IL-15 overcomes CD4+ T cell deficiency in vitro in induction of recall CD8+ T cell responses after CD4 depletion or HIV infection.
Keywords: primary cells, dendritic cells, lymphoid cell-mediated immunity
Abstract
CD4+ Th cells are important for the induction and maintenance of antigen-specific CD8+ T cell function, so their loss or dysfunction in HIV-infected or cancer patients could reduce the patients' ability to control viral infection. Previous work in murine systems indicated that IL-15 codelivered with vaccines could overcome CD4+ Th cell deficiency for induction of functionally efficient CD8+ T cells and maintenance of viral-specific CTLs, but its efficacy in helping primary human CD8+ T cell responses is unknown. In the present study, a peptide-pulsed, DC-based human coculture ex vivo system was used to study the role of IL-15 in overcoming CD4+ Th deficiency to elicit CD8+ T cell responses in CD4-depleted PBMCs from healthy individuals and PBMCs from HIV-1-infected patients. We found that IL-15 could overcome CD4+ Th deficiency to induce primary and recall memory CD8+ T cell responses in healthy individuals. Moreover, in CD4-deficient, HIV-1-infected patients with diminished CD8+ T cell responses, IL-15 greatly enhanced CD8+ T cell responses to alloantigen. These results suggest that IL-15 may be useful in the development of therapeutic and preventive vaccines against cancers and viral infections in patients defective in CD4+ Th cell.
Introduction
Effective antigen-specific CD8+ T cells, often functioning as CTLs, are critical for establishment of protective immunity against a number of pathogens and cancers [1]. CD4+ Th cells are essential for the induction and maintenance of these antigen-specific CD8+ T cell functions [2–4]. Loss and dysfunction of CD4+ T cells often occur in chronic viral infections such as lymphocytic choriomeningitis virus, HCV, and HIV infection, as well as in cancer. CD4+ T cell deficiency results in the generation of dysfunctional CD8+ T cells and impairs viral clearance, further leading to disease progression [5–7]. It is therefore important to explore ways to overcome CD4+ Th cell deficiency to induce efficient CTL responses.
IL-15, one of the common cytokine receptor γ-chain family cytokines, displays multiple immunomodulatory activities in innate and adaptive immune systems [8, 9]. Our previous work in murine systems indicated that IL-15, codelivered with vaccines, could overcome CD4+ Th cell deficiency for induction of long-lived CD8+ T cells resistant to TRAIL-mediated apoptosis during secondary responses [10]. In another influenza virus challenge mouse model, administration of an optimized plasmid encoding IL-15 could significantly enhance the function and longevity of CD8+ T cells that are partially independent of CD4+ T cell help [11]. These results highlight the crucial role of IL-15 in the generation of efficient CTL function in murine systems. However, whether the finding that IL-15 could overcome CD4+ Th deficiency to induce efficient primary CD8+ T cell responses can be translated to humans remains to be investigated. For vaccines, it is especially important to be able to induce a primary rather than a recall response.
In the present study, a peptide-pulsed, DC-based coculture ex vivo system was used to explore the role of IL-15 in overcoming CD4+ Th deficiency for the induction of recall and primary antigen-specific CD8+ T cell responses in CD4-depleted PBMCs from healthy individuals. DC-pulsed influenza antigen for induction of recall CD8+ T cell responses and DC-pulsed HCV antigen for induction of primary CD8+ T cell responses were used in our system. In parallel with the healthy individuals, the ability of IL-15 to overcome CD4+ Th deficiency for the induction of the CD8+ T cell responses to alloantigen in HIV-1-infected individuals was also investigated.
MATERIALS AND METHODS
Reagents and mAb
HLA class I-restricted, HCV-specific peptides C7A2 (DLMGYIPLV) and influenza-specific peptide matrix (GILGFVFTL) were from NeoMPS (San Diego, CA, USA) [12, 13]. Tet 830-modified Th epitope from tetanus toxoid for CD4+ T cell stimulation was from AnaSpe (San Jose, CA, USA). TLR4 ligands–LPS were purchased from Calbiochem (San Diego, CA, USA). TLR3 ligand and TLR7/8 ligands were purchased from Invivogen (San Diego, CA, USA). A commercial ELISA kit for IL-15 detection was from R&D Systems (Minneapolis, MN, USA).
