Abstract
OBJECTIVE
The development of obesity-related metabolic disorders varies with ethnicity. We examined whether ethnicity modifies the relationship between BMI and three metabolic pathways (insulin resistance, inflammation, and adiponectin) that are involved in the pathogenesis of diabetes and cardiovascular disease (CVD).
RESEARCH DESIGN AND METHODS
We analyzed data from 4,804 Chinese, Malay, and Asian-Indian residents of Singapore with complete data on insulin resistance (IR), C-reactive protein (CRP), and total adiponectin levels. Linear regression models with an interaction term ethnicity*BMI were used to evaluate whether ethnicity modifies the association between BMI and IR, CRP, and adiponectin.
RESULTS
In both uni- and multivariate analyses, BMI was directly associated with IR and CRP and inversely with adiponectin across all ethnic groups. When compared with Chinese and Malays, Asian-Indians had higher IR and CRP and lower adiponectin levels. The associations between BMI and its metabolic pathways were significantly stronger in Chinese than in other ethnic groups. The increase in IR and CRP and the decrease in adiponectin for each unit increase in BMI were greater in Chinese than in other ethnic groups. The findings were similar when waist circumference was used in the analyses instead of BMI.
CONCLUSIONS
The impact of BMI on IR, CRP, and adiponectin appears greater in Chinese as compared with other major Asian ethnic groups. This may partly explain the rapid increase in the prevalence of diabetes and CVD in Chinese populations and highlights the importance of weight management in Asian ethnic groups despite the apparently low levels of obesity.
Type 2 diabetes and cardiovascular disease (CVD) are important causes of mortality and morbidity in developing countries. Increasing levels of obesity are an important contributor to the increasing rates of type 2 diabetes and CVD (1). Insulin resistance (IR) represents a fundamental defect in type 2 diabetes and precedes the disease for many years (2). However, not all obese people are insulin resistant. Studies have shown that obesity only explains about 22% variability in IR, and individuals can have very different levels of IR for the same level of obesity (3).
Individuals from different ethnic groups show different levels of IR. In particular, it has been observed that individuals of Asian ethnicity experience high rates of type 2 diabetes and CVD despite much lower levels of obesity compared with their Caucasian counterparts. This has led the World Health Organization to recommend that a BMI above 23 kg/m2 should be a trigger point for public health action in Asian populations (4). Several studies have shown that, when compared with Caucasians, South Asians (Asian-Indians) have higher levels of IR for the same level of BMI (5). In our own studies, we have shown that within Asia, different ethnic groups exhibit different levels of IR, even after adjusting for the level of obesity (6).
The pathophysiological impact of obesity extends beyond IR. It has become evident that obesity is a state of subclinical systemic inflammation. Using C-reactive protein (CRP) as a measure of inflammation, studies have shown that higher BMI is associated with higher CRP levels (7). In turn, atherosclerosis is also an inflammatory process. Elevated CRP levels predicted higher future cardiovascular events in individuals with and without CVD (8).
Finally, it is now established that adipocytes play an active role in energy homeostasis by secreting various important adipokines that regulate metabolism. One of these adipokines, adiponectin, is a potent regulator of glucose and lipid metabolism and possesses antiatherogenic and anti-inflammatory properties. In humans, adiponectin levels decrease with increasing levels of obesity, and several prospective studies have demonstrated that low adiponectin levels are associated with increased risk of CVD and type 2 diabetes (9). Adiponectin levels also differ between ethnic groups and are lower in Asian-Indians compared with Europeans (5).
What is unclear is whether the effects of ethnicity are direct, and independent of obesity, or whether ethnicity alters the relationship between obesity and IR. The distinction is important because the latter would suggest that efforts to prevent or treat obesity may be particularly important and efficacious in reducing the burden of type 2 diabetes in some populations. This is particularly relevant in Asia, where the most rapid increase in type 2 diabetes and CVD is being experienced (10). To the best of our knowledge, only one study has examined the association of ethnicity on the relationship between obesity and CRP (11), and this has not been examined in Asians. In another study, the inverse relationship between obesity and adiponectin appeared to be attenuated in Asian-Indians compared with other ethnic groups (5).
We therefore compared the association between obesity (measured by BMI) and 1) IR; 2) CRP; and 3) adiponectin in Chinese, Malays, and Asian-Indians living in Singapore. We also tested the hypothesis that ethnicity might modify the relationship between obesity and the three pathogenic pathways in an Asian population.
