Abstract
In the marine mollusk Aplysia, the CCAAT/enhancer-binding protein, ApC/EBP, serves as an immediate early gene in the consolidation of long-term facilitation in the synaptic connection between the sensory and motor neurons of the gill-withdrawal reflex. To further examine the role of ApC/EBP as a molecular switch of a stable form of long-term memory, we cloned the full-length coding regions of two alternatively spliced forms, the short and long form of ApC/EBP. Overexpression of each isoform by DNA microinjection resulted in a l6-fold increase in the expression of the coinjected luciferase reporter gene driven by an ERE promoter. In addition, when we overexpressed ApC/EBP in Aplysia sensory neurons, we found that the application of a single pulse of 5-HT that normally induced only short-term facilitation now induced long-term facilitation. Conversely, when we attempted to block the synthesis of native ApC/EBP by microinjecting double-strand RNA or antisense RNA, we blocked long-term facilitation in a sequence-specific manner. These data support the idea that ApC/EBP is both necessary and sufficient to consolidate short-term memory into long-term memory. Furthermore, our results suggest that this double-strand RNA interference provides a powerful tool in the study of the genes functioning in learning and memory in Aplysia by specifically inhibiting both the constitutive and induced expression of the genes.
Memory storage has two forms, that is, a short-term memory lasting seconds to minutes, and a long-term memory lasting days to weeks and sometimes even the lifetime of the organism. Whereas short-term memory depends on a covalent modification of pre-existing proteins, long-term memory requires new protein and mRNA synthesis and is associated with the growth of new synaptic connections (Bailey et al. 1983; Montarolo et al. 1986; Glanzman et al. 1990). This requirement suggests that transcription is critical for long-lasting memory. Among a number of transcription factors expressed in neuronal cell nuclei, the CCAAT/enhancer binding protein (C/EBP) involved in terminal differentiation of a variety of non-neuronal cells has been implicated as being essential for memory storage (Alberini et al. 1994). In the hippocampus, which is involved in the formation of explicit long-term memory, the expression of C/EBP members of the C/EBP family, is enhanced in response to cAMP and Ca2+ and is critical for inductive signals for memory formation (Yukawa et al. 1998). Furthermore, in the hippocampus, two isoforms are induced by inhibitory avoidance learning (Taubenfeld et al. 2001). The ApC/EBP cloned in Aplysia has been shown to be an immediate early gene in the consolidation of long-term memory that is required for the structural changes that stabilize long-term facilitation. The ApC/EBP contains basic and leucine zipper domains involved in DNA binding and homo- or heterodimerization, respectively (Lekstrom-Himes et al. 1998).
Serotonin (5-HT) released by sensitizing stimuli binds to the 5-HT receptor on the sensory neurons, leading through Gs proteins to activation of adenylyl cyclase and to a rise in cAMP and the activation of protein kinase A (PKA). With repeated pulses of 5-HT, PKA recruits MAP kinase and both translocate to the nucleus where they phosphorylate the CREB (cAMP response element-binding protein). This phosphorylation results in the activation of cAMP inducible genes (Kaang et al. 1993). In response to five repeated pulses of 5-HT, ApC/EBP is rapidly and transiently induced so that the mRNA reaches its peak level of expression 2 h after 5-HT application. The expression of ApC/EBP mRNA was increased by a cAMP signal (Alberini et al. 1994; Bailey et al. 1996). Induction of ApC/EBP is believed to produce expression of downstream effector genes essential for stable long-term facilitation. Injection of antisense ApC/EBP RNA or specific antibody blocked selectively long-term facilitation without affecting short-term facilitation (Alberini et al. 1994).
To further characterize the role of ApC/EBP as a transcription factor required in long-term facilitation, we determined the effects on long-term synaptic facilitation of either overexpression of cloned ApC/EBP by DNA microinjection or repression of ApC/EBP by introduction of double-strand RNA of ApC/EBP in Aplysia sensory neurons.
RESULTS
Cloning of cDNA Encoding ApC/EBP from Aplysia kurodai
We obtained a full-length clone of ApC/EBP by screening an Aplysia kurodai cDNA library. We also found a splice variant containing an insertion of 156 bp in the coding region. As shown in Figure 1, the predicted amino acid sequence of A. kurodai was 97% homologous to those of Aplysia californica, but it contains two more amino acids. The basic region, leucine zipper domain, and putative phosphorylation sites are highly conserved in two species.
Figure 1.
Comparison of amino acid sequences of ApC/EBP from A. kurodai and A. californica. Amino acid sequences of cloned ApC/EBP are ∼97% homologous to those of A. californica. Unconserved amino acids are indicated by blackened squares. Basic region and leucine zipper domain are indicated by thick line. The long form of ApC/EBP has 52-amino acid insert marked by a shaded box.
