There is a pressing need for immunotherapy to halt the progression of β-cell damage after the onset of diabetes, and the hope is to find a means for reversal of the disease. However, prevention of diabetes is equally important. Given the strength and diversification of a fully developed autoimmune response, it is likely to be easier to protect from disease progression and prevent onset of diabetes at a much earlier time point in the disease process. The nonobese diabetic (NOD) mouse model of human type 1 diabetes is often criticized for the fact that many manipulations in the early phases of disease process are successful in disease prevention. Note that far fewer treatments are able to protect the NOD mice at later stages of prediabetes, and even fewer reverse diabetes after onset of hyperglycemia (rev. in 1). However, some treatments that have reversed diabetes in the NOD mouse, for example anti-CD3 monoclonal antibody (2), heat shock protein 60 peptide (3), anti-CD20 monoclonal antibody (4), and antithymocyte globulin (5), have reached clinical trials and had partial success. However, currently, as we cannot know whether some preventive strategies identified for early disease in the NOD mouse may ultimately be useful in humans, it is important to continue to study early treatments and the mechanisms by which they work.
Although in recent years many studies have focused on regulatory T cells that produce the immunoregulatory cytokines interleukin (IL)-10 and transforming growth factor-β (6), it should not be forgotten that there are other regulatory pathways that may be harnessed for protection against Th1-dominated autoimmune disease, in particular a skewing to Th2 pathways. Glutamic acid decarboxylase (GAD)-specific T-cell responses are among the earliest noted in NOD mice (7,8). A number of investigators have previously shown that administration of GAD/GAD peptides have protected NOD mice from diabetes, particularly when administered at a young age (9–12). A focus on GAD is particularly interesting given the recent success in immunotherapy of diabetes (13) using a GAD-alum preparation in which mechanistic studies have shown immune changes that included an increase in IL-5, IL-10, IL-13, IL-17, interferon-γ (IFN-γ), and tumor necrosis factor-α. What these changes mean in the light of a decrease in the loss of C-peptide with time is not clear. In the NOD mouse, a variety of therapeutic strategies using GAD and GAD peptides have also invoked a variety of protective mechanisms, although it is clear that from all these studies that a deviation toward a Th2 profile has been most prominent.
IL-13 is an important part of a Th2 response. However, it has unique effector functions that are distinct from those of IL-4 both in its protective qualities against gastrointestinal parasites as well as in its deleterious effects in airway hyperresponsiveness (14). There are two IL-13 receptors—IL-13Rα1 and IL-13Rα2. IL-13Rα1 is a low-affinity receptor for IL-13 when it binds IL-13 as a homodimer. However, when IL-13Rα1 is found as heterodimer with IL-4R, it is a high-affinity receptor (15), the binding to which leads to phosphorylation of signal transducer and activator of transcription (STAT) 6 (16). IL-13Rα2 does not apparently signal through janus kinase/STATs (17) and is thought to be a decoy receptor important for regulation of IL-13 (14).
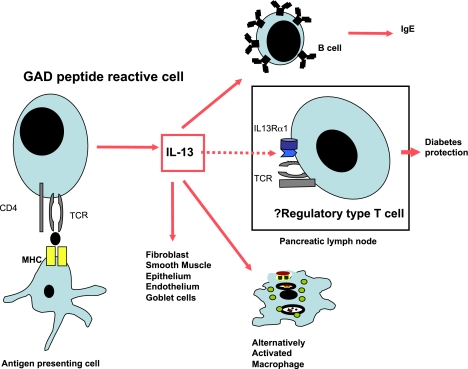
In this issue, Rasche et al. (18) report a novel means by which very early administration of GAD peptide 524-543 protects from diabetes in NOD mice. They show that in very young NOD mice (2 weeks of age) immunized with GAD peptide and incomplete Freund’s adjuvant, cells producing IL-5 and IL-13 are induced, and the IL-13 reduces spontaneous GAD responses in NOD mice. IL-13 was shown to reduce responsiveness of polyclonal CD4 T cells and islet-reactive BDC2.5 T cells to anti-CD3 stimulation. Of particular interest is their other observation of a subset of cells (about 5%) in the pancreatic lymph nodes (PLN), found when the mice are in the early stages of insulitis, that express IL-13Rα1. It was previously thought that T cells did not express this receptor, although more recently IL-13Rα1 has been identified on a subset of IL-17–producing cells (16,19). However, these IL-13Rα1–positive T cells identified by Rasche et al. do not produce IL-17 in this context and thus appear to represent a newly identified novel population of cells. It is intriguing that treatment with GAD peptide 524-538 or 524-543 in very young mice promotes T cells that produce IL-13 and appears to maintain the population of IL-13Rα1 T cells in the PLN. Under normal circumstances, the percentage of IL-13Rα1–positive cells is high in the PLNs of 4-week-old NOD mice and it reduces with time in the later stages of diabetes development in female NOD mice. Interestingly, GAD-induced IL-13–producing cells are distinct from the IL-13Rα1–positive T cells. Thus, it is conceivable that diabetes protection was mediated by IL-13 that acts on the IL-13Rα1–positive T cells, which have a protective effect (Fig. 1).
FIG. 1.