RESEARCH DESIGN AND METHODS
Study design and population
Essentially, 10,445 participants from four previously conducted population-based cross-sectional surveys carried out in Singapore (1982–1998) were invited to participate in a repeat examination from 2004 to 2007. The four studies included the Thyroid and Heart Study 1982–1984, the National Health Survey 1992, the National University of Singapore Heart Study 1993–1995, and the National Health Survey 1998. Detailed methodologies for these studies have been previously described (12). Briefly, all studies were a random sample of individuals from the Singapore population, with oversampling of the minority ethnic groups (Malays and Asian-Indians). The recruitment and exclusion process is summarized in Supplementary Fig. A1. There were 517 participants who were deceased at the time of follow-up, 6 had emigrated, and 85 had errors in the identity card numbers. Remaining participants were contacted to obtain an appointment for investigators to administer a questionnaire at the participant’s homes. Three home visits were made on three different occasions including 1 weekend and weekday before a participant was deemed noncontactable. After this procedure, 2,673 participants were noncontactable. Of those participants who were contactable, 30 participants (0.3%) refused to participate. All participants were invited to attend a health examination for additional tests and collection of biological specimens shortly after the home visit. A total of 8,411 participants completed the questionnaire, of which 5,815 (69.1% of those who completed the questionnaire or 49.6% of all eligible participants) also attended the health examination. We also excluded participants who reported their ethnic group as other than Chinese, Malay, or Asian-Indian (n = 13) and participants with missing laboratory information (n = 380). Thus our final analyses included 4,804 participants.
Ethics approval was obtained from two Institutional Review Boards (National University of Singapore and Singapore General Hospital). Informed consent was obtained before conducting the study.
Data collection
Demographic data were collected using interviewer-administered questionnaires. Information on socio-demographic history, lifestyle (smoking history), medical history, and medication use was recorded. The methodologies for the measurement of height, weight, and blood pressure (BP) have been previously described (12). Following a 10-h overnight fast, venous blood was drawn and collected in plain and fluoride oxalate tubes and stored at 4°C for a maximum of 4 h before processing.
All biochemical analyses were carried out at the National University Hospital Referral Laboratory, which was accredited by the College of American Pathologists. Plasma glucose was assayed using enzymatic methods (ADVIA 2400; Bayer Diagnostics). Serum total cholesterol, triglycerides, and HDL cholesterol (HDL-C) were measured using an automated autoanalyzer (ADVIA 2400; Bayer Diagnostics). LDL cholesterol (LDL-C) levels were calculated using the Friedewald formula. Serum CRP was measured using the hs-CRP immunoturbidimetric assay implemented on a Roche Integra 400 (intra- and interassay CV of 0.6–1.3 and 2.3–3.1%, respectively). The detection limit of this assay is 0.07 mg/L, and the CV is 2.9% at 6.3 mg/L and 3.9% at 108 mg/L mean value. Insulin was assayed by microparticle enzyme immunoassay using the Bayer ADVIA Centaur chemiluminescent assay (intra- and interassay CV of 4.0 and 4.5%, respectively). IR was assessed by the homeostasis model assessment. Fasting serum adiponectin were determined using a sandwich enzyme-linked immunosorbent assay using an antibody specific for all multimeric forms of human adiponectin (Daiichi Pure Chemicals, Japan) with intra- and interassay CV of 3.3 and <1.5%, respectively.
Statistical methods
All values were given as means ± SD unless stated otherwise. The statistical analyses were carried out using the SPSS software version 17 (SPSS, Chicago, IL). All statistical tests were two-sided, and a P value < 0.05 was considered significant.
IR, CRP, and adiponectin were log-transformed to improve normality. Analyses of the CRP levels were limited to a maximum of 10 mg/L (n = 212 for CRP >10 mg/L), as suggested by the Centers for Disease Control and the American Heart Association, because extremely elevated values were of indeterminate significance without accompanying clinical correlation (13). BMI was computed using weight in kilograms divided by the square of height in meters. To compare continuous variables between ethnic groups and/or sex, the Student t test was used for two-group comparisons of continuous variables, whereas ANOVA was used for comparisons in more than two groups, with Bonferroni corrections applied for pairwise comparisons. The χ2 tests were used to compare proportions. Pearson correlation analysis was used to assess relationships between ln-IR, ln-CRP, and ln-adiponectin with other continuous variables.
