Abstract
OBJECTIVE
T cells and level of the cytokine interferon-γ (IFN-γ) are increased in adipose tissue in obesity. Hedgehog (Hh) signaling has been shown to potently inhibit white adipocyte differentiation. In light of recent findings in neurons that IFN-γ and Hh signaling cross-talk, we examined their potential interaction in the context of adipogenesis.
RESEARCH DESIGN AND METHODS
We used Hh reporter cells, cell lines, and primary adipocyte differentiation models to explore costimulation of IFN-γ and Hh signaling. Genetic dissection using Ifngr1−/− and Stat1−/− mouse embryonic fibroblasts, and ultimately, anti–IFN-γ neutralization and expression profiling in obese mice and humans, respectively, were used to place the findings into the in vivo context.
RESULTS
T-cell supernatants directly inhibited hedgehog signaling in reporter and 3T3-L1 cells. Intriguingly, using blocking antibodies, Ifngr1−/− and Stat1−/− cells, and simultaneous activation of Hh and IFN-γ signaling, we showed that IFN-γ directly suppresses Hh stimulation, thus rescuing adipogenesis. We confirmed our findings using primary mouse and primary human (pre)adipocytes. Importantly, robust opposing signals for Hh and T-cell pathways in obese human adipose expression profiles and IFN-γ depletion in mice identify the system as intact in adipose tissue in vivo.
CONCLUSIONS
These results identify a novel antagonistic cross-talk between IFN-γ and Hh signaling in white adipose tissue and demonstrate IFN-γ as a potent inhibitor of Hh signaling.
The World Health Organization (WHO) currently estimates that more than 1 billion individuals worldwide are overweight. Almost one-third of these individuals are clinically obese, markedly raising their chances of cardiovascular disease, type 2 diabetes, cancer, and stroke (1).
Interestingly, not all obesity results in metabolic disease, and thus, it is not adiposity alone that contributes to adipose tissue abnormalities (2–9). For instance, large, lipid-loaded fat cells appear to be particularly important for the development of obesity-related cardiovascular and metabolic disorders. Increases in adipocyte size correlate with higher production of inflammatory adipokines, and increased circulating inflammatory markers are clinically observed in patients with hypertrophic adipocytes compared with fat mass–matched control subjects with smaller fat cells. Exactly why this is the case remains to be proven; one suggestion has been that the impaired function of large adipocytes results from the relative hypoxia of the microenvironment (2,3).
Poor expandability of the adipocyte pool, combined with chronic low-grade inflammation, is thought to initiate a vicious cycle that ultimately culminates in obesity with full metabolic dysfunction, including insulin resistance (10–13). Several studies have shown that in addition to macrophages, the number of CD3+ T cells is increased in adipose tissue in obesity (14,15). Furthermore, genetic or diet-induced obese (DIO) mouse models exhibit a prominent and early influx of cytotoxic CD8+ T cells (16–19). Local mRNA levels of the activated T-cell cytokine interferon-γ (IFN-γ) are increased in adipose tissue of DIO mice compared with lean controls, and IFN-γ–deficient animals show significantly decreased proinflammatory gene expression and macrophage accumulation in adipose tissue in obesity (20). In addition, IFN-γ decreases insulin sensitivity and suppresses differentiation in human adipocytes (21,22). However, whether T-cell activation and associated increases in IFN-γ per se cause insulin resistance in adipose tissue has been questioned (23). Indeed, macrophage infiltration is the most robust discriminant between insulin-sensitive (IS) and insulin-resistant (IR) individuals with morbid obesity (24).
Hedgehog (Hh) signaling is an ancestral developmental process directing embryonic differentiation and adult tissue homeostasis through stem cell regulation and orchestration of complex differentiation programs (25–28). Activation of the Hh pathway is initiated by the Hh ligands, which release inhibition of the Smoothened (Smo)-Patched (Ptch) dual-receptor system at the cell surface, ultimately culminating in translocation of the Gli transcription factors to the nucleus and modulation of their target genes. Activation reinforces the signaling system as promoters of a number of the signaling constituents themselves represent Gli-targets, including Gli1 and Ptch1. We and others have demonstrated that Hh signaling is important in adipose tissue differentiation in vivo (29,30), specifically blocking white but not brown adipocyte differentiation at an early stage (30). Of note, expression of Hh target genes is significantly decreased in adipose tissue of genetic and DIO mouse models, suggesting persistent inhibition of the pathway in obesity (29).
Cross-talk between Hh and IFN-γ signaling has been described in some cells of the central nervous system (31–33). Canonical Hh signaling has been shown to mediate neuronal and oligodendroglial differentiation (34–37). Interestingly, IFN-γ induces sonic Hh (Shh) mRNA expression in astroglia and neuronal stem cells (32) and has been shown to contribute to medulloblastoma development by reactivating Hh signaling via induction of Shh (38). IFN-γ stimulation induces Shh gene expression in cultured primary granular neuronal precursor cells, with subsequent induction of the Hh target gene Gli1 (33). However, IFN-γ might also be capable of inhibiting canonical Hh-signaling and Gli1 expression in neuronal stem cells in multiple sclerosis and associated animal models (32).
Considering that CD8+ T cells that produce IFN-γ are among the first invaders of adipose tissue in obesity and that Hh blocks white fat expansion in vivo (30), we hypothesized that IFN-γ might interfere with ongoing Hh signaling and support increased adipocyte turnover. Using a combination of in vitro and in vivo models we show that IFN-γ– and Hh-signaling pathways independently impair white adipocyte differentiation. Surprisingly, costimulation of both signaling cascades resulted in reciprocal cosuppression, relieving their individual antiadipogenic actions and permitting adipogenesis. Our data demonstrate for the first time inhibition of Hh signaling via the IFN-γ/Janus kinase (Jak)/signal transducer and activator of transcription (Stat1) axis in adipose tissue and suggest a novel antagonistic regulatory module to optimize white adipocyte turnover according to the metabolic state.
RESEARCH DESIGN AND METHODS
Screening of Hh pathway activities using Shh-LIGHT2 and Ptch−/− cells.