The following antihuman mAb reagents were purchased from eBioscience (San Diego, CA, USA): PE anti-human CTLA-4, PE-Cy7 anti-human TNF-α, and allophycocyanin anti-human program death-1. Anti-human IL-15 fluorescein mAb was from R&D Systems. MDDCs were stained with CD1a, CD11c, CD14, HLA-DR, CD80, CD86, CD83, and CD40. For intracellular staining, cells were stained with mAb to IL-2, TNF-α, IFN-γ, and perforin. All mAb were purchased from BioLegend (San Diego, CA, USA) unless indicated otherwise.
Patient samples and cell cultures
Blood samples were obtained from healthy blood donors and HIV-1-infected patients. HLA-A*0201-positive PBMC concentrates were collected using a CS3000 Plus blood cell separator (Baxter Healthcare, Fenwal Division, Deerfield, IL, USA) from healthy donors in the DTM, Clinical Center, NIH (Bethesda, MD, USA). All donors signed an informed consent approved by a NIH Institutional Review Board. Monocytes were isolated from the PBMC concentrates on the day of collection by elutriation (Elutra, Gambro BCT, Lakewood, CO, USA), according to the manufacturer's recommendations. Heparinized research blood specimens from HIV-1-infected individuals were obtained on HIV and AIDS Malignancy Branch, Protocol 01-C-0038, with informed consent. PBMCs were separated by Ficoll density centrifugation (GE Healthcare, Waukesha, WI, USA). Naïve CD8+ T cells with phenotype CD8+CD45RA+ were isolated from PBMCs by using a naïve CD8+ T cell isolation kit from Miltenyi Biotec (Auburn, CA, USA). CD4+ T cells were depleted from PBMCs using CD4 microbeads kit (Miltenyi Biotec). The selected cell populations contained <1% CD4+ T cells, as measured by FACS analysis. In our ex vivo coculture system, the CD4+ T cell number did not change during the culture in the CD4+ T cell-depleted condition in the presence or absence of IL-15, as measured by FACS analysis. Cells were cultured in RPMI 1640 with L-glutamine containing 10% FCS and antibiotics. Human rIL-2 (20 U/ml) and 10 ng/ml IL-15 (PeproTech, Rocky Hill, NJ, USA) were added to the culture system as indicated in each experiment.
Preparation of MDDCs
Elutriated monocytes were cultured at 1 ×106 cell/ml in six-well plates or 25 cm2 cell-culture flasks. Human rIL-4 and GM-CSF (2000 U/ml, PeproTech) were added at Days 0 and 2. On Day 3, MDDCs were matured with LPS (100 ng/ml) in combination with IFN-γ (1000 U/ml) for 24 h before use. The imDC and mDC were used as APCs.
Induction of antigen-specific CD8+ T cell responses using allogeneic MDDCs or peptide-pulsed, autologous MDDCs
Induction of recall antigen-specific T cell responses in autologous PBMC-DC coculture systems.
MDDCs were pulsed with the specific HLA-A2.1-restricted FMP (GILGFVFTL) at 2 μg/ml for 2 h at 37°C [13]. To provide a stimulus to CD4+ T cells, these MDDCs were also pulsed with a specific Th epitope from tetanus toxoid at 4 μg/ml. Pulsed or nonpulsed MDDCs (1×105)/well were seeded in 96-round-bottom well plates. Autologous PBMCs, unfractionated (1×106/well) or CD4+ T cell-depleted (4×105/well), were added to MDDCs at a 10:1 ratio. The percentage of CD8+ T cells within total PBMCs and CD4+ T cell-depleted PBMCs was determined by FACS analysis so that an equal input of CD8+ T cells could be plated whether unfractionated or CD4+ T cell-depleted. Peptide-pulsed MDDCs were cocultured with PBMCs or CD4+ T cell-depleted PBMCs for 7 days. Cytokine IL-2 (20 U/ml) or IL-15 (10 ng/ml) was added in CD4+ T cell-depleted experiments where indicated.
Induction of primary antigen-specific T cell responses in autologous PBMC-DC coculture systems.