We then performed ANCOVA to examine the effect of ethnicity on the relationship between 1) BMI and IR, 2) BMI and CRP, and 3) BMI and adiponectin. Analyses were performed with and without adjustment for age, smoking status, history of CVD (myocardial infarction, stroke, or transient ischemic attack), history of type 2 diabetes, use of aspirin, or a statin. For these analyses, ln-IR, ln-CRP, and ln-adiponectin were entered as dependent variables in separate analyses, with ethnicity (three levels) and BMI as covariates. The cross-product interaction term, ethnicity*BMI, was included as an independent variable to test for ethnic differences in the relationship between BMI and IR, CRP, or adiponectin. We then calculated the predicted values for ln-IR, ln-CRP, and ln-adiponectin based on the regression model. The predicted values were plotted against BMI for each ethnic group. All analyses were stratified by sex because of previous data that showed that the associations between BMI and CRP and between BMI and adiponectin were modified by sex (14), and this sex interaction was also observed in the current study (see Supplementary Fig. A2).
RESULTS
Our study population included 4,804 participants, comprising 65.3% Chinese, 18.1% Malays, and 16.6% Asian-Indians, with a slight predominance of women (55.1%). The mean age and BMI were 49.4 ± 12.2 years and 24.0 ± 4.4 kg/m2, respectively. Baseline demographic, clinical, and biochemical characteristics are summarized in Table 1. Asian-Indians had the least favorable cardiovascular risk profiles. Asian-Indians were significantly older and had higher fasting glucose, IR, and CRP but lower HDL-C and adiponectin levels (all P values < 0.01, with Bonferroni correction). However, Malays had significantly higher systolic BP and LDL-C levels compared with Chinese and Asian-Indians.
Table 1.
Demographic characteristics of study participants by sex and ethnic group
| Men |
Women |
*PANOVA men | †PANOVA women | |||||
|---|---|---|---|---|---|---|---|---|
| Chinese | Malays | Asian-Indians | Chinese | Malays | Asian-Indians | |||
| Age (years) | 50.4 ± 12.3 | 50.3 ± 11.5 | 52.9 ± 11.7 | 49.1 ± 11.6 | 49.5 ± 11.6 | 51.5 ± 10.6 | 0.001 | 0.001 |
| BMI (kg/m2) | 23.5 ± 3.4 | 25.3 ± 4.0 | 24.8 ± 3.7 | 22.4 ± 3.8 | 26.9 ± 4.9 | 26.6 ± 4.6 | <0.001 | <0.001 |
| Waist circumference (cm) | 87.4 ± 9.3 | 90.3 ± 10.2 | 93.2 ± 10.8 | 77.2 ± 9.4 | 86.0 ± 11.5 | 88.3 ± 10.8 | <0.001 | <0.001 |
| BP (mmHg) | ||||||||
| Systolic | 134.3 ± 18.8 | 135.7 ± 18.6 | 133.3 ± 17.9 | 127.5 ± 21.5 | 139.1 ± 22.9 | 134.6 ± 24.4 | 0.173 | <0.001 |
| Diastolic | 81.3 ± 10.5 | 81.89 ± 9.8 | 81.4 ± 10.0 | 73.44 ± 9.7 | 77.39 ± 10.8 | 76.12 ± 10.3 | 0.760 | <0.001 |
| Total cholesterol (mmol/L) | 5.15 ± 0.9 | 5.45 ± 1.00 | 5.12 ± 0.92 | 5.22 ± 0.94 | 5.50 ± 1.02 | 5.22 ± 0.93 | <0.001 | <0.001 |
| Triglycerides (mmol/L) | 1.50 ± 0.95 | 1.68 ± 0.91 | 1.55 ± 0.81 | 1.14 ± 0.71 | 1.40 ± 1.45 | 1.35 ± 0.66 | <0.001 | <0.001 |
| HDL-C (mmol/L) | 1.32 ± 0.29 | 1.23 ± 0.27 | 1.15 ± 0.26 | 1.61 ± 0.36 | 1.48 ± 0.31 | 1.32 ± 0.29 | <0.001 | <0.001 |
| LDL-C (mmol/L) | 3.14 ± 0.81 | 3.45 ± 0.93 | 3.26 ± 0.84 | 3.09 ± 0.83 | 3.38 ± 0.99 | 3.29 ± 0.77 | <0.001 | <0.001 |