Ectopic Expression of ApC/EBP in Aplysia Sensory Neurons
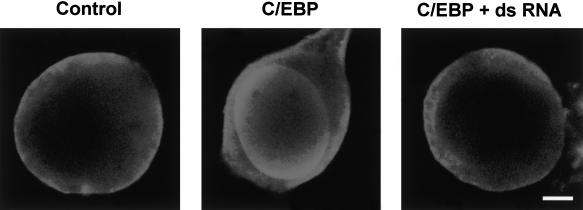
To examine a cellular expression of ApC/EBP, we microinjected pNEXδ-ApC/EBP and pNEXδ-GFP in cultured sensory neurons and stained the cells with anti-ApC/EBP antibody 24 h after microinjection. As shown in Figure 2, the injected ApC/EBP is detected in the nucleus. Within the nucleus, the ectopically expressed ApC/EBP seemed to be localized at specific peripheral regions.
Figure 2.
Ectopic expression of ApC/EBP in cultured sensory neurons. Cultured sensory cells were injected with pNEXδ-ApC/EBP in the absence (middle) and presence (right) of double-strand RNA and were treated with five pulses of 5-HT 1 h after microinjection. (Left) A control cell without injection. The cultures were fixed and stained with anti-ApC/EBP antibody and Cy3-conjugated anti-mouse antibody 24 h after microinjection. Scale bar, 10 μm.
Transcriptional Activity of Cloned ApC/EBP
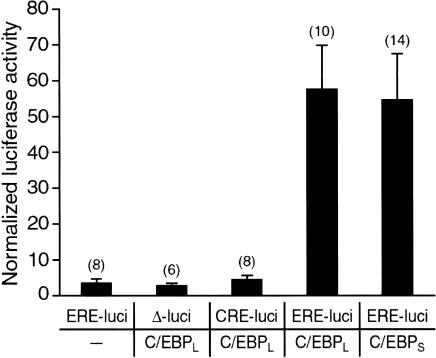
To investigate whether one or the other of the two cloned ApC/EBPs encodes a functional transcription factor capable of activating an enhancer response element (ERE) promoter, we microinjected pNEXδ-ApC/EBP DNA into the Aplysia neurons together with an ERE-luciferase reporter. Twenty-four hours after DNA injection, reporter expression was increased about l6-fold by introduction of either short-form or long-form ApC/EBP constructs compared with the control expression in which only the ERE-luciferase reporter was introduced (Fig. 3). In contrast, ApC/EBP did not activate the expression of a luciferase reporter driven by cAMP response element (CRE) (Fig. 3). These data indicate that in Aplysia neurons the cloned ApC/EBP isoforms encode functional transcription factors acting through a ERE-enhancer sequence.
Figure 3.
Transactivation by ApC/EBP of luciferase reporter gene driven by ERE. The expression of the luciferase reporter gene driven by ERE was increased by introduction of either the short form (ApC/EBPS) or long form (ApC/EBPL) of ApC/EBP constructs in Aplysia neurons. Normalized luciferase activity was obtained by dividing the luciferase activity by β-galactosidase activity. Results are expressed as mean ± SEM. Numbers in parentheses indicate the numbers of ganglia tested. (ERE-luci) ERE-luciferase; (CRE-luci) CRE-luciferase; (Δ-luci) enhancerless luciferase; (C/EBPL) pNEXδ-ApC/EBPL; (C/EBPS) pNEXδ-ApC/EBPS.
Overexpression of ApC/EBP Induces Long-Term Facilitation in Response to a Single Pulse of 5-HT That Normally Induces Only Short-Term Facilitation
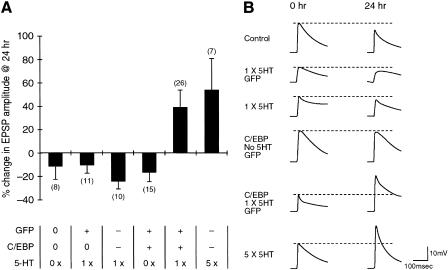
We next examined the role of cloned ApC/EBP in the long-term facilitation by overexpressing ApC/EBP by microinjecting pNEXδ-ApC/EBP into sensory neurons that were synaptically connected to motor cells in sensory-motor coculture. Transgenic expression of ApC/EBP in the sensory neurons had no significant effect on basal synaptic strength (−16.5 ± 8.0%, n = 15) and was comparable with uninjected control cells (−11.5 ± 10.7%, n = 8), measured 24 h after ApC/EBP transfection (Fig. 4). Interestingly, however, when ApC/EBP-overexpressing sensory cells were treated with one pulse of 5-HT (10 μM) for 5 min, which normally induced only short-term facilitation, the synaptic efficacy between sensory and motor neurons was significantly increased as measured by the amplitude of synaptic potential 24 h after 5-HT treatment (38.9 ± 14.6%, n = 26), showing that overexpression of ApC/EBP can transform short-term facilitation into long-term facilitation (Fig. 4). In control experiments, five pulses of 5-HT produced long-term facilitation with a normal increase (53.5 ± 27.0%, n = 7) in synaptic efficacy, whereas one pulse did not produce long-term facilitation (−23.5 ± 7.3%, n = 10) in the sensory cells injected with DNAs, but showed a failure in ApC/EBP overexpression. Furthermore, sensory cells injected with GFP DNA instead of ApC/EBP and then exposed to 10 μM 5-HT for 5 min had no effect on long-term synaptic efficacy (−9.5 ± 7.4%, n = 11) (Fig. 4). Taken together, these data showed that the specific overexpression of ApC/EBP could consolidate short-term facilitation into long-term facilitation.
