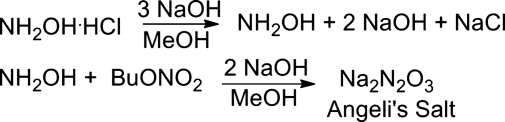Abstract
Nitroxyl (HNO) demonstrates a diverse and unique biological profile compared to nitric oxide, a redox-related compound. Although numerous studies support the use of HNO as a therapeutic agent, the inherent chemical reactivity of HNO requires the use of donor molecules. Two general chemical strategies currently exist for HNO generation from nitrogen-containing molecules: (i) the disproportionation of hydroxylamine derivatives containing good leaving groups attached to the nitrogen atom and (ii) the decomposition of nitroso compounds (X-N=O, where X represents a good leaving group). This review summarizes the synthesis and structure, the HNO-releasing mechanisms, kinetics and by-product formation, and alternative reactions of six major groups of HNO donors: Angeli's salt, Piloty's acid and its derivatives, cyanamide, diazenium diolate-derived compounds, acyl nitroso compounds, and acyloxy nitroso compounds. A large body of work exists defining these six groups of HNO donors and the overall chemistry of each donor requires consideration in light of its ability to produce HNO. The increasing interest in HNO biology and the potential of HNO-based therapeutics presents exciting opportunities to further develop HNO donors as both research tools and potential treatments. Antioxid. Redox Signal. 14, 1637–1648.
Introduction
The International Union of Pure and Applied Chemistry designates the simple triatomic molecule nitroxyl (HNO) as hydrogen oxonitrate, which also has been referred to as nitrosyl hydride and commonly as HNO, especially with regard to its biological actions. HNO demonstrates a distinct chemical and biological profile compared to other redox-related nitrogen oxides such as hydroxylamine (NH2OH), nitric oxide (NO), nitrite (NO2−), and nitrate (NO3−) (32, 42, 64). These chemical and biological differences, particularly in contrast to the well-known signaling agent NO, have focused attention on HNO as a potential endogenous biochemical mediator and therapeutic entity for various physiological disorders.
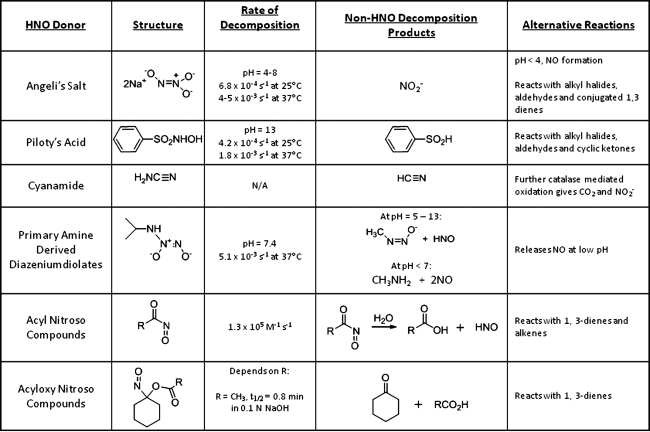
Despite the recent interest in HNO biology and chemistry, endogenous HNO production eludes conclusive description (32, 42). The biochemical conversion of L-arginine to L-N-hydroxyarginine to NO, which is catalyzed by NO synthase (NOS), provides a potential HNO-forming pathway. In the absence of the redox cofactor tetrahydrobiopterin, NOS catalyzes HNO formation from L-arginine (in vitro) supporting the possibility of this route for endogenous HNO formation (91). The distinct and controlled biological activity observed with HNO donors suggests a natural role for HNO, but HNO reactivity and the lack of specific detection methods hinder the confirmation of in vivo HNO production (32). Even though endogenous HNO production remains undefined, the identification of HNO formation from both cyanamide and hydroxyurea, clinically used drugs, coupled with the specifically observed responses of the cardiovascular system to HNO donors clearly identifies the potential of HNO donors as new therapeutics (22, 44). Indeed, the recent patent literature reflects the developing interest of new HNO donors as therapeutics as well as HNO-based strategies for disease treatment (31, 45, 85, 86). Given this dual importance and interest in HNO donors as basic chemical, biochemical, and pharmacological tools, as well as potential therapeutics, this review summarizes the general chemistry of six known classes of HNO donors (Fig. 1). Specifically, the preparation, structure, and HNO release mechanism (including reaction by-products) and kinetics of each donor will be considered. In addition, any known alternative reactions of the HNO donor, which could interfere with its HNO release properties, will be summarized. As interest in HNO as a therapeutic agent continues to grow, a complete understanding of the chemistry of HNO donor compounds will become increasingly important in the development of new HNO-based treatments.
FIG. 1.
Six classes of nitroxyl donors.
Chemistry and Biology of HNO
Excellent reviews summarize the known chemistry of HNO (11, 55). In particular, experimental and theoretical studies indicate that HNO predominantly exists in the protonated (HNO) form in aqueous solution at physiological pH with an approximate pKa of 11.4 (Fig. 1) (7, 8, 74). Analogous to oxygen, theory predicts the nitroxyl anion (NO−) to possess a triplet ground state, which kinetically retards HNO deprotonation (7, 8, 75). The relatively high pKa of HNO and slow deprotonation, combined with the thermodynamically unfavorable reduction of NO to 3NO−, permits HNO to exist as a chemically reactive species in biological systems (Fig. 2) (59). Similar to C-nitroso compounds and lower molecular weight aldehydes, such as formaldehyde, HNO dimerizes with a rate constant of k=8×106 M−1s−1 to yield hyponitrous acid (H2N2O2) that dehydrates to nitrous oxide (N2O) (Fig. 2) (11). This reaction directly and irreversibly consumes HNO preventing the storage or direct use of HNO solutions and necessitates the use of HNO donors or HNO-generating systems for the examination of HNO chemistry or biology.
FIG. 2.
Basic nitroxyl (HNO) chemistry.
The structure of HNO permits reaction with a number of important biomolecules and these reactions provide the chemical basis of the observed biological actions of HNO (7, 59). The N=O bond dipole and small size allow HNO to act as a potent electrophile and HNO readily reacts with many nucleophiles, including amines, thiols, and phosphines to generate diazenes, disulfides or sulfinamides, and aza-ylides, respectively (24, 50, 69). The electron lone pairs on both nitrogen and oxygen permit HNO to interact with various Lewis acids, particularly metals, and HNO exhibits diverse chemistry with many metal-containing proteins. Deoxygenated ferrous heme proteins, such as myoglobin (Mb), directly complex HNO to give coordination complexes demonstrating HNO's ability to act as a ligand in these systems (29). The Mb-HNO complex shows excellent stability under anaerobic conditions and rapidly reacts with oxygen, nitrite, or NO to give oxidized ferric Mb (83). HNO reductively nitrosylates ferric heme proteins, such as ferric Mb, yielding the corresponding ferrous–nitrosyl complex (9, 24, 55). HNO rapidly reacts with oxygenated ferrous heme proteins, such as oxygenated hemoglobin (Hb) or Mb, to generate the oxidized ferric heme protein and nitrate (24). The known chemical reactivity of HNO including its reaction as an electrophile, antioxidant, and with heme proteins has been demonstrated through the use of HNO donors and these reactions have been reviewed (46, 55, 58). In addition to providing the basis of the observed biological actions of HNO, these reactions also form the foundation of the most commonly utilized HNO detection methods.
These reactions (including dimerization) of HNO necessitate the use of donors to unravel HNO's distinct biological action and the biology and pharmacology of HNO has been extensively reviewed (32, 42, 64).
HNO Donor Chemistry
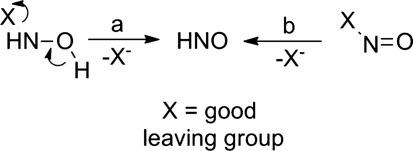
Understanding current HNO donors and the development of new HNO-liberating entities requires consideration of both basic nitrogen and HNO chemistry. Currently, two general principal strategies exist for HNO formation from organic and inorganic molecules (Fig. 3) (46). First, hydroxylamine derivatives that possess good leaving groups attached to the nitrogen atom have traditionally found use as HNO donors (Angeli's salt and Piloty's acid, vide infra, Fig. 3, path a). The nitrogen atom of HNO possesses a formal+1 oxidation state (NO has a+2 nitrogen oxidation state), but hydroxylamine (NH2OH) contains a nitrogen atom with a −1 oxidation state. Disproportionation directly converts these compounds from the N=− 1 to the N=+ 1 oxidation state. Second, the decomposition of nitroso compounds (X-N=O, where X again represents a reasonable leaving group, vide infra, Fig. 3, path b) generates HNO. These compounds already contain N in the formal+1 oxidation state and do not require further redox chemistry but undergo other nonredox reactions (such as hydrolysis) to generate HNO. The formation of the nitroso compound itself may occur through other multiple mechanistic pathways that include disproportionation, oxidation, and hydrolysis. Two-electron oxidation of hydroxylamine (NH2OH) provides a direct pathway to HNO absent of other donor-derived by-products. These chemical approaches apply to currently known HNO donors and remain applicable to the design and development of new HNO-releasing compounds (46).
FIG. 3.
Chemical strategies for HNO generation. a, HNO formation from hydroxylamine derivatives. b, HNO formation from nitroso compounds.