| Fasting glucose (mmol/L) | 5.15 ± 1.23 | 5.52 ± 2.26 | 5.78 ± 2.10 | 4.85 ± 1.11 | 5.38 ± 2.00 | 5.65 ± 2.20 | <0.001 | <0.001 |
| Fasting insulin (μU/L) | 7.44 ± 6.51 | 8.73 ± 7.57 | 11.45 ± 11.13 | 7.01 ± 5.04 | 9.23 ± 5.60 | 12.00 ± 8.82 | <0.001 | <0.001 |
| HOMA-IR | 1.76 ± 1.72 | 2.23 ± 2.54 | 3.02 ± 3.26 | 1.58 ± 1.43 | 2.28 ± 1.84 | 3.18 ± 3.18 | <0.001 | <0.001 |
| CRP (mg/L) | 2.11 ± 5.89 | 2.82 ± 4.23 | 3.25 ± 7.10 | 2.02 ± 4.33 | 3.64 ± 4.89 | 5.71 ± 8.36 | 0.001 | <0.001 |
| Total adiponectin (μg/mL) | 3.10 ± 1.79 | 2.97 ± 1.80 | 2.97 ± 1.75 | 4.60 ± 2.50 | 4.28 ± 2.52 | 3.83 ± 1.95 | 0.268 | <0.001 |
| CVD (%) | 5.1 | 5.1 | 13.4 | 1.4 | 2.0 | 3.9 | <0.001 | 0.003 |
| Diabetes (%) | 7.3 | 10.7 | 19.8 | 5.0 | 12.4 | 18.6 | <0.001 | <0.001 |
| Statin use (%) | 10.4 | 10.5 | 18.0 | 6.8 | 9.5 | 11.5 | <0.001 | 0.003 |
| Aspirin use (%) | 5.3 | 5.1 | 13.7 | 1.4 | 2.0 | 3.9 | <0.001 | 0.003 |
| Current smokers (%) | 19.5 | 34 | 21.8 | 2.6 | 2.7 | 0.7 | <0.001 | 0.001 |
Data are expressed as mean ± SD or as % (proportion to the group). The χ2 test was used for categorical variables to compare differences between the ethnic groups. For men, n = 1,390 Chinese, 428 Malays, and 388 Asian-Indians. For women, n = 1,745 Chinese, 444 Malays, and 409 Asian-Indians. HOMA-IR, homeostasis model assessment-IR. CVD was defined as history of coronary heart disease, stroke, or transient ischemic attack. Diabetes was defined as history of diabetes with or without treatment.
*P value for comparison between the ethnic groups in men;
†P value for comparison between the ethnic groups in women.
Table 2 shows the correlation between measures of obesity (BMI and waist circumference); metabolic profiles; and IR, CRP, and adiponectin for males and females in our study population. After adjustment for age and ethnicity, BMI was positively correlated with IR and CRP and inversely correlated with adiponectin. Both IR and CRP were significantly correlated with systolic BP, diastolic BP, and triglycerides and inversely with HDL-C (all P values < 0.001). On the contrary, adiponectin has a significant inverse correlation with diastolic BP, fasting insulin, fasting glucose, and triglycerides but positive correlation with HDL-C (all P values < 0.001).
Table 2.
Correlation between ln-IR, ln-CRP, and ln-adiponectin with cardiovascular risk factors by sex
| Ln-IR |
Ln-CRP |
Ln-adiponectin |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men |
Women |
Men |
Women |
Men |
Women |
|||||||
| Correlation coefficient (r) | P value | Correlation coefficient (r) | P value | Correlation coefficient (r) | P value | Correlation coefficient (r) | P value | Correlation coefficient (r) | P value | Correlation coefficient (r) | P value | |
| Age (years) | 0.108 | <0.001 | 0.233 | <0.001 | 0.119 | <0.001 | 0.222 | <0.001 | 0.166 | <0.001 | 0.084 | <0.001 |
| BMI (kg/m2) | 0.539 | <0.001 | 0.582 | <0.001 | 0.366 | <0.001 | 0.544 | <0.001 | −0.280 | <0.001 | −0.307 | <0.001 |
| Waist circumference (cm) | 0.572 | <0.001 | 0.611 | <0.001 | 0.402 | <0.001 | 0.548 | <0.001 | −0.268 | <0.001 | −0.314 | <0.001 |
