted to 2 × 106 cells/well in 24-well plates for Western blot assays or 5 × 105 cells/well in 48-well plates for the remaining assays. The remaining adherent cells were highly enriched for MΦ, with flow cytometry analysis revealing 90% F4/80+ cells.
Culture of naive pMΦ
Macrophages obtained from non-infected BALB/c mice were stimulated with FhESP at a concentration of 20 μg/ml for 48 hr. To investigate the role of receptors in the FhESP effects, we pre-incubated the cells with Mannan (1 mg/ml), Laminarin (0·5 mg/ml), Syk inhibitor (20 μm), anti-Dectin-1 (0·5 μg/ml) or anti-MR (10 μg/ml) for 30 min at 37° in 5% CO2. Then, after being washed the cells were stimulated with FhESP at a concentration of 20 μg/ml for 48 hr.
Culture of pMΦ derived from infected animals
Mice were orally infected with 10 metacercariae of F. hepatica (Baldwin Aquatics Inc., Monmouth, OR). Forty-eight hours after being infected, we carried out peritoneal lavages and the adherent cells were cultivated for a further 48 hr.
To evaluate the participation of MR and Dectin-1 during the infection, mice were intraperitoneally injected with Mannan (1 mg/ml), Laminarin (0·5 mg/ml) or PBS 4 hr before being infected.52 The blocker concentrations used were based on in vitro experiments, and we used a volume of 2 ml for the peritoneal cavity.
Cytokine assays
Culture supernatants were collected after 48 hr in the in vitro as well as in the in vivo experiments and assayed for the presence of TGF-β and IL-10 according to the manufacture's protocol, using a capture ELISA kit purchased from Biosource and e-Bioscience, respectively.
Arginase activity assay
Briefly, the arginase activity was measured as previously described by Corraliza et al.53 After lysis of the cells in Triton X-100 containing 5 μg pepstatin, 5 μg aprotinin and 5 μg antipain as protease inhibitors, the mixture was stirred for 30 min at room temperature. Then, 50 μl of 10 mm MnCl2 and 50 mm Tris–HCl were added to lysed cells to activate the enzyme by heating for 10 min at 56°. The arginine hydrolysis was initiated by the addition of 25 μl 0·5 m l-arginine, pH 9·7, at 37° for 45 min. The reaction was stopped with 400 μl H2SO4 (96%)/H3PO4 (85%)/H2O (1/3/7, volume/volume/volume). Then, after the addition of 25 μl α-isonitrosopropiophenone, followed by heating at 95° for 45 min, the urea concentration was measured at 540 nm, with the results expressed as μg of urea.
SDS–PAGE and Western blot analysis
For Western blot analysis, 20 μg pMφ lysate were separated on 10% SDS–polyacrylamide gels and transferred to a nitrocellulose membrane (Amersham Bioscience, Amersahm place, Little Chalfont, UK). Non-specific binding was blocked with 5% non-fat dry milk in Tris–HCl saline buffer containing 0·01% Tween 20 (TBS-T) for 60 min at room temperature. The membranes were incubated overnight with specific primary antibodies at 4°, washed three times with TBS-T, and then incubated with secondary horseradish peroxidase-conjugated antibodies for 1 hr at room temperature. The specific bands were revealed by a chemiluminescence reaction (Amersham Biosciences) with autoradiographic or maximum performance light films (Kodak, Rochester, NY) and quantified by densitometric analysis using image software (SCION Version free online, Scion Corporation, Frederick, MD).
MR and Dectin-1 binding assays
To demonstrate binding of FhESP to MR and Dectin-1, we performed inhibition assays. Total peritoneal cells were pre-incubated with Mannan (1 mg/ml), Laminarin (0·5 mg/ml), Galactose (1 mg/ml) or specific blocking antibodies to MR (10 μg/ml) and Dectin-1 (0·5 μg/ml) for 30 min at 37°, and washed before being incubated with FITC-labelled FhESP for 1 hr (different time-points were used, and the most representative was chosen). The reaction was terminated by the addition of cold PBS, and the cells were washed and finally fixed with 1%p-formaldehyde solution after being analysed by flow cytometry. To discriminate the specific population of pMΦ, we considered the F4/80+ cells, which corresponded to the pMΦ population.
Statistical analysis
Data are expressed as means ± SEMs. The two-tailed Student's t-test was used, and a one-way analysis of variance with Tukey–Kramer's post-hoc test was used to determine the statistical significance for all pairwise multiple-comparison procedures. A P-value of 0·05 was considered significant. All experiments were performed in triplicate and equivalent results were obtained in each experiment.
Results
Products from F. hepatica are able to induce immunomodulatory effects on pMΦ in the early stage of infection as well as after in vitro stimulation
To evaluate the behaviour of pMΦ during the early stage of the infection we challenged BALB/c mice with F. hepatica metacercariae for 48 hr, and for the in vitro studies we stimulated pMΦ derived from naive mice with FhESP for 48 hr. It was observed that high levels of arginase activity and Arginase I (Arg I) expression with respect to the control group were induced in both cases, after the infection (Fig. 1a, P<0·018 and P<0·000035, respectively) as well as in the in vitro stimulation with FhESP (Fig. 1c, P<0·001 and P < 0·020, respectively). In addition, high levels of IL-10 and TGF-β production with respect to the basal group were observed in pMΦ derived from infected mice (Fig. 1b, P<0·020 and P < 0·027, respectively), as well as in pMΦ stimulated in vitro with FhESP (Fig. 1d, P<0·00061 and P<0·028, respectively).
