Abstract
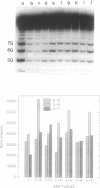
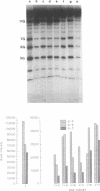
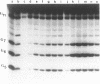
The detection of abnormal DNA base pairing arrangements and conformations is chemically probed in synthetic 32P-end-labeled deoxyribonucleotide oligomers using N-methyl-N-nitrosourea (MNU) and 2,12,-dimethyl-3,7,11,17-tetraazabicyclo-[11.3.1]heptadeca-1 -[17],2,11,13,15 pentaene-Ni (II) (Ni-complex) with KHSO5. The DNA targets studied are single-stranded (s-s) DNA, double-stranded (d-s) DNA, d-s DNA with G-G, G-A and G-T mismatches, d-s DNA with a single bulged G and d-s DNA with two bulged G's. The effect of the non-Watson--Crick structures on the formation of N7-methylguanine (N7-MeG) by MNU and the oxidation of G by Ni-complex is reported along with the Tm's and circular dichroism spectra of the different duplex oligomers. The results for MNU and Ni-complex show that the qualitative and quantitative character of the cleavage patterns at a G3 run change with the nature of the abnormal base pairing motif. Based on the DNA substrates studied, the results indicate that a combination of reagents which report electronic and steric perturbations can be a useful approach to monitor DNA mismatches and bulges.
Full text
PDF

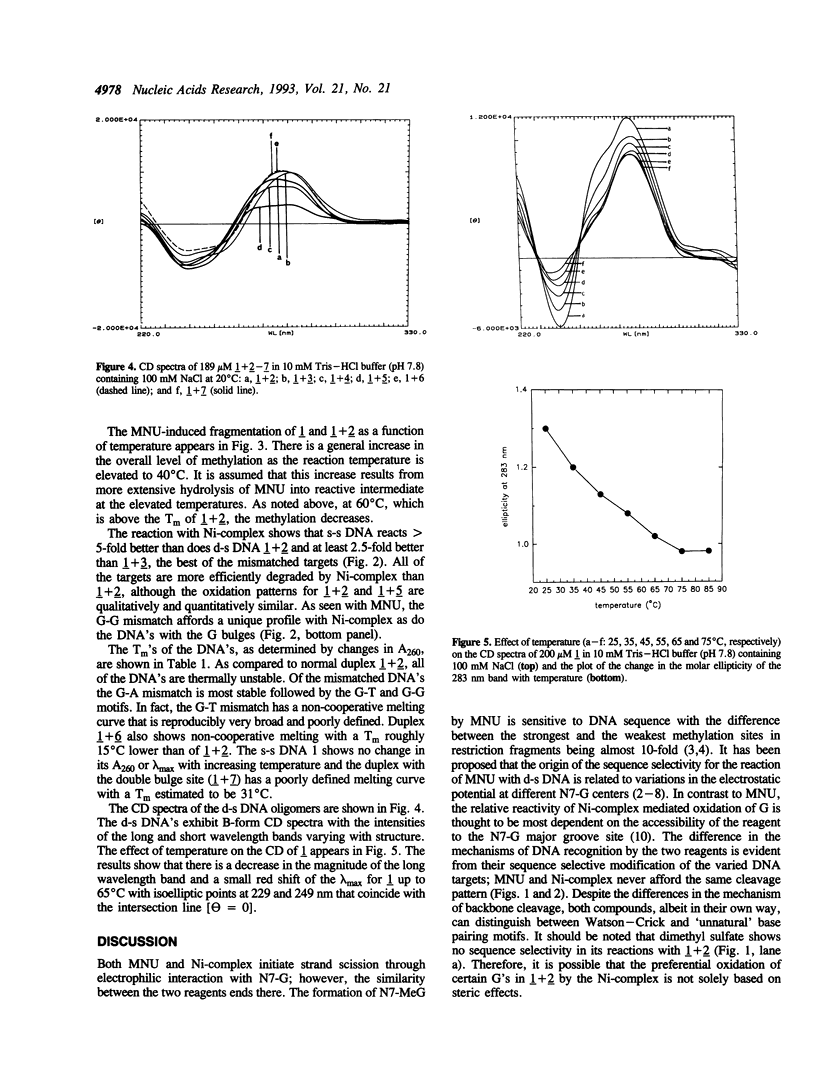


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beranek D. T., Weis C. C., Swenson D. H. A comprehensive quantitative analysis of methylated and ethylated DNA using high pressure liquid chromatography. Carcinogenesis. 1980 Jul;1(7):595–606. doi: 10.1093/carcin/1.7.595. [DOI] [PubMed] [Google Scholar]
- Bodell W. J., Singer B. Influence of hydrogen bonding in DNA and polynucleotides on reaction of nitrogens and oxygens toward ethylnitrosourea. Biochemistry. 1979 Jun 26;18(13):2860–2863. doi: 10.1021/bi00580a029. [DOI] [PubMed] [Google Scholar]
- Borden K. L., Jenkins T. C., Skelly J. V., Brown T., Lane A. N. Conformational properties of the G.G mismatch in d(CGCGAATTGGCG)2 determined by NMR. Biochemistry. 1992 Jun 16;31(23):5411–5422. doi: 10.1021/bi00138a024. [DOI] [PubMed] [Google Scholar]
- Briscoe W. T., Cotter L. E. DNA sequence has an effect on the extent and kinds of alkylation of DNA by a potent carcinogen. Chem Biol Interact. 1985 Dec 31;56(2-3):321–331. doi: 10.1016/0009-2797(85)90014-6. [DOI] [PubMed] [Google Scholar]
- Brown T., Hunter W. N., Kneale G., Kennard O. Molecular structure of the G.A base pair in DNA and its implications for the mechanism of transversion mutations. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2402–2406. doi: 10.1073/pnas.83.8.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T., Leonard G. A., Booth E. D., Kneale G. Influence of pH on the conformation and stability of mismatch base-pairs in DNA. J Mol Biol. 1990 Apr 5;212(3):437–440. doi: 10.1016/0022-2836(90)90320-L. [DOI] [PubMed] [Google Scholar]