Angeli's Salt
Structure/synthesis
The Italian scientist Angeli first synthesized sodium trioxodinitrate (Na2N2O3, CAS 13826-64-7) in 1896 and this inorganic salt that bears his name is commercially available (3). Condensation of hydroxylamine with an organic nitrate allows the direct synthesis of Angeli's salt (Fig. 4) (46). Treatment of hydroxylamine hydrochloride with excess sodium hydroxide to yield hydroxylamine followed by the addition of butyl nitrate gives Angeli's salt as a white solid (mp=284°C, Fig. 4) (46). Angeli's salt displays a UV-vis absorbance with a λmax at 248 nm (ɛ=8.2×103 M−1 cm−1) (46, 52). X-ray crystallography shows Angeli's salt contains a N=N double bond making the overall molecule planar (41). A procedure for the preparation of 15N-labeled Angeli's salt (O15NNO2)2− has been reported (13). Numerous studies of HNO chemistry, biology, and medicine utilize Angeli's salt as an HNO source and its chemistry has been reviewed (55, 58).
FIG. 4.
Synthesis of Angeli's salt.
Mechanism/rate of HNO release/by-products
Figure 5 shows the generally accepted mechanism for the decomposition of Angeli's salt to singlet HNO in aqueous solution from pH 4 to 8 (13, 26, 39–41, 56). Initial protonation occurs at the nitroso group oxygen followed by a rate-limiting tautomerization to give a monoanion protonated at the nitroso nitrogen atom, a structure supported by 15N NMR and Raman spectroscopy (Fig. 5) (10). Heterolytic cleavage of the N–N bond yields the observed products HNO and nitrite (Fig. 5), and this decomposition represents an example of HNO formation from the disproportionation of a hydroxylamine derivative with a good leaving group attached to N (Fig. 3, path a) (13, 26, 39, 52, 56). Theoretical studies support such a pathway and further labeling studies using 15N Angeli's salt show that HNO derives from the nitroso nitrogen atom and nitrite from the nitro group (13). Excess nitrite slightly inhibits this reaction revealing the reversibility of Angeli's salt decomposition (82). As nitrite demonstrates its own biological profile, nitrite formation from Angeli's salt decomposition must be considered in experiments using this HNO donor (33).
FIG. 5.
Decomposition of Angeli's salt.
Angeli's salt decomposes in a first-order and pH-dependent fashion related to the two pKa's of oxyhyponitrous acid (2.5 and 9.7, respectively) (82). From pH 4–8, Angeli's salt decomposes by first-order kinetics with rate constants of 6.8×10−4 s−1 at 25°C and 4–5×10−3 s−1 at 37°C (52). Above pH 8, the rate of Angeli's salt decomposition decreases, allowing the preparation of alkaline Angeli's salt stock solutions for short-term storage (55). UV-vis spectroscopy allows direct kinetic measurement of Angeli's salt decomposition by monitoring the disappearance of the absorbance at λmax=248 nm (ɛ=8.2×103 M−1 cm−1) for N2O32− or the disappearance of the absorbance at λmax=237 nm (ɛ=5.5 or 6.1×103 M−1 cm−1) for HN2O3−. A manganese (III) porphyrin complex, designed as an HNO trap, directly interacts with Angeli's salt (dianion form) catalyzing HNO and nitrite formation (53). Overall, the kinetic properties, water-solubility, commercial availability, and ease of handling make Angeli's salt the most popular choice as a donor to examine HNO chemistry and biology.
Alternative reactions
Below pH 4, Angeli's salt does not form HNO and nitrite but generates NO as the only nitrogen-containing product (87). Mechanistically, NO formation may arise from an unstable di-protonated isomer of H2N2O3 that decomposes to water and excited state NO dimer or through subsequent reactions of nitrous acid (HNO2) with H2N2O3 (26, 39). Regardless of mechanism, NO formation from Angeli's salt only occurs at low pH. Angeli's salt also directly reacts with various metal complexes, such as hexaammineruthenium (III) or hexacyanoferrate (III), to generate NO under basic conditions (2).
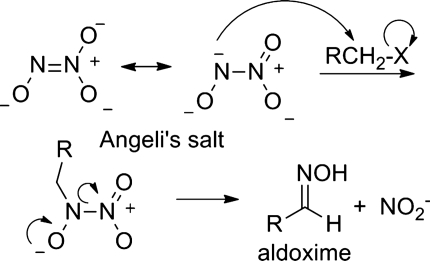
Angeli's salt demonstrates reactivity with a number of organic compounds, including alkyl halides, aldehydes, and conjugated 1,3-dienes. Early work to determine the reactivity of HNO with alkyl halides and aldehydes actually reveals that Angeli's salt directly reacts with these functional groups to give the observed products (aldoximes and hydroxamic acids) (79). Figure 6 shows the proposed reaction of Angeli's salt and a generic primary alkyl halide to generate an aldoxime. Direct N-alkylation of Angeli's salt (with the reactive resonance species shown) gives the N-alkyl nitro intermediate that decomposes to generate a C-nitroso compound that immediately tautomerizes to the aldoxime and nitrite. Angeli's salt directly reacts with aldehydes through a similar mechanism to generate an unstable tetrahedral intermediate that decomposes to the hydroxamic acid and nitrite (79). A Diels-Alder cycloaddition between Angeli's salt and a reactive 1,3-diene (1,3-diphenylisobenzofuran) generates an N-O-containing cycloadduct characterized by X-ray crystallography (84). These authors suggest that the reaction occurs from the monoprotonated form of Angeli's salt, which would indicate alternative reactivity besides HNO release (84). While these reactions occur in organic solvents or basic aqueous conditions and under nonphysiological conditions, they clearly demonstrate that Angeli's salt possesses other chemistries that should be considered.
FIG. 6.
Reaction of Angeli's salt with alkyl halides.
Piloty's Acid
Structure/synthesis
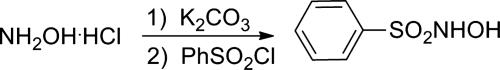
First reported in 1896, Piloty's acid (N-hydroxybenzenesulfonamide, C6H5SO2NHOH, CAS 599-71-3) is available commercially and can also be synthesized by condensation of hydroxylamine with benzenesulfonyl chloride to give a solid product in 45%–55% yield after column chromatographic purification (Fig. 7) (12, 71). A similar procedure yields 15N-labeled Piloty's acid (12). Various reviews address Piloty's acid's chemistry and HNO release properties (55, 58).
FIG. 7.
Synthesis of Piloty's acid.
Mechanism/rate of HNO release/by-products
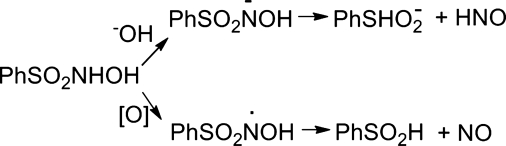
Figure 8 depicts the generally accepted mechanism for the decomposition of Piloty's acid in basic conditions to singlet HNO and benzenesulfinate, and provides another example of HNO formation from a hydroxylamine derivative with a good leaving group at nitrogen (Fig. 3, path a) (12). Kinetic studies and 15N NMR experiments show that initial deprotonation occurs at the nitrogen atom to give the N-anion (structurally similar to monoprotonated Angeli's salt) that undergoes S–N bond heterolysis to yield the products (12). The benzenesulfinate ion, the organic by-product, should act as a good nucleophile and modest reducing agent and these properties should be considered in biological experiments. In base, Piloty's acid decomposes by a first-order process with rate constants at pH 13 of 4.2×10−4 s−1 at 25°C and 1.8×10−3 s−1 at 37°C, which are comparable to the rate constant for decomposition of Angeli's salt at neutral pH (12, 72). At neutral pH, the rate of HNO release significantly decreases, and under aerobic conditions Piloty's acid undergoes oxidation to the nitroxide radical that forms NO rather than HNO (Fig. 8) (95). The slow rate of HNO release and production of NO severely limit the use of Piloty's acid as an HNO donor under neutral aerobic conditions.
FIG. 8.
Decomposition of Piloty's acid at basic pH.
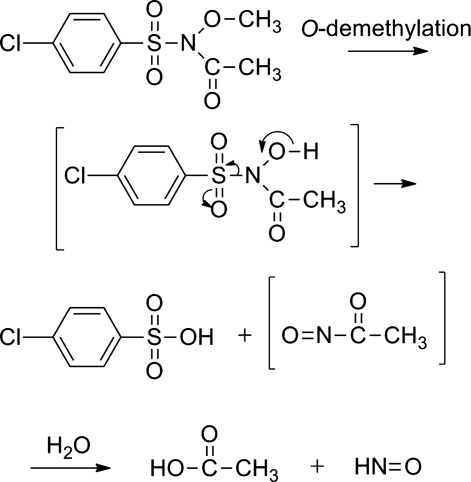
Other simple N-hydroxysulfonamides, such as N-hydroxymethanesulfonamide (CH3SO2NHOH), release HNO under basic conditions and find some use as HNO donors (76). Recent patents describe the HNO-releasing properties of a large number of Piloty's acid derivatives with various aromatic substitutions at different positions or based upon different aromatic heterocycles (85, 86). Gas chromatographic headspace analysis of nitrous oxide release shows that some of these derivatives release significant amounts of HNO at neutral pH under anaerobic conditions (85, 86). A number of N-, O-diacylated or alkylated derivatives of Piloty's acid have been prepared as HNO pro-drugs and potential inhibitors of aldehyde dehydrogenase (ALDH) (58). Following ester hydrolysis or O-dealkylation, these compounds generate a reactive acyl nitroso compound and release HNO through hydrolysis, therefore providing an example of HNO generation from hydrolysis of a nitroso compound (Figs. 9 and 3, path b) (58). For example, O-demethylation of the chlorpropamide derivative shown in Figure 9 yields an N-hydroxy intermediate that decomposes to the sulfinic acid and an acyl nitroso compound that undergoes hydrolysis to HNO (58). Similar O-alkylated or O-acylated precursors of N-hydroxysaccharin also release HNO under basic conditions through a similar pathway (Fig. 9) (58). Attempts to produce N-, O-diacylated Piloty's acid derivatives with different acyl groups lead to the formation of carboximidic acid derivatives that also release HNO upon esterase-mediated hydrolysis (76). Finally, N-hydroxysulfonimidamides and N-(diphenylphosphinoyl)hydroxylamine, the phosphorus analog of Piloty's acid, both release HNO through pathways similar to Piloty's acid, further supporting HNO formation through decomposition of properly constructed hydroxylamine derivatives (Fig. 3, path a) (65, 90).