Figure 1.
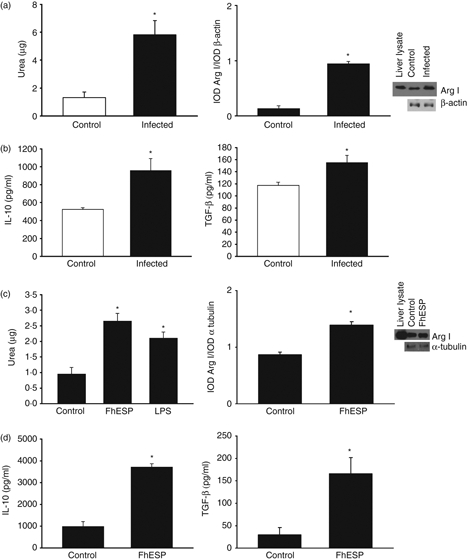
Products from Fasciola hepatica are able to induce immunomodulatory effects on peritoneal macrophages (pMΦ) in the early stage of infection as well as after in vitro stimulation. Expression levels of Arginase I (Arg I) by Western blot and arginase activity in pMΦ derived from infected BALB/c mice, 48 hr after metacercariae challenge and further cultivation for 48 hr, as well as in naive mice derived pMΦ stimulated with F. hepatica excretory–secretory products (FhESP; 20 μg/ml) for 48 hr (a, d). Transforming growth factor-β (TGF-β) and interleukin-10 (IL-10) levels were measured by ELISAs in culture supernatants in the respective pMΦ of the ex vivo (b) and in vitro (d) experiments. In the infection experiment, four mice/group were analysed. Data are mean ± SEM of three independent experiments, analysed in triplicate. *P<0·05 respect to pMΦ from non-infected mice (a–c) or pMΦ in medium alone (c–d).
These results suggest that products from F. hepatica are able to induce immunomodulatory effects on pMΦ during the early stage of infection as well as after in vitro stimulation.
Mannose receptors participate in the interaction of FhESP with pMΦin vitro
To investigate the role of MR in the FhESP effects induced on pMΦ, these cells were pre-incubated with Mannan or with a specific blocking antibody to MR for 30 min at 37° before being stimulated with FhESP for 48 hr. As shown in Fig. 2(a), a partial inhibition in the increased arginase activity was detected in pMΦ pre-incubated with Mannan or anti-MR and then stimulated with FhESP (P<0·01 and P < 0·05 anti-MR, respectively) compared with cells stimulated with FhESP. Similar results for Arg I expression were obtained in pMΦ pre-incubated with Mannan and then stimulated with FhESP with respect to cells stimulated with FhESP (Fig. 2b, P < 0·00032). Also, a partial reduction of the high levels of IL-10 and TGF-β induced by FhESP when pMΦ were pre-incubated with Mannan or anti-MR was found compared with cells stimulated with FhESP alone (P < 0·05 and P<0·01, respectively, employing Mannan; P < 0·05 employing anti-MR) (Fig. 2c).
Figure 2.
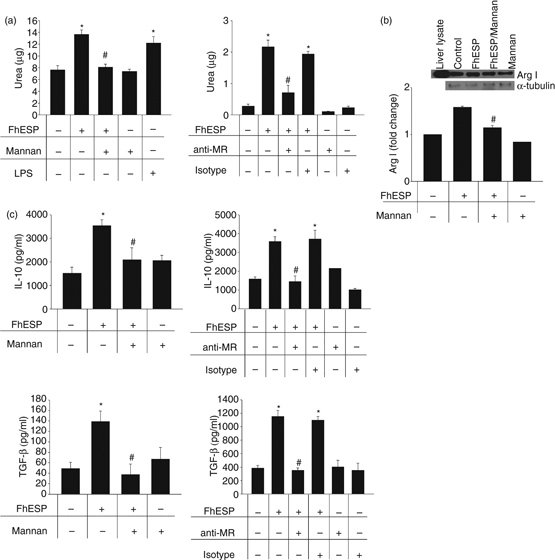
Mannose receptor (MR) participates in the induction of the increased arginase expression and activity, as well as transforming growth factor-β (TGF-β) and interleukin-10 (IL-10) production exerted by Fasciola hepatica excretory–secretory products (FhESP) on peritoneal macrophages (pMΦ). Arginase activity (a), Arginase I (Arg I) expression (b), as well as TGF-β and IL-10 production (c), were determined in pMΦ pre-incubated with mannan (1 mg/ml) or anti-MR for 30 min at 37° before being stimulated with FhESP (20 μg/ml) for 48 hr. Data are mean ± SEM of three independent experiments, analysed in triplicate. *P<0·05 respect to pMΦ in medium alone; #P<0·05 respect to pMΦ stimulated with FhESP.
To demonstrate the specific binding of FhESP to MR, we performed an inhibition assay in which cells were pre-incubated with Mannan or anti-MR for 30 min at 37° before being incubated with FITC-labelled FhESP for 1 hr. As shown in Fig. 3(a,b), the binding of FhESP to pMΦ was significantly less than that of the respective controls (P<0·0011 and P < 0·046, respectively). Based on these above findings we suggest that MR participates in the interaction of FhESP with pMΦ.
Figure 3.