Figure 4.
Effect of overexpression of ApC/EBP on long-term facilitation in the sensory-motor synapse. (A) Bar graph representing the effect of overexpression of ApC/EBP on long-term facilitation. Overexpression of ApC/EBP by injection of pNEXδ-ApC/EBPL into the sensory cell produced long-term facilitation when it was combined with the application of 10 μM 5-HT for 5 min (one pulse, 1 ×) that can induce only short-term facilitation. The height of each bar corresponds to the mean percentage of change ± SEM in EPSP amplitude tested 24 h after 5-HT (10 μM) treatment. Numbers in parentheses represent the number of the sensory-to-motor synaptic connections tested. Plus (+) symbol in GFP and C/EBP rows indicates the successful expression of the microinjected DNAs (pNEXδ-GFPS65TΔC9 and pNEXδ-ApC/EBPL) in the sensory cells as verified by GFP fluorescence, whereas minus (−) symbol indicates the failure of expression following the microinjection. In some experiments, the DNA construct(s) were not injected, indicated by the zero (0) symbol in the figure. 5-HT row indicates the number of 5-HT pulses treated in the cultures. A one-way analysis of variance and Duncan's multiple range test were used to determine the significance of the EPSP changes (F = 4.57, df = 5, P <0.002). (B) Examples of EPSPs recorded in motor neuron LFS after the stimulation of sensory neurons before (0 h) and 24 h after 5-HT treatment. The recording traces shown in the order of top to bottom in B are the representatives of the corresponding bars shown from left to right in A.
Microinjection of Double-Strand RNA of ApC/EBP Blocked Long-Term Facilitation
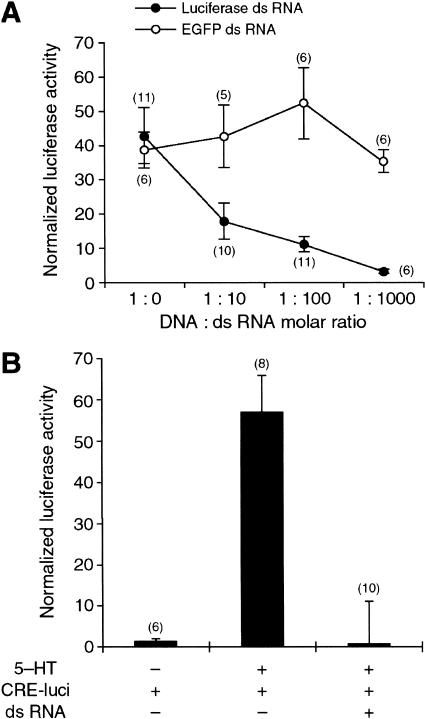
To demonstrate an essential role of cloned ApC/EBP in long-term facilitation, we attempted to block the synthesis of native ApC/EBP by microinjecting double-strand RNA of ApC/EBP. Double-strand RNA has been shown to inhibit gene expression specifically in various organisms through a mechanism called RNA interference (RNAi) (Montgomery et al. 1998). To our knowledge, RNAi has not been reported in the Aplysia nervous system. Therefore, we carried out a control experiment to determine whether constitutive expression of luciferase from microinjected pNEXδ-luciferase DNA construct was inhibited by coinjected sequence-specific luciferase double-strand RNA in Aplysia neurons. When pNEXδ-luciferase and luciferase double-strand RNA were coinjected with the molar ratio of 1:10, 1:100, and 1:1000, the activity of luciferase detected in the cell extracts was decreased to 17.7 ± 5.3% (n = 10), 10.9 ± 2.2% (n = 11), and 3.2 ± 0.6% (n = 6), respectively (Fig. 5A). In contrast, nonspecific EGFP double-strand RNA did not reduce the expression of luciferase activity in a dose-dependent manner (Fig. 5A). These data showed that luciferase double-strand RNA inhibited expression of the luciferase reporter driven by a constitutive pNEXδ-luciferase construct in a dose-dependent manner.