- Carbonnaux C., Fazakerley G. V., Sowers L. C. An NMR structural study of deaminated base pairs in DNA. Nucleic Acids Res. 1990 Jul 25;18(14):4075–4081. doi: 10.1093/nar/18.14.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognet J. A., Gabarro-Arpa J., Le Bret M., van der Marel G. A., van Boom J. H., Fazakerley G. V. Solution conformation of an oligonucleotide containing a G.G mismatch determined by nuclear magnetic resonance and molecular mechanics. Nucleic Acids Res. 1991 Dec 25;19(24):6771–6779. doi: 10.1093/nar/19.24.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognet J. A., Gabarro-Arpa J., Le Bret M., van der Marel G. A., van Boom J. H., Fazakerley G. V. Solution conformation of an oligonucleotide containing a G.G mismatch determined by nuclear magnetic resonance and molecular mechanics. Nucleic Acids Res. 1991 Dec 25;19(24):6771–6779. doi: 10.1093/nar/19.24.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fersht A. R., Knill-Jones J. W., Tsui W. C. Kinetic basis of spontaneous mutation. Misinsertion frequencies, proofreading specificities and cost of proofreading by DNA polymerases of Escherichia coli. J Mol Biol. 1982 Mar 25;156(1):37–51. doi: 10.1016/0022-2836(82)90457-0. [DOI] [PubMed] [Google Scholar]
- Gray D. M., Ratliff R. L., Vaughan M. R. Circular dichroism spectroscopy of DNA. Methods Enzymol. 1992;211:389–406. doi: 10.1016/0076-6879(92)11021-a. [DOI] [PubMed] [Google Scholar]
- Hartley J. A., Gibson N. W., Kohn K. W., Mattes W. B. DNA sequence selectivity of guanine-N7 alkylation by three antitumor chloroethylating agents. Cancer Res. 1986 Apr;46(4 Pt 2):1943–1947. [PubMed] [Google Scholar]
- Hartley J. A., Mattes W. B., Vaughan K., Gibson N. W. DNA sequence specificity of guanine N7-alkylations for a series of structurally related triazenes. Carcinogenesis. 1988 Apr;9(4):669–674. doi: 10.1093/carcin/9.4.669. [DOI] [PubMed] [Google Scholar]
- Hunter W. N., Brown T., Kneale G., Anand N. N., Rabinovich D., Kennard O. The structure of guanosine-thymidine mismatches in B-DNA at 2.5-A resolution. J Biol Chem. 1987 Jul 25;262(21):9962–9970. doi: 10.2210/pdb113d/pdb. [DOI] [PubMed] [Google Scholar]
- Kalnik M. W., Kouchakdjian M., Li B. F., Swann P. F., Patel D. J. Base pair mismatches and carcinogen-modified bases in DNA: an NMR study of G.T and G.O4meT pairing in dodecanucleotide duplexes. Biochemistry. 1988 Jan 12;27(1):108–115. doi: 10.1021/bi00401a018. [DOI] [PubMed] [Google Scholar]
- Kohn K. W., Hartley J. A., Mattes W. B. Mechanisms of DNA sequence selective alkylation of guanine-N7 positions by nitrogen mustards. Nucleic Acids Res. 1987 Dec 23;15(24):10531–10549. doi: 10.1093/nar/15.24.10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane A. N., Jenkins T. C., Brown D. J., Brown T. N.m.r. determination of the solution conformation and dynamics of the A.G mismatch in the d(CGCAAATTGGCG)2 dodecamer. Biochem J. 1991 Oct 1;279(Pt 1):269–281. doi: 10.1042/bj2790269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Moe J. G., Russu I. M. Kinetics and energetics of base-pair opening in 5'-d(CGCGAATTCGCG)-3' and a substituted dodecamer containing G.T mismatches. Biochemistry. 1992 Sep 15;31(36):8421–8428. doi: 10.1021/bi00151a005. [DOI] [PubMed] [Google Scholar]