| BP (mmHg) | ||||||||||||
| Systolic | 0.243 | <0.001 | 0.358 | <0.001 | 0.166 | <0.001 | 0.280 | <0.001 | −0.010 | 0.644 | −0.058 | 0.003 |
| Diastolic | 0.227 | <0.001 | 0.273 | <0.001 | 0.167 | <0.001 | 0.245 | <0.001 | −0.119 | <0.001 | −0.122 | <0.001 |
| Total cholesterol (mmol/L) | 0.036 | 0.092 | 0.109 | <0.001 | 0.105 | <0.001 | 0.135 | <0.001 | −0.050 | 0.018 | 0.014 | 0.485 |
| Triglycerides (mmol/L) | 0.276 | <0.001 | 0.347 | <0.001 | 0.189 | <0.001 | 0.243 | <0.001 | −0.292 | <0.001 | −0.281 | <0.001 |
| HDL-C (mmol/L) | −0.310 | <0.001 | −0.410 | <0.001 | −0.263 | <0.001 | −0.333 | <0.001 | 0.395 | <0.001 | 0.425 | <0.001 |
| LDL-C (mmol/L) | 0.010 | 0.630 | 0.130 | <0.001 | 0.113 | <0.001 | 0.177 | <0.001 | −0.048 | 0.025 | −0.031 | 0.119 |
| Fasting glucose (mmol/L) | 0.438 | <0.001 | 0.516 | <0.001 | 0.139 | <0.001 | 0.246 | <0.001 | −0.114 | <0.001 | −0.190 | <0.001 |
| Fasting insulin (μU/L) | 0.769 | <0.001 | 0.859 | <0.001 | 0.236 | <0.001 | 0.382 | <0.001 | −0.263 | <0.001 | −0.322 | <0.001 |
| Ln-IR | 1 | — | 1 | — | 0.339 | <0.001 | 0.474 | <0.001 | −0.344 | <0.001 | −0.387 | <0.001 |
| Ln-CRP (mg/L) | 0.339 | <0.001 | 0.474 | <0.001 | 1 | — | 1 | — | −0.212 | <0.001 | −0.307 | <0.001 |
| Ln-adiponectin (ng/mL) | −0.344 | <0.001 | −0.387 | <0.001 | −0.212 | <0.001 | −0.307 | <0.001 | 1 | — | 1 | — |
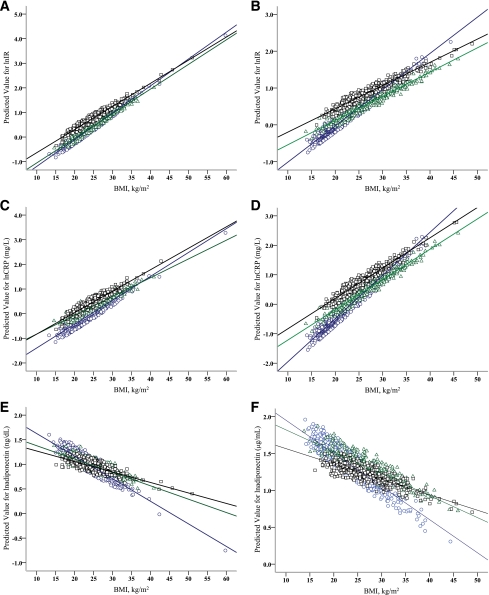
Effects of ethnicity on the relationship between BMI and IR, CRP, and adiponectin are shown in Fig. 1 and in Supplementary Table A1. The association between BMI and IR differed significantly between ethnic groups. Although increasing levels of BMI were associated with increased IR and CRP and reduced adiponectin in all three ethnic groups, each unit increase in BMI was associated with a larger increase in IR and CRP, and a larger decline in adiponectin in Chinese compared with Malays and Asian Indians. This interaction was particularly pronounced in women (P interaction < 0.001 for IR, CRP, and adiponectin). In men, interactions were in the same direction as for women, but reached statistical significance only for adiponectin. Further adjustment for age, smoking status, history of CVD, type 2 diabetes, use of aspirin, or statins did not substantially alter the effect of ethnicity on the relationship between BMI and IR, CRP, and adiponectin (data not shown). In addition, when we used waist circumference instead of BMI in the analyses, the interactions between waist circumference and ethnicity were essentially the same as for BMI and ethnicity (see Supplementary Table A2 and Supplementary Fig. A3).
Figure 1.
Age-adjusted fitted regression plots showing relationships between BMI and IR, CRP, and adiponectin in Chinese (○), Malays (△), and Asian-Indians (□), stratified by sex. A: Relationship of IR to BMI in men (P interaction = 0.282). B: Relationship of IR to BMI in women (P interaction < 0.001). C: Relationship of CRP to BMI in men (P interaction = 0.304). D: Relationship of CRP to BMI in women (P interaction < 0.001). E: Relationship of adiponectin to BMI in men (P interaction < 0.001). F: Relationship of adiponectin to BMI in women (P interaction < 0.001).