Figure 5.
Inhibition of reporter gene expression by double-strand RNA. (A) Giant neurons in the abdominal ganglion were coinjected with pNEXδ-lacZ, pNEXδ-luciferase, and double-strand RNA of either luciferase or EGFP. Injection of luciferase double-strand RNA reduced luciferase activity in a sequence-specific and dose-dependent manner. pNEXδ-luciferase and double-strand RNA were mixed with the molar ratio of 1:0, 1:10, 1:100, and 1:1000 in the injection solutions. (•) Luciferase double-strand RNA; (○) EGFP double-strand RNA. (B) Sensory cells in the pleural ganglion were coinjected with CRE-lucifease and double-strand RNA luciferase and were treated by five pulses of 5-HT. CRE-driven luciferase gene expression, induced by five pulses of 5-HT, was completely blocked by luciferase double-strand RNA. Bars, mean ± SEM. Numbers in parentheses indicate the number of ganglia tested.
Double-strand RNA also blocked not only the constitutive expression but also the induced expression of the reporter gene. When CRE-luciferase DNA construct was injected into the sensory cells, five pulses of 5-HT produced a 40-fold induction of luciferase activity in the sensory cells. This CRE-driven luciferase expression induced by the long-term facilitation protocol was completely blocked by coinjected luciferase double-strand RNA (Fig. 5B). These data suggest that double-strand RNA could be an effective way to block specifically, not only constitutive, but also induced expression of the genes.
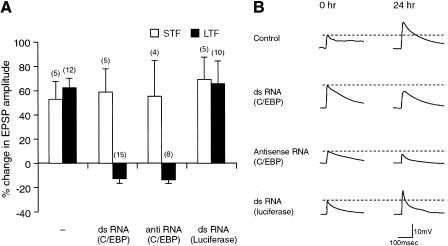
Next, we attempted to block the expression of ApC/EBP of the Aplysia nervous system by microinjecting double-strand RNA in sensory-motor coculture. We injected either ApC/EBP double-strand RNA, ApC/EBP antisense RNA, or luciferase double-strand RNA (control) into sensory neurons. These cells were then exposed to five pulses of 5-HT to induce long-term synaptic facilitation. The cells that were injected with either double-strand RNA or antisense RNA showed no significant increase in amplitude of synaptic potential 24 h after 5-HT treatment (double-strand RNA, −12.9 ± 3.8%, n = 15; antisense, −13.5 ± 3.4%, n = 8, respectively) (Fig. 6). In contrast, the noninjected cells and cells injected with luciferase double-strand RNA showed long-term facilitation with a normal increase (62.2 ± 7.7%, n = 12, and 65.5 ± 18.2%, n = 10, respectively) in synaptic strength 24 h after treatment with 5-HT (Fig. 6). Microinjection of ApC/EBP double-strand RNA, ApC/EBP antisense RNA, or luciferase double-strand RNA did not affect short-term facilitation (59.9 ± 19.0%, n = 5; 55.0 ± 29.6%, n = 4 and 69.0 ± 18.0%, n = 5, respectively) induced by a single pulse of 5-HT (Fig. 6)
Figure 6.
Inhibition by double-strand RNA of ApC/EBP expression blocks long-term facilitation in the sensory-motor synapse. (A) Bar graph representing the effect of ApC/EBP double-strand RNA on short-term (STF, open bars) and long-term facilitation (LTF, solid bars). Injection of either ApC/EBP double-strand RNA or antisense RNA blocked long-term facilitation induced by five pulse of 5-HT, but injection of luciferase double-strand RNA did not. In contrast, injection of ApC/EBP double-strand RNA, ApC/EBP antisense RNA, or luciferase double-strand RNA did not affect short-term facilitation induced by one pulse of 5-HT. The height of each bar corresponds to the mean percentage change ± SEM in EPSP amplitude tested after 5-HT treatment. (−) No injection of double-strand RNA. A one-way analysis of variance and Duncan's multiple range test were used to determine the significance of the EPSP changes by long-term facilitation (F = 21.99, df = 3, P <0.001). (B) Examples of EPSP recorded in motor neuron LFS after stimulation of the sensory neurons before (0 h) and 24 h after five pulses of 5-HT (10 μM) treatment. Control, no injection of double-strand RNA.