MDDCs were pulsed with the specific HLA-A2.1-restricted HCV peptide C7A2 at 10 μg/ml and Th epitope from tetanus toxoid at 4 μg/ml for 2 h at 37°C. The peptide-pulsed MDDCs were then cocultured with autologous PBMC for 7 days. The cocultures were restimulated with C7A2-pulsed MDDCs on Day 7 and incubated for another 7 days.
Induction of primary allogeneic antigen-specific T cells in an allogeneic PBMC-DC coculture system.
For allogeneic, mature MDDC stimulation, unfractionated or CD4+ T cell-depleted PBMCs were added to allogeneic, mature MDDCs at a 10:1 ratio and cultured for 4 days. The cultures were restimulated overnight by the addition of 5 × 104 freshly thawed allogeneic monocytes in the presence of Brefeldin A, followed by intracellular staining.
Intracellular staining and FACS analysis
The cocultures of autologous PBMC-DCs were restimulated by the addition of 5 × 104 freshly thawed, autologous peptide (10 μg/ml)-pulsed monocytes in the presence of 10 μg/ml Brefeldin A (BioLegend) and were then incubated overnight. The cultures were harvested and tested for CD8+ T cell responses by intracellular cytokine staining.
After cell surface staining with anti-CD8 and anti-CD3, cells were permeabilized with Cytofix/Cytoperm (PharMingen, San Diego, CA, USA) in accordance with the manufacturer's recommendations. Intracellular staining was performed with anti-IFN-γ, anti-IL-2, and anti-TNF-α. Cells were analyzed on a FACSCalibur, FACSAria, or LSR II (BD Biosciences, San Jose, CA, USA) flow cytometer and analyzed by using CellQuest software (BD Biosciences) or FlowJo software (TreeStar, San Carlos, CA, USA). In all cases, at least 200,000 live events were collected for analysis.
Statistics
The data were compared for significance (P<0.05) with the nonparametric Mann-Whitney test. The correlations between fold increase of CD8+ T responses by addition of IL-15 and the CD4+ T cell counts were calculated using Spearman's correlation.
RESULTS
MDDCs matured with TLR4 ligands induced more efficient CD8+ T cell responses than immature MDDCs
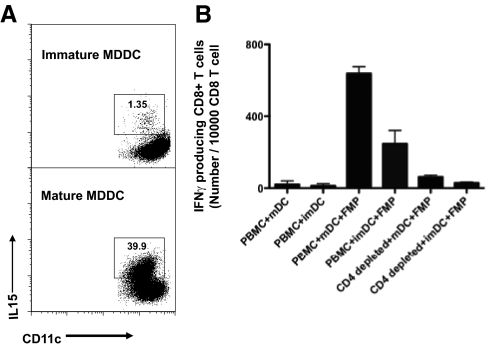
LPS is U.S. Food and Drug Administration-approved to be used as an ancillary agent to mature DCs in vitro for clinical use and is currently being used with IFN-γ by the NIH Clinical Center, DTM, to mature DCs for clinical trials [14]. We first confirmed whether this regimen, in clinical use of LPS (a TLR4 ligand) in combination with IFN-γ, could induce maturation of MDDCs, as this was reported to be one of the most effective ways to mature DCs [14]. Compared with immature MDDCs, a variety of DC maturation markers, such as CD83, MHC class II, CD80, and CD40, was up-regulated in LPS and IFN-γ-matured MDDCs (Supplemental Fig. 1). Moreover, high levels of IL-12 production and CCR7 expression were also observed previously [14]. Furthermore, more cells expressing surface IL-15 were also observed among matured MDDCs (ranging from 7% to 40%; Fig. 1A), suggesting that they had also up-regulated the IL-15Rα, as surface IL-15 must be bound to that receptor. Indeed, it has been demonstrated previously that the combination of LPS and IFN-γ strongly up-regulates expression of the IL-15Rα on human DCs [15]. We reasoned that IL-15Rα expression on DCs, if not all occupied by endogenous IL-15, could enhance the effect of exogenous IL-15 further via transpresentation to increase CD8+ T cell responses, thus making the mDCs more effective in this system. These findings are consistent with observations that DCs empowered by TLR4 ligands to enhance CTL responses could be used as immunotherapy for HIV-1 infection and cancers [16, 17]. As shown in Fig. 1B, when pulsed with FMP, matured MDDCs elicited more efficient antigen-specific CD8+ T cell responses compared with immature MDDCs (Fig. 1B). The frequency of recall, influenza-specific CD8+ T cells measured by intracellular IFN-γ staining was more than twofold greater in the PBMC + mDC + FMP group than in the PBMC + imDC + FMP group. In contrast, low antigen-specific CD8+ T cell responses were observed in the CD4+ T cell-depleted PBMCs with either type of DC. This result in agreement with previous studies suggests that in the ex vivo culture system, CD4+ T cells play a critical role for optimal stimulation to induce recall responses of CD8+ T cells [2, 18]. Of note, neither mDCs nor imDCs alone could substitute for CD4+ T cell help to induce antigen-specific CD8+ T cell responses. Thus, it appears that even these mDCs that express some IL-15, which we have detected, must not make enough IL-15 without CD4+ T cell help, as exogenous IL-15 is needed in the absence of help (see below).