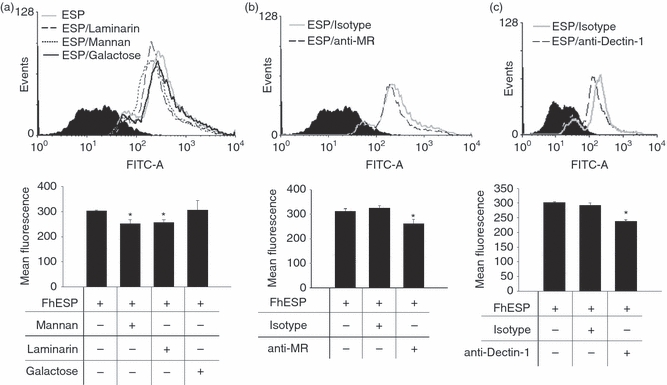
Mannose receptors (MR) and Dectin-1 participate in the interaction of Fasciola hepatica excretory–secretory products (FhESP) with peritoneal macrophages (pMΦ). Binding assays to determine the union of FhESP–FITC to MR and Dectin-1 on pMΦ were performed by employing blockers to the respective receptors. The binding was evaluated by flow cytometry in cells gated on F4/80+ cells, with the histograms showing control (solid histogram); FhESP–FITC stimulated cells and isotype controls (solid grey line); pre-treatment with Mannan (1 mg/ml) or Laminarin (0·5 mg/ml) (a), anti-MR (10 μg/ml) (b), anti-Dectin-1 (0·5 μg/ml) (c) (open line); pre-treatment with galactose (1 mg/ml) (a) (solid black line). Data are mean ± SEM of three independent experiments, analysed in triplicate. *P<0·05 with respect to pMΦ stimulated with FhESP–FITC.
Dectin-1 participate in the interaction of FhESP with pMΦin vitro
Although there is no previous evidence suggesting the involvement of Dectin-1 in parasite product recognition by innate immune cells, in our preliminary inhibition experiments employing blockers to different receptors we found that, in a similar way to Mannan, Laminarin also inhibited the effects exerted by FhESP on pMΦ. As shown in Fig. 4(a), when we performed the experiments in the presence of Laminarin or of a specific blocking antibody to Dectin-1 for 30 min at 37°, we observed a partial inhibition in the high levels of arginase activity induced by FhESP respect to cells stimulated with FhESP alone (P<0·001 and P < 0·05, respectively). In addition, an inhibition of the increased Arg I expression was observed when cells were pre-incubated with anti-Dectin-1 (Fig. 4b, P < 0·0003). When the involvement of Dectin-1 was evaluated in the production of regulatory cytokines, we observed a reduction of the high levels of IL-10 and TGF-β induced by FhESP in pMΦ pre-incubated with Laminarin or anti-Dectin-1 compared with cells stimulated with FhESP alone (Fig. 4c, P<0·01 and P<0·05, respectively, employing Laminarin; P<0·01 and P<0·05, respectively, employing anti-Dectin-1).
Figure 4.
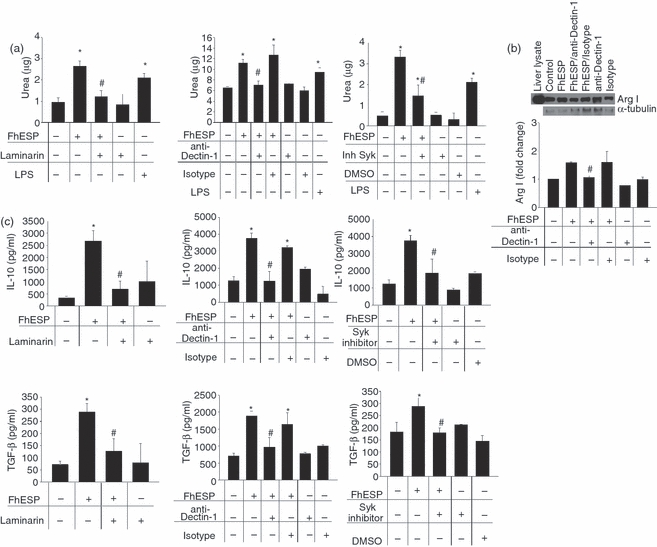
Dectin-1 participates in the increased arginase expression and activity, as well as in the higher Transforming growth factor-β (TGF-β) and interleukin-10 (IL-10) production induced by Fasciola hepatica excretory–secretory products (FhESP) on peritoneal macrophages (pMΦ). Arginase activity (a), Arginase I (Arg I) expression by Western blot (b), as well as TGF-β and IL-10 levels (c) were determined in pMΦ pre-incubated with Laminarin (0·5 mg/ml), anti-Dectin-1 specific antibody (0·5 μg/ml), or Syk inhibitor (20 μm) for 30 min at 37° before being stimulated with FhESP (20 μg/ml) for 48 hr. Data are mean ± SEM of three independent experiments, analysed in triplicate. *P<0·05 respect to pMΦ in medium alone; #P<0·05 respect to pMΦ stimulated with FhESP.
Dectin-1 signals through a novel pathway, including an interaction with Syk (spleen tyrosine kinase) which then activates downstream signalling components, including the transcription factor nuclear factor-κB.27,29,54 When we evaluated the role of Syk by employing an inhibitor of this kinase, a partial inhibition of the increase in arginase activity (Fig. 4a, P<0·05) as well as in the IL-10 and TGF-β levels (Fig. 4c, P<0·001 and P<0·01, respectively) induced by FhESP was observed with respect to cells stimulated with FhESP alone.