In a control experiment, we attempted to detect immunohistochemically the change in the level of ApC/EBP proteins. Our antibody against ApC/EBP was not sensitive enough to detect the low level of nuclear ApC/EBP induced by 5-HT (Fig. 2, left). Therefore, we increased the level of ApC/EBP protein by micoinjection of pNEXδ-ApC/EBP. The overexpressed ApC/EBP protein was localized to the nucleus at certain peripheral regions, as was also shown by Alberini et al. (1994) (Fig. 2, middle). However, if ApC/EBP double-strand RNA was coinjected with pNEXδ-ApC/EBP, this expression of ApC/EBP proteins was no longer detected (Fig. 2, right). These data again show that double-strand RNA interference is effective in blocking even the expression of genes driven by an ectopic overexpression system. Taken together, these results show that double-strand RNA of ApC/EBP, like antisense RNA, interfered with the expression of endogenous ApC/EBP in a sequence-specific manner, therefore interrupting the consolidation phase of long-term facilitation.
DISCUSSION
In a previous study, Alberini et al. (1994) showed that ApC/EBP, an immediate-early gene encoding a transcription factor, was induced rapidly by 5-HT and cAMP and was critically involved in long-term facilitation. Injection of antisense RNA or antibody against ApC/EBP into sensory neurons blocked the action of ApC/EBP, indicating that ApC/EBP was required for the consolidation of long-term facilitation in A. californica.
In our study, we further characterized the role of ApC/EBP in the induction of long-term facilitation by either overexpressing or inhibiting ApC/EBP through a DNA or RNA microinjection method, respectively. We cloned ApC/EBP from an Asian species, A. kurodai, which has very similar anatomy and function of the central nervous system to that of A. californica (Lim et al. 1997). Our transcription assay data showed that the cloned ApC/EBP from Aplysia encodes a functional transcription factor that transactivates the genes via a CCAAT enhancer sequence such as ERE.
Bartsch et al. (1998) have shown that phosphorylated CREB-1a microinjected into sensory neurons was sufficient in inducing long-term facilitation. Because ApC/EBP is a downstream target gene of CREB-1a, it raises the following questions: Is overexpression of ApC/EBP sufficient for long-term facilitation? Can overexpressed ApC/EBP replace the function of the phosphorylated and active CREB? Our data showed that ectopic overexpression of ApC/EBP did not mimic CREB activity and was not sufficient in triggering long-term facilitation. However, overexpression of ApC/EBP reduced the threshold for long-term facilitation such that one pulse of 5-HT that would normally induce only short-term facilitation now produced long-term facilitation. Various stimuli impinged upon the sensory neurons can induce an immediate-early gene ApC/EBP. It may be possible that the sensory neurons primed by these stimuli can undergo the long-term synaptic changes by 5-HT released by a short-term sensitization protocol. Failure of inducing long-term facilitation by ApC/EBP alone suggests that proteins other than ApC/EBP that are activated or induced by the cAMP/PKA/CREB pathway may be required to induce long-term facilitation. For example, there may be other types of immediate early genes such as ubiquitin C-terminal hydrolase that are induced by activated CREB. Finally, other activators such as ApAF (Bartsch et al. 2000) may also be required.
Double-strand RNA interference (dsRNAi) has become a powerful tool to inhibit a specific target gene in a variety of organisms from Caenorhabiditis elegans to mammalian species. However, to our knowledge, it has not been determined whether dsRNAi is effective in the molluscan species or in particular in the nervous system of Aplysia. Therefore, we tried to block the function of ApC/EBP by introducing double-strand RNA of ApC/EBP to see whether it blocks the long-term facilitation process. Alberini et al. (1994) showed that long-term facilitation was blocked by antisense ApC/EBP microinjected into sensory cells. Consistent with their results, our data showed that not only antisense but also double-strand RNA of ApC/EBP inhibited long-term facilitation in a nucleotide sequence-specific manner. In a control experiment with a luciferase reporter gene, double-strand RNA also inhibited the expression of the reporter gene in a dose-dependent manner. Although we have not shown that double-strand RNA introduced into Aplysia neurons underwent exactly the same RNAi mechanism through the processing of double-strand RNA into short 21- to 23-nucleotide segments that has been reported in other systems (Zamore et al. 2000), double-strand RNA seemed to block the gene expression as effectively as antisense RNA injection. dsRNAi can be used as a powerful tool to inhibit the specific target gene in Aplysia neurons to study the function of neuronal proteins that are thought to be important in synaptic transmission and plasticity.