FIG. 9.
HNO release from N-, O- modified Piloty's acid derivatives.
Alternative reactions
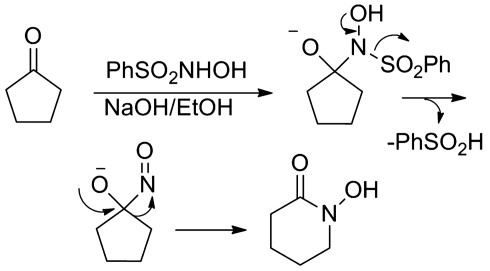
In addition to oxidation at neutral pH to form NO via the nitroxide radical (Fig. 8), Piloty's acid directly reacts under basic conditions with alkyl halides and aldehydes to give aldoximes and hydroxamic acids (79, 95). These reactions appear to proceed through a similar mechanism as the reactions of these functional groups with Angeli's salt (Fig. 6) with the final step being the tautomerism of a C-nitroso compound to the aldoxime or hydroxamic acid (79). These reactions form the basis of the Angeli-Rimini colorimetric test for aldehydes and since they occur under basic conditions, directly compete with HNO generation (70). Under basic conditions, Piloty's acid also reacts with cyclic ketones to give the cyclic hydroxamic acid and a recent study explores the synthetic potential of this transformation with substituted cyclobutanone and cyclopentanone substrates (6, 63). Figure 10 provides a mechanism for the reaction of Piloty's acid and a cyclic ketone. Addition of the N-anion of Piloty's acid generates a tetrahedral intermediate that eliminates benzenesulfinic acid to give a C-nitroso intermediate (Fig. 10) (6). This C-nitroso intermediate may eliminate HNO (regenerating the ketone) or rearrange to the cyclic hydroxamic acid (Fig. 10). Treatment of aldehydes under neutral conditions in methanol with Piloty's acid yields the dimethyl acetals, revealing that Piloty's acid acts as an acidic catalyst under these nonaqueous conditions (34). Piloty's acid H-bonding, acid–base, and redox properties should be kept in mind when using Piloty's acid or its derivatives as HNO donors.
FIG. 10.
Reaction of Piloty's acid with cyclic ketones.
Cyanamide
Structure/synthesis
Cyanamide (H2N-CN, CAS 420-04-2) has been used to treat alcoholism in Europe, Canada, and Japan (32). A number of studies show that cyanamide elicits its effects by acting as a pro-drug that requires oxidative bioactivation to release HNO, which modifies the active-site thiol of ALDH inhibiting normal ethanol metabolism (22, 60). Cyanamide can be purchased commercially and the addition of water to calcium cyanamide gives hydrogen cyanamide as a solid (mp=42°C–44°C) (49). The syntheses of both 13C-labeled and 15N-labeled cyanamide have been reported (60).
Mechanism/rate of HNO release/by-products
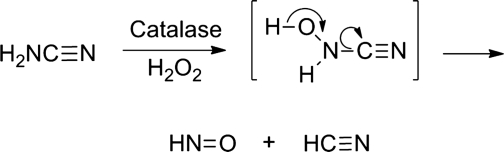
Detailed chemical and biochemical studies outline the mechanistic pathway of HNO release from cyanamide (22, 60, 77). Oxidation of cyanamide with catalase and a hydrogen peroxide-generating system forms unstable N-hydroxycyanamide through a catalase-mediated N-hydroxylation (Fig. 11) (60). N-Hydroxycyanamide directly decomposes to HNO and HCN (Fig. 11), another example of a hydroxylamine derivative with a good leaving group attached to nitrogen decomposing to HNO (Fig. 3, path a) (61). 13C NMR spectroscopy studies using 13C-labeled cyanamide clearly identify cyanide as a by-product of catalase-mediated cyanamide oxidation (60). While metabolic activation of cyanamide forms the cyanide ion, evidence of cyanide toxicity during cyanamide use has generally not been reported (32). Gas chromatographic analysis of the reaction headspace using both 14N- and 15N-labeled cyanamide identifies nitrous oxide as evidence of HNO formation and the nitrogen atoms of cyanamide as the ultimate source of HNO (60). Although N-hydroxycyanamide provides an alternative structural platform for new HNO donors, the possibility of cyanide formation severely limits the development of these compounds as new therapeutics. Few cyanamide derivatives have been reported, but the treatment of N-, O-dibenzoyl hydroxylamine with cyanogen bromide gives N-, O-dibenzoyl-N-hydroxycyanamide (58). Although this N-hydroxycyanamide derivative would be expected to release HNO upon ester hydrolysis, the HNO release properties of this compound have not been reported.
FIG. 11.
Bioactivation of cyanamide to HNO.
Alternative reactions
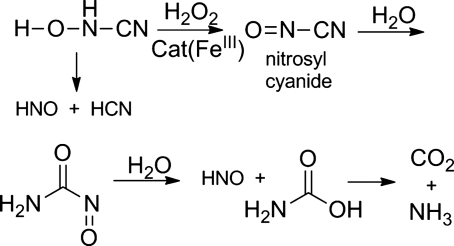
Further work on the mechanism of cyanamide bioactivation reveals that carbon dioxide and nitrite also form during the catalase-mediated oxidation of cyanamide (77). These studies indicate that this system further oxidizes the N-hydroxycyanamide intermediate to nitrosyl cyanide (ONCN), which reacts with water at the cyano group to form an acyl nitroso compound (77). The acyl nitroso compound hydrolyzes to HNO and a carbamic acid, which decomposes to carbon dioxide and ammonia (Fig. 12) (77). In a separate pathway, water adds to the nitroso group directly generating nitrite and cyanide without HNO formation (77). These further reactions reveal more complexity in HNO formation from cyanamide, including the production of nitrite (similar to Angeli's salt), and may partially explain the lack of cyanide toxicity in patients taking cyanamide.
FIG. 12.
Catalase-mediated oxidation of N-hydroxycyanamide.
Diazeniumdiolate-Based Donors
Structure/synthesis
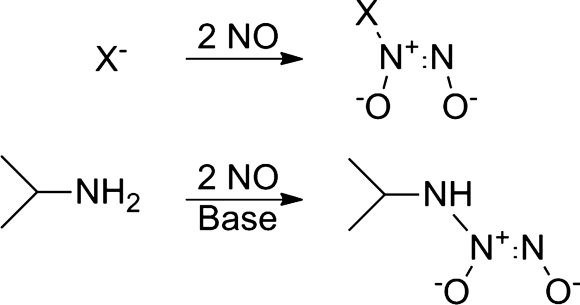
Complexes of two equivalents of NO with a carbon, oxygen, sulfur, or nitrogen nucleophile generate diazeniumdiolates (NONOates), which contain the N(O)NO− moiety (Fig. 13) (25). Secondary amine-derived NONOates generally release NO under neutral and acidic conditions and have found wide use as NO donors with therapeutic promise (57). Angeli's salt, which releases HNO at physiological pH and NO below pH 4, possesses the formal NONOate structure when the nucleophile is oxide (O2−) (57). Complexes of primary amines and the NO dimer also generate NONOates and Figure 13 shows the isopropylamine NO adduct (isopropylamine diazeniumdiolate [IPA/NO]). IPA/NO releases both HNO and NO at pH 7.4, as judged by chemiluminescent and electrochemical studies, and only NO below pH 3 (57). Under physiological conditions, IPA/NO primarily acts as an HNO donor by reductively nitrosylating ferric Mb in a thiol-sensitive fashion (57). In dogs, IPA/NO demonstrates a pharmacological profile similar to Angeli's salt by producing positive inotropic effects and increased plasma levels of calcitonin gene-related peptide (CGRP) but not cyclic guanylate monophosphate (57). Treatment of isopropylamine with NO under pressure in the presence of base gives IPA/NO as a solid (mp=71°C), which displays a UV-vis absorbance λmax at 252 nm, ɛ=8.7×103 M−1 cm−1 (25, 52).
FIG. 13.
Basic structure/synthesis of diazeniumdiolates including isopropylamine diazeniumdiolate (IPA/NO).
