Abstract
Central in respiration or photosynthesis, the cytochrome bc1 and b6f complexes are regarded as functionally similar quinol oxidoreductases. They both catalyse a redox loop, the Q-cycle, which couples electron and proton transfer. This loop involves a bifurcated electron transfer step considered as being mechanistically mandatory, making the Q-cycle indispensable for growth. Attempts to falsify this paradigm in the case of cytochrome bc1 have failed. The rapid proteolytic degradation of b6f complexes bearing mutations aimed at hindering the Q-cycle has precluded so far the experimental assessment of this model in the photosynthetic chain. Here we combine mutations in Chlamydomonas that inactivate the redox loop but preserve high accumulation levels of b6f complexes. The oxidoreductase activity of these crippled complexes is sufficient to sustain photosynthetic growth, which demonstrates that the Q-cycle is dispensable for oxygenic photosynthesis.
 The Q-cycle is thought to be an essential energetic component of the photosynthetic electron-transfer chain. Here, Chlamydomonas mutants with an inactive Q-cycle but normal levels of b6f complexes are shown to display photosynthetic growth, demonstrating the dispensability of the Q-cycle in the oxygenic photosynthetic chain.
The Q-cycle is thought to be an essential energetic component of the photosynthetic electron-transfer chain. Here, Chlamydomonas mutants with an inactive Q-cycle but normal levels of b6f complexes are shown to display photosynthetic growth, demonstrating the dispensability of the Q-cycle in the oxygenic photosynthetic chain.
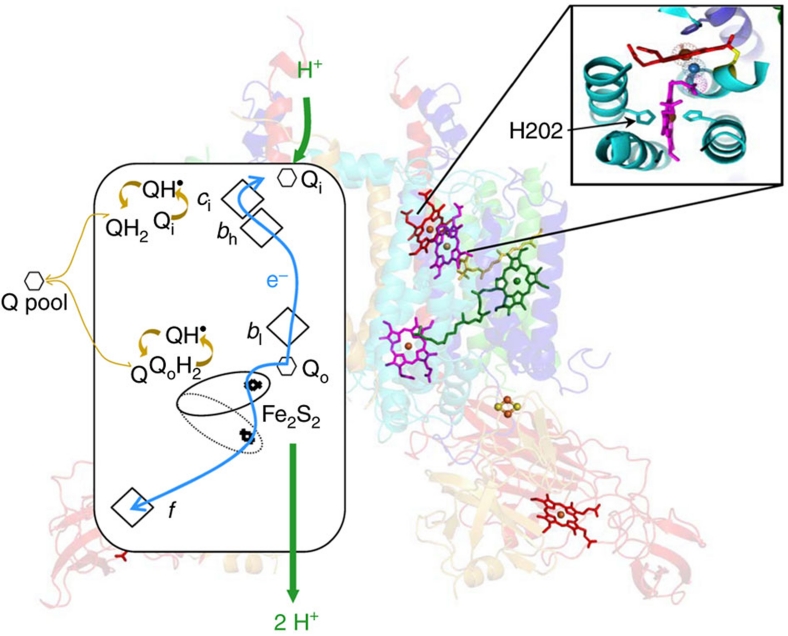
Cytochrome b6f and bc1 are homologous protein complexes having a major role in photosynthetic and respiratory electron transport chains. They contribute to building up the proton motive force via the Q-cycle1,2 depicted in Figure 1 and Supplementary Figure S1. This redox loop couples the consecutive oxidation of two quinols at the Qo site to the reduction of one quinone at the Qi site through the low-potential chain and of two plastocyanins along the high-potential chain. It increases the ratio of H+ pumped per electron transferred and thus the overall energetic efficiency of the complex. In cytochrome b6f, the low-potential chain involves two b haems, bl and bh (the subscripts l and h stand for low and high midpoint potential), and a single covalently bound c-type haem, ci3,4,5,6,7,8, in close vicinity with the bh haem as depicted in Figure 1. In the conditions tested so far, the inactivation of the Qi site of the cytochrome bc1 of purple photosynthetic bacteria forbids photosynthetic growth9,10,11. In the oxygenic photosynthetic chain, attempts to inactivate the Q-cycle by knocking out the bh haem with mutation of its histidine axial ligand have failed until now because, at variance with the bc1 case9, mutation of His202 dramatically decreases the accumulation of the b6f complex12.
Figure 1. The cytochrome b6f dimer operates through a modified Q-cycle.
Left, box schematic; the b6f complex transfers two protons (green arrows) per electron transferred (blue arrows) along high (Fe2S2 cluster, cytochrome f) and low potential chains (bl, bh, ci haems). Quinol (QH2) oxidation at Qo site, Quinone (Q) reduction at Qi site. Right, structure (redrawn from ref. 35) depicting haems b (purple), ci and f (red), Fe2S2 cluster (yellow and orange ball-and-stick model), cytochrome b6 (cyan), subunit IV (blue), Rieske subunit (yellow), cytochrome f (red), PetG, L, M and N subunits (green). Magnification of Qi site comprising bh and ci haems.