To demonstrate the specific binding of FhESP to Dectin-1, we performed an inhibition assay in which cells were pre-incubated with Laminarin or anti-Dectin-1 for 30 min at 37° before being incubated with FITC-labelled FhESP for 1 hr. As shown in Fig. 3(a,c), the binding of FhESP to pMΦ was significantly less than for the respective controls (P<0·036 and P < 0·017, respectively). Based on the above observations we suggest that Dectin-1 is involved in the interaction of FhESP with pMΦ.
MR and Dectin-1 participate in the phenotypic changes observed in the pMΦ during the early stage of F. hepatica infection
To determine if the participation of MR and Dectin-1 observed in the in vitro experiments may be extrapolated to in vivo events, mice were intraperitoneally injected with Mannan or Laminarin 4 hr before infection. Forty-eight hours after infection, peritoneal lavages were carried out and the adherent cells were cultivated for 48 hr. As shown in Fig. 5, there was a partial inhibition in the increased arginase activity and Arg I expression (P<0·05 and P<0·00045, respectively, with intraperitoneal Laminarin injection; P < 0·05 and P < 0·0000039, respectively, with intraperitoneal Mannan injection), as well as in the high levels of IL-10 and TGF-β production (P<0·05 and P < 0·05, respectively, with intraperitoneal Laminarin injection; P<0·05 and P<0·001, respectively, with intraperitoneal Mannan injection). Therefore, taking into account the in vitro as well as the in vivo results, we can conclude that the participation of at least two CLRs, MR and Dectin-1, occurred in the interaction of F. hepatica products with pMΦ.
Figure 5.
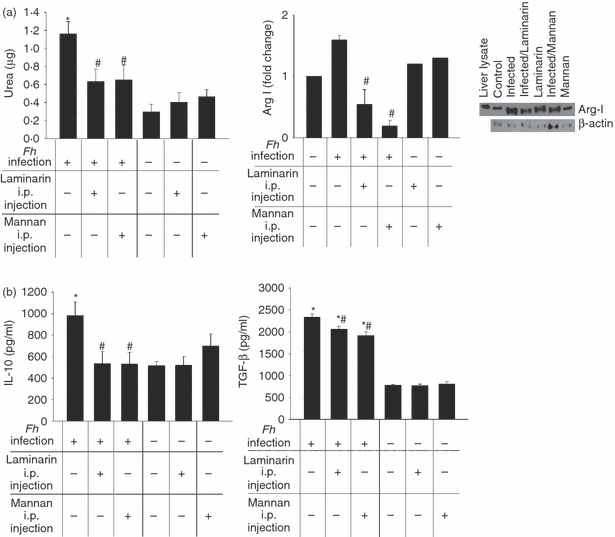
Mannose receptor (MR) and Dectin-1 participate in the in vivo Fasciola hepatica-induced effects on peritoneal macrophages (pMΦ) during the early stages of the infection. Mice were intraperitoneally injected with Mannan (1 mg/ml) or Laminarin (0·5 mg/ml) 4 hr before being challenged with F. hepatica metacercariae. Forty-eight hours after the infection, pMΦ were cultivated for 48 hr more. Arginase activity and Arginase I (Arg I) expression were determined in the respective cell lysates (a), and levels of transforming growth factor-β (TGF-β) and interleukin-10 (IL-10) were measured in the culture supernatants (b). Four mice/group were analysed, and data are mean ± SEM of two independent experiments, analysed in triplicate. *P<0·05 with respect to pMΦ from non-infected mice; #P<0·05 respect to pMΦ from infected mice.
Discussion
Macrophages are innate immune cells that can respond to endogenous and exogenous stimuli. These cells are rapidly generated following injury or infection, by recognizing danger signals through receptors capable of inducing specialized activation programmes, which finally influence their physiology and functions.55–58 Some microorganisms can interfere with MΦ activation or induce the development of regulatory MΦ, resulting in killing defects in cells and enhanced survival and spread of parasites in the host. In agreement with previous results in different experimental models,41–45 we have observed that F. hepatica-released products induced a pMΦ population with immunoregulatory properties during the early stage of infection as well as after in vitro stimulation. In this regard, we observed increased levels of arginase activity and Arg I expression, and high levels of IL-10 and TGF-β production. In addition, we observed an increased MR expression, low expression of MHC class II molecules, and a down-modulated response to the activating molecule LPS in pMΦ stimulated with FhESP (unpublished data, L. Guasconi and D. T. Masih).
Immunomodulatory properties have been described for carbohydrates present in the FhESP. For example, previous studies in our group have indicated a possible role of carbohydrate components of FhESP in the induction of eosinophil apoptosis, because the treatment of these products with sodium metaperiodate markedly diminished their effects.50 Furthermore, the oxidation of FhESP suggests that glycan residues played a role in the parasite-MΦ interactions evaluated by Flynn and Mulcahy43 in a bovine model. Nevertheless, little is known about the PRRs involved in the recognition of F. hepatica PAMPs by MΦ or the subsequent immunoregulatory effects induced on these cells. Considering that some pathogens have evolved immunosuppressive activities through their interaction with CLRs (a group of PRRs which are best known for their ability to recognize specific pathogen-associated carbohydrate structures4,5) together with previous evidence suggesting a possible role of glycan residues in the interaction of FhESP with innate immune cells such as eosinophils and MΦ, we decided to evaluate the role of the CLRs expressed in pMΦ in the interaction with parasite products. These results demonstrated the participation of MR in the interaction of FhESP with pMΦ, and related to this, when these cells were pre-incubated with Mannan as a competitive ligand or a specific blocking antibody to MR before being stimulated with FhESP, the increase in arginase activity or Arg I expression as well as the high levels of TGF-β and IL-10 were partially inhibited. Similar effects were observed when mice were intraperitoneally injected with Mannan before being infected.