Figure 1. Characterization and function of DCs used.
(A) Up-regulated expression of IL-15 on mature MDDCs was observed as compared with immature MDDCs. MDDCs were stained with allophycocyanin anti-human CD11c and FITC anti-human IL-15, analyzed by FACS. (B) Mature MDDCs could induce more efficient antigen-specific recall CD8+ T cell responses as compared with immature MDDCs when DCs were pulsed with HLA-A2.1-restricted FMPs. Data represent means ± sem of the triplicate cultures. The experiment was repeated with similar results.
IL-15 substituted for CD4+ T cell help to induce high-quality recall CD8+ T cell responses
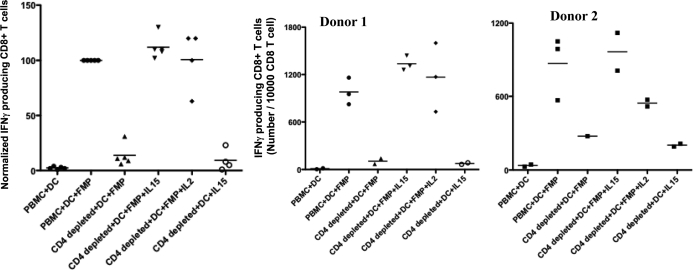
Using the above ex vivo cell culture system, the role of IL-15 in overcoming CD4+ T deficiency to induce recall CD8+ T cell responses was addressed. The cells were isolated from healthy HLA-A*0201+ donors who had likely been exposed historically to influenza virus infection. Fig. 2 shows results of several independent cultures under each condition of cells from two different representative healthy HLA-A*0201+ blood donors. Under CD4+ T cell-depleted conditions, the addition of IL-15 was able to increase IFN-γ-producing, antigen-specific CD8+ T cell responses to the level seen with unfractionated PBMCs (Fig. 2 and Supplemental Fig. 2). The antigen-specific, IFN-γ-producing CD8+ T cells were negligible in the coculture of PBMC with nonpeptide-pulsed DCs (pulsed only with serum containing multiple irrelevant peptides; Fig. 2), confirming the specificity of the responses. There was a significant decrease in the CD4+ T cell-depleted cultures versus undepleted PBMC cultures. The addition of IL-15 to CD4+ T cell-depleted cultures significantly restored the antigen-specific, IFN-γ-producing CD8+ T cells in all of the cultures from both donors. Although the degree of enhancement was variable among the different donors, the ability of IL-15 to restore CD8+ T cell responses under the CD4+ T cell-depleted condition was consistent in all of the experiments. In addition to IL-15, IL-2 could substitute for CD4+ T cell help to promote recall CD8+ T cell responses in agreement with a previous study [18].
Figure 2. Cytokine substitution for CD4+ T cell help in recall CD8+ T cell responses.