Here we engineered in the green alga Chlamydomonas reinhardtii a strain restoring the accumulation of b6f complexes although lacking haems bh and ci. We show that it sustains photosynthetic growth and propose a mechanism accounting for this growth despite a broken Q-cycle.
Results
The QiKO strain has b6f complexes but lacks haems bh and ci
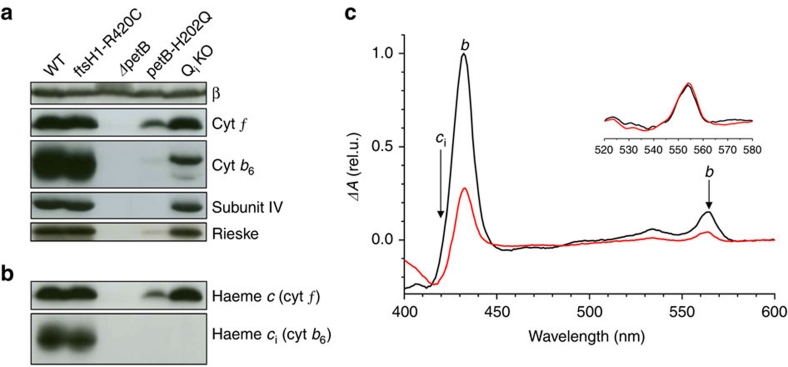
To overcome the accumulation defect resulting from the H202Q mutation in the petB gene encoding cytochrome b6, we genetically combined it with the R420C mutation13,14 in the chloroplast protease FtsH1. The double mutant, referred to as QiKO, contains a wild-type level of cytochrome b6f complex (Fig. 2a,b), which is in marked contrast with the parental single mutant petB-H202Q. We purified by affinity chromatography5 the QiKO b6f complex, which contains, expectedly, a decreased amount of b haem (30%) as shown by the UV–visible spectra in Figure 2c. This lower-than-expected content (30 versus 50%) stems from the instability of the solubilized complex. Indeed, we assessed the amount of remaining b haem in vivo and found that it matched the amount of bl haem from the control strain (Fig. 3a, black filled symbols). Importantly, in addition to lacking the bh haem, the rescued b6f complex also lacked the ci haem as evidenced by Figure 2b,c, as suggested for petB-H202Q6. We thus successfully recovered, in the QiKO strain, a b6f complex with a fully inactivated Qi site without affecting the other cofactors.
Figure 2. Characterization of cytochrome b6f complex in the QiKO strain.
(a) Immunoblot chemiluminescence analysis of the major subunits of cytochrome b6f. Subunit β of chloroplast ATPase as a loading control. QiKO shows wild-type level of all cytochrome b6f subunits with the doublet signature for cytochrome b6 missing the ci haem after SDS-urea PAGE. (b) Covalent haem peroxidasic activity confirms the absence of the ci haem in QiKO strain. (c) Dithionite minus ascorbate spectra from purified b6f complexes. Black, WT; red, QiKO. Haems b (peaks at 434 and 564 nm) and ci (broad band at 425 nm) components36; in QiKO the decreased amplitude at 434 and 564 nm demonstrates the absence of bh and the trough at 420 nm the absence of ci. The spectra have been normalized to the cytochrome f content (inset).
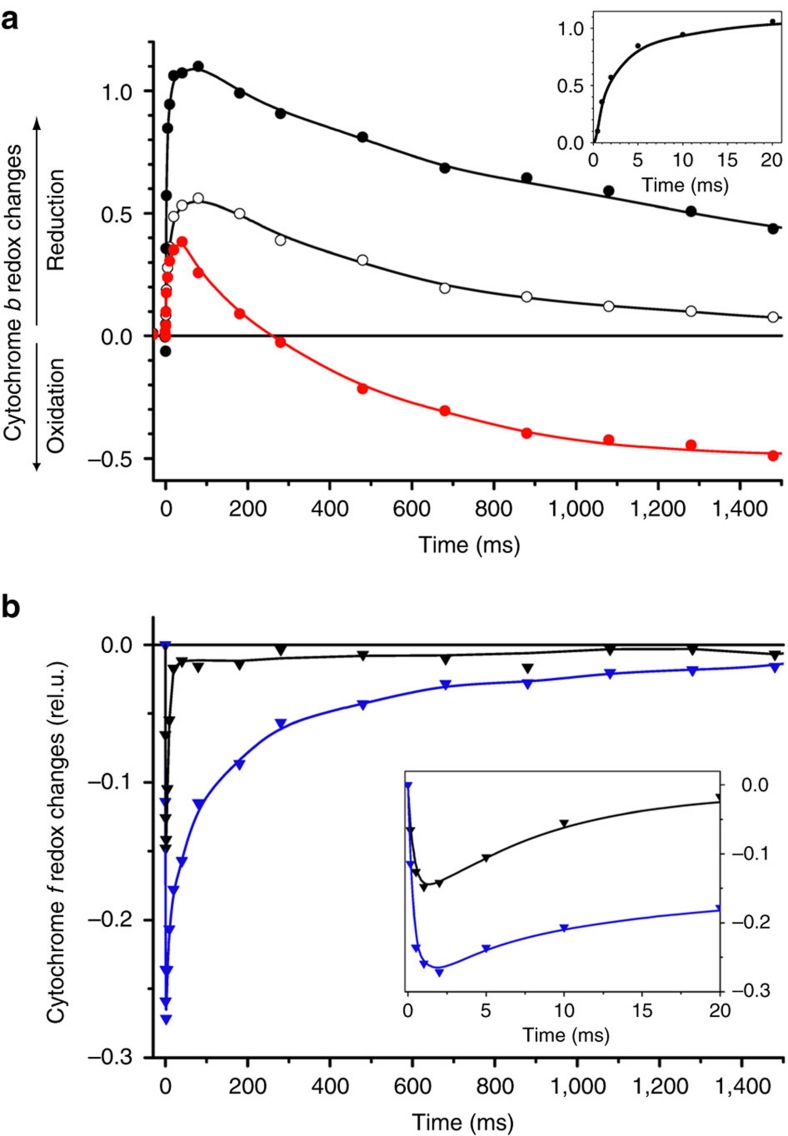
Figure 3. Probing electron transfer in vivo.