Shh-LIGHT2 cells, a NIH-3T3–derived cell line generated to screen for Hh pathway activities (39) were obtained from the American Type Culture Collection (cat. CRL-2795, Manassas, VA) and cultured according to recommended protocols (39). After reaching confluence, cells were starved in Dulbecco’s modified Eagle’s medium containing 0.5% FCS. Starved cells were stimulated for 48 h with the synthetic Smoothened AGonist (SAG; ALEXIS Biochemicals, San Diego, CA) or recombinant sonic Hh (Shh) protein (R&D Systems, Minneapolis, MN) and/or IFN-γ (BD Pharmingen, San Diego, CA), or were left untreated. Jak proteins were inhibited by Jak Inhibitor I (Calbiochem, San Diego, CA). Primary fibroblasts lacking functional Ptch (Ptch−/−) were derived from Ptch−/− mouse embryos (strain 003081, The Jackson Laboratory, Bar Harbor, ME). After the cells had reached confluence, they were maintained in low-serum medium (0.5% FCS) and treated with IFN-γ or left untreated for 2 days. Hh signaling activities in Shh-LIGHT2 and Ptch−/− cells were measured using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI) or the Tropix galacto-light plus assay system (Tropix, Bedford, MA), respectively. Signal intensities were normalized to Renilla luciferase (Shh-LIGHT2 cells) or total protein content (Ptch−/− cells), as described (39).
Preparation and activation of T cells.
CD8+ lymphocytes were purified from spleens from C57BL/6J mice using negative selection with IMag Streptavidin Particles Plus-DM (BD Biosciences, Bedford, MA) according to the manufacturer’s protocol. T cells were added to 48-well plates coated with 1 mg/mL anti-Cd3e and 3 mg/mL anti-Cd28 (both BD Pharmingen). Supernatants of stimulated T cells were harvested after 48 h.
Cell culture and adipocyte differentiation.
Mouse 3T3-L1 preadipocytes, primary mouse, and human cells were isolated, propagated, and differentiated as described ([30] and Supplementary Methods). Recombinant Shh (R&D Systems), SAG (ALEXIS Biochemicals), and/or recombinant IFN-γ (BD Pharmingen) were added to the cells, as indicated in the text or Figure legends.
Oil Red O staining.
For staining of neutral lipids, cells were fixed and stained with Oil Red O (OrO; Sigma-Aldrich, St. Louis, MO) according to standard procedures. In brief, cells were washed with PBS and fixed with 2% paraformaldehyde in PBS at room temperature for 10 min. Fixed cells were washed again with PBS and stained with OrO (1% w/v isopropanol, diluted 3:2 in PBS) for 1 h at room temperature. Nuclei were counterstained with bromophenol blue. Stained cells were washed and photographed.
Quantitative RT-PCR.
Transcript levels were quantified as described in the Supplementary Appendix.
Western blot analysis.
Antibodies and detailed methods are described in the Supplementary Appendix.
Isolation of primary mouse embryonic fibroblasts.
Primary mouse embryo fibroblasts (MEFs) were derived from day 13.5 embryos obtained from wild-type, Stat1−/− (40) and Ifngr1−/− (41) mice. MEFs were prepared as described (42). All mice were of C57BL/6 background. The animal experiments were discussed and approved by the ethics committee at the Institute of Animal Breeding and Genetics, University of Veterinary Medicine Vienna and the Austrian laws (GZ 68.205/0204-C/GT/2007 and GZ 68.205/0233-II/10b/2009).
Animal handling and treatment of mice with anti–IFN-γ monoclonal antibody.
C57BL/6J mice were purchased from The Jackson Laboratory. At age 6 weeks, male littermates were placed for 24 weeks on a high-fat (HF) diet (HF group, n = 15, 60 kcal% fat, D12492) to induce obesity and on a low-fat (LF) diet (LF group, n = 10, 10 kcal% fat, D12450B) to serve as lean controls (both diets from Research Diets, New Brunswick, NJ). For the last 2 weeks of diets, mice fed the HF diet were randomly split into two groups: HF and HF treated with antibodies (HF+Ab, n = 5). HF+Ab mice were injected with 0.5 mg i.p. anti–IFN-γ monoclonal antibodies (BD Pharmigen) three times within the last 2 weeks of the diet. Control LF and HF animals received the same amount of rat IgG (Sigma-Aldrich). All experimental protocols were approved by the Institutional Animal Care and Use Committee at Medical University Vienna (protocol no. BMWF-66.009/0066-II/106/2009), and all studies were performed according to the methods approved in the protocol.
IFN-γ and adiponectin ELISA.
IFN-γ and adiponectin levels in mouse serum were determined by ELISA (R&D Systems). For details see Supplementary Methods.
Adipose tissue fractionation.
Digestion of perigonadal white fat pads was performed as described previously (30). Adipose tissue macrophages were isolated from stromal vascular fraction (SVF) cells using an antibody directed against the panmacrophage marker F4/80 (eBiosciences, San Diego, CA) coupled to magnetic beads (Miltenyi Biotech, Auburn, CA) according to the manufacturer’s protocol.
Human samples and clinical parameters.
The study included 25 obese patients and 21 nonobese control subjects who underwent weight-reducing surgery or elective surgical procedures such as cholecystectomy. Participants were included if they had fasting plasma glucose levels <7.0 mmol/L, no history of diabetes or use of blood glucose–lowering medications, no weight changes >3% during the previous 2 months, and C-reactive protein levels <20 mg/L. To reduce confounding by insulin resistance, obese subjects with a homeostasis model assessment (HOMA) index >2.5 were excluded. All study subjects provided informed consent, and the local ethics committee approved the study protocols. Tissue biopsy specimens from visceral adipose tissue, obtained during surgery, were collected in RNA-later (Ambion, Austin, TX) and stored at −80°C until further processing. Plasma glucose, insulin, C-reactive protein concentrations, and the HOMA index were determined as described (43).
Gene set enrichment analysis.
Gene set enrichment analysis methods were done as described in the Supplementary Appendix.
Statistical analyses.
All data are shown as mean ± SEM, unless otherwise indicated. The significance of differences between means was assessed by two-tailed Student t test or ANOVA with the Tukey honestly significance difference test post hoc, where appropriate. Differences between human study groups were ascertained by ANOVA. We used multiple regression to adjust all measurements for concomitant effects of sex and age. Adjustments for CD68 and CD144 were performed as indicated. Categoric data were summarized by frequencies and analyzed by the χ2 test. All reported P values are two-tailed.