Among the numerous microorganisms recognized by MR are other helminth parasites such as Trichinella spiralis12 and Trichuris muris.13 According to Matthew et al.,13 components of T. muris bind to the MR in a redundant way, hence generating an effective immune response that leads to MΦ activation, parasite expulsion and tissue repair, in a similar way to the results observed in mice lacking this receptor and infected with Candida albicans,14???Pneumocystis carinii15 or Leishmania sp.,16 which all showed a normal immune response. On the other hand, the carbohydrate components of Taenia crassiceps may favour a Th2 response, with MR being a candidate receptor in this effect.59 In this regard, the engagement of MR with certain fungal pathogens and selected ligands or antibodies may result in an immunosuppressive function characterized by a profile of anti-inflammatory and tolerogenic cytokines, which could prevent the generation of Th1-polarized responses.60,61 Therefore, we support the idea that MR can be exploited by F. hepatica to influence the activation of the immune system, specifically by modulating the pMΦ phenotype and function.
Dectin-1, on the other hand, has been conclusively demonstrated to function as a signalling PRR,26 which regulates the expression of innate response genes including those encoding co-stimulatory molecules, and pro-inflammatory cytokines and chemokines.27–29 Paradoxically, the recognition of fungal β-glucans by Dectin-1 also triggers the production of non-protective cytokines, such as IL-23 and IL-10, although the reasons for this are not fully understood. However, it is likely that Dectin-1 plays a central role in the immunomodulatory activities of these carbohydrates.27,29,30 Recent studies suggest that internalization of Dectin-1 following interaction with ligand leads to attenuation of the signalling pathways involved in innate gene induction. This may be particularly relevant for understanding the role of innate immune receptors such as C-type lectins, which play a dual role in microbe phagocytosis and in inducing inflammation.62,63 The findings presented in this work are novel results because Dectin-1 has still not been associated with an innate immune response to any parasite, and are in agreement with reports describing an immunomodulatory response for this receptor. In this regard, when pMΦ were pre-incubated with Laminarin as a competitive ligand or a specific blocking antibody to Dectin-1 before being stimulated with FhESP, the increase in arginase activity or Arg I expression was partially inhibited, and the high levels of TGF-β and IL-10 were lowered, with similar effects being observed when mice were intraperitoneally injected with Laminarin before being infected.
Dectin-1 signals via a novel hemITAM motif that becomes phosphorylated by Src family kinases on receptor engagement,27,64 permitting recruitment and activation of the spleen tyrosine kinase (Syk). Then, Syk couples to downstream pathways and, via the adaptor CARD9, to the activation of nuclear factor-κB,27,29,54 which together with nuclear factor of activated T cells and the transcription factors activated by mitogen-activated protein kinases downstream of Syk, regulates the expression of the innate response genes.27–29 To evaluate the role plays by Syk employing an inhibitor of this kinase, we observed that the increase in arginase activity was partially inhibited and the TGF-β and IL-10 levels were lowered. This effect observed in the IL-10 production is in agreement with that reported in the bibliography, which demonstrated that IL-10 induction through the ligand/Dectin-1 interaction is Syk-dependent.27,65
Based on the above findings, together with the results that show the interaction of FhESP with MR and Dectin-1 observed in the binding assays, we propose that these CLRs would be highly employed by F. hepatica. We suggest that both CLRs are important in the modulation of pMΦ activation, which are among the first innate immune cells to come into contact with the parasite on entering the host. However, we considerer that the pathways used by these receptors for immunomodulation are not exactly the same. In relationship with this, in preliminary assays we observed that the suppressor phenomenon induced by pMΦ stimulated with FhESP in the naive T-cell proliferative response, is Dectin-1-dependent but not MR-dependent (in vitro assays, unpublished data, L. Guasconi and D. T. Masih). In this regard, we have begun to evaluate potential signalling pathways involved on each receptor's effects. Finally, we considered that both CLRs cooperate for the same purpose: modulating the anti-parasite immune response. The summary of the effects of MR and Dectin-1 would be part of the strength that finally prevails over the immunostimulatory signals from other PRRs, which ultimately determine the survival of this parasite in the host.
However, considering that little is known about the extracellular parasite PAMPs, their recognition by antigen-presenting cells or the link to initiate the Th2 response and cytokine production, further studies are required to improve the understanding of host defence against such microorganisms. Ultimately, identification of the molecules which interact with these CLRs could help to clarify how they direct the immune response.
Acknowledgments
The present work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica (PICT 33326); Consejo Nacional de Investigaciones Científicas y Técnicas de Argentina (PIP 6327); Secretaría de Ciencia y Tecnología (SeCyT), Universidad Nacional de Córdoba (Grant 69/08); and Ministerio de Ciencia y Tecnología de la Provincia de Córdoba (Grant 2008). L. Guasconi and M.C. Serradell are PhD fellows of Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). D.T. Masih is a member of the Research Career of CONICET. We would like to thank native speaker, Dr Paul Hobson, for revision of the manuscript.
Disclosures
The authors have no conflict of interest to disclose.