MATERIALS AND METHODS
Screening of ApC/EBP
To clone ApC/EBP from A. kurodai, we performed RT–PCR using the sense and antisense primers (ApC/EBP 500-sense, 5′-CTGGGGT GGATGCTCCC-3′; ApC/EBP 850-antisense, 5′-CGTACTCCTGAGTT CCC-3′) designed on the basis of ApC/EBP sequences (Alberini et al. 1994) of A. californica. The PCR reaction yielded a DNA fragment of 363 bp, which was subsequently used as probe to isolate cDNA library. We screened ∼1.5 × 105 clones and 12 positive signals were obtained after the second screening. Four of these clones were analyzed by DNA sequencing and all contained the ApC/EBP coding region. One clone revealed a 156-bp insertion that is specific for long-form ApC/EBP. The sequences of both forms of ApC/EBPL, S were separately subcloned into HindIII/SmaI-digested pNEXδ (Kaang et al. 1993) to create pNEXδ-ApC/EBPL (long form) and pNEXδ-ApC/EBPS (short form), respectively.
Luciferase Reporter DNA Constructs
ERE (Metz et al. 1991; Alberini et al. 1994) and CRE sequences were inserted into the BglII site of the pGL3-promotor vector (Promega), producing ERE-luciferase and CRE-luciferase, respectively. The inserted sequences are 5′-CGCGCGCATATTAGGACATGCGGGGC CCAGATC-3′ (ERE) and 5′-TGGGCCCTGACGTCACGCGCGGATC 3′ (CRE) (core enhancer sequences are underlined). The inserted sequences were confirmed by DNA sequencing. pGL3-promoter vector was used as enhancerless luciferase (Δ-luciferase, negative control).
RNA Synthesis by In Vitro Transcription
To make template DNAs for in vitro transcription, the cDNAs for ApC/EBPL, luciferase, and EGFP (Clontech) were inserted into pBluescript II (SK) vector using BamHI/KpnI, EcoRV/HindIII, and BamHI/EcoRI digestions, respectively. Sense and antisense RNAs were synthesized by in vitro transcription of linearized template DNAs using T3 and T7 RNA polymerase (mRNA capping kits, Stratagene). Double-strand RNA was prepared as described previously (Kennerdell and Carthew 1998).
Microinjection
Microinjection into Aplysia neurons was done by air pressure as described elsewhere (Kaang et al. 1992; Kaang 1996a,b). To examine transcriptional activity of ApC/EBP isoforms, pNEXδ-ApC/EBP was coinjected with ERE-luciferase, CRE-luciferase, or enhancerless luciferase into ∼20 giant neurons of the abdominal ganglion. As an internal control for gene expression, pNEX2-lacZ (Kaang 1996a) was included in the injection solution (Kaang 1996b). The DNA concentrations (mg/mL) in the injection solution of pNEXδ-ApC/EBP, the luciferase constructs, and pNEX2-lacZ were 0.1, 0.7, and 0.1, respectively. The injected ganglia were incubated at 18°C for 24 h, until reporter gene assays were done.
For immunocytochemistry, pNEXδ-ApC/EBP (100 μg/mL) and double-strand RNA were injected into the cultured sensory neurons with the molar ratio of 1:10 in injection buffer. As control, pNEXδ-GFPS65TΔC9 (200 μg/mL) (Kim et al. 1998) also was included in the injection solution as a marker of gene transfer.
To examine the effect of ApC/EBP overexpression on synaptic facilitation, sensory cells were injected with the injection buffer containing pNEXδ-ApC/EBPL (500 μg/mL) and pNEXδ-GFPS65TΔC9 (500 μg/mL) 1 d before 5-HT training.
To examine the effect of double-strand RNA on constitutive gene expression, pNEXδ-luciferase (10 μg/mL) (Kaang 1996b) and double-strand RNA (up to 700 μg/mL) were injected into ∼20 giant neurons of the abdominal ganglion with the molar ratio of 1:10, 1:100, and 1:1000 in the injection buffer. To examine the effect of double-strand RNA on inducible gene expression, CRE-luciferase (10 μg/mL) and double-strand RNA were injected into ∼30 sensory neurons per sensory cluster of pleural ganglion with the molar ratio of 1:100 in injection buffer. As an internal control for gene expression, pNEXδ-lacZ (50 μg/mL) (Kaang et al. 1993) was included in the injection solution.
To examine the effect of double-strand RNA and antisense RNA of ApC/EBP on synaptic facilitation, the sensory cells in sensory-to-motor cocultures were injected with the injection buffer containing 500 μg/mL of pNEXδ-EGFP and 300 μg/mL of double-strand RNA or antisense RNA 1 h before 5-HT training. pNEXδ-EGFP was constructed by inserting EGFP into HindIII/SmaI sites of pNEXδ.
Successful RNA or DNA microinjections in sensory-to-motor cocultures were determined 24 h after microinjection into the sensory cells by the expression of GFP DNA construct. Only GFP-positive fluorescent cells were included in the data.