Mechanism/rate of HNO release/by-products
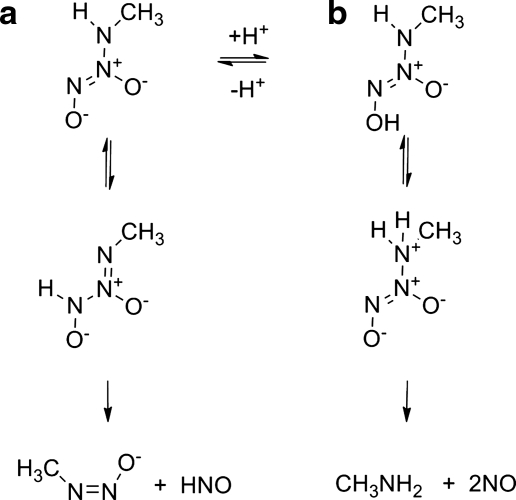
Kinetic studies show that IPA/NO decomposes in a first-order manner with a rate constant at pH 7.4 of 5.1×10−3 s−1 at 37°C (comparable to the rate constant for decomposition of Angeli's salt at pH 4 to 8) (52). Figure 14 gives the proposed mechanism, based on theoretical calculations using methylamine diazeniumdiolate (MA/NO) as a model, for IPA/NO decomposition to HNO (and NO under acidic conditions) (27). At pH 5–13, HNO release occurs through the decomposition of an unstable tautomer [MeNN+(O−)NHO−] of IPA/NO in a process that also generates the N-nitrosated primary amine (Fig. 14, path a) (27). The decomposition of this tautomer fits the pattern of HNO release from a hydroxylamine derivative substituted with a good leaving group at the nitrogen atom (Fig. 3, path a). At lower pH (below 7), protonation of IPA/NO at the amine nitrogen gives an unstable intermediate that decomposes to two equivalents of NO and the primary amine (Fig. 14, path b) (27).
FIG. 14.
pH-dependent decomposition of IPA/NO to (a) HNO and (b) NO.
Alternative reactions
While IPA/NO generates both HNO and NO formation at neutral pH, the biological profile of this primary amine-derived NONOate appears quite similar to Angeli's salt and consistent with an HNO-based effect. The current mechanism suggests that manipulation of the basicity of the starting amine used to generate the NONOate may control the proportion of HNO to NO generated. The release of a primary amine N-nitroso compound may be of toxicological concern (57). The large amount of structural variety available within this class (primary amine starting materials) further warrants detailed exploration.
Acyl Nitroso Compounds
Structure/synthesis
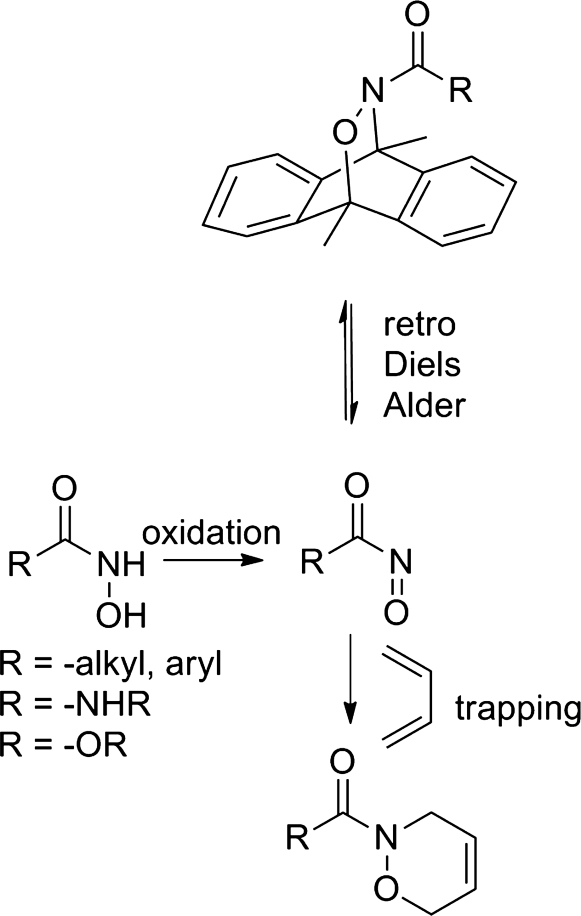
Acyl nitroso compounds [RC(=O)NO] are highly reactive species and no stable or isolable examples of acyl nitroso compounds have been reported (Fig. 15). Time-resolved infrared spectroscopic measurements give evidence (infrared resonances at 1735, 1590, 1560, and 1235 cm−1) for the first direct observation of an acyl nitroso compound in solution (18). The observed decay of these resonances gives a lifetime on the order of 1 ms for these species in organic solvent (18). Oxidation of N-acyl hydroxylamine derivatives (hydroxamic acids, N-hydroxyureas, or N-hydroxycarbamates) with various reagents (sodium periodate, tetra-alkyl ammonium periodate, Swern, Dess-Martin periodinane, and metal complexes) provides the most direct method of acyl nitroso compound formation (17, 36). Acyl nitroso compounds rapidly react with conjugated 1,3-dienes as an N-O- heterodienophile to produce cycloadducts, whose structure strongly supports the intermediacy of the acyl nitroso compound (Fig. 15) (47). The horseradish peroxidase (HRP) and catalase-mediated oxidation of hydroxyurea in the presence of hydrogen peroxide forms the corresponding acyl nitroso compound, which has been trapped using 1,3-cyclohexadiene (38). This sequence indicates that these reactive intermediates can be enzymatically generated in aqueous conditions. Cycloadducts of different acyl nitroso compounds and 9,10-dimethylanthracene undergo a retro-Diels-Alder reaction with half-lives between 0.25 and 2.6 h at 40°C. This method provides a nonoxidative method for the formation of acyl nitroso compounds (Fig. 15) (17, 92). Modification of the hydroxyurea portion of these cycloadducts gives water-soluble and photoactivatable compounds with enhanced acyl nitroso (and HNO)-generating capabilities (1, 96). Other reactions that generate acyl nitroso species include the photolysis or thermolysis of 1,2,4-oxadiazole-4-oxides, exposure of 1,2,4-oxadiazole-4-oxides to a nitrile oxide, the addition of N-methyl morpholine N-oxide to nitrile oxides and the decomposition of N-, O-diacylated or alkylated N-hydroxyarylsulfonamides and have been reviewed (Fig. 9) (58).
FIG. 15.
Generation of acyl nitroso compounds.
Mechanism/rate of HNO release/by-products
Reaction of an acyl nitroso compound with a nucleophile presumably generates HNO through a nucleophilic acyl substitution mechanism that includes a tetrahedral intermediate with decomposition to HNO and the corresponding carboxylic acid derivative as the by-product (Fig. 16). Time-resolved infrared spectroscopic measurements show that an acyl nitroso compound (R=− Ph) reacts with diethyl amine to give the corresponding amide with a derived second order rate constant of 1.3×105 M−1 s−1 (18). In support of this pathway (Fig. 3, pathway b), the oxidation of a hydroxamic acid in water or in the presence of an amine yields carboxylic acids and amides, respectively (4, 78). The identification of nitrous oxide in these reactions provides strong evidence for HNO generation. Oxidation of hydroxyurea with aqueous sodium periodate in the absence of any 1,3-diene rapidly produces nitrous oxide, carbon dioxide, and ammonia (93). Catalase and HRP-mediated oxidation of hydroxyurea generate an acyl nitroso compound that hydrolyzes to HNO and carbamic acid, which decomposes to carbon dioxide and ammonia (Fig. 16) (37, 38). The nascent HNO dimerizes and dehydrates to nitrous oxide in the HRP system but reductively nitrosylates catalase distinguishing these ferric heme proteins (37, 38). HNO formation through ferric heme protein-mediated oxidation of nitrogen-containing substrates has recently been reviewed (68).
FIG. 16.
HNO formation from acyl nitroso compounds.
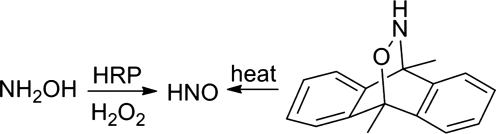
Application of similar strategies (oxidation/retro Diels-Alder reactions) to hydroxylamine or HNO-derived cycloadducts present methods for the direct generation of HNO (Fig. 17). Hydroxylamine rapidly reacts with heme proteins, including oxy, deoxy, and methemoglobin to produce NO (44, 51, 80, 89). Treatment of hydroxylamine with HRP in the presence of hydrogen peroxide generates HNO as determined by HNO trapping with glutathione and high-performance liquid chromatography/mass spectrometric identification of glutathione sulfinamide (23). Gas chromatographic identification of nitrous oxide in the headspace of this reaction further confirms HNO formation. In this system, hydrogen peroxide activates HRP to a reactive intermediate that oxidizes hydroxylamine to HNO (Fig. 17) (23). Hydroxylamine, in the presence of catalase and a hydrogen peroxide-generating system, activates sGC (20). EPR spectroscopic examination of this reaction reveals the formation of the Cat(FeII)NO complex, which forms during the reaction of HNO and ferric catalase (20). Given the inability of NO to reduce ferric catalase, these results strongly imply initial HNO formation followed by reductive nitrosylation (35). A cycloadduct of HNO and 9,10-dimethylanthracene, produced by the Diels-Alder reaction of an acyl nitroso compound followed by amide hydrolysis, undergoes a retro-Diels-Alder reaction at high temperature to directly generate HNO (19). Subsequent work reveals the ability of a catalytic antibody to catalyze HNO formation from the retro-Diels-Alder reaction of a cycloadduct providing a nonoxidative means to HNO (5).
FIG. 17.
Formation of HNO from hydroxylamine.
Alternative reactions
Acyl nitroso compounds react with 1,3-dienes as N-O-heterodienophiles to give cycloadducts and with alkenes as N-O-enophiles (ene reactions) to give N-substituted hydroxamic acids (43, 81, 88). Such reactions often form the basis for the total synthesis of nitrogen-containing molecules and have been performed in water, indicating that these cycloadditions compete with HNO formation from hydrolysis (81, 88). The relatively rare occurrence of 1,3-dienes and low concentration of alkenes should limit such reactions of acyl nitroso compounds in biological systems. Similar to HNO, the high reactivity of acyl nitroso compounds requires their generation from precursor molecules and as such, the chemistry of each acyl nitroso donor and their by-products must be considered. For example, a variety of ferric heme proteins act as competent oxidants for N-acyl hydroxylamines providing a potential in vivo pathway for acyl nitroso compound formation but the carcinogenic/mutagenic properties of 9,10-dimethylanthracene likely limit the use of the acyl nitroso cycloadducts derived from this 1,3-diene to in vitro studies of HNO biology/chemistry (30).