(a) Light-induced redox changes of cytochrome b in QiKO. Black, filled symbols, mildly reducing conditions; open symbols, after pre-illumination to get similar contents of pre-reduced and pre-oxidized haem b; red, strongly reducing conditions. Inset: reduction component on a smaller time scale. (b) Light-induced redox changes of cytochrome f in strongly reducing conditions. Black, WT; blue, QiKO, normalized on the photosystem I amount. Re-reduction of cytochrome f in QiKO is rate-limited by the re-oxidation of the bl haem. The reduction of cytochrome f in QiKO is biphasic, with the fast component being similar to the WT one (t1/2∼3 ms) (see inset), and the slow component being concomitant with the oxidation of bl (t1/2∼250 ms).
QiKO has a disabled Qi site but retains a wild-type Qo site
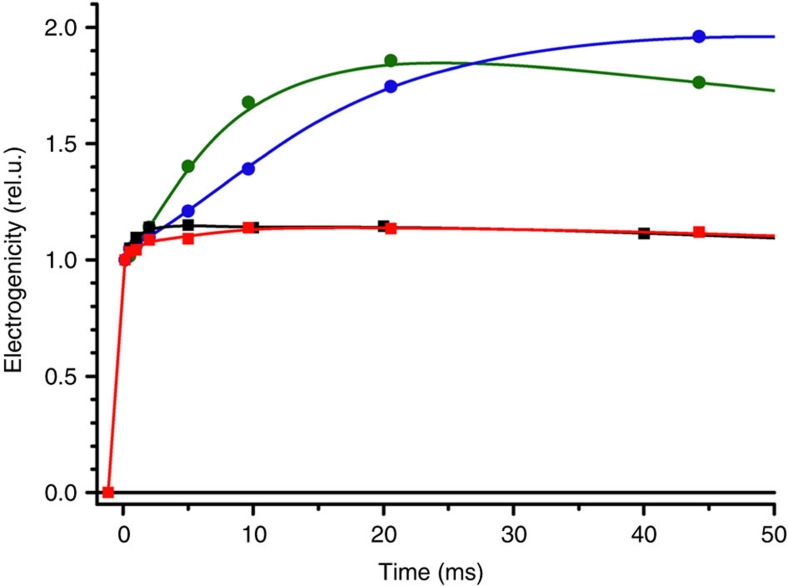
This was further demonstrated by the functional characterization of the cytochrome b6f variant in vivo. The oxidation kinetics of cytochrome f was identical to the wild-type one (Supplementary Fig. S2). Figure 3a shows the transient absorbance changes associated with the redox changes of the b haem. Under mildly reducing conditions (Fig. 3a, black trace and inset), the reduction of a b haem is similar to that of the wild type, with a half-time of ∼2 ms (ref. 15). Thus, the Qo site is not impaired. However, contrary to the wild-type case (Fig. 4, green trace), this reduction is not electrogenic (Fig. 4, black trace), showing that the reduced b haem is on the lumenal side of the membrane and that, as a corollary, the bh haem and the Qi site are indeed knocked out. The QiKO b6f complex is thus genuinely the long-sought variant, inactivated in its Qi site yet retaining a wild type like Qo site, required to assess the dispensable character of the Q-cycle in the photosynthetic chain.
Figure 4. Light-induced electrogenicity in QiKO and WT (520–546 nm).
QiKO (squares) and WT (circles), black and green, mildly reducing conditions; red and blue, strongly reducing conditions.