RESULTS
T-cell activation blocks Hh signaling in an IFN-γ–dependent manner.
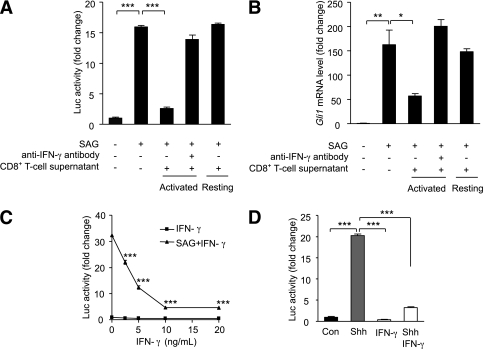
Previous studies have shown that Hh signaling activity is diminished in the adipose tissue of obese mice (29) and that CD8+ T-cell infiltration is an early hallmark of obese adipose tissue in both mice and humans (17). To test whether the two phenomena are causally related, we examined the effects of activated T-cell supernatants on the Hh-responsive luciferase reporter cell line Shh-LIGHT2 (39). Addition of the Hh-activator SAG to Shh-LIGHT2 cells robustly induces luciferase activity (Fig. 1A). Intriguingly, cotreatment with supernatants from CD8+ T cells activated in vitro with anti-CD3 and anti-CD28 antibodies markedly diminished the SAG-induced luciferase response. Importantly, the supernatant from resting T cells showed no inhibitory effect (Fig. 1A). To test whether the observed effect was unique to Shh-LIGHT2 cells, we exposed 3T3-L1 preadipocytes to parallel treatment conditions. In direct corroboration of the Shh-LIGHT2 findings, the activated CD8+ T-cell supernatant substantially reduced SAG-induced Gli1 mRNA expression in 3T3-L1 cells (Fig. 1B). Once again, the addition of supernatant from resting, rather than activated cells, showed no effect. Importantly, the addition of anti–IFN-γ–blocking antibodies abolished the marked inhibitory effect of the T-cell supernatant on Hh activation in both experimental systems (Fig. 1A and B), directly implicating IFN-γ in the inhibition of Hh signaling in Shh-LIGHT2 cells and in preadipocytes. Characterization of the potency of IFN-γ–mediated Hh inhibition revealed significant effects at the low nmol/L level in Shh-LIGHT2 cells (Fig. 1C). Indeed, IFN-γ alone was sufficient to affect a nearly total block of SAG-induced Hh activity. Importantly, these findings were confirmed pharmacologically using the endogenous Shh ligand as well as genetically using fibroblasts derived from Ptch knockout mouse embryos (39) harboring constitutive activation of Hh signaling and downstream target genes (Fig. 1D and Supplementary Fig. 1). Thus, activated CD8+ T cells inhibit Hh signaling in an IFN-γ–dependent manner.
FIG. 1.
Suppression of Shh signaling by activated T cells is IFN-γ–dependent. A: Shh-LIGHT2 cells were cultured for 48 h with no stimulus, SAG (200 nmol/L), SAG in the presence or absence of supernatant from resting or activated CD8+ T cells, with or without anti–IFN-γ monoclonal antibody (25 ng/µL) and assayed for luciferase activity. B: 3T3-L1 preadipocytes were treated in the same way as described for the Shh-LIGHT2 cells above (SAG 20 nmol/L). Gli1 mRNA was measured by quantitative RT-PCR and normalized for ribosomal protein, large, P0, (Rplp0). C: Shh-LIGHT2 cells were cultured for 48 h in the absence or presence of SAG plus the indicated concentrations of IFN-γ and assayed for luciferase activity. D: Confluent Shh-LIGHT2 reporter cells were left untreated (Con), or treated with recombinant Shh (200 ng/mL), IFN-γ (10 ng/mL), or both, for 48 h. Data represent means ± SEM of triplicate samples. *P < 0.05; **P < 0.01; ***P < 0.001.
IFN-γ rescues adipogenesis in vitro by overriding the strong inhibitory effect of Hh signaling.
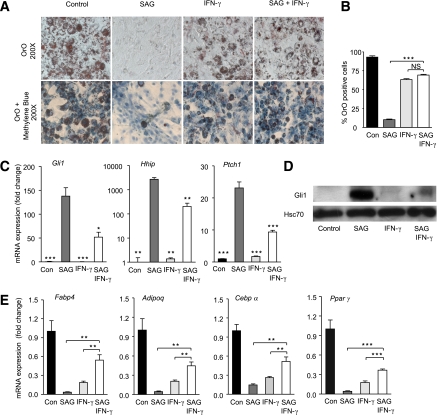
To functionally assess the inhibitory role of IFN-γ on Hh signaling in adipocytes, we induced 3T3-L1 cells to undergo adipogenesis in the presence or absence of SAG, IFN-γ, or vehicle for 10 days. In line with our previous data (30), SAG treatment of 3T3-L1 cells completely blocked adipogenesis. These effects were observed macroscopically with OrO staining for lipid droplets (Fig. 2A and B), by quantitative PCR-based expression profiling of the hallmark Hh target genes Gli1, Hhip, and Ptch1 (Fig. 2C), and on corresponding protein levels of Gli1 and Hhip (Fig. 2D and Supplementary Fig. 2).
FIG. 2.
IFN-γ prevents SAG-induced inhibition of adipocyte differentiation. A: 3T3-L1 cells were induced to differentiate into adipocytes in the presence of 20 nmol/L SAG and/or 50 ng/mL IFN-γ or were left untreated (Con) for 10 days. OrO staining was performed 10 days after induction. B: Adipocytes were counted under a bright-field microscope and presented as the percentage of the total number of methylene blue–stained nuclei. C: mRNA levels of Hh target genes (Gli1, Hhip, and Ptch1) were measured by quantitative RT-PCR analysis and normalized to Rplp0. D: Gli1 Western blot of whole-cell lysates. Heat shock protein 70 was used as the loading control. E: mRNA levels of marker genes of adipocyte differentiation (aP2/Fabp4, Adipoq, Cebpα, and Pparγ) were measured by quantitative RT-PCR analysis. Data represent means ± SEM of triplicate samples. *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant. (A high-quality color representation of this figure is available in the online issue.)