Autologous MDDCs were pulsed with HLA-A2.1-restricted FMP at 2 μg/ml and Th epitope from tetanus toxoid at 4 μg/ml for 2 h at 37°C. FMP-pulsed or unpulsed MDDCs were cocultured with total PBMC, CD4+ T cell-depleted PBMC, or CD4+ T cell-depleted PBMC plus IL-15 or IL-2, respectively, for 7 days. The cocultures were restimulated by autologous peptide (10 μg/ml)-pulsed monocytes and cultured overnight. The cocultures were harvested and tested for CD8+ T cell responses by intracellular IFN-γ staining. The panels show results for two representative blood donors, representative of five tested for IL-15 and four tested for IL-2 with similar results. Each point represents a separate independent culture with the stimulation conditions shown. The left panel shows normalized results for all donors combined, with the result for PBMC + DC + FMP taken as 100% for each donor.
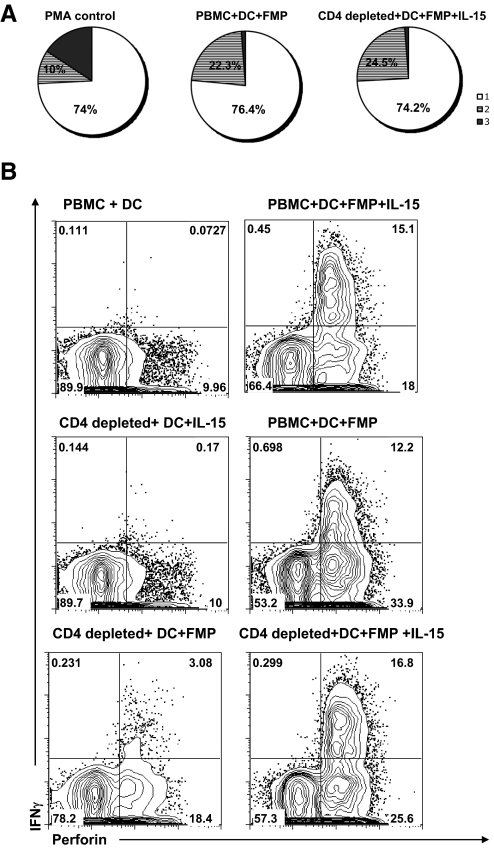
The above results indicated that the IL-15 could promote induction of antigen-specific CD8+ T cell recall responses in the absence of the CD4+ T cell help. We further asked whether IL-15 could substitute for help in inducing a similar quality of antigen-specific CD8+ T cells producing multiple cytokines such as IFN-γ, IL-2, and TNF-α. As shown in Fig. 3A, the profile of single, double, and triple cytokine (IFN-γ, IL-2, and TNF-α) production by the CD8+ T cells in the presence of CD4+ T cell help was virtually the same as that of CD4-depleted PBMC with exogenous IL-15. The distribution of IFN-γ, TNF-α, and IL-2 is equivalent among the groups, and the rank order of cytokine production is IFN-γ >TNF-α > IL-2 in all of the groups. Lack of a response precluded the measurement of this profile in PBMCs without help or IL-15, but at least we can conclude that the T cell quality induced when IL-15 is substituted for help is not different from that induced by Th cells. As perforin produced by CD8+ T cells could mediate apoptosis in target cells, its production was used to assess the cytotoxic capacity of human CD8+ T cells [19]. As shown in Fig. 3B, a high frequency of IFN-γ and perforin-producing CD8+ T cells was detected in the total PBMC and in CD4+ T cell-depleted PBMC given exogenous IL-15. These results further confirmed that IL-15 can substitute for CD4+ T cell help to stimulate recall CD8+ T cell responses, as the pattern of IFN-γ+Perforin+ cells in the different groups in Fig. 3B parallels that for IFN-γ responses in the donors shown in Fig. 2. The results also indicate that the ability to replace CD4+ T cell help applies to a variety of response measurements, not just IFN-γ production, and that IL-15 induces a similar quality of CD8+ response as does CD4+ T cell help.
Figure 3. IL-15 substitutes for CD4+ T cell help to stimulate high-quality recall CD8+ T cell responses.