CONCLUSIONS
Based on our analysis of data from a large cross-sectional study, which included three major Asian ethnic groups (Chinese, Malays, and Asian-Indians) residing in Singapore, ethnic differences were observed for IR, CRP, and adiponectin. Some of these differences were independent of BMI, with greater IR, higher CRP, and lower adiponectin observed in Malays and Asian-Indians as compared with Chinese. However, the relationship between BMI and these parameters also differed between ethnic groups such that increases in BMI were associated with greater changes in all three parameters (IR, CRP, and adiponectin) in Chinese as compared with Malays and Asian-Indians.
We confirmed that Asian-Indians and Malays have more unfavorable risk profiles for type 2 diabetes and CVD when compared with Chinese. This is consistent with data from other studies that have shown that Malays and Asian-Indians exhibit higher rates of these disorders than Chinese (15). We have previously shown that IR was greater in Asian-Indians when compared with Chinese or Malays across the range of glucose tolerance, and this was associated with more unfavorable cardiovascular risk profiles (16). Other studies have also shown greater IR in Asian-Indians (5). This study has now extended these findings to include CRP and adiponectin levels. We showed that Asian-Indians have the highest CRP levels, followed by Malays and Chinese. Several previous studies have shown that CRP levels differ between populations (17). In most Asian populations, CRP levels are lower than in Western populations (18). It is of some interest that in the Singapore population, which has a rate of mortality from CVD similar to or higher than that in many developed countries, the CRP levels are similar, if not higher, than those in the U.S. (19). This suggests that low CRP levels are not intrinsic to any ethnic group but more likely reflect reduced exposure to environmental factors that would raise CRP. Total adiponectin levels were the lowest in Asian-Indians, a finding that is consistent with the greater degree of IR previously reported in this ethnic group (20).
In addition, we also observed that ethnicity modifies the relationship between BMI and IR, CRP, and adiponectin in a manner that is highly consistent across all three parameters. To our knowledge, this has only been examined in two previous studies. In the National Health and Nutrition Examination Survey (NHANES) study, it was shown that that the relationship between BMI and CRP did not differ between white Americans, African Americans, and Hispanic Americans (11). However, this study did not include individuals of Asian ethnicity. One previous study by Mente et al. (5) examined the association between obesity and adiponectin or IR and included significant numbers of Asians. This study found that Chinese had similar levels of IR to Europeans, whereas Asian-Indians had greater IR. In addition, they also found that the association between BMI and adiponectin was weaker in Asian-Indians than in any of the other ethnic groups studied. Our study corroborates these findings for the difference between Asian-Indians and Chinese and extends them to include IR and CRP, which were not reported in the study by Mente et al. (5). Furthermore, we found that Malays, similar to Asian-Indians, also exhibited a weaker association between BMI and these measures. This is important because most studies in Asia have focused on Japanese, Chinese, and Asian-Indians. Asia also comprises a large number of islands (such as Indonesia, East Timor, and Philippines), which have been jointly referred to as “Other Asian Islands.” The populations of these islands are ethnically distinct from those of Chinese, Japanese, and Asian-Indians and can be expected to experience a greater increase in the burden from CVD than either India or China (21). Malays, and other related ethnic groups, represent 300–400 million people inhabiting these Other Asian Islands. Data from Singapore suggest that this ethnic group is at high risk of diabetes and CVD and represents a large understudied population.