In keeping with the macroscopic findings, we observed substantially reduced mRNA expression levels of the key adipogenic markers Fabp4 and Adipoq as well as the master regulators of adipogenesis, Cebpα and Pparγ (Fig. 2E). In line with published data, IFN-γ also partially blocked differentiation of 3T3-L1 cells with a reduction of OrO-positive adipocytes and a decrease of key adipose-specific markers ([20–22]; Fig. 2A, B, and E). Intriguingly, whereas treatment of 3T3-L1 cells with IFN-γ alone induced a partial inhibitory effect on adipogenesis, simultaneous activation of Hh and IFN-γ signaling pathways in 3T3-L1 preadipocytes rescued any observable Hh-induced block and restored nearly complete adipogenesis (Fig. 2A–E). Restoration of differentiation capacity by coaddition of IFN-γ and SAG was associated with substantial reductions in Hh target gene expression relative to SAG alone on both the protein and the mRNA levels (Gli1 and Hhip; Fig. 2C and D and Supplementary Fig. 2) as well as recovery of mRNA expression for all key differentiation markers (Fabp4, Adipoq, Cebpα, and Pparγ; Fig. 2E). Further, IFN-γ treatment blunted the SAG-induced increase of chicken ovalbumin upstream promoter-transcription factor 2 (Coup-TF2) and Gata2 (Supplementary Fig. 3), direct downstream mediators of Hh-induced adipogenic block (30,44). Thus, IFN-γ rescues Hh-induced adipogenic block.
IFN-γ–mediated inhibition of Hh responses requires activation of Jak-Stat signaling.
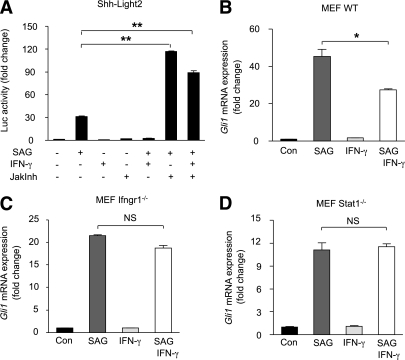
Among others, IFN-γ activates the Jak-Stat pathway (45). To test whether activated Jak-Stat signaling was responsible for inhibition of SAG responses, we treated Shh-LIGHT2 cells with SAG and/or IFN-γ in the presence or absence of a pan-Jak inhibitor (JakInh) (46). As expected, of the single-compound control wells, only SAG elicited a substantial luciferase response (Fig. 3A). IFN-γ and JakInh alone or combined showed no significant induction of Hh signaling. Surprisingly, simultaneous treatment of cells with SAG (200 nmol/L) and JakInh (2 μmol/L) resulted in a marked potentiation of the Gli-induced luciferase activity compared with SAG treatment alone (Fig. 3A). Critically, the addition of JakInh to IFN-γ/SAG costimulated wells also resulted in a potentiated response compared with SAG alone, indicating that IFN-γ requires downstream Jak activity to inhibit Hh signaling (Fig. 3A). Of note, there is evidence that IFN-γ can regulate the expression of select downstream genes even in the absence of Stat1 (47). To address this issue, we tested whether IFN-γ suppression of Gli1 expression was maintained in MEFs isolated from IFN-γ receptor 1–deficient (Ifngr1−/−) and Stat1-deficient (Stat1−/−) mice. Importantly, we observed that the inhibitory effect of IFN-γ on Hh signaling was abolished in MEFs isolated from both strains, direct genetic evidence implicating Ifngr1 and Stat1 in Hh signaling control (Fig. 3B–D). Thus, IFN-γ inhibits Hh signaling via Infgr1-induced activation of Stat1.
FIG. 3.
IFN-γ prevents SAG-induced inhibition of adipocyte differentiation via Jak-Stat signaling. A: Shh-LIGHT2 cells were treated with 200 nmol/L SAG and/or 25 ng/µL IFN-γ in the absence or presence of JakInh. Simultaneous treatment of cells with SAG and JakInh resulted in an additional increase in relative Gli1 luciferase activity compared with SAG only–treated cells. B–D: We also tested whether effects of IFN-γ on Gli1 expression could persist in MEFs from Ifngr1−/− and Stat1−/− mice. The inhibitory effect of IFN-γ on Shh signaling was cancelled in Ifngr1−/− and Stat1−/− MEFs. These experiments demonstrate that IFN-γ acts by means of the Jak-Stat pathway to abrogate the effects of Shh signaling. Data represent means ± SEM of triplicate samples. *P < 0.01; **P < 0.001; NS, not significant.
To corroborate the 3T3-L1 studies, we attempted to test Ifn-γ-Hh cross-talk in human and mouse primary white preadipocytes. Because the key differentiation factors 3-isobutyl-1-methylxanthin (IBMX) and dexamethasone (Dex) block Hh activation (30), 3T3-L1 and primary preadipocytes were induced using a minimal induction medium containing insulin and troglitazone only. We previously used this differentiation cocktail to successfully explore Hh signaling effects of adipogenesis in 3T3-L1 cells (30). In contrast to 3T3-L1 cells, induction of primary mouse and human preadipocytes by the minimal induction medium was insufficient to induce substantial differentiation, even after 10 days.
Primary cells cultured in parallel under classical conditions differentiated efficiently (Supplementary Fig. 4A and B). IBMX/Dex completely blocked Hh activation by SAG (Supplementary Fig. 4D). These findings were supported by OrO staining of the same cells (Supplementary Fig. 4E and F). We confirmed this IBMX/Dex Hh–neutralizing effect by qPCR of the Hh target gene Gli1 and the early adipogenic marker peroxisome proliferator–activated receptor-γ (Ppar-γ) 48 h after induction (Supplementary Fig. 4G and H). Looking instead at preadipocytes, IFN-γ efficiently inhibited SAG-induced Gli1 mRNA expression in both human and mouse primary cultures (Fig. 4A and Supplementary Fig. 4I), providing strong evidence that the appropriate signaling framework exists and is functional in the human and mouse progenitor pool. Importantly, in direct agreement with the findings in 3T3-L1 cells above, addition of IFN-γ attenuated SAG-induced Hh activation and relieved Hh suppression of Pparγ expression (Fig. 4B and Supplementary Fig. 4J).