Acyloxy Nitroso Compounds
Structure/synthesis
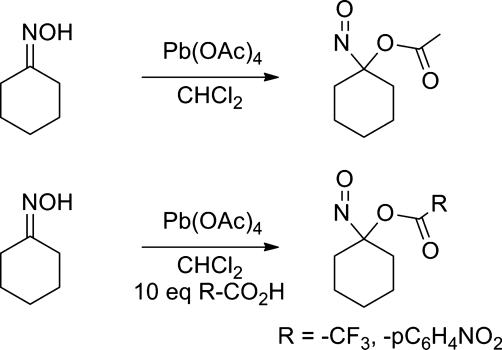
Many acyloxy nitroso compounds, which typically demonstrate a brilliant bright blue color due to their n→π* electronic transition, have been prepared and studied (15, 21, 66, 97). Direct oxime oxidation with lead tetra-acetate gives 1-nitrosocyclohexyl acetate and addition of an excess amount of the appropriate acid allows variation of the acyl group (Fig. 18) (66, 67). These methods yield various acyloxy nitroso compounds, including 1-nitrosocyclohexylacetate, 1-nitrosocyclohexyl-4-nitrobenzoate, and 1-nitrosocyclohexyl trifluroroacetate (Fig. 18) (73). 1-Nitrosocyclohexyl acetate displays a UV-vis absorbance λmax at 667 nm (ɛ=20.7 M−1 cm−1) and infrared absorbances for C=O at ν=1750 cm−1 and N=O at ν=1561 cm−1 (73). A crystal structure of 1-nitrosocyclohexyl 4-nitrobenzoate reveals the C-N-O bond angle as 114.2° that is consistent with a HNO-like bent configuration and a N=O bond length of 1.183 Å (73). Hypervalent iodine compounds oxidize oximes to acyloxy nitroso compounds and p-bromo(diacetoxyiodo)benzene converts the oximes of acetone, cyclohexanone, and cyclopentanone to the corresponding 1-nitrosoacetoxy derivatives (97). Acylation of nitronate anions, generated by the treatment of alkyl nitro compounds with base, followed by a [2, 3] sigmatropic rearrangement, provides another alternative synthetic route to acyloxy nitroso compounds (21).
FIG. 18.
Synthesis of acyloxy nitroso compounds.
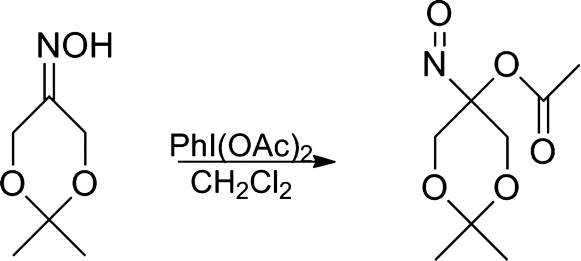
Similarly, oxime oxidation with iodobenzene diacetate gives the acyloxy nitroso compound, 2,2-dimethyl-5-nitroso-1,3-dioxan-5-yl acetate (Fig. 19) (16). 2,2-Dimethyl-5-nitroso-1,3-dioxan-5-yl benzoate and 5-nitroso-1,3-dioxan-5-yl benzoate have also been synthesized, crystallized, and characterized by X-ray crystallography (14).
FIG. 19.
Synthesis of 2,2-dimethyl-5-nitroso-1,3-dioxan-5-yl acetate.
Mechanism/rate of HNO release/by-products
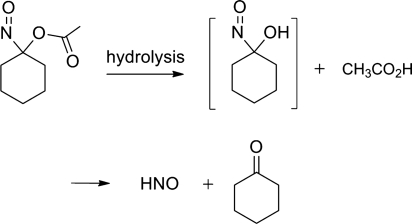
Acyloxy nitroso compounds undergo hydrolysis to release HNO, the corresponding ketone and acid (Fig. 20) (73). Acid- or base-catalyzed ester hydrolysis gives the acid and an unstable nitroso alcohol derivative that decomposes to HNO and the ketone (Fig. 20). Gas chromatographic headspace analysis of the basic decomposition of these compounds shows thiol and ferric iron sensitive formation of nitrous oxide, strong evidence for HNO formation (73). Nitrous oxide does not form from acyloxy nitroso compounds upon incubation in alcohol solutions, but 1-nitrosocyclohexyl acetate generates nitrous oxide (24%) in a mixture of methanol and neutral phosphate buffer after 2 h, indicating HNO formation (66, 67, 73). 1-Nitrosocyclohexyl acetate generates small amounts (0.4%–0.5%) of NO and (3%–4%) of nitrite over this same time period (73). 1-Nitrosocyclohexyl acetate demonstrates relative stability under buffered conditions (t1/2>2 h) but rapidly decomposes under basic conditions (t1/2=0.8 min in 0.1 N NaOH) (73). The rate of hydrolysis depends on the structure of the acyloxy nitroso compound: 1-nitrosocyclohexyl trifluoroacetate releases more nitrous oxide (34% yield, phosphate buffer pH 7.4, 2 h) compared to 1-nitrosocyclohexyl acetate (28% yield) (73).
FIG. 20.
Hydrolysis of acyloxy nitroso compounds to release HNO.
Early work shows acyloxy nitroso compounds inhibit platelet aggregation as well as thrombus formation in vitro (66, 67). Both 1-nitrosocyclohexyl acetate and trifluoroacetate relax preconstricted rat aortic rings in a dose-dependent fashion (EC50=10,000 and 885 nM, respectively) similar to Angeli's salt (EC50=82 nM) (73). 1-Nitrosocyclohexyl trifluoroacetate enhances sGC activity 60-fold providing support for HNO activation of this important heme protein (54). Further biological studies are required to clearly delineate the activity of this class of HNO donors.
Alternative reactions
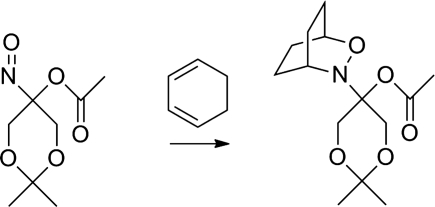
2,2-Dimethyl-5-nitroso-1,3-dioxan-5-yl acetate acts as an N–O dienophile in Diels-Alder reactions (Fig. 21) in both organic and aqueous media (15, 16). Lewis acids catalyze this reaction resulting in improved yields and the cycloadducts can be opened to give 1,4-allylic amino alcohols (Fig. 21) (15).
FIG. 21.
Diels-Alder reaction of acyloxy nitroso compounds.
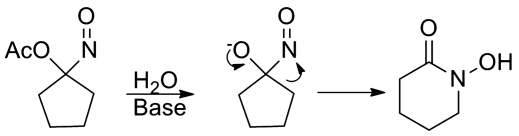
Surprisingly, acyloxy nitroso compounds derived from cyclopentanone oximes do not generate HNO upon hydrolysis but rather rearrange to the cyclic hydroxamic acid (Fig. 22) (6). Expansion of the cyclopentane apparently relieves eclipsing interactions not present in the cyclohexane-derived compounds. This pathway converges with the direct reaction of Piloty's acid anion with a cyclic ketone to yield the ring-expanded cyclic hydroxamic acid (Fig. 10) (79).
FIG. 22.
Ring expansion of cyclopentane-derived acyloxy nitroso compounds.
Acyloxy nitroso compounds bear structural similarity to C-nitroso compounds, such as nitrosobenzene and 1-nitrosoadamantane, which react as potent electrophiles with various nucleophiles. For example, nitrosobenzene and other C-nitroso compounds react with enol ethers and enolates to generate substituted hydroxylamines with further synthetic utility (94). Nitrosobenzene also forms an adduct with glutathione both in vitro and within red blood cells that ultimately rearranges to a glutathione sulfinanilide that hydrolyzes to aniline. Further investigation into reactions of nitrosobenzene with 1-thioglycerol reveals that sulfinic and sulfenic phenylamides form, which also hydrolyze to aniline (48). Para-substituted nitrosobenzenes react with the thiol groups of human Hb trapping these adducts within red blood cells (28). C-Nitroso compounds also inhibit ALDH through modification of the active site thiol (62). Taken together, these results clearly show that C-nitroso compounds react with thiols to yield oxidized sulfur products, and such reactivity may compete with the HNO-releasing properties of acyloxy nitroso compounds and demands further investigation.
Conclusion
The distinct biological properties of HNO compared to NO have markedly increased interest in this triatomic molecule over the last 10 years. The inherent chemical reactivity of HNO requires the use of donor molecules for the study of its chemical and biological processes and interest in the development of new molecular entities, as well as better understanding HNO's reactivity, has paralleled the increase in interest in its biology. Two general chemical strategies currently exist for the generation of HNO from nitrogen-containing molecules: (i) the disproportionation of hydroxylamine derivatives containing good leaving groups attached to the nitrogen atom and (ii) the decomposition of nitroso compounds (X–N=O, where X again represents a good leaving group). This review examines the synthesis/structure, HNO-releasing mechanisms, and alternative reactions of six major groups of HNO donors: Angeli's salt, Piloty's acid and its derivatives, cyanamide, diazenium diolate-derived compounds, acyl nitroso compounds, and acyloxy nitroso compounds (Fig. 1). The large body of work already performed on these six groups of HNO donors supports much of our current understanding of HNO chemical reactivity and biology and presents opportunities to further develop new molecules as both research tools and potential therapeutics.