QiKO sustains phototrophic growth
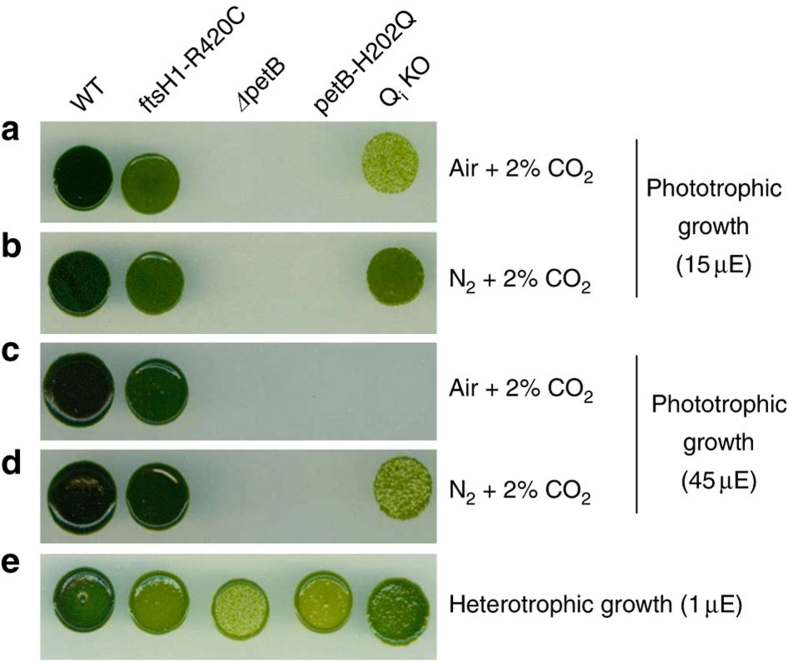
In vitro assay (reduction of plastocyanin in the presence of excess plastoquinol16) showed that the QiKO b6f complex sustains a notable electron-transfer flux. The turnover rate is, taking into account that only 30% of b haem is present, 20±4 s−1, 5% that of wild type (WT)15. Although faint, this flux proved to be vital in essence. Indeed, it sustained photosynthetic growth (Fig. 5 and Supplementary Fig. S3). Figure 5 shows growth efficiencies under moderate illumination, in the presence (a) and absence (b) of oxygen. As expected from the block in the photosynthetic electron-transfer chain, a b6f-lacking mutant showed no phototrophic growth under either condition. This was in contrast to the QiKO strain, which grew moderately but markedly under phototrophic conditions (Fig. 5). Although this phototrophic growth is oxygen sensitive, the b6f-lacking and QiKO strains grew at a rate similar to that of the wild type in the presence of oxygen, which allows mitochondrial respiration, under heterotrophic conditions (acetate) (Fig. 5e). Oxygen per se is thus not detrimental, but the combination of light and oxygen is (Fig. 5c), suggesting that photosynthetic activity over-produces reactive oxygen species in the QiKO strain, as found in the H212N bc1 case11.
Figure 5. Remaining electron flow in QiKO devoid of Q-cycle sustains phototrophic growth with light enhanced oxygen sensitivity.
Cells were plated on minimal medium and grown for 10 days under 15 (a, b) or 45 (c, d) μE m−2 s−1 of light and a controlled atmosphere combining 2% CO2 and 98% air (a, c) and or 98% N2 (b, d) to test phototrophic growth. (e) Cells were plated on acetate medium as heterotrophic growth control at very low light fluences (1 μE m−2 s−1).
The light-induced oxidation of pre-reduced bl
What mechanism underlies the unexpected finding that, despite its inactivated Q-cycle, the QiKO b6f complex sustains a flux compatible with photosynthetic growth? In the mechanistic framework of the Q-cycle, the oxidation of a quinol at the Qo site relies on the bifurcated electron transfer to the oxidized Fe2S2 cluster of the Rieske subunit and to the oxidized bl haem. In the wild type, bl is quickly reoxidized by the bh haem, and thus made available as an electron acceptor for the next quinol oxidation. Consequently, in QiKO, the long-lived reduction of the bl haem (Fig. 3a, black trace) should only allow a single turnover of the Qo site and not a steady flux as observed here.
We thus studied in vivo the function of the Qo site in conditions where a significant fraction of the bl haem was reduced (∼60%, Fig. 3a, red trace) prior to the light activation of the complex. As a fraction of bl haem was pre-reduced, the relative amplitude of the flash-induced reduction phase was smaller than that under oxidizing conditions. Saliently, we observed a net oxidation of a b haem that developed with a ∼250 ms half-time. The Qi site being knocked out, this net oxidation of a b haem was not electrogenic (Fig. 4, red trace). It must thus reflect the electron transfer from the reduced b haem to an electron acceptor produced by the light-induced injection of an oxidizing equivalent, or hole, in the high-potential chain.
In principle, this hole may be borne by cytochrome f, the Fe2S2 cluster or the semiquinone produced at the Qo site. The oxidized cytochrome f can be excluded as it is separated from bl by too large a distance (more than 30 Å) to allow electron transfer in a few hundred ms (ref. 17). The edge-to-edge distance between bl and the Fe2S2 cluster (∼23 Å) is compatible with such an electron-transfer rate. However, 2-iodo-2′,4′,4′-trinitro-3-methyl-6-isopropyl diphenyl ether (DNP-INT), specifically inhibiting quinol access to the Qo site while permitting Fe2S2 head domain movement (Supplementary Fig. S4), prevented bl oxidation (Supplementary Fig. S5). The semiquinone thus stands as the most likely candidate (Fig. 6).