FIG. 4.
IFN-γ inhibits Hh signaling in mouse primary preadipocytes. Mouse primary preadipocytes were cultured in medium containing insulin and troglitazone for induction of adipogenic markers or in medium without any adipogenic inducers (Pre). Induced cells were stimulated with the Hh agonist SAG (200 nmol/L) and/or 50 ng/mL IFN-γ or left untreated (Con) for 48 h. A–B: Quantitative RT-PCR monitoring of Hh target gene Gli1 and adipogenic marker Pparγ. mRNA levels of the genes of interest were normalized to Rplp0. Data represent means ± SEM of triplicate samples. *P < 0.05; ***P < 0.001; NS, not significant.
IFN-γ depletion restores Hh signaling in adipose tissue stromal vascular cells in vivo.
Genetic-induced and diet-induced obesity both decrease expression levels of Hh target genes in perigonadal white fat pads of mice (29). This is in accordance with antiadipogenic properties of Hh signaling and the substantial generation of new adipocytes during weight gain. However, the underlying cause of this deregulation of Hh targets is not understood. We examined the effects of IFN-γ depletion on the expression of Hh target genes in obese adipose tissue using an in vivo neutralizing antibody strategy. Male C57BL/6J mice exposed to a HF diet for 22 weeks were randomly assigned to a control group (HF; n = 10) or a group that received intraperitoneal anti-IFN-γ–neutralizing antibody (HF+IFN-γ-Ab; n = 5). Control animals were injected in parallel with a control IgG antibody. Consistent with previous findings (20) IFN-γ serum levels in the HF group were significantly higher than in animals fed normal chow (Supplementary Fig. 5). Immunoneutralization in the treated animals was similarly confirmed by ELISA.
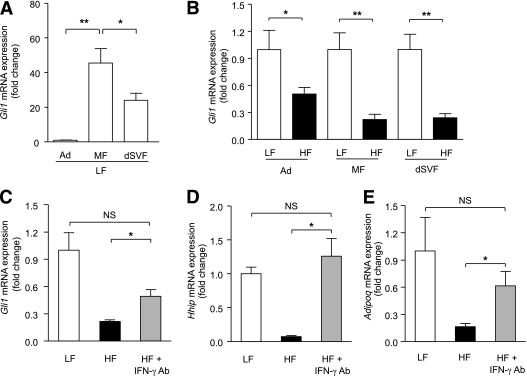
As expected, body weight, fasted blood glucose levels, perigonadal fat mass, adipose tissue infiltration with Mac2+ macrophages, and adipocyte size were significantly higher in HF fed mice relative to LF fed controls (Supplementary Table 1 and Supplementary Fig. 6A–K). Anti–IFN-γ–neutralizing antibody treatment for 2 weeks did not significantly affect any of these gross metabolic parameters (Supplementary Table 1 and Supplementary Fig. 6A–K). To characterize the system, we compared Hh signaling activity in adipose tissue fractionated into mature adipocytes, macrophages, and macrophage-depleted stromal vascular cells. In line with the role of Hh signaling in inhibiting fat formation, the Hh target Gli1 showed low expression in mature adipocytes compared with macrophage- and macrophage-depleted stromal vascular fractions (dSVF). Indeed, the highest Gli1 mRNA expression levels were found in the macrophage fraction (Fig. 5A). Further, Gli1 expression in all three adipose tissue compartments was significantly lower in HF-treated mice relative to the LF-treated control animals, suggesting that the HF diet imposed a broad suppressive effect on Hh activity in adipose tissue (Fig. 5B).
FIG. 5.
IFN-γ inhibits Hh signaling in obesity in vivo. A–B: C57BL/6J mice were fed a LF or HF diet for 24 weeks (n = 10 or 15 mice per group, respectively). For the last 2 weeks of diets, mice fed the HF diet were randomly split into HF and HF+Ab groups. The HF+Ab (n = 5) group was treated with monoclonal antibodies against IFN-γ. Adipose tissue was separated into adipocytes (Ad), macrophage fraction (MF), and macrophage-dSVF using magnetic immunoaffinity isolation. Cell fractions from mice fed LF and HF diets were analyzed for Gli1 mRNA expression. C–E: dSVF from mice fed LF and HF diets was analyzed for Gli1, Hhip, and Adipoq. *P < 0.05; **P < 0.01.
Intriguingly, examination of expression profiles from IFN-γ–depleted animals suggested that IFN-γ mediates Hh-suppression in adipose tissue in vivo. Immunoneutralization substantially reversed HF-induced suppression of the downstream Hh target Gli1 (greater than twofold), and impressively, completely restored expression of the target Hhip to normal chow levels (∼20-fold increase; Fig. 5C and D). Critically, the Hh target Nr2f2/Coup-Tf2 also showed restoration of expression (Supplementary Fig. 7), as did the key insulin-sensitizing adipokine adiponectin (Adipoq; Fig. 5E). This latter finding correlated directly with increased serum adiponectin levels in immunoneutralized animals (Supplementary Fig. 8).
Together, these data identify Hh signaling as a target of IFN-γ in adipose tissue in vivo and support a functional role for their cross-talk in balancing adipose tissue function. Thus, IFN-γ mediates functional suppression of Hh signaling by a HF diet in vivo.
Hh signaling is downregulated in human obesity.
Next, we determined GLI1 transcript levels in human intraperitoneal adipose tissue of lean and insulin sensitive (IS) obese subjects. This design allowed us to detect potential associations of Hh signaling activity with intraperitoneal adipose tissue expandability in humans without confounding interference of insulin resistance. Despite a slight age and sex variation in this unique patient set, no differences were observed in plasma glucose and insulin levels or in the HOMA index (Supplementary Table 2). GLUT4 expression values were similar in both groups, reflecting the equal presence of glucose transporters (Table 1). In line with current knowledge, IS obese subjects did not show increased CD68 expression levels or alterations in the endothelial marker VE-cadherin (CD144). However, and in line with our hypothesis that increased Hh activity inhibits adipose tissue expansion, GLI1 expression was significantly decreased in adipose tissue samples of IS obese subjects (P = 0.0237). Importantly, adjusting for age, sex, CD68, and CD144 did not affect any of the statistical results, suggesting that decreased GLI1 levels in IS obese subjects are not due to changes in vascularization or macrophage infiltration (Table 1). These findings highlight reduced Hh activity in patients with a likely intact expandable progenitor pool and no evidence of immune cell infiltration.