(A) The intracellular cytokine staining of IFN-γ, IL-2, and TNF-α was measured in our FMP-pulsed MDDC coculture system. The frequency of each response based on all possible combinations of IFN-γ, IL-2, and TNF-α expression was created with Boolean gating using FlowJo software and plotted as a pie chart (1, □, single cytokine-positive; 2, shaded, double cytokine-positive; 3, ■, triple cytokine-positive). PMA- and ionomycin-stimulated PBMCs served as a positive control. The profile of cytokine production is similar among the groups PBMC + DC + FMP and CD4-depleted + DC+ FMP + IL-15. The data represent results of two independent experiments from two different healthy donors. (B) The intracellular cytokine staining for IFN-γ and perforin was measured in our FMP-pulsed DC coculture system. The cells in our coculture system were surface-stained for CD3 and CD8, followed by intracellular IFN-γ and perforin staining. Events shown were gated on CD3+CD8+ T cells. The double-positive staining of IFN-γ and perforin is 0.073 in the PBMC + DC cocultures; 12.2 in PBMC + DC + FMP cocultures; 3.08 in CD4-depleted + DC + FMP cocultures; and 16.8 in CD4-depleted + DC + FMP + IL-15 cocultures. These results suggested that IL-15 not only substitutes for CD4+ T cell help to recall the antigen-specific CD8+ T cell responses but also maintains their high quality. The data are a representative of three different donors.
IL-15 can overcome CD4+ Th cell deficiency to induce primary CD8+ T cell responses
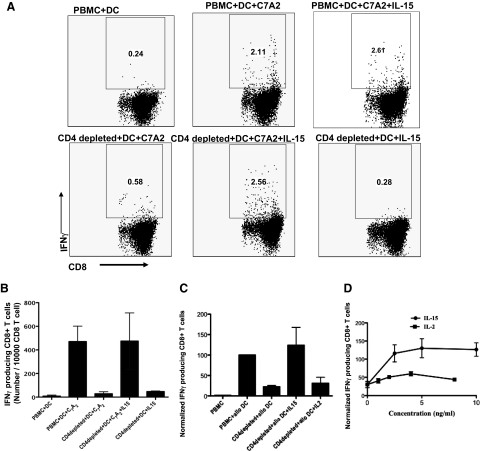
Our murine study indicated that IL-15 codelivered with vaccines could overcome CD4+ Th cell deficiency for induction of primary and secondary CD8+ T cell responses [10]. For vaccines, induction of primary immune responses by naïve T cells is most important. In the present study, the ability of IL-15 to replace CD4+ T cell help to stimulate primary responses in human CD8+ T lymphocytes was examined using allogeneic MDDCs and HCV peptide-pulsed, autologous MDDCs. As the normal blood bank donors were never exposed to HCV infection (based on blood bank screening), HLA-A2.1-restricted, HCV-specific peptide C7A2 (DLMGYIPLV) was considered as a new antigen to which the blood donors were naïve [12]. After the CD8+ T cell population was stimulated twice with HCV peptide-pulsed, autologous MDDCs, antigen-specific, primary CD8+ T cell responses were detectable (Fig. 4A and B). For the primary responses, purified, naïve CD8+ T cells were used to rule out responses by cross-reactive memory T cells (Supplemental Fig. 3). The naïve CD8+ T cells were isolated by using a naïve CD8+ T cell isolation kit from Miltenyi Biotec with purity >90% (data not shown). The antigen-specific, IFN-γ-producing CD8+ T cells were negligible in the coculture of CD4+-depleted PBMC with nonpeptide-pulsed DCs (pulsed with serum containing irrelevant peptides) in the presence of IL-15 (Fig. 4A). In parallel, as allogeneic, mature MDDC-primed CD8+ T cell responses could be detected with one stimulation, we also examined primary allogeneic responses (Fig. 4C). In experimental allogeneic and autologous HCV antigen models, antigen-specific CD8+ T cell responses were observed in the presence of CD4+ T cell help. In the absence of CD4 help, antigen-specific CD8+ T cell responses were reduced significantly but were fully restored by exogenous IL-15 (Fig. 4A–C). CD8+ T cell responses to autologous mature, unpulsed MDDCs were negligible and similar to those of PBMC alone. Of note, in the primary allogeneic response, in contrast to the recall response to FMP, IL-2 was much less effective than IL-15 over a range of titrated concentrations compared consistently with a more stringent requirement for help to induce primary responses of naïve T cells (Fig. 4D). These results indicated that IL-15, but not IL-2, could overcome CD4+ T cell helper deficiency to induce primary CD8+ T cell responses to alloantigen. To our knowledge, this is the first demonstration of the superiority of IL-15 over IL-2 in such primary in vitro human CD8+ T cell responses.