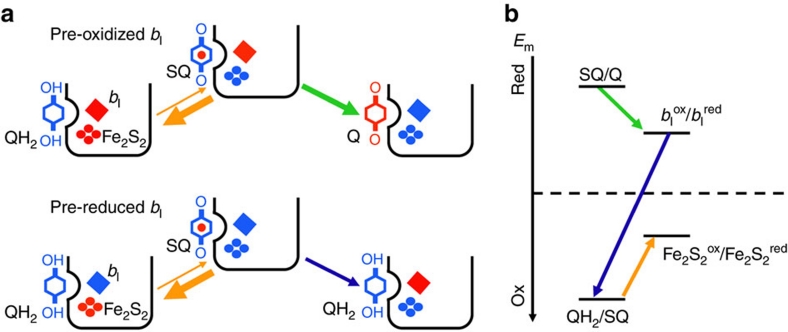
Figure 6. Proposed mechanism for the turnover of the Qo site in QiKO.
(a) Sequence of electron-transfer reactions depending on redox state of bl: blue, reduced and red, oxidized. For simplicity, the semiquinone is depicted as being fully deprotonated. (b) Relative midpoint potential of the different redox couples. The arrows start from the redox couple, which provides the electron donor and point towards the redox couple providing the electron acceptor. The thicker the arrow, the larger the rate of the corresponding reaction.
The dual role of the semiquinone
As depicted in Figure 6, the oxidation of the quinol by the Fe2S2 cluster is thought to be endergonic18,19. Recently, Zhang et al.18 located the midpoint potential of the quinol/semiquinone couple 200 mV above that of the Fe2S2 (at pH 9.0). This makes the equilibrium constant of the forward electron transfer from the quinol to the Fe2S2 cluster much lower than 118,19. Consequently, the concentration of the reactive semiquinone is kept extremely low (see ref. 20) and has remained undetectable under functional conditions21 or barely detectable (0.1–10%) under extreme ones18,19. Notably, in a cytochrome bc1 mutant lacking the bl haem, that is, under even harsher conditions than those described here, the semiquinone has kept elusive11. In most of the currently considered scenarios, even though they may cover different mechanistic details, the formation of the semiquinone resulting from the oxidation of the quinol by the Fe2S2 is strongly uphill in energy and is thus pulled forward by the depletion of its semiquinone product through the subsequent downhill electron transfer to the bl haem18,19,22. As a strong support to this mechanistic scenario, changing the driving force for quinol oxidation by changing the redox properties of the Fe2S2 cluster results in linear changes in the activation energy of the Q-cycle with a slope near unity23. In this sequential scenario (see ref. 22), the injection of an electron into the high-potential chain is effectively driven by the second step, that is, the consumption of the semiquinone, and both reactions are concurrent18,22,24. Importantly, this behaviour is not restricted to the regular function of the Qo site but it also holds when, as we propose, the consumption of the semiquinone involves its reduction by the pre-reduced bl haem. We found accordingly that, the injection of an electron into the high-potential chain, as probed by the redox changes of cytochrome f, paralleled in time the redox changes of the bl haem irrespective of the redox poise, or, in other words, was concomitant with the redox changes of the bl haem (Fig. 3b).
Altogether these findings show that a pre-reduced haem bl hampers, but does not preclude, the injection of an electron in the high-potential chain, that is, the quinol-plastocyanin oxidoreductase activity. A parsimonious mechanistic model accounting for these observations is a ping-pong play in which the semiquinone and the bl haem act, one after the other, as the electron donor and electron acceptor (Fig. 6). This mechanism relies on the dual properties of the semiquinone species, which can act either as an electron-acceptor-yielding quinol or as an electron-donor-yielding quinone. In the currently accepted energy landscape of the bc1 and b6f complexes, the semiquinone is a much stronger electron acceptor than the quinone18,21,22,24 and can thus, on thermodynamic grounds, readily oxidize the bl haem to form the quinol species (Fig. 6b). Notably, it is yet a sluggish process with an overall rate (4 s−1) being several orders of magnitude slower than the theoretically predicted rate (106 s−1)20,25. It is thus kinetically limited, bringing experimental supports to a hypothesized gating mechanism (see refs 19, 20, 25, 26, 27). Interestingly, the overall electron-transfer flux sustained by the QiKO b6f is similar to that found with the antimycin-inhibited cytochrome bc128,29 or the homodimer H212N bc110,30. This suggests that the mechanism proposed here may also apply to the bc1 complex, as considered in refs 28, 29 as one among other possible scenarios.
Discussion
As any energy-converting enzymes, cytochrome bc1 and b6f are prone to undergo short circuits in their reaction pathways. Although expected on thermodynamic grounds, appropriate mechanistic control can relegate these to extremely slow processes and thus make them negligible with regard to their yield. In keeping with this framework, we propose that a short-circuit reaction between the reduced bl and a semiquinone may occur, but that its rate would make it a poor competitor with the forward-productive electron-transfer reactions. Yet, under conditions inactivating the redox loop, the occurrence of such short circuit would provide an 'emergency exit' pathway bypassing the Q-cycle and making it dispensable. It would thereby rescue its quinol-plastocyanin oxidoreductase activity and thus the function of the entire photosynthetic chain.