Reporter Gene Assays
Ganglionic cell extracts were made 24 hr after micoinjection using 30 μL of 1 × Reporter Lysis buffer per each ganglion (Promega), as described previously (Kaang 1996a). Luciferase and β-galactosidase assays were done as described previously (Kaang 1996a). Normalized luciferase activity was obtained by dividing luciferase activity by β-galactosidase activity. Results are expressed as mean ± SEM.
Cell Cultures, Electrophysiology, and Induction of Long-Term Facilitation by 5-HT
Culture dishes and medium were prepared as described previously (Schacher et al. 1983; Montarolo et al. 1986). Sensory cells isolated from the pleural ganglia of adult animal (100–150 g) were cocultured with the motor neuron LFS obtained from the abdominal ganglia of adult animals as described previously (Montarolo et al. 1986). Coculture was maintained for 4–5 d at 18 °C to allow the formation and stabilization of synaptic connections. The motor cell was impaled intracellularly with a glass microelectrode filled with 2 M K-acetate, 0.5 M KCl, 10 mM K-HEPES (10–15 MΩ), and the membrane potential was held at −30 mV below its resting value. The excitatory postsynaptic potential was evoked in LFS by stimulating the sensory neurons with a brief depolarizing stimulus by use of an extracellular electrode. After the initial excitatory postsynaptic potential (EPSP) value was measured, the cultures received one pulse of 10 μM 5-HT for 5 min or five pulses at 15-min intervals to induce long-term facilitation. The amount of synaptic facilitation was calculated as the percentage change in EPSP amplitude recorded 24 h after the 5-HT treatment, compared with its initial value before the treatment. All data are presented as the mean percentage change ± SEM in EPSP amplitude. Statistical analysis was performed using one-way ANOVA followed by Duncan's multiple range test.
Immunocytochemistry
To generate polyclonal mouse antisera against the ApC/EBP, the gene encoding full-length ApC/EBPL was fused to the downstream of His-tag in pRSETa vector (Invitrogen). Purified His-ApC/EBP fusion protein from Escherichia coli was used to immunize a mouse strain Balb/c as described in Harlow et al. (1998). Polyclonal anti-ApC/EBP antibody was purified from the antisera according to the method described by Gruber et al. (1995).
Immunocytochemistry was carried out as described in Martin et al. (1997). Cultured sensory cells were microinjected with pNEXδ-ApC/EBP in the presence and absence of ApC/EBP double-strand RNA and were received by five pulses of 5-HT 1 h after microinjection. The cultures were incubated at 18°C for 24 h and were fixed for immunohistochemistry. The primary anti-C/EBP antibody and the secondary Cy3-conjugated anti-mouse IgG antibody (Amersham Pharmacia Biotech) were used with dilution ratios of 1:100 and 1:500, respectively. Fluorescent images were obtained through a confocal laser-scanning microscope (Radiance 2000, Bio-Rad).
Acknowledgments
We thank Drs. E. Kandel and C. Alberini for DNA constructs and valuable comments on a previous version of the manuscript. We are also grateful to Dr. H. Suh (Ajou University) for the generous gift of a reporter construct and Dr. J. Lee (Yonsei University) for technical comments on dsRNAi. This work was supported by grant no. 1999-1-213-002-5 from the Basic Research Program of the Korea Science & Engineering Foundation and partly by the Korean Ministry of Science and Technology under the Brain Science Research Program. J.-A. L., J.-H. H., Y.-S. L., and C.-S. L. were supported by BK21 Research Fellowships from the Korean Ministry of Education. The sequence of ApC/EBP from A. kurodai has been deposited in GenBank (accession no. AY024341).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL kaang@snu.ac.kr; FAX 82-2-874-1206.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.40201.