TABLE 1.
Comparison of intraperitoneal adipose mRNA expression levels in lean vs. obese study subjects (n = 46)
| Gene | Lean (n = 21) | Obese (n = 25) | Adjusted for | P* |
|---|---|---|---|---|
| GLUT4 | 5.77 (0.70) | 4.78 (0.43) | Sex, age | NS |
| CD68 | 3.38 (0.39) | 3.03 (0.29) | Sex, age | NS |
| CD144 | 8.18 (0.81) | 10.16 (0.77) | Sex, age | NS |
| GLI1 | 7.46 (0.79) | 5.17 (0.39) | Sex, age | 0.0237 |
| GLI1 | 7.54 (0.79) | 5.32 (0.39) | Sex, age, CD68 | 0.0224 |
| GLI1 | 7.80 (0.79) | 5.06 (0.39) | Sex, age, CD144 | 0.0049 |
| GLI1 | 7.85 (0.79) | 5.27 (0.39) | Sex, age, CD68, CD144 | 0.0065 |
Data are arbitrary units and presented as mean ± SEM.
*P values obtained from ANOVA.
NS, not significant.
Next, we sought additional, more generalized evidence where inflammation is present. We used gene set enrichment to analyze publically available adipose tissue expression arrays from two of the best genetically controlled human obesity cohorts, Pima Indians (GEO Reference Series GSE2508) and a set of 13 pairs of monozygotic twins discordant for adiposity (EBI-EMBL Reference Series E-MEXP-1425). Not without surprise, pathway enrichment for Th1/Th2 T-cell differentiation markers, a surrogate for T-cell infiltration, exhibited robust enrichment in obese Pima Indian and obese MZ-twin adipose tissues (FDR 0.08 and 0.16, respectively; nom. P = 0.006 and P = 0.01, respectively; Supplementary Fig. 9). Intriguingly, and with equally robust signal intensity to that of the Th1/Th2 pathway, Hh signaling exhibited an enrichment signal in the control subjects of both genetically distinct cohorts, indicating consistent downregulation of the pathway in both obesity cohorts (FDR 0.08 and 0.12, respectively; nom. P = 0.08 and P = 0.12, respectively).
These findings indicate that in the absence of inflammation, low Hh signaling is associated with insulin sensitivity and, presumably, a highly plastic progenitor pool, and that T-cell infiltration correlates highly with suppressed Hh signaling in human adipose tissue.
DISCUSSION
Understanding the molecular mechanisms involved in adipocyte differentiation is of great importance because it may lead to new ways of treating obesity and, in particular, its associated metabolic complications. Hh signaling and IFN-γ already have an established role in inhibiting adipogenesis (21,22,29,30).
Here we demonstrate for the first time that inhibition of adipocyte differentiation by Hh signaling can be balanced by the antagonistic actions of IFN-γ. The effect was observed in 3T3-L1 adipocytes (Fig. 2A–E) as well as in three different primary culture systems—mouse embryonic fibroblasts (Fig. 3B–D), primary mouse preadipocytes (Fig. 4A and B), and primary human preadipocytes (Supplementary Fig. 4I and J). These data, as well as recapitulation of the phenotype using the endogenous Shh ligand, rule out cell-line or chemical artifacts. The difficulty associated with recapitulating these studies in mature primary adipocytes is caused by the direct inhibition of Hh signaling by both IBMX and Dex, required constituents of primary adipocyte differentiation cocktails.
Cross-talk between the Hh signaling pathway and IFN-γ has already been shown to influence cell differentiation and proliferation in specific neuronal settings (31–33). IFN-γ indirectly regulates proliferation of neuronal precursor cells through mechanisms involving elevated expression of Shh and its major target genes, Gli1 and Ptch (31). Discordant upregulation of Shh by IFN-γ, while suppressing its target Gli1, was observed in studies on the differentiation of neuronal stem cells (32). Interestingly, our studies demonstrate that IFN-γ–mediated inhibition of Hh target genes (Gli1, Ptch, Hhip, and Nr2f2/Coup-Tf2) in 3T3-L1 cells promotes adipocyte differentiation. IFN-γ not only rescued adipocyte differentiation but also decreased Coup-Tf2 and Gata2 levels, further supporting an antiadipogenic role of both genes (32). Thus, it appears that IFN-γ signaling can have very context-dependent effects on Hh signaling.
After binding of IFN-γ to its receptor, Jak1 and Jak2 are activated and regulate downstream phosphorylation of Stat1 (46). Although Stat1 is required for many IFN-γ–dependent actions, evidence shows that in the absence of Stat1, IFN-γ can still regulate the expression of some genes (46,47). Because IFN-γ–mediated inhibition of Hh signaling did not occur in Stat1−/− MEFs, we conclude that this cross-talk depends on Stat1. Interestingly, blocking constitutive Jak-Stat activation by a pan-Jak inhibitor resulted in greatly increased Gli1 luciferase activities in SAG-treated Shh-LIGHT2 cells, even in the absence of IFN-γ. This suggests that basal Jak activity exerts an inhibitory tone on Hh pathway activation.
Because the concentration of IFN-γ is elevated in obesity and the Hh signaling pathway was less active in obese compared with lean mice, we hypothesized that IFN-γ might inhibit Hh signaling in vivo. Interestingly, when examining adipose tissue fractions for differences in Gli1 expression in the lean and obese state, we found low Gli1 expression in mature adipocytes. Levels of mRNA in dSVF were >20-fold and in macrophages >40-fold higher than in the adipocyte fraction. This is in accordance with previous data that show decreased expression of positively acting Hh components (Smo and the Glis) during adipogenesis (29,30). To determine whether reduced expression of Hh target genes in white fat of obese animals depends on higher IFN-γ concentrations in obesity, we treated obese mice with a neutralizing antibody against IFN-γ. Our results demonstrate that obesity-associated repression of Hh-target genes like Gli1 and Hhip in the dSVF was partially or completely relieved by inhibiting IFN-γ elicited Jak/Stat signaling. This suggests that IFN-γ opposes inhibition of Hh signaling in obese adipose tissue in vivo.