As mentioned above, the finding that the Q-cycle is dispensable from a mechanistic point of view also applies to the bc1 complex, as such complex with an inhibited Qi site can sustain an overall electron-transfer flux11,28,29,30. However, it is also dispensable from an energetic standpoint in the oxygenic photosynthetic chain, whereas similar mutants of the bc1 complex from photosynthetic purple bacteria forbid photosynthetic growth. A rationale behind this physiological difference may lie in the relative contribution of the two complexes to their respective energy-converting chain. With a fully active Q-cycle, the ratio of H+ transferred across the membrane per electron transferred through the high potential chain increases from 1 to 2. The total H+/e− ratio being 2 in the photosynthetic chain of purple bacteria and 3 in the oxygenic photosynthetic chain (see Supplementary Fig. S1), inactivating the low-potential chain and its associated H+ transfer impacts 'only' a third of the H+/e− in the latter case and up to 50% in the former. In addition, whereas the photosynthetic chain of purple bacteria promotes a cyclic electron transfer, the oxygenic photosynthetic chain allows the linear electron transfer from water to NADP+. As the mechanism we propose preserves linear electron transfer, at least partially, the impaired photosynthetic chain still yields oxygen and reducing power that can fuel the respiratory chain and thereby compensate the decreased production of ATP resulting from the inactivation of the Q-cycle and meet the requirement of the Benson–Calvin cycle in terms of ATP and reduced nicotinamide adenine dinucleotide phosphate (NADPH).
To conclude, the present data show that, as in the bc1 complex case, a disabled Qi site does not completely inhibit the function of the Qo site, which can still sustain an electron-transfer flux. In addition, we show that, at odds with the bc1 complex case, this flux is large enough to allow photosynthetic growth, thus demonstrating that, in the oxygenic photosynthetic chain, the Q-cycle is dispensable from an energetic standpoint.
Methods
Strains and growth conditions
The following C. reinhardtii strains were grown heterotrophically in continuous white light (5 μE m−2 s−1) in Tris–acetate–phosphate medium, pH 7.2 at 25 °C: wild type, deletion of chloroplast petB gene encoding cytochrome b6 ΔpetB31, substitution of bh haem ligand petB-H202Q6,12, His-tag addition in chloroplast petA gene encoding cytochrome f petA-CterH65), substitution of ATP-dependent FtsH protease arginine finger that is essential for ATPase and protease activity13 in nuclear-encoded FtsH1 gene ftsH1-R420C14, and combinations isolated by sexual crosses32 and chloroplast transformation ftsH1-R420C{petB-H202Q} (QiKO), ftsH1-R420C{petA-CterH6} and ftsH1-R420C{petA-CterH6;petB-H202Q}. Growth tests were initiated by spotting 105 cells of log-phase cultures onto agar plates. Plates were placed in tight-sealed chambers applying 2% CO2 and 98% N2 with a gas flowmeter (Aalborg) for anaerobiosis.
Immunoblot analysis
Cell proteins were separated on 12–18% SDS–polyacrylamide gels containing 8 M urea, electrotransferred onto polyvinylidene fluoride membranes, revealed for haem peroxidase activity by femto chemiluminescence, and immunodetected using antibodies against C. reinhardtii proteins by chemiluminescence6.
His-tagged QiKO strain construct
Plasmid pB202QK was constructed by introducing the EcoRV–SmaI fragment of plasmid pUC-atpX-AAD bearing the aadA cassette conferring spectinomycin resistance33 downstream and in the same orientation as the petB gene at the unique NsiI site of plasmid pB202Q carrying the mutation petB-H202Q, and was transformed12 in strain ftsH1-R420C{petA-CterH6} to generate a His-tagged QiKO.
In vitro analysis
Cytochrome b6f complex was purified as in ref.5 and its in vitro activity was assessed as in ref.16 using petA-CterH6 and ftsH1-R420C{petA-CterH6;petB-H202Q} strains after proper non-specific activity subtraction.
Spectroscopic analysis
Electrogenicity of electron transfers and redox changes of cytochromes b and f were assessed by monitoring absorbance at 520, 546, 554, 564 and 573 nm with a JTS10 spectrophotometer (BioLogic). Cytochrome b redox changes were measured at 564 nm with a baseline drawn between 546 and 573 nm. Cytochrome f redox changes were measured at 554 nm with a baseline drawn between 546 and 573 nm and the intensity of the actinic was tuned to hit 30% photosystem I. In addition, the latter were corrected for the contribution of the electrochromic bandshift by subtracting 5% of the absorption changes measured at 520 nm. The cytochrome absorbance changes were calibrated on the basis of normalization to the cytochrome f content in the WT.