References
- 1.Drickamer K. Engineering galactose-binding activity into a C-type mannose-binding protein. Nature. 1993;360:183–6. doi: 10.1038/360183a0. [DOI] [PubMed] [Google Scholar]
- 2.Iobst AT, Drickamer K. Binding of sugar ligands to Ca2+-dependent animal lectins: II. Generation of high-affinity galactose binding by site-directed mutagenesis. J Biol Chem. 1994;269:15512–9. [PubMed] [Google Scholar]
- 3.Weis WI, Taylor ME, Drickamer K. The C-type lectin superfamily in the immune system. Immunol Rev. 1998;163:19–34. doi: 10.1111/j.1600-065x.1998.tb01185.x. [DOI] [PubMed] [Google Scholar]
- 4.McGreal EP, Martinez-Pomares L, Gordon S. Divergent roles for C-type lectins expressed by cells of the innate immune system. Mol Immunol. 2004;41:1109–21. doi: 10.1016/j.molimm.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 5.McGreal EP, Miller JL, Gordon S. Ligand recognition by antigen-presenting cell C-type lectin receptors. Curr Opin Immunol. 2005;17:18–24. doi: 10.1016/j.coi.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linehan SA, Martinez-Pomares L, Stahl PD, Gordon S. Mannose receptor and its putative ligands in normal murine lymphoid and nonlymphoid organs: in situ expression of mannose receptor by selected macrophages, endothelial cells, perivascular microglia, and mesangial cells, but not dendritic cells. J Exp Med. 1999;189:1961–72. doi: 10.1084/jem.189.12.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Pomares L, Linehan SA, Taylor PR, Gordon S. Binding properties of the mannose receptor. Immunobiology. 2001;204:527–35. doi: 10.1078/0171-2985-00089. [DOI] [PubMed] [Google Scholar]
- 8.Schlesinger LS, Kaufman TM, Iyer S, Hull SR, Marchiando LK. Differences in mannose receptor-mediated uptake of lipoarabinomannan from virulent and attenuated strains of Mycobacterium tuberculosis by human macrophages. J Immunol. 1996;157:4568–75. [PubMed] [Google Scholar]
- 9.Kahn S, Wleklinski M, Aruffo A, Farr A, Coder D, Kahn M. Trypanosoma cruzi amastigote adhesion to macrophages is facilitated by the mannose receptor. J Exp Med. 1995;182:1243–58. doi: 10.1084/jem.182.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zamze S, Martinez-Pomares L, Jones H, Taylor PR, Stillion RJ, Gordon S, Wong SY. Recognition of bacterial capsular polysaccharides and lipopolysaccharides by the macrophage mannose receptor. J Biol Chem. 2002;277:41613–23. doi: 10.1074/jbc.M207057200. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen DG, Hildreth JE. Involvement of macrophage mannose receptor in the binding and transmission of HIV by macrophages. Eur J Immunol. 2003;33:483–93. doi: 10.1002/immu.200310024. [DOI] [PubMed] [Google Scholar]
- 12.Gruden-Movsesijan A, Milosavljevic LS. The involvement of the macrophage mannose receptor in the innate immune response to infection with parasite Trichinella spiralis. Vet Immunol Immunopathol. 2006;109:57–67. doi: 10.1016/j.vetimm.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 13.Matthew L, deSchoolmeester ML, Martinez-Pomares L, Gordon S, Else KJ. The mannose receptor binds Trichuris muris excretory/secretory proteins but is not essential for protective immunity. Immunology. 2008;126:246–55. doi: 10.1111/j.1365-2567.2008.02893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SJ, Zheng NY, Clavijo M, Nussenzweig MC. Normal host defense during systemic candidiasis in mannose receptor-deficient mice. Infect. Immun. 2003;71:437–45. doi: 10.1128/IAI.71.1.437-445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swain SD, Lee SJ, Nussenzweig MC, Harmsen AG. Absence of the macrophage mannose receptor in mice does not increase susceptibility to Pneumocystis carinii infection in vivo. Infect Immun. 2003;71:6213–21. doi: 10.1128/IAI.71.11.6213-6221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akilov OE, Kasuboski RE, Carter CR, McDowell MA. The role of mannose receptor during experimental leishmaniasis. J Leukoc Biol. 2007;81:1188–96. doi: 10.1189/jlb.0706439. [DOI] [PubMed] [Google Scholar]
- 17.Taylor PR, Brown GD, Reid DM, Willment JA, Martinez-Pomares L, Gordon S, Wong SYC. The β-glucan receptor, Dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J Immunol. 2002;169:3876–82. doi: 10.4049/jimmunol.169.7.3876. [DOI] [PubMed] [Google Scholar]
- 18.Brown GD, Gordon S. Immune recognition: a new receptor for beta-glucans. Nature. 2001;413:36–7. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 19.Brown GD, Taylor PR, Reid DM, Willment JA, Willismas DL, Martinez-Pomares L, et al. Dectin-1 is a major beta-glucan receptor on macrophages. J Exp Med. 2002;296:407–12. doi: 10.1084/jem.20020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ariizumi K, Shen GL, Shikano S, Xu S, Ritter R, Kumamoto T, et al. Identification of a novel, dendritic cell-associated molecule, Dectin-1 by subtractive cDNA cloning. J Biol Chem. 2000;275:20157–67. doi: 10.1074/jbc.M909512199. [DOI] [PubMed] [Google Scholar]