Increases in fat mass can involve both hypertrophy and hyperplasia of adipocytes. Obesity in adults is typically associated with adipocyte hypertrophy, and an additional adipocyte hyperplasia occurs in morbidly obese humans and rodents (48–50). Adiposity-induced cellular and tissue hypoxia is an important contributor to adipose tissue immunopathies and adipocyte and adipose tissue dysfunction (2,8,9,16). Data suggest that the initial hypertrophic response of adipocytes promotes necrotic-like death, after which the lipid storage capacity of adipose tissue can be efficiently maintained or increased only by adipocyte hyperplasia. Interestingly, current concepts support that early adipose tissue expandability prevents metabolic consequences like insulin resistance, even in morbid obesity (9).
Some have proposed that the generation of new adipocytes may be balanced by adipocyte death, with the constant and tightly regulated total number set by early adulthood (50). Our findings suggest a model where the inhibition of Hh signaling by IFN-γ in obesity contributes to the development of new adipocytes (Fig. 6). At steady-state in lean individuals, basal Hh-pathway activity prevents the formation of new adipocytes. T-cell infiltration, as an early event in obesity development, leads to elevated IFN-γ production in adipose tissue and inhibition of Hh-signaling, a scenario that is permissive for the recruitment and development of new adipocyte populations. Such an early inhibition of Hh signaling may be a prerequisite for compensatory increases in total adipocyte numbers, although that is speculation.
FIG. 6.
Model shows a novel cross-talk mechanism between IFN-γ and Hh signaling maintains adipogenesis in adipose tissue. Activation of Hh signaling blocks white adipocyte differentiation. T cells infiltrating white adipose tissue early during the development of obesity secrete IFN-γ and thus inhibit ongoing Hh signaling. Decreased Hh signaling activity in (pre)adipocytes rescues adipogenesis.
Our observation that the main Hh target gene GLI1 is downregulated in intraperitoneal adipose tissue from metabolically healthy IS obese subjects supports the concept that early inactivation of Hh signaling and subsequent release of the intracellular progenitor pool to expansion might alleviate obesity associated insulin resistance. Clearly though, the system is more complex. Indeed, robust opposing signals for T-cell infiltration (upregulated) and Hh signaling (downregulated) in the adipose tissue of MZ twins as well as in Pima Indian cohorts support the idea that inhibition of Hh signaling alone is insufficient to block pathologies associated with obesity and chronic inflammation (Supplementary Fig. 9). It is almost certain that additional positive expansion signals are required for proper expansion. A deeper understanding of these concepts will be aided by long-term genetic dissections in vivo, using for instance inducible adipose tissue specific IFN-γ receptor–deficient mice.
In conclusion, we have established for the first time that IFN-γ directly inhibits Hh signaling in (pre)adipocytes in vitro and in adipose tissue in vivo. Our results suggest that the cross-talk between IFN-γ and the Hh-signaling pathway is essential in the maintenance of optimal adipocyte differentiation. The ability to control the plasticity of adipocyte turnover would be a powerful tool in alleviating many complications associated with obesity. That Hh antagonists will one day be used for such purposes in the clinic is perhaps a stretch. However, understanding the regulatory architecture that controls the progenitor cell decision “To differentiate, or not to differentiate?” will be critical to the development of any such therapies. We believe we have added one of the first pieces with which to construct this complex puzzle.
ACKNOWLEDGMENTS
This work was supported by research grants to J.A.P. and H.E. from the Vienna Science and Technology Fund (project LS07-058) and to J.T. from the Austrian Society for Laboratory Medicine. B.S. and M.M. were supported by the FWF-funded SFB F28 and by BM.W_fa GEN-AU III (Austromouse). F.K. and W.P. were supported by the Bundesland Salzburg (grant 2005-20089).
No potential conflicts of interest relevant to this article were reported.
J.T. researched data, contributed to discussion, and drafted the manuscript. B.S. researched data and contributed to discussion. A.J. researched data. N.B. researched data and contributed to discussion. M.Ba., S.A., J.L., R.T., G.P., and M.Bi. researched data. W.E. contributed to discussion and edited the manuscript. F.K. researched data and contributed to discussion. M.M. and O.W. contributed to discussion and edited the manuscript. W.P. researched data and contributed to discussion. J.A.P. and H.E. researched data, contributed to discussion, and wrote and edited the manuscript. The contribution by M.Ba. constitutes part of her PhD work.
The authors are indebted to the service departments and Maria Ozsvar of the Medical University of Vienna for excellent technical help.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-1628/-/DC1.