Control of the redox poise
Cells were dark-adapted in 20 mM Hepes, 20% Ficoll, pH 7.2. A 5-ns and 1-mJ cm−2 laser flash was used to activate light reactions. To achieve mildly reducing conditions, cells were thoroughly mixed and aerated before absorbance measurement for each wavelength probed. Cells were kept in the sample cell in the dark for a time ranging from 1 to 3 min depending on the strain used and their respiration rate. Strongly reducing conditions were obtained by adding 20 mM glucose, 2 mg ml−1 glucose oxidase to achieve anoxia, and mediators anthraquinone (−100 mV) and anthraquinone-2-sulphonate (−225 mV) at 1 μM to promote redox poising of the cells and thus bl haem reduction34. The sample was kept in the dark for 25 min under complete anoxia. We have checked (not shown) that the first quinone acceptor of photosystem II was fully reduced under such conditions. The time between two consecutive actinic flashes was set at 5 min, to allow the redox equilibration of the samples between flashes. To obtain a similar content of pre-reduced and pre-oxidized b haem in the QiKO strain, the cells were first dark adapted for 25 min under complete anoxia, yielding the strongly reducing case described above, and then submitted to a series of 5 pre-illuminating flashes at 0.2 Hz.
Inhibitors
Two distinct Qo site inhibitors were used: tridecyl-stigmatellin and 2-iodo-2′,4′,4′-trinitro-3-methyl-6-isopropyl diphenyl ether. PSII was inhibited by 1 mM hydroxylamine and 10 μM 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) to determine the PSII:PSI ratio for normalization.
Author contributions
A.M. and C.V. isolated Chlamydomonas reinhardtii strains and performed genetic, molecular, biochemical and physiological analyses; A.M. and F.R. performed spectroscopic analyses; A.M., F.A.W., C.V. and F.R. designed the study, analysed the data and wrote the paper.
Additional information
How to cite this article: Malnoë, A. et al. Photosynthetic growth despite a broken Q-cycle. Nat. Commun. 2:301 doi: 10.1038/ncomms1299 (2011).
Supplementary Material
Supplementary Figures S1-S5 and Supplementary References.
Acknowledgments
We are grateful to J. Girard-Bascou for contributing to ftsH1-R420C mutant isolation, M. Goldschmidt-Clermont and R. Kuras for plasmids pUC-atpX-AAD and pB202Q, Y. Choquet for cloning advice, F. Zito for antibody against subunit IV, Y. Pierre and D. Picot for sharing their expertise in b6f purification, F. Giusti and A. Trebst for Qo site inhibitors TDS and DNP-INT, G. Finazzi for assistance, J. Alric, F. Baymann, D. Picot and F. Zito for discussions, and W.A. Cramer and A. Osyczka for critical reading of the paper. This work was supported by Agence Nationale de la Recherche ANR-07-BLAN-0114 (C.V.) and by Centre National de la Recherche Scientifique/Université Pierre et Marie Curie (UMR 7141).
References
- Mitchell P. The protonmotive Q cycle: a general formulation. FEBS Lett. 59, 137–139 (1975). [DOI] [PubMed] [Google Scholar]
- Crofts A. R. & Meinhardt S. W. A Q-cycle mechanism for the cyclic electron-transfer chain of Rhodopseudomonas sphaeroides. Biochem. Soc. Trans. 10, 201–203 (1982). [DOI] [PubMed] [Google Scholar]
- Lavergne J. Membrane potential-dependent reduction of cytochrome b-6 in an algal mutant lacking Photosystem I centers. Biochim. Biophys. Acta 725, 25–33 (1983). [Google Scholar]
- Kurisu G., Zhang H., Smith J. L. & Cramer W. A. Structure of the cytochrome b6f complex of oxygenic photosynthesis: tuning the cavity. Science 302, 1009–1014 (2003). [DOI] [PubMed] [Google Scholar]
- Stroebel D., Choquet Y., Popot J. L. & Picot D. An atypical haem in the cytochrome b6f complex. Nature 426, 413–418 (2003). [DOI] [PubMed] [Google Scholar]
- de Vitry C. et al. Biochemical and spectroscopic characterization of the covalent binding of heme to cytochrome b6. Biochemistry 43, 3956–3968 (2004). [DOI] [PubMed] [Google Scholar]
- Zatsman A. I. et al. Heme-heme interactions in the cytochrome b6f complex: EPR spectroscopy and correlation with structure. J. Am. Chem. Soc. 128, 14246–14247 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baymann F., Giusti F., Picot D. & Nitschke W. The ci/bH moiety in the b6f complex studied by EPR: a pair of strongly interacting hemes. Proc. Natl Acad. Sci. USA 104, 519–524 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun C. H., Crofts A. R. & Gennis R. B. Assignment of the histidine axial ligands to the cytochrome bH and cytochrome bL components of the bc1 complex from Rhodobacter sphaeroides by site-directed mutagenesis. Biochemistry 30, 6747–6754 (1991). [DOI] [PubMed] [Google Scholar]
- Lanciano P. et al. Intermonomer electron transfer between the low-potential b hemes of cytochrome bc1. Biochemistry 50, 1651–1663 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Ma H. W., Yu L. & Yu C. A. On the mechanism of quinol oxidation at the QP site in the cytochrome bc1 complex: studied using mutants lacking cytochrome bL or bH. J. Biol. Chem. 283, 28767–28776 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuras R. et al. Molecular genetic identification of a pathway for heme binding to cytochrome b6. J. Biol. Chem. 272, 32427–32435 (1997). [DOI] [PubMed] [Google Scholar]