Abbreviations Used
- ALDH
aldehyde dehydrogenase
- CGRP
calcitonin gene-related peptide
- Hb
hemoglobin
- HNO
nitroxyl
- HRP
horseradish peroxidase
- IPA/NO
isopropylamine diazeniumdiolate
- MA/NO
methylamine diazeniumdiolate
- Mb
myoglobin
- NO
nitric oxide
- NONOates
diazeniumdiolates
- NOS
nitric oxide synthase
Acknowledgments
Financial support for work accomplished in the author's laboratory was provided by the National Institutes of Health (HL62198), the American Heart Association (963031N and 0140020N), the Petroleum Research Fund of the American Chemical Society (PRF 48660-AC1), and Wake Forest University. The authors wish to thank Ms. Julie A. Reisz for discussion and proofreading of this article.
References
- 1.Adachi Y. Nakagawa H. Matsuo K. Suzuki T. Miyata N. Photoactivatable HNO-releasing compounds using the retro-Diels-Alder reaction. Chem Commun. 2008;41:5149–5150. doi: 10.1039/b811985f. [DOI] [PubMed] [Google Scholar]
- 2.Akhtar MJ. Lutz CA. Bonner FT. Decomposition of sodium trioxodinitrate (Na2N2O3) in the presence of added nitrite in aqueous solution. Inorg Chem. 1979;18:2369–2375. [Google Scholar]
- 3.Angeli A. Rimini E. Nitrohydroxylamine. Gazz Chim Ital. 1896;26:17–25. [Google Scholar]
- 4.Atkinson RN. Storey BM. King SB. Reactions of acyl nitroso compounds with amines: production of nitroxyl (HNO) with the preparation of amides. Tetrahedron Lett. 1996;37:9287–9290. [Google Scholar]
- 5.Bahr N. Guller R. Reymond J-L. Lerner RA. A nitroxyl synthase catalytic antibody. J Am Chem Soc. 1996;118:3550–3555. [Google Scholar]
- 6.Banerjee R. King SB. Synthesis of cyclic hydroxamic acids through -NOH insertion of ketones. Org Lett. 2009;11:4580–4583. doi: 10.1021/ol9018198. [DOI] [PubMed] [Google Scholar]
- 7.Bartberger MD. Fukuto JM. Houk KN. On the acidity and reactivity of HNO in aqueous solution and biological systems. Proc Natl Acad Sci USA. 2001;98:2194–2198. doi: 10.1073/pnas.041481598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartberger MD. Liu W. Ford E. Miranda KM. Switzer C. Fukuto JM. Farmer PJ. Wink DA. Houk KN. The reduction potential of nitric oxide (NO) and its importance to NO biochemistry. Proc Natl Acad Sci USA. 2002;99:10958–10963. doi: 10.1073/pnas.162095599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bazylinski DA. Goretski J. Hollocher TC. On the reaction of trioxodinitrate(II) with hemoglobin and myoglobin. J Am Chem Soc. 1985;107:7986–7989. [Google Scholar]
- 10.Bonner FT. Degani H. Akhtar MJ. N-15 magnetic-resonance spectroscopy of trioxodinitrate - N-protonation of an oxoanion. J Am Chem Soc. 1981;103:3739–3742. [Google Scholar]
- 11.Bonner FT. Hughes MN. The aqueous solution chemistry of nitrogen in low positive oxidation states. Comments Inorg Chem. 1988;7:215–234. [Google Scholar]
- 12.Bonner FT. Ko YH. Kinetic, isotopic, and N-15 NMR-study of N-hydroxybenzenesulfonamide decomposition—an HNO source reaction. Inorg Chem. 1992;31:2514–2519. [Google Scholar]
- 13.Bonner FT. Ravid B. Thermal-decomposition of oxyhyponitrite (sodium trioxodinitrate(II)) in aqueous-solution. Inorg Chem. 1975;14:558–563. [Google Scholar]
- 14.Calvet G. Blanchard N. Kouklovsky C. Guillot R. 2,2-Dimethyl-5-nitroso-1,3-dioxan-5-yl benzoate, 2,2-dimethyl-5-nitroso-1,3-dioxan-5-yl 4-chlorobenzoate and 5-nitroso-1,3-dioxan-5-yl 4-chlorobenzoate. Acta Crystallogr C. 2007;C63:o365–o368. doi: 10.1107/S0108270107021804. [DOI] [PubMed] [Google Scholar]
- 15.Calvet G. Dussaussois M. Blanchard N. Kouklovsky C. Lewis acid-promoted hetero Diels-Alder cycloaddition of α-acetoxynitroso dienophiles. Org Lett. 2004;6:2449–2451. doi: 10.1021/ol0491336. [DOI] [PubMed] [Google Scholar]
- 16.Calvet G. Guillot R. Blanchard N. Kouklovsky C. Intermolecular nitroso Diels-Alder cycloaddition of α-acetoxynitroso derivatives in aqueous medium. Org Biomol Chem. 2005;3:4395–4401. doi: 10.1039/b513397a. [DOI] [PubMed] [Google Scholar]
- 17.Christie CC. Kirby GW. McGuigan H. Mackinnon JWM. C-Nitrosoformamides, a new class of transient dienophiles formed by oxidation of N-hydroxyureas. JSC Perkin Trans. 1985;I:2469–2473. [Google Scholar]
- 18.Cohen AD. Zeng BB. King SB. Toscano JP. Direct observation of an acyl nitroso species in solution by time-resolved IR spectroscopy. J Am Chem Soc. 2003;125:1444–1445. doi: 10.1021/ja028978e. [DOI] [PubMed] [Google Scholar]
- 19.Corrie JET. Kirby GW. Laird AE. Mackinnon LW. Tyler JK. Detection by microwave spectroscopy of HNO produced by a retro-Diels-Alder reaction. J Chem Soc Chem Commun. 1978;6:275–276. [Google Scholar]
- 20.Craven PA. Derubertis FR. Pratt DW. Electron-spin resonance study of the role of NO. Catalase in the activation of guanylate-cyclase by NaN3 and NH2OH—modulation of enzyme responses by heme-proteins and their nitrosyl derivatives. J Biol Chem. 1979;254:8213–8222. [PubMed] [Google Scholar]
- 21.Daineko VI. Proskurnina MV. Skornyakov YV. Trofimov BA. Zefirov NS. [3,2]-sigmatropic rearrangement of acyloxy nitronic acids into geminal acyloxy nitroso compounds. Russ J Org Chem. 2002;38:1431–1433. [Google Scholar]
- 22.DeMaster EG. Redfern B. Nagasawa HT. Mechanisms of inhibition of aldehyde dehydrogenase by nitroxyl, the active metabolite of the alcohol deterrent agent cyanamide. Biochem Pharmacol. 1998;55:2007–2015. doi: 10.1016/s0006-2952(98)00080-x. [DOI] [PubMed] [Google Scholar]
- 23.Donzelli S. Espey MG. Flores-Santana W. Switzer CH. Yeh GC. Huang JM. Stuehr DJ. King SB. Miranda KM. Wink DA. Generation of nitroxyl by heme protein-mediated peroxidation of hydroxylamine but not N-hydroxy-L-arginine. Free Radic Biol Med. 2008;45:578–584. doi: 10.1016/j.freeradbiomed.2008.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doyle MP. Mahapatro SN. Broene RD. Guy JK. Oxidation and reduction of hemoproteins by trioxodinitrate(II)—the role of nitrosyl hydride and nitrite. J Am Chem Soc. 1988;110:593–599. [Google Scholar]
- 25.Drago RS. Karstetter BR. The reactions of nitrogen (II) oxide with various primary and secondary amines. J Am Chem Soc. 1960;83:1819–1822. [Google Scholar]
- 26.Dutton AS. Fukuto JM. Houk KN. Mechanisms of HNO and NO production from Angeli's salt: density functional and CBS-QB3 theory predictions. J Am Chem Soc. 2004;126:3795–3800. doi: 10.1021/ja0391614. [DOI] [PubMed] [Google Scholar]
- 27.Dutton AS. Suhrada CP. Miranda KM. Wink DA. Fukuto JM. Houk KN. Mechanism of pH-dependent decomposition of monoalkylamine diazeniumdiolates to form HNO and NO, deduced from the model compound methylamine diazeniumdiolate, density functional theory, and CBS-QB3 calculations. Inorg Chem. 2006;45:2448–2456. doi: 10.1021/ic051505z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eyer P. Ascherl M. Reactions of para-substituted nitrosobenzenes with human-hemoglobin. Biol Chem Hoppe Seyler. 1987;368:285–294. doi: 10.1515/bchm3.1987.368.1.285. [DOI] [PubMed] [Google Scholar]
- 29.Farmer PJ. Sulc F. Coordination chemistry of the HNO ligand with hemes and synthetic coordination complexes. J Inorg Biochem. 2005;99:166–184. doi: 10.1016/j.jinorgbio.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Fluckiger-Isler S. Baumeister A. Braun K. Gervais V. Hasler-Nguyen N. Reimann R. Van Gompel J. Wunderlich HG. Engelhardt G. Assessment of the performance of the Ames II (TM) assay: a collaborative study with 19 coded compounds. Mutat Res. 2004;558:181–197. doi: 10.1016/j.mrgentox.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Frost LM. Courtney SM. Brookfield FA. Kalish VJ. Novel Nitroso Compounds Including Carboxylic Acid and Phosphoric Acid Esters as Nitroxyl Donors and Methods of Use Thereof in Treating Diseases Such as Heart Failure, Ischemia/Reperfusion Injury and Cancer. Chapel Hill, NC: Cardioxyl Pharmaceuticals, Inc.; 2009. p. 112. [Google Scholar]