REFERENCES
- 1.World Health Organization Global Strategy on Diet, Physical Activity and Health. Geneva, Switzerland, World Health Organization, 2009 [Google Scholar]
- 2.Blüher M. Adipose tissue dysfunction in obesity. Exp Clin Endocrinol Diabetes 2009;117:241–250 [DOI] [PubMed] [Google Scholar]
- 3.Karelis AD, St-Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET. Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab 2004;89:2569–2575 [DOI] [PubMed] [Google Scholar]
- 4.Allende-Vigo MZ. Pathophysiologic mechanisms linking adipose tissue and cardiometabolic risk. Endocr Pract 2010;16:692–698 [DOI] [PubMed] [Google Scholar]
- 5.Ballantyne CM. T cells, macrophages, chemokines, and adiposopathy in diet-induced obesity [Internet], 2010. NIH Report. Lambertville, NJ. Available from http://www.labome.org. Accessed 1 February 2011
- 6.Heilbronn L, Smith SR, Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int J Obes Relat Metab Disord 2004;28(Suppl. 4):S12–S21 [DOI] [PubMed] [Google Scholar]
- 7.Ravussin E, Smith SR. Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type 2 diabetes mellitus. Ann N Y Acad Sci 2002;967:363–378 [DOI] [PubMed] [Google Scholar]
- 8.Bays HE, González-Campoy JM, Bray GA, et al. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev Cardiovasc Ther 2008;6:343–368 [DOI] [PubMed] [Google Scholar]
- 9.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011;11:85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 2005;115:1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest 2008;118:2992–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006;116:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Phys 2010;72:219–246 [DOI] [PubMed] [Google Scholar]
- 14.Wu H, Ghosh S, Perrard XD, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation 2007;115:1029–1038 [DOI] [PubMed] [Google Scholar]
- 15.Kintscher U, Hartge M, Hess K, et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol 2008;28:1304–1310 [DOI] [PubMed] [Google Scholar]
- 16.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 2008;32:451–463 [DOI] [PubMed] [Google Scholar]
- 17.Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009;15:914–920 [DOI] [PubMed] [Google Scholar]
- 18.Winer S, Chan Y, Paltser G, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med 2009;15:921–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lumeng CN, Maillard I, Saltiel AR. T-ing up inflammation in fat. Nat Med 2009;15:846–847 [DOI] [PubMed] [Google Scholar]
- 20.Rocha VZ, Folco EJ, Sukhova G, et al. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res 2008;103:467–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGillicuddy FC, Chiquoine EH, Hinkle CC, et al. Interferon gamma attenuates insulin signaling, lipid storage, and differentiation in human adipocytes via activation of the JAK/STAT pathway. J Biol Chem 2009;284:31936–31944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duffaut C, Zakaroff-Girard A, Bourlier V, et al. Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arterioscler Thromb Vasc Biol 2009;29:1608–1614 [DOI] [PubMed] [Google Scholar]
- 23.Sultan A, Strodthoff D, Robertson AK, et al. T cell-mediated inflammation in adipose tissue does not cause insulin resistance in hyperlipidemic mice. Circ Res 2009;104:961–968 [DOI] [PubMed] [Google Scholar]
- 24.Klöting N, Fasshauer M, Dietrich A, et al. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab 2010;299:E506–E515 [DOI] [PubMed] [Google Scholar]
- 25.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev 2001;15:3059–3087 [DOI] [PubMed] [Google Scholar]
- 26.Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev 2008;22:2454–2472 [DOI] [PubMed] [Google Scholar]
- 27.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature 2004;432:324–331 [DOI] [PubMed] [Google Scholar]
- 28.Hooper JE, Scott MP. Communicating with hedgehogs. Nat Rev Mol Cell Biol 2005;6:306–317 [DOI] [PubMed] [Google Scholar]
- 29.Suh JM, Gao X, McKay J, McKay R, Salo Z, Graff JM. Hedgehog signaling plays a conserved role in inhibiting fat formation. Cell Metab 2006;3:25–34 [DOI] [PubMed] [Google Scholar]
- 30.Pospisilik JA, Schramek D, Schnidar H, et al. Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate. Cell 2010;140:148–160 [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Lin W, Popko B, Campbell IL. Inducible production of interferon-gamma in the developing brain causes cerebellar dysplasia with activation of the sonic hedgehog pathway. Mol Cell Neurosci 2004;27:489–496 [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Imitola J, Rasmussen S, O’Connor KC, Khoury SJ. Paradoxical dysregulation of the neural stem cell pathway sonic hedgehog-Gli1 in autoimmune encephalomyelitis and multiple sclerosis. Ann Neurol 2008;64:417–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun L, Tian Z, Wang J. A direct cross-talk between interferon-gamma and sonic hedgehog signaling that leads to the proliferation of neuronal precursor cells. Brain Behav Immun 2010;24:220–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tekki-Kessaris N, Woodruff R, Hall AC, et al. Hedgehog-dependent oligodendrocyte lineage specification in the telencephalon. Development 2001;128:2545–2554 [DOI] [PubMed] [Google Scholar]
- 35.Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci 2003;6:21–27 [DOI] [PubMed] [Google Scholar]
- 36.Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to sonic hedgehog. Nature 2005;437:894–897 [DOI] [PubMed] [Google Scholar]
- 37.Gao L, Miller RH. Specification of optic nerve oligodendrocyte precursors by retinal ganglion cell axons. J Neurosci 2006;26:7619–7628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin W, Kemper A, McCarthy KD, et al. Interferon-gamma induced medulloblastoma in the developing cerebellum. J Neurosci 2004;24:10074–10083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taipale J, Chen JK, Cooper MK, et al. Effects of oncogenic mutations in smoothened and patched can be reversed by cyclopamine. Nature 2000;406:1005–1009 [DOI] [PubMed] [Google Scholar]
- 40.Durbin JE, Hackenmiller R, Simon MC, Levy DE. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell 1996;84:443–450 [DOI] [PubMed] [Google Scholar]
- 41.Huang S, Hendriks W, Althage A, et al. Immune response in mice that lack the interferon-gamma receptor. Science 1993;259:1742–1745 [DOI] [PubMed] [Google Scholar]
- 42.Todaro GJ, Green H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol 1963;17:299–313 [DOI] [PMC free article] [PubMed]
- 43.Oberkofler H, Linnemayr V, Weitgasser R, et al. Complex haplotypes of the PGC-1alpha gene are associated with carbohydrate metabolism and type 2 diabetes. Diabetes 2004;53:1385–1393 [DOI] [PubMed] [Google Scholar]
- 44.Xu Z, Yu S, Hsu CH, Eguchi J, Rosen ED. The orphan nuclear receptor chicken ovalbumin upstream promoter-transcription factor II is a critical regulator of adipogenesis. Proc Natl Acad Sci USA 2008;105:2421–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol 2005;5:375–386 [DOI] [PubMed] [Google Scholar]
- 46.Aaronson DS, Horvath CM. A road map for those who don’t know JAK-STAT. Science 2002;296:1653–1655 [DOI] [PubMed] [Google Scholar]
- 47.Ramana CV, Gil MP, Schreiber RD, Stark GR. Stat1-dependent and -independent pathways in IFN-gamma-dependent signaling. Trends Immunol 2002;23:96–101 [DOI] [PubMed] [Google Scholar]
- 48.Maumus M, Sengenès C, Decaunes P, et al. Evidence of in situ proliferation of adult adipose tissue-derived progenitor cells: influence of fat mass microenvironment and growth. J Clin Endocrinol Metab 2008;93:4098–4106 [DOI] [PubMed] [Google Scholar]
- 49.Arner P, Spalding KL. Fat cell turnover in humans. Biochem Biophys Res Commun 2010;396:101–104 [DOI] [PubMed] [Google Scholar]
- 50.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature 2008;453:783–787 [DOI] [PubMed] [Google Scholar]