- Karata K. et al. Dissecting the role of a conserved motif (the second region of homology) in the AAA family of ATPases. Site-directed mutagenesis of the ATP-dependent protease FtsH. J. Biol. Chem. 274, 26225–26232 (1999). [DOI] [PubMed] [Google Scholar]
- Malnoë A. et al. Photosynthesis with simplified cytochrome b6f complexes: are all hemes required? Biochim. Biophys. Acta 1797, 19 (2010). [Google Scholar]
- de Lacroix de Lavalette A. et al. Is the redox state of the ci heme of the cytochrome b6f complex dependent on the occupation and structure of the Qi site and vice versa? J. Biol. Chem. 284, 20822–20829 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre Y., Breyton C., Kramer D. & Popot J. L. Purification and characterization of the cytochrome b6f complex from Chlamydomonas reinhardtii. J. Biol. Chem. 270, 29342–29349 (1995). [DOI] [PubMed] [Google Scholar]
- Moser C. C. et al. Nature of biological electron transfer. Nature 355, 796–802 (1992). [DOI] [PubMed] [Google Scholar]
- Zhang H., Osyczka A., Dutton P. L. & Moser C. C. Exposing the complex III Qo semiquinone radical. Biochim. Biophys. Acta 1767, 883–887 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cape J. L., Bowman M. K. & Kramer D. M. A semiquinone intermediate generated at the Qo site of the cytochrome bc1 complex: importance for the Q-cycle and superoxide production. Proc. Natl Acad. Sci. USA 104, 7887–7892 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts A. R. et al. Proton pumping in the bc1 complex: a new gating mechanism that prevents short circuits. Biochim. Biophys. Acta 1757, 1019–1034 (2006). [DOI] [PubMed] [Google Scholar]
- Junemann S., Heathcote P. & Rich P. R. On the mechanism of quinol oxidation in the bc1 complex. J. Biol. Chem. 273, 21603–21607 (1998). [DOI] [PubMed] [Google Scholar]
- Cape J. L., Bowman M. K. & Kramer D. M. Understanding the cytochrome bc complexes by what they don't do. The Q-cycle at 30. Trends Plant Sci. 11, 46–55 (2006). [DOI] [PubMed] [Google Scholar]
- Forquer I. et al. Similar transition states mediate the Q-cycle and superoxide production by the cytochrome bc1 complex. J. Biol. Chem. 281, 38459–38465 (2006). [DOI] [PubMed] [Google Scholar]
- Crofts A. R. & Wang Z. How rapid are the internal reactions of the ubiquinol:cytochrome c2 oxidoreductase? Photosynth. Res. 22, 69–87 (1989). [DOI] [PubMed] [Google Scholar]
- Osyczka A., Moser C. C., Daldal F. & Dutton P. L. Reversible redox energy coupling in electron transfer chains. Nature 427, 607–612 (2004). [DOI] [PubMed] [Google Scholar]
- Rich P. R. The quinone chemistry of bc complexes. Biochim. Biophys. Acta 1658, 165–171 (2004). [DOI] [PubMed] [Google Scholar]
- Ransac S. & Mazat J.- P. How does antimycin inhibit the bc1 complex? A part-time twin. Biochim. Biophys. Acta 1797, 1849–1857 (2010). [DOI] [PubMed] [Google Scholar]
- Muller F., Crofts A. R. & Kramer D. M. Multiple Q-cycle bypass reactions at the Qo site of the cytochrome bc1 complex. Biochemistry 41, 7866–7874 (2002). [DOI] [PubMed] [Google Scholar]
- Borek A., Sarewicz M. & Osyczka A. Movement of the iron-sulfur head domain of cytochrome bc1 transiently opens the catalytic Qo site for reaction with oxygen. Biochemistry 47, 12365–12370 (2008). [DOI] [PubMed] [Google Scholar]
- Swierczek M. et al. An electronic bus bar lies in the core of cytochrome bc1. Science 329, 451–454 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuras R. & Wollman F. A. The assembly of cytochrome b6/f complexes: an approach using genetic transformation of the green alga Chlamydomonas reinhardtii. EMBO J. 13, 1019–1027 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. H. The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use (Academic Press, 1989). [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont M. Transgenic expression of aminoglycoside adenine transferase in the chloroplast: a selectable marker of site-directed transformation of Chlamydomonas. Nucleic Acids Res. 19, 4083–4089 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss D. A. & Rich P. R. The effect of pre-reduction of cytochrome b563 on the electron-transfer reactions of the cytochrome bf complex in higher-plant chloroplasts. Biochim. Biophys. Acta 894, 189–197 (1987). [Google Scholar]
- de Vitry C. & Kuras R. in The Chlamydomonas Sourcebook: Organellar and Metabolic Processes Vol. 2 (ed. Stern, D.) 603–637 (Academic Press, 2008). [Google Scholar]
- Alric J. et al. Spectral and redox characterization of the heme ci of the cytochrome b6f complex. Proc. Natl Acad. Sci. USA 102, 15860–15865 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures S1-S5 and Supplementary References.