- Oda Y., Uesugi S., Ikehara M., Kawase Y., Ohtsuka E. NMR studies for identification of dI:dG mismatch base-pairing structure in DNA. Nucleic Acids Res. 1991 Oct 11;19(19):5263–5267. doi: 10.1093/nar/19.19.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privé G. G., Heinemann U., Chandrasegaran S., Kan L. S., Kopka M. L., Dickerson R. E. Helix geometry, hydration, and G.A mismatch in a B-DNA decamer. Science. 1987 Oct 23;238(4826):498–504. doi: 10.1126/science.3310237. [DOI] [PubMed] [Google Scholar]
- Pullman A., Pullman B. Molecular electrostatic potential of the nucleic acids. Q Rev Biophys. 1981 Aug;14(3):289–380. doi: 10.1017/s0033583500002341. [DOI] [PubMed] [Google Scholar]
- Quignard E., Fazakerley G. V., van der Marel G., van Boom J. H., Guschlbauer W. Comparison of the conformation of an oligonucleotide containing a central G-T base pair with the non-mismatch sequence by proton NMR. Nucleic Acids Res. 1987 Apr 24;15(8):3397–3409. doi: 10.1093/nar/15.8.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radman M., Wagner R. Effects of DNA methylation on mismatch repair, mutagenesis, and recombination in Escherichia coli. Curr Top Microbiol Immunol. 1984;108:23–28. doi: 10.1007/978-3-642-69370-0_3. [DOI] [PubMed] [Google Scholar]
- Singer B., Fraenkel-Conrat H. Chemical modification of viral ribonucleic acid. VII. The action of methylating agents and nitrosoguanidine on polynucleotides including tobacco mosaic virus ribonucleic acid. Biochemistry. 1969 Aug;8(8):3260–3266. doi: 10.1021/bi00836a019. [DOI] [PubMed] [Google Scholar]
- Skelly J. V., Edwards K. J., Jenkins T. C., Neidle S. Crystal structure of an oligonucleotide duplex containing G.G base pairs: influence of mispairing on DNA backbone conformation. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):804–808. doi: 10.1073/pnas.90.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S. S., Lahue R. S., Au K. G., Modrich P. Mispair specificity of methyl-directed DNA mismatch correction in vitro. J Biol Chem. 1988 May 15;263(14):6829–6835. [PubMed] [Google Scholar]
- Webster G. D., Sanderson M. R., Skelly J. V., Neidle S., Swann P. F., Li B. F., Tickle I. J. Crystal structure and sequence-dependent conformation of the A.G mispaired oligonucleotide d(CGCAAGCTGGCG). Proc Natl Acad Sci U S A. 1990 Sep;87(17):6693–6697. doi: 10.1073/pnas.87.17.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson S. A., Crothers D. M. Preferential location of bulged guanosine internal to a G.C tract by 1H NMR. Biochemistry. 1988 Jan 12;27(1):436–445. doi: 10.1021/bi00401a065. [DOI] [PubMed] [Google Scholar]
- Wurdeman R. L., Gold B. The effect of DNA sequence, ionic strength, and cationic DNA affinity binders on the methylation of DNA by N-methyl-N-nitrosourea. Chem Res Toxicol. 1988 May-Jun;1(3):146–147. doi: 10.1021/tx00003a003. [DOI] [PubMed] [Google Scholar]