- 21.Willment JA, Gordon S, Brown GD. Characterisation of the human β-glucan receptor and its alternatively spliced isoforms. J Biol Chem. 2001;276:43818–23. doi: 10.1074/jbc.M107715200. [DOI] [PubMed] [Google Scholar]
- 22.Yadav M, Schorey JS. The beta-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood. 2006;108:3168–75. doi: 10.1182/blood-2006-05-024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothfuchs AG, Bafica A, Feng CG, Egen JG, Williams DL, Brown GD, et al. Dectin-1 interaction with Mycobacterium tuberculosis leads to enhanced IL-12p40 production by splenic dendritic cells. J Immunol. 2007;179:3463–71. doi: 10.4049/jimmunol.179.6.3463. [DOI] [PubMed] [Google Scholar]
- 24.Shin DM, Yang CS, Yuk JM, Lee JY, Kim KH, Shin SJ, et al. Mycobacterium abscessus activates the macrophage innate immune response via a physical and functional interaction between TLR2 and dectin-1. Cell Microbiol. 2008;10:1608–21. doi: 10.1111/j.1462-5822.2008.01151.x. [DOI] [PubMed] [Google Scholar]
- 25.Weck MM, Appel S, Werth D, Sinzger C, Bringmann A, Grünebach F, et al. hDectin-1 is involved in uptake and crosspresentation of cellular antigens. Blood. 2008;111:4264–72. doi: 10.1182/blood-2006-10-051375. [DOI] [PubMed] [Google Scholar]
- 26.Brown GD. Dectin-1: a signaling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 27.Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 2005;22:507–17. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Goodridge HS, Simmons RM, Underhill DM. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J Immunol. 2007;178:3107–15. doi: 10.4049/jimmunol.178.5.3107. [DOI] [PubMed] [Google Scholar]
- 29.LeibundGut-Landmann S, Gro O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–8. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 30.Tsoni SV, Brown GD. β-glucans and Dectin-1. Ann NY Acad Sci. 2008;1143:45–60. doi: 10.1196/annals.1443.019. [DOI] [PubMed] [Google Scholar]
- 31.Wang LJ, Cao Y, Shi HN. Helminth infections and intestinal inflammation. World J Gastroenterol. 2008;14:5125–32. doi: 10.3748/wjg.14.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cervi L, Rubinstein H, Masih DT. Involvement of excretion–secretion products from Fasciola hepatica inducing suppression of the cellular immune responses. Vet Parasitol. 1996;61:97–111. doi: 10.1016/0304-4017(95)00816-0. [DOI] [PubMed] [Google Scholar]
- 33.Cervi L, Masih DT. Inhibition of spleen cell proliferative response to mitogens by excretory–secretory antigens of Fasciola hepatica. Int J Parasitol. 1997;27:573–9. doi: 10.1016/s0020-7519(96)00188-9. [DOI] [PubMed] [Google Scholar]
- 34.Cervi L, Rossi G, Cejas H, Masih DT. Fasciola hepatica-induced immune suppression of spleen mononuclear cell proliferation: role of nitric oxide. Clin Immunol Immunopathol. 1998;87:145–54. doi: 10.1006/clin.1997.4499. [DOI] [PubMed] [Google Scholar]
- 35.Cervi L, Rossi G, Masih DT. Potential role for excretory–secretory forms of glutathione-S-transferase (GST) in Fasciola hepatica. Parasitology. 1999;119:627–33. doi: 10.1017/s003118209900517x. [DOI] [PubMed] [Google Scholar]
- 36.Cervi L, Cejas H, Masih DT. Cytokines involved in the immunosuppressor period in experimental fasciolosis in rats. Int J Parasitol. 2001;31:1467–73. doi: 10.1016/s0020-7519(01)00275-2. [DOI] [PubMed] [Google Scholar]
- 37.Chapman CB, Mitchell GF. Proteolytic cleavage of immunoglobulin by enzymes released by Fasciola hepatica. Vet Parasitol. 1982;11:165–78. doi: 10.1016/0304-4017(82)90039-5. [DOI] [PubMed] [Google Scholar]
- 38.Duffus WP, Franks D. In vitro effect of immune serum and bovine granulocytes on juvenile Fasciola hepatica. Clin Exp Immunol. 1980;41:430–40. [PMC free article] [PubMed] [Google Scholar]
- 39.Heffernan M, Smith A, Curtain D, McDonnell S, Ryan J, Dalton JP. Characterisation of a cathepsin-B proteinase released by Fasciola hepatica (liver fluke) Biochem Soc Trans. 1991;19:27S. doi: 10.1042/bst019027s. [DOI] [PubMed] [Google Scholar]
- 40.Masih DT, Cervi L, Casado JM. Modification of accessory activity of peritoneal cells from Fasciola hepatica infected rats. Vet Immunol Immunopathol. 1996;53:257–68. doi: 10.1016/S0165-2427(96)05611-5. [DOI] [PubMed] [Google Scholar]
- 41.Donnelly S, O'Neill SM, Sekiya M, Mulcahy G, Dalton JP. Thioredoxin peroxidase secreted by Fasciola hepatica induces the alternative activation of macrophages. Infect Immun. 2005;73:166–73. doi: 10.1128/IAI.73.1.166-173.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flynn RJ, Irwin JA, Olivier M, Sekiya M, Dalton JP, Mulcahy G. Alternative activation of ruminant macrophages by Fasciola hepatica. Vet Immunol Immunopathol. 2007;120:31–40. doi: 10.1016/j.vetimm.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Flynn RJ, Mulcahy G. Possible role for toll-like receptors in interaction of Fasciola hepatica excretory/secretory products with bovine macrophages. Infect Immun. 2008;76:678–84. doi: 10.1128/IAI.00732-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donnelly S, Stack CM, O'Neill SM, Sayed AA, Williams DL, Dalton JP. Helminth 2-Cys peroxiredoxin drives Th2 responses through a mechanism involving alternatively activated macrophages. FASEB J. 2008;22:4022–32. doi: 10.1096/fj.08-106278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walsh KP, Brady MT, Finlay CM, Boon L, Mills KHG. Infection with a helminth parasite attenuates autoimmunity through TGF-β mediated suppression of Th17 and Th1 responses. J Immunol. 2009;183:1577–86. doi: 10.4049/jimmunol.0803803. [DOI] [PubMed] [Google Scholar]