- 32.Fukuto JM. Bianco CL. Chavez TA. Nitroxyl (HNO) signaling. Free Radic Biol Med. 2009;47:1318–1324. doi: 10.1016/j.freeradbiomed.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Gladwin MT. Schechter AN. Kim-Shapiro DB. Patel RP. Hogg N. Shiva S. Cannon RO. Kelm M. Wink DA. Espey MG. Oldfield EH. Pluta RM. Freeman BA. Lancaster JR. Feelisch M. Lundberg JO. The emerging biology of the nitrite anion. Nat Chem Biol. 2005;1:308–314. doi: 10.1038/nchembio1105-308. [DOI] [PubMed] [Google Scholar]
- 34.Hassner A. Wiederkehr R. Kascheres AJ. Reaction of aldehydes with N-hydroxybenzenesulfonamide. Acetal formation catalyzed by nucleophiles. J Org Chem. 1970;35:1962–1964. [Google Scholar]
- 35.Hoshino M. Maeda M. Konishi R. Seki H. Ford PC. Studies on the reaction mechanism for reductive nitrosylation of ferrihemoproteins in buffer solutions. J Am Chem Soc. 1996;118:5702–5707. [Google Scholar]
- 36.Howard JAK. Ilyashenko G. Sparkes HA. Whiting A. Development of new transition metal catalysts for the oxidation of a hydroxamic acid with in situ Diels-Alder trapping of the acyl nitroso derivative. Dalton Trans. 2007;21:2108–2111. doi: 10.1039/b704728b. [DOI] [PubMed] [Google Scholar]
- 37.Huang JM. Kim-Shapiro DB. King SB. Catalase-mediated nitric oxide formation from hydroxyurea. J Med Chem. 2004;47:3495–3501. doi: 10.1021/jm030547z. [DOI] [PubMed] [Google Scholar]
- 38.Huang JM. Sommers EM. Kim-Shapiro DB. King SB. Horseradish peroxidase catalyzed nitric oxide formation from hydroxyurea. J Am Chem Soc. 2002;124:3473–3480. doi: 10.1021/ja012271v. [DOI] [PubMed] [Google Scholar]
- 39.Hughes MN. Wimbledon PE. Chemistry of trioxodinitrates. 1. Decomposition of sodium trioxodinitrate (Angelis Salt) in aqueous-solution. J Chem Soc Dalton Trans. 1976;8:703–707. [Google Scholar]
- 40.Hughes MN. Wimbledon PE. Chemistry of trioxodinitrates. 2. Effect of added nitrite on stability of sodium trioxodinitrate in aqueous-solution. J Chem Soc Dalton Trans. 1977;17:1650–1653. [Google Scholar]
- 41.Hunt HR. Cox JR. Ray JD. The heat of formation of crystalline sodium α-oxyhyponitrite. The structure of aqueous α-oxyhyponitrite ion. Inorg Chem. 1962;1:938–941. [Google Scholar]
- 42.Irvine JC. Ritchie RH. Favaloro JL. Andrews KL. Widdop RE. Kemp-Harper BK. Nitroxyl (HNO): the Cinderella of the nitric oxide story. Trends Pharmacol Sci. 2008;29:601–608. doi: 10.1016/j.tips.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Keck GE. Webb RR. Yates JB. A versatile method for carbon-nitrogen bond formation via ene reactions of acylnitroso compounds. Tetrahedron. 1981;37:4007–4016. [Google Scholar]
- 44.King SB. N-hydroxyurea and acyl nitroso compounds as nitroxyl (HNO) and nitric oxide (NO) donors. Curr Top Med Chem. 2005;5:665–673. doi: 10.2174/1568026054679362. [DOI] [PubMed] [Google Scholar]
- 45.King SB. Oral or Parenteral C-Nitroso-Derived Nitroxyl Donors for Treatment of Cardiovascular and other Disorders. Winston-Salem, NC: Wake Forest University Health Sciences; 2007. p. 24. [Google Scholar]
- 46.King SB. Nagasawa HT. Chemical approaches toward generation of nitroxyl. In: Packer L, editor. Nitric Oxide, Part C. San Diego, CA: Academic Press; 1999. pp. 211–220. [DOI] [PubMed] [Google Scholar]
- 47.Kirby GW. McGuigan H. Mackinnon JWM. McLean D. Sharma RP. Formation and reactions of C-nitrosoformate esters, a new class of transient dienophiles. JSC Perkin Trans. 1985;I:1437–1442. [Google Scholar]
- 48.Klehr H. Eyer P. Schafer W. On the mechanism of reactions of nitrosoarenes with thiols—formation of a common intermediate semimercaptal. Biol Chem Hoppe Seyler. 1985;366:755–760. doi: 10.1515/bchm3.1985.366.2.755. [DOI] [PubMed] [Google Scholar]
- 49.Lawson A. Stevens JO. Preparation of cyanamide. J Chem Soc. 1958;2903 [Google Scholar]
- 50.Lemal DM. Rave TW. Diazenes from Angeli's Salt. J Am Chem Soc. 1965;87:393–394. [Google Scholar]
- 51.Lockamy VL. Shields H. Kim-Shapiro DB. King SB. Iron nitrosyl hemoglobin formation from the reaction of hydroxylamine and hemoglobin under physiological conditions. Biochim Biophys Acta Gen Subj. 2004;1674:260–267. doi: 10.1016/j.bbagen.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 52.Maragos CM. Morley D. Wink DA. Dunams TM. Saavedra JE. Hoffman A. Bove AA. Isaac L. Hrabie JA. Keefer LK. Complexes of NO with nucleophiles as agents for the controlled biological release of nitric-oxide—vasorelaxant effects. J Med Chem. 1991;34:3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- 53.Marti MA. Bari SE. Estrin DA. Doctorovich F. Discrimination of nitroxyl and nitric oxide by water-soluble Mn(III) porphyrins. J Am Chem Soc. 2005;127:4680–4684. doi: 10.1021/ja044632n. [DOI] [PubMed] [Google Scholar]
- 54.Miller TW. Cherney MM. Lee AJ. Francoleon NE. Farmer PJ. King SB. Hobbs AJ. Miranda KM. Burstyn JN. Fukuto JM. The effects of nitroxyl (HNO) on soluble guanylate cyclase activity interactions at ferrous heme and cysteine thiols. J Biol Chem. 2009;284:21788–21796. doi: 10.1074/jbc.M109.014282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miranda KM. The chemistry of nitroxyl (HNO) and implications in biology. Coord Chem Rev. 2005;249:433–455. [Google Scholar]
- 56.Miranda KM. Dutton AS. Ridnour LA. Foreman CA. Ford E. Paolocci N. Katori T. Tocchetti CG. Mancardi D. Thomas DD. Espey MG. Houk KN. Fukuto JM. Wink DA. Mechanism of aerobic decomposition of Angeli's salt (sodium trioxodinitrate) at physiological pH. J Am Chem Soc. 2005;127:722–731. doi: 10.1021/ja045480z. [DOI] [PubMed] [Google Scholar]
- 57.Miranda KM. Katori T. de Holding CLT. Thomas L. Ridnour LA. MeLendon WJ. Cologna SM. Dutton AS. Champion HC. Mancardi D. Tocchetti CG. Saavedra JE. Keefer LK. Houk KN. Fukuto JM. Kass DA. Paolocci N. Wink DA. Comparison of the NO and HNO donating properties of diazeniumdiolates: Primary amine adducts release HNO in vivo. J Med Chem. 2005;48:8220–8228. doi: 10.1021/jm050151i. [DOI] [PubMed] [Google Scholar]
- 58.Miranda KM. Nagasawa HT. Toscano JP. Donors of HNO. Curr Top Med Chem. 2005;5:647–664. doi: 10.2174/1568026054679290. [DOI] [PubMed] [Google Scholar]
- 59.Miranda KM. Paolocci N. Katori T. Thomas DD. Ford E. Bartberger MD. Espey MG. Kass DA. Feelisch M. Fukuto JM. Wink DA. A biochemical rationale for the discrete behavior of nitroxyl and nitric oxide in the cardiovascular system. Proc Natl Acad Sci USA. 2003;100:9196–9201. doi: 10.1073/pnas.1430507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nagasawa HT. Demaster EG. Redfern B. Shirota FN. Goon JW. Evidence for nitroxyl in the catalase-mediated bioactivation of the alcohol deterrent agent cyanamide. J Med Chem. 1990;33:3120–3122. doi: 10.1021/jm00174a001. [DOI] [PubMed] [Google Scholar]
- 61.Nagasawa HT. Kawle SP. Elberling JA. Demaster EG. Fukuto JM. Prodrugs of nitroxyl as potential aldehyde dehydrogenase inhibitors vis-a-vis vascular smooth-muscle relaxants. J Med Chem. 1995;38:1865–1871. doi: 10.1021/jm00011a005. [DOI] [PubMed] [Google Scholar]
- 62.Nagasawa HT. Yost Y. Elberling JA. Shirota FN. Demaster EG. Nitroxyl analogs as inhibitors of aldehyde dehydrogenase - C-nitroso compounds. Biochem Pharmacol. 1993;45:2129–2134. doi: 10.1016/0006-2952(93)90026-s. [DOI] [PubMed] [Google Scholar]
- 63.Panizzi L. Di Maio G. Tardella PA. d'Abbiero L. Action of nitroxyl on ketonic compounds. I. Cyclic ketones. Ricerca Sci. 1961;1:312–318. [Google Scholar]