We considered several possible explanations as to why increasing levels of BMI were associated with greater increases in IR and CRP and greater decline in adiponectin in Chinese. First, it is well recognized that despite a lower BMI, Asian-Indians have a greater degree of adiposity than Caucasians (4). Using a four-compartment model, Deurenberg et al. (22) have shown that for the same age, sex, and BMI, Asian-Indians had the highest body fat percentage followed by Malays and Chinese. However, the relationship between BMI and adiposity was similar between ethnic groups with no interaction between ethnicity and BMI. As such, we believe that ethnic differences in the relationship between BMI and adiposity per se do not account for the observed interactions. Instead, the interactions might represent ethnic-specific sensitivity to the effects of increasing adiposity. This may relate to differences in fat distribution with increasing levels of adiposity, particularly in the visceral compartment. Lear et al. (23), in the Multicultural Community Health Assessment Trial (M-CHAT), compared the relation between abdominal adipose tissue and total body fat between people living in Canada of aboriginal, Chinese, and Asian-Indian origin with people of European origin. They showed that ethnicity has an effect on fat distribution between the subcutaneous and visceral compartments with increasing levels of adiposity. However, this study did not directly compare Chinese with Asian-Indians, making it difficult to determine whether this could explain the differences between Chinese and Asian-Indians observed in our study. To explore this further, we also extended our analyses to use waist circumference instead of BMI because waist circumference has been shown to be more strongly related to visceral adiposity than BMI. We observed that the ethnic differences in regard to the relationship between adiposity and IR, CRP, and adiponectin were essentially the same for waist circumference as for BMI. Third, it has recently been suggested that the total adipose tissue in an individual might have an upper limit capacity for storing fat optimally. Once this capacity is exceeded, any further nutrient excess will result in metabolic perturbations such as IR and inflammation (24). We postulate that Chinese may have a lower capacity for fat storage and an excess caloric intake may result in greater metabolic perturbations. It is also tempting to implicate genetic differences between the ethnic groups. However, to date, we do not have any evidence for specific genetic differences that may contribute to ethnic differences in metabolic responses to excess adiposity. Finally, we also considered the possibility that differential patterns of immigration between the ethnic groups could have resulted in differential exposure to important environmental influences on inflammation and obesity. Unfortunately, we do not have detailed information on immigration for our study participants. In our study, the participants from the four population-based cross-sectional surveys were all Singapore residents when they were first studied. Because this follow-up was carried out 5 to 20 years after the initial studies, all participants had resided in Singapore for at least 5 to 20 years before participating in this study and have been exposed to the similar urbanized environment. Nevertheless, we cannot exclude the possibility that earlier exposure to different environments (such as during childhood) may underlie some of the ethnic differences that we have observed.
Our study has several strengths. This study included three major ethnic groups (Chinese, Malays, and Asian-Indians) that represent the majority of ethnic groups living in Asia, a region where the prevalence of type 2 diabetes and CVD is expected to increase over the next several decades and the highest population in the world. The large number of participants with data collected in a standardized manner minimizes measurement error and potential biases. There are also limitations in our study. The cross-sectional nature of the study does not allow us to infer causality. In addition, stratified sampling for the minority ethnic groups means that the study population may not be fully representative of the Singapore population. However, we feel that the nonrepresentativeness of the study population does not impair our ability to make these biologically relevant observations. We also accept that BMI and waist circumference are imperfect measures of adiposity and that more definitive measures of adiposity, including an assessment of fat distribution, would have provided a better understanding of the interactions observed. Finally, our study does not provide a mechanistic basis for the interactions observed. Mechanistic studies to explore these ethnic differences are important. Nevertheless, we believe that this is an important hypothesis generating study and provides some insight as to the rapid increase in the rates of type 2 diabetes in Chinese populations (1).
In conclusion, the relationships between BMI and three pathogenic pathways (IR, CRP, and adiponectin) are modified by ethnicity. The negative impact of increasing BMI on IR, CRP, and adiponectin levels is more pronounced in Chinese than the other ethnic groups. This may have contributed to the rapid increase in the prevalence of type 2 diabetes observed in China, which has been accompanied by increasing levels of overweight and obesity (1). These findings suggest that, as a public health strategy, weight reduction or prevention of weight gain is particularly important in many Asian countries despite the relatively low levels of obesity. In addition, studies between ethnic groups may provide insight as to the interindividual differences in susceptibility to obesity-related diseases.
Supplementary Material
Acknowledgments
This work was supported by grants from the Biomedical Research Council (grant 03/1/27/18/216) and National Medical Research Council (grants 0838/2004, NMRC/CSI/0002/2005, and 1111/2007).
E.S.T. has served on advisory boards for Merck Sharp and Dohme (IA Corp), AstraZeneca, and Novo Nordisk. He is the principle investigator of an investigator-initiated research grant funded by Pfizer, which seeks to identify biological, lifestyle, and psychosocial factors associated with individual trajectories of cardiovascular risk factors in South East Asia. No other potential conflicts of interest relevant to this article were reported.
C.M.K. helped conduct the literature review and data analysis and wrote the manuscript. S.S. helped with data analysis. S.T. and D.G. contributed to discussion. Y.W. helped with data analysis. J.L. helped in the design of the study and contributed to the final edition of the manuscript. R.M.v.D. contributed to discussion. E.S.T. helped in the design of the study and contributed to the final edition of the manuscript.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-2097/-/DC1.