- 46.Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites–masters of regulation. Immunol Rev. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 47.Harn DA, McDonald J, Atochina O, Da'dara AA. Modulation of host immune responses by helminth glycans. Immunol Rev. 2009;230:247–57. doi: 10.1111/j.1600-065X.2009.00799.x. [DOI] [PubMed] [Google Scholar]
- 48.Everts B, Perona-Wright G, Smits HH, et al. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J Exp Med. 2009;206:1673–80. doi: 10.1084/jem.20082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Terrazas CA, Gómez-García L, Terrazas LI. Impaired pro-inflammatory cytokine production and increased Th2-biasing ability of dendritic cells exposed to Taenia excreted/secreted antigens: a critical role for carbohydrates but not for STAT6 signaling. Int J Parasitol. 2010;40:1051–62. doi: 10.1016/j.ijpara.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 50.Serradell MC, Guasconi L, Cervi L, Chiapello LS, Masih DT. Excretory–secretory products from Fasciola hepatica induce eosinophil apoptosis by a caspase-dependent mechanism. Vet Immunol Immunopathol. 2007;117:197–208. doi: 10.1016/j.vetimm.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 51.Diaz A, Espino AM, Marcet R, Otero O, Torres D, Finlay CM, et al. Partial characterization of the epitope on excretory–secretory products of Fasciola hepatica recognized by monoclonal antibody ES78. J Parasitol. 1998;84:55–61. [PubMed] [Google Scholar]
- 52.Ozment-Skelton TR, Goldman MP, Gordon S, Brown GD, Williams DL. Prolonged reduction of leukocyte membrane-associated Dectin-1 levels following β-glucan administration. J Pharmacol Exp Ther. 2006;318:540–6. doi: 10.1124/jpet.106.102293. [DOI] [PubMed] [Google Scholar]
- 53.Corraliza IM, Campo ML, Soler G, Modollel M. Determination of arginase activity in macrophages: a micromethod. J Immunol Methods. 1994;174:231–5. doi: 10.1016/0022-1759(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 54.Gross O, Gewies A, Finger K, Schafer M, Sparwasser T, Peschel C, et al. CARD9 controls a non-TLR signaling pathway for innate anti-fungal immunity. Nature. 2006;442:651–6. doi: 10.1038/nature04926. [DOI] [PubMed] [Google Scholar]
- 55.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 56.Mantovani A, Sica A, Locati M. New vistas on macrophage differentiation and activation. Eur J Immunol. 2007;37:14–6. doi: 10.1002/eji.200636910. [DOI] [PubMed] [Google Scholar]
- 57.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 59.Gómez-García L, Rivera-Montoya I, Rodríguez-Sosa M, Terrazas LI. Carbohydrate components of Taenia crassiceps metacestodes display Th2-adjuvant and anti-inflammatory properties when co-injected with bystander antigen. Parasitol Res. 2006;99:440–8. doi: 10.1007/s00436-006-0159-2. [DOI] [PubMed] [Google Scholar]
- 60.Chieppa M, Bianchi G, Doni A, Del Prete A, Sironi M, Laskarin G, et al. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol. 2003;171:4552–60. doi: 10.4049/jimmunol.171.9.4552. [DOI] [PubMed] [Google Scholar]
- 61.Zhang J, Zhu J, Bu X, Cushion M, Kinane TB, Avraham H, et al. Cdc42 and RhoB activation are required for mannose receptor-mediated phagocytosis by human alveolar macrophages. Mol Biol Cell. 2005;16:824–34. doi: 10.1091/mbc.E04-06-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosas M, Liddiard K, Kimberg M, Faro-Trindade I, McDonald JU, Williams DL, et al. The induction of inflammation by dectin-1 in vivo is dependent on myeloid cell programming and the progression of phagocytosis. J Immunol. 2008;181:3549–57. doi: 10.4049/jimmunol.181.5.3549. [DOI] [PubMed] [Google Scholar]
- 63.Hernanz-Falcón P, Joffre O, Williams DL, Reis e Sousa C. Internalization of Dectin-1 terminates induction of inflammatory responses. Eur J Immunol. 2009;39:507–13. doi: 10.1002/eji.200838687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Underhill DM, Rossnagle E, Lowell CA, Simmons RM. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood. 2005;106:2543–50. doi: 10.1182/blood-2005-03-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferwerda G, Meyer-Wentrup F, Kullberg B, Netea MG, Adema GJ. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell Microbiol. 2008;10:2058–66. doi: 10.1111/j.1462-5822.2008.01188.x. [DOI] [PubMed] [Google Scholar]