REFERENCES
- Alberini CM, Ghiradi M, Metz R, Kandel ER. ApC/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplyisa. Cell. 1994;76:1099–1114. doi: 10.1016/0092-8674(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1983;5:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Bartsch D, Kandel ER. Toward a molecular definition of long-term memory storage. Proc Natl Acad Sci. 1996;93:13445–13452. doi: 10.1073/pnas.93.24.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch D, Casadio A, Karl CA, Serodio P, Kandel ER. CREB1 encodes a nuclear activator, repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell. 1998;95:211–223. doi: 10.1016/s0092-8674(00)81752-3. [DOI] [PubMed] [Google Scholar]
- Bartsch D, Ghirardi M, Casadio A, Giustetto M, Karl KA, Zhu H, Kandel ER. Enhancement of memory-related long-term facilitation by ApAF, a novel transcription factor that acts downstream from both CREB1 and CREB2. Cell. 2000;103:1–20. doi: 10.1016/s0092-8674(00)00163-x. [DOI] [PubMed] [Google Scholar]
- Glanzman DL, Kandel ER, Schacher S. Target dependent structural changes accompanying long-term synaptic facilitation in Aplysia sensory neurons. Science. 1990;249:799–802. doi: 10.1126/science.2389145. [DOI] [PubMed] [Google Scholar]
- Gruber A, Zingales B. Alternative method to remove antibacterial antibodies from antisera used for screening of expression library. Biotechnique. 1995;19:27–28. [PubMed] [Google Scholar]
- Harlow E, Lane D. Immunization, antibodies: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1998. pp. 53–135. [Google Scholar]
- Kaang B-K, Pfaffinger PJ, Grant SGN, Kandel ER, Furukawa Y. Overexpression of an Aplysia shaker K+ channel gene modifies the electrical properties and synaptic efficacy of identified Aplysia neuron. Proc Natl Acad Sci. 1992;89:1133–1137. doi: 10.1073/pnas.89.3.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaang B-K, Kandel ER, Grant SGN. Activation of cAMP-response genes by stimuli that produce long-term facilitation in Aplysia sensory neurons. Neuron. 1993;10:427–435. doi: 10.1016/0896-6273(93)90331-k. [DOI] [PubMed] [Google Scholar]
- Kaang B-K. Parameters influencing ectopic gene expression in Aplysia neurons. Neurosci Lett. 1996a;221:29–32. doi: 10.1016/s0304-3940(96)13279-1. [DOI] [PubMed] [Google Scholar]
- ————— Neuronal expression of reporter genes in the intact nervous system of Aplysia. Mol Cells. 1996b;6:285–295. [Google Scholar]
- Kennerdell JR, Carthew RW. Use of ds RNA-mediated genetic interference to demonstrate that frizzled and frizzled2 act in the wingless pathway. Cell. 1998;95:1017–1026. doi: 10.1016/s0092-8674(00)81725-0. [DOI] [PubMed] [Google Scholar]
- Kim H-K, Kaang B-K. Truncated green fluorescent protein mutants and their expression in Aplysia neurons. Brain Res Bull. 1998;47:35–41. doi: 10.1016/s0361-9230(98)00020-3. [DOI] [PubMed] [Google Scholar]
- Lekstrom-Himes J, Xanthopoulos KG. Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J Biol Chem. 1998;273:28545–28548. doi: 10.1074/jbc.273.44.28545. [DOI] [PubMed] [Google Scholar]
- Lim C-S, Chung D-Y, Kaang B-K. Partial anatomical and physiological characterization and dissociated cell culture of the nervous system of the marine mollusk Aplysia kurodai. Mol Cell. 1997;7:399–407. [PubMed] [Google Scholar]
- Martin KC, Michael D, Rose JC, Barad M, Casadio A, Zhu H, Kandel ER. MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia. Neuron. 1997;18:899–912. doi: 10.1016/s0896-6273(00)80330-x. [DOI] [PubMed] [Google Scholar]
- Metz R, Ziff E. The helix-loop-helix protein rE12 and the C/EBP-related factor rNFIL-6 bind to neighboring sites within the c-fos serum response element. Oncogene. 1991;6:2165–2178. [PubMed] [Google Scholar]
- Montarolo PG, Goelet P, Castellucci VF, Morgan J, Kandel ER. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. 1986;234:1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- Montgomery M, Kostas SA, Fire A, Driver SE, Mello CC. Potent and specific genetic interference by double stranded RNA in C. elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Schacher S, Proshansky E. Neurite generation by Aplysia neurons in dissociated cell culture: Modulation by Aplysia hemolymph and the presence of the initial axonal segment. J Neurosci. 1983;3:2403–2413. doi: 10.1523/JNEUROSCI.03-12-02403.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenfeld SM, Wiing KA, Monti B, Dolan B, Pollonini G, Alberini CM. Fornix-dependent induction of hippocampal CCAAT enhancer-binding protein β and δ co-localizes with phosphorylated cAMP response element-binding protein and accompanies long-term memory consolidation. J Neurosci. 2001;21:84–91. doi: 10.1523/JNEUROSCI.21-01-00084.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukawa K, Tanaka T, Tsuji S, Akira S. Expression of CCAAT/enhancer-binding proteins β and δ and intensified by cAMP signaling as well as Ca2+/Calmodulin kinase activation in hippocampal neurons. J Biol Chem. 1998;273:31345–31351. doi: 10.1074/jbc.273.47.31345. [DOI] [PubMed] [Google Scholar]
- Zamore PD, Tusch T, Sharp PA, Bartel DP. RNAi: Double-strand RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]