References
- 1.Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009;301:2129–2140 [DOI] [PubMed] [Google Scholar]
- 2.Lillioja S, Mott DM, Howard BV, et al. Impaired glucose tolerance as a disorder of insulin action. Longitudinal and cross-sectional studies in Pima Indians. N Engl J Med 1988;318:1217–1225 [DOI] [PubMed] [Google Scholar]
- 3.Abbasi F, Brown BW, Jr, Lamendola C, McLaughlin T, Reaven GM. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol 2002;40:937–943 [DOI] [PubMed] [Google Scholar]
- 4.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–163 [DOI] [PubMed] [Google Scholar]
- 5.Mente A, Razak F, Blankenberg S, et al. ; Study of the Health Assessment And Risk Evaluation; Study of the Health Assessment And Risk Evaluation in Aboriginal Peoples Investigators Ethnic variation in adiponectin and leptin levels and their association with adiposity and insulin resistance. Diabetes Care 2010;33:1629–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tai ES, Lim SC, Chew SK, Tan BY, Tan CE. Homeostasis model assessment in a population with mixed ethnicity: the 1992 Singapore National Health Survey. Diabetes Res Clin Pract 2000;49:159–168 [DOI] [PubMed] [Google Scholar]
- 7.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999;282:2131–2135 [DOI] [PubMed] [Google Scholar]
- 8.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004;350:1387–1397 [DOI] [PubMed] [Google Scholar]
- 9.Szmitko PE, Teoh H, Stewart DJ, Verma S. Adiponectin and cardiovascular disease: state of the art? Am J Physiol Heart Circ Physiol 2007;292:H1655–H1663 [DOI] [PubMed] [Google Scholar]
- 10.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation 2001;104:2746–2753 [DOI] [PubMed] [Google Scholar]
- 11.Wee CC, Mukamal KJ, Huang A, Davis RB, McCarthy EP, Mittleman MA. Obesity and C-reactive protein levels among white, black, and Hispanic US adults. Obesity (Silver Spring) 2008;16:875–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nang EE, Khoo CM, Tai ES, et al. Is there a clear threshold for fasting plasma glucose that differentiates between those with and without neuropathy and chronic kidney disease? The Singapore Prospective Study Program. Am J Epidemiol 2009;169:1454–1462 [DOI] [PubMed] [Google Scholar]
- 13.Pearson TA, Mensah GA, Alexander RW, et al. ; Centers for Disease Control and Prevention; American Heart Association Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107:499–511 [DOI] [PubMed] [Google Scholar]
- 14.Lakoski SG, Cushman M, Criqui M, et al. Gender and C-reactive protein: data from the Multiethnic Study of Atherosclerosis (MESA) cohort. Am Heart J 2006;152:593–598 [DOI] [PubMed] [Google Scholar]
- 15.Mak KH, Chia KS, Kark JD, et al. Ethnic differences in acute myocardial infarction in Singapore. Eur Heart J 2003;24:151–160 [DOI] [PubMed] [Google Scholar]
- 16.Ang LW, Ma S, Cutter J, Chew SK, Tan CE, Tai ES. The metabolic syndrome in Chinese, Malays and Asian Indians. Factor analysis of data from the 1998 Singapore National Health Survey. Diabetes Res Clin Pract 2005;67:53–62 [DOI] [PubMed] [Google Scholar]
- 17.McDade TW, Rutherford JN, Adair L, Kuzawa C. Population differences in associations between C-reactive protein concentration and adiposity: comparison of young adults in the Philippines and the United States. Am J Clin Nutr 2009;89:1237–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee YJ, Lee JH, Shin YH, Kim JK, Lee HR, Lee DC. Gender difference and determinants of C-reactive protein level in Korean adults. Clin Chem Lab Med 2009;47:863–869 [DOI] [PubMed] [Google Scholar]
- 19.Rifai N, Ridker PM. Population distributions of C-reactive protein in apparently healthy men and women in the United States: implication for clinical interpretation. Clin Chem 2003;49:666–669 [DOI] [PubMed] [Google Scholar]
- 20.Smith J, Al-Amri M, Sniderman A, Cianflone K. Leptin and adiponectin in relation to body fat percentage, waist to hip ratio and the apoB/apoA1 ratio in Asian Indian and Caucasian men and women. Nutr Metab (Lond) 2006;3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 22.Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat percent relationship. Obes Rev 2002;3:141–146 [DOI] [PubMed] [Google Scholar]
- 23.Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, Birmingham CL. Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT). Am J Clin Nutr 2007;86:353–359 [DOI] [PubMed] [Google Scholar]
- 24.Frayn KN, Arner P, Yki-Järvinen H. Fatty acid metabolism in adipose tissue, muscle and liver in health and disease. Essays Biochem 2006;42:89–103 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.