- 64.Paolocci N. Jackson MI. Lopez BE. Miranda K. Tocchetti CG. Wink DA. Hobbs AJ. Fukuto JM. The pharmacology of nitroxyl (HNO) and its therapeutic potential: Not just the Janus face of NO. Pharmacol Ther. 2007;113:442–458. doi: 10.1016/j.pharmthera.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pennington RL. Sha X. King SB. N-hydroxy sulfonimidamides as new nitroxyl (HNO) donors. Bioorg Med Chem Lett. 2005;15:2331–2334. doi: 10.1016/j.bmcl.2005.02.082. [DOI] [PubMed] [Google Scholar]
- 66.Rehse K. Herpel M. New NO-donors with antithrombotic and vasodilating activities, part 20—azodioxides activated by electron acceptors in geminal or vicinal position. Arch Pharm (Weinheim) 1998;331:104–110. doi: 10.1002/(sici)1521-4184(199803)331:3<104::aid-ardp104>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 67.Rehse K. Herpel M. New NO-donors with antithrombotic and vasodilating activities, part 21—pseudonitrosites and other azodioxides with vicinal electron acceptors. Arch Pharm (Weinheim) 1998;331:111–117. doi: 10.1002/(sici)1521-4184(199803)331:3<111::aid-ardp111>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 68.Reisz J. Bechtold E. King SB. Oxidative heme protein-mediated nitroxyl (HNO) generation. Dalton Trans. 2010;39:5203–5212. doi: 10.1039/c000980f. [DOI] [PubMed] [Google Scholar]
- 69.Reisz JA. Klorig EB. Wright MW. King SB. Reductive phosphine-mediated ligation of nitroxyl (HNO) Org Lett. 2009;11:2719–2721. doi: 10.1021/ol900914s. [DOI] [PubMed] [Google Scholar]
- 70.Rimini E. Over a new reaction of the aldehydes. Atti R Accad dei Lincei Roma. 1901;10:355–362. [Google Scholar]
- 71.Scholz JN. Engel PS. Glidewell C. Whitmire KH. Reaction of hydroxylamine with benzenesulfonyl chloride—X-ray crystal-structure of pilotys acid and other benzenesulfonylhydroxylamines. Tetrahedron. 1989;45:7695–7708. [Google Scholar]
- 72.Seel FB. C. Mechanism of the decomposition of sodium benzenesulfohydroxamate in aqueous solution. Z Anorg Allg Chem. 1972;394:187–196. [Google Scholar]
- 73.Sha X. Isbell TS. Patel RP. Day CS. King SB. Hydrolysis of acyloxy nitroso compounds yields nitroxyl (HNO) J Am Chem Soc. 2006;128:9687–9692. doi: 10.1021/ja062365a. [DOI] [PubMed] [Google Scholar]
- 74.Shafirovich V. Lymar SV. Nitroxyl and its anion in aqueous solutions: spin states, protic equilibria, and reactivities toward oxygen and nitric oxide. Proc Natl Acad Sci USA. 2002;99:7340–7345. doi: 10.1073/pnas.112202099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shafirovich V. Lymar SV. Spin-forbidden deprotonation of aqueous nitroxyl (HNO) J Am Chem Soc. 2003;125:6547–6552. doi: 10.1021/ja034378j. [DOI] [PubMed] [Google Scholar]
- 76.Shirota FN. DeMaster EG. Lee MJC. Nagasawa HT. Generation of nitric oxide and possibly nitroxyl by nitrosation of sulfohydroxamic acids and hydroxamic acids. Nitric Oxide. 1999;3:445–453. doi: 10.1006/niox.1999.0257. [DOI] [PubMed] [Google Scholar]
- 77.Shirota FN. Goon DJW. DeMaster EG. Nagasawa HT. Nitrosyl cyanide, a putative metabolic oxidation product of the alcohol-deterrent agent cyanamide. Biochem Pharmacol. 1996;52:141–147. doi: 10.1016/0006-2952(96)00174-8. [DOI] [PubMed] [Google Scholar]
- 78.Sklarz B. Al-Sayyar AF. The oxidation of hydroxamic acids: a synthesis of amines. J Chem Soc. 1964:1318–1320. [Google Scholar]
- 79.Smith PAS. Hein GE. The alleged role of nitroxyl in certain reactions of aldehydes and alkyl halides. J Am Chem Soc. 1960;82:5731–5740. [Google Scholar]
- 80.Stolze K. Nohl H. Detection of free-radicals as intermediates in the methemoglobin formation from oxyhemoglobin induced by hydroxylamine. Biochem Pharmacol. 1989;38:3055–3059. doi: 10.1016/0006-2952(89)90015-4. [DOI] [PubMed] [Google Scholar]
- 81.Streith J. Defoin A. Hetero-Diels-Alder reactions with nitroso dienophiles - application to the synthesis of natural product derivatives. Synthesis. 1994:1107–1117. [Google Scholar]
- 82.Sturrock PE. Ray JD. McDowell J. Hunt HR. The dissociation constant of α-oxyhyponitrous acid. Inorg Chem. 1963;2:649–650. [Google Scholar]
- 83.Sulc F. Immoos CE. Pervitsky D. Farmer PJ. Efficient trapping of HNO by deoxymyoglobin. J Am Chem Soc. 2004;126:1096–1101. doi: 10.1021/ja0376184. [DOI] [PubMed] [Google Scholar]
- 84.Torun L. Mohammad T. Morrison H. Cycloaddition reactions of sodium dinitroxytrioxide (Angeli's salt) Tetrahedron Lett. 1999;40:5279–5282. [Google Scholar]
- 85.Toscano JP. Brookfield FA. Cohen AD. Courtney SM. Frost LM. Kalish VJ. Preparation of N-Hydroxylsulfonamide Derivatives as Nitroxyl (HNO) Donors. Baltimore, MD: Johns Hopkins University; Chapel Hill, NC: Cardioxyl Pharmaceuticals, Inc.; 2007. p. 83. [Google Scholar]
- 86.Toscano JP. Brookfield FA. Cohen AD. Courtney SM. Frost LM. Kalish VJ. Preparation of Aryl N-Hydroxysulfonamides as Physiologically Useful Nitroxyl Donors. Baltimore, MD: Johns Hopkins University; Chapel Hill, NC: Cardioxyl Pharmaceuticals, Inc.; 2009. p. 24. [Google Scholar]
- 87.Veprek-Siska J. Pliska V. Smirous F. Visely F. Inorganic nitrogen compounds. II. Mechanism of decomposition of aqueous solutions of Na2N2O3. Collect Czech Chem Commun. 1959;24:687–693. [Google Scholar]
- 88.Vogt PF. Miller MJ. Development and applications of amino acid derived chiral acylnitroso hetero Diels-Alder reactions. Tetrahedron. 1998;54:1317–1348. [Google Scholar]
- 89.Wang PG. Xian M. Tang XP. Wu XJ. Wen Z. Cai TW. Janczuk AJ. Nitric oxide donors: chemical activities and biological applications. Chem Rev. 2002;102:1091–1134. doi: 10.1021/cr000040l. [DOI] [PubMed] [Google Scholar]
- 90.Ware RW. King SB. N-phosphinoylnitroso compounds: new asymmetric N-O heterodienophiles and nitroxyl delivery agents. J Am Chem Soc. 1999;121:6769–6770. [Google Scholar]
- 91.Wei CC. Wang ZQ. Hemann C. Hille R. Stuehr DJ. A tetrahydrobiopterin radical forms and then becomes reduced during N-omega-hydroxyarginine oxidation by nitric-oxide synthase. J Biol Chem. 2003;278:46668–46673. doi: 10.1074/jbc.M307682200. [DOI] [PubMed] [Google Scholar]
- 92.Xu YP. Alavanja MM. Johnson VL. Yasaki G. King SB. Production of nitroxyl (HNO) at biologically relevant temperatures from the retro-Diels-Alder reaction of N- hydroxyurea-derived acyl nitroso-9,10-dimethylanthracene cycloadducts. Tetrahedron Lett. 2000;41:4265–4269. [Google Scholar]
- 93.Xu YP. Mull CD. Bonifant CL. Yasaki G. Palmer EC. Shields H. Ballas SK. Kim-Shapiro DB. King SB. Nitrosylation of sickle cell hemoglobin by hydroxyurea. J Org Chem. 1998;63:6452–6453. doi: 10.1021/jo981151b. [DOI] [PubMed] [Google Scholar]
- 94.Yamamoto H. Kawasaki M. Nitroso and azo compounds in modern organic synthesis: late blooming but very rich. Bull Chem Soc Jpn. 2007;80:595–607. [Google Scholar]
- 95.Zamora R. Grzesiok A. Weber H. Feelisch M. Oxidative release of nitric-oxide accounts for guanylyl cyclase stimulating, vasodilator and antiplatelet activity of Pilotys acid—a comparison with Angelis salt. Biochem J. 1995;312:333–339. doi: 10.1042/bj3120333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zeng BB. Huang JM. Wright MW. King SB. Nitroxyl (HNO) release from new functionalized N-hydroxyurea-derived acyl nitroso-9,10-dimethylanthracene cycloadducts. Bioorg Med Chem Lett. 2004;14:5565–5568. doi: 10.1016/j.bmcl.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 97.Zhutov EV. Skornyakov YV. Proskurina MV. Zefirov NS. p-bromo(diacetoxyiodo)benzene, an efficient oxidant for conversion of oximes into nitroso compounds. Russ J Org Chem. 2003;39:1672–1673. [Google Scholar]