GAD-reactive cells stimulated on immunization of young NOD mice with incomplete Freund’s adjuvant and GAD peptide 524-543 produce IL-13. This may have many effects on a variety of tissues that include possible stimulation of a putative regulatory population of cells that expresses IL-13Rα1. Figure includes information adapted from Wynn (14) and Rasche et al. (18). MHC, major histocompatibility complex; TCR, T-cell receptor.
Recombinant human IL-13 protects from diabetes in the NOD mouse (20) and attests to the potential usefulness of IL-13 in counteracting the Th1 responses leading to diabetes. However, systemic administration of a beneficial cytokine that could have wide-ranging deleterious effects will not be translatable to human therapy in this form. The fact that an antigen-specific peptide therapy could have a very focused effect locally in the PLN by producing IL-13 and maintaining a hitherto unidentified, possibly protective, subset of cells as a mechanism of protection should be further investigated.
Rasche et al. (18) do not comment on the effects of later administration of GAD peptide, and they have shown previously that the treatment has greatest effect when given early in diabetes development. It will, however, be important to know whether such treatment could be harnessed at a later point in the development of diabetes. More importantly, the suggestion that this treatment may maintain a possibly protective cell population that normally decreases with time will certainly need further exploration. It will perhaps be particularly important to further study the IL-13Rα1–expressing subset of T cells for regulatory properties in NOD mice and to test whether such a population may also be important in humans.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 1716.
REFERENCES
- 1.Shoda LK, Young DL, Ramanujan S, et al. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity 2005;23:115–126 [DOI] [PubMed] [Google Scholar]
- 2.Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci USA 1994;91:123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elias D, Cohen IR. Peptide therapy for diabetes in NOD mice. Lancet 1994;343:704–706 [DOI] [PubMed] [Google Scholar]
- 4.Hu CY, Rodriguez-Pinto D, Du W, et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest 2007;117:3857–3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parker MJ, Xue S, Alexander JJ, et al. Immune depletion with cellular mobilization imparts immunoregulation and reverses autoimmune diabetes in nonobese diabetic mice. Diabetes 2009;58:2277–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong FS, Dayan CM. Regulatory T cells in autoimmune endocrine diseases. Trends Endocrinol Metab 2008;19:292–299 [DOI] [PubMed] [Google Scholar]
- 7.Kaufman DL, Clare-Salzler M, Tian J, et al. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature 1993;366:69–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tisch R, Yang XD, Singer SM, Liblau RS, Fugger L, McDevitt HO. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature 1993;366:72–75 [DOI] [PubMed] [Google Scholar]
- 9.Tisch R, Yang XD, Liblau RS, McDevitt HO. Administering glutamic acid decarboxylase to NOD mice prevents diabetes. J Autoimmun 1994;7:845–850 [DOI] [PubMed] [Google Scholar]
- 10.Tian J, Atkinson MA, Clare-Salzler M, et al. Nasal administration of glutamate decarboxylase (GAD65) peptides induces Th2 responses and prevents murine insulin-dependent diabetes. J Exp Med 1996;183:1561–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian J, Clare-Salzler M, Herschenfeld A, et al. Modulating autoimmune responses to GAD inhibits disease progression and prolongs islet graft survival in diabetes-prone mice. Nat Med 1996;2:1348–1353 [DOI] [PubMed] [Google Scholar]
- 12.Quinn A, McInerney B, Reich EP, Kim O, Jensen KP, Sercarz EE. Regulatory and effector CD4 T cells in nonobese diabetic mice recognize overlapping determinants on glutamic acid decarboxylase and use distinct V beta genes. J Immunol 2001;166:2982–2991 [DOI] [PubMed] [Google Scholar]
- 13.Ludvigsson J, Faresjö M, Hjorth M, et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med 2008;359:1909–1920 [DOI] [PubMed] [Google Scholar]
- 14.Wynn TA. IL-13 effector functions. Annu Rev Immunol 2003;21:425–456 [DOI] [PubMed] [Google Scholar]
- 15.Hilton DJ, Zhang JG, Metcalf D, Alexander WS, Nicola NA, Willson TA. Cloning and characterization of a binding subunit of the interleukin 13 receptor that is also a component of the interleukin 4 receptor. Proc Natl Acad Sci USA 1996;93:497–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newcomb DC, Zhou W, Moore ML, et al. A functional IL-13 receptor is expressed on polarized murine CD4+ Th17 cells and IL-13 signaling attenuates Th17 cytokine production. J Immunol 2009;182:5317–5321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donaldson DD, Whitters MJ, Fitz LJ, et al. The murine IL-13 receptor alpha 2: molecular cloning, characterization, and comparison with murine IL-13 receptor alpha 1. J Immunol 1998;161:2317–2324 [PubMed] [Google Scholar]
- 18.Rasche SS, Phillips M, McInerney MF, Sercarz EE, Quinn A. IL-13Rα1 expression on β-cell–specific T cells in NOD mice. Diabetes 2011;60:1716–1725 [DOI] [PMC free article] [PubMed]
- 19.Newcomb DC, Boswell MG, Zhou W, et al. Human T(H)17 cells express a functional IL-13 receptor and IL-13 attenuates IL-17A production. J Allergy Clin Immunol 2011;127:1006–1013 [DOI] [PMC free article] [PubMed]
- 20.Zaccone P, Phillips J, Conget I, et al. Interleukin-13 prevents autoimmune diabetes in NOD mice. Diabetes 1999;48:1522–1528 [DOI] [PubMed] [Google Scholar]