Figure 4. Cytokine substitution for CD4+ T cell help in primary CD8+ T cell responses.
(A and B) IL-15 overcomes CD4+ Th cell deficiency to induce primary CD8+ T cell responses to naïve antigen C7A2. (A) MDDCs were pulsed with HLA-A2.1-restricted HCV peptide (C7A2) at 10 μg/ml and Th epitope from tetanus toxoid at 4 μg/ml for 2 h at 37°C. After 7 days coculture of C7A2-pulsed MDDCs or unpulsed MDDC with PBMC, CD4+ T cell-depleted PBMC, or CD4+ T cell-depleted PBMC + IL-15, respectively, the cells were restimulated with MDDCs and cocultured for another 7 days. Intracellular IFN-γ staining was performed. (B) Number of IFN-γ-producing CD8+ T cells to naïve antigen C7A2/10,000 CD8+ T cells. Data represent means ± sem of three independent experiments from three different healthy donors. (C and D) IL-15, but not IL-2, overcomes CD4+ Th cell deficiency to induce primary CD8+ T cell responses to allogeneic antigen. (C) Allogeneic MDDCs were cocultured with intact PBMCs or CD4+ T cell-depleted PBMCs in the presence or absence of IL-15 (10 ng/ml) or IL-2 (20 U/ml=2 ng/ml) for 4 days. The cultures were restimulated overnight by the addition of freshly thawed allogeneic monocytes, followed by intracellular IFN-γ staining. Data represent means ± sem of three donors, each stimulated twice. Similar results of IL-15 were reproductive in three independent experiments. (D) Titration of IL-15 and IL-2 in overcoming CD4+ Th cell deficiency to induce primary CD8+ T cell responses to allogeneic antigen, performed as in C with different concentrations of IL-2 and IL-15 is indicated (two donors each repeated twice). To compare and combine donors in one plot, the data were normalized. For normalization, the IFN-γ-producing CD8 T cells in the group PBMC + alloDC, among the different donors, were considered as 100%. The number of the IFN-γ-producing CD8 T from different groups was normalized relative to the group of PBMC+ allo DC.
IL-15 enhanced CD8+ T cell responses from HIV-1-infected patients to allogeneic antigen
CD4+ Th cell deficiency often accompanies chronic viral infections and is a hallmark of HIV-1-infected individuals. CD4+ T cell help is necessary to maintain effective CD8+ T cell responses to HIV-1 [5–7]. To address whether the CD4+ Th cell deficiency could be compensated by IL-15, we examined six HIV-1-infected individuals who were being treated with effective cART and who had plasma HIV-1 RNA levels below 50 copies/ml. Nevertheless, most of these patients had low CD4+ T cell counts (Table 1). We compared these with 10 healthy controls.
Table 1. CD4+ T Cell Counts from HIV-Infected Individuals and Their CD8+ T Cell Responses to Alloantigen.
| Patient | Number of positive IFN-γ-producing cells/104 CD8+ T cells |
CD4+ T cell count/μl | Fold increased with IL-15 | |
|---|---|---|---|---|
| PBMC + DC | PBMC + DC + IL-15 | |||
| 1 | 444 | 764 | 233 | 1.7 |
| 2 | 320 | 727 | 189 | 2.3 |
| 3 | 302 | 673 | 274 | 2.2 |
| 4 | 307 | 1065 | 144 | 3.5 |
| 5 | 450 | 700 | 899 | 1.6 |
| 6 | 950 | 1290 | 363 | 1.4 |
| Healthy | ||||
| 7 | 1280 | 1980 | 899 | 1.5 |
| 8 | 1140 | 1700 | 860 | 1.5 |
| 9 | 701 | 680 | 990 | 0.97 |
| 10 | 431 | 513 | 998 | 1.2 |
| 11 | 1660 | 1640 | 800 | 0.99 |
| 12 | 623 | 600 | 920 | 0.96 |
| 13 | 636 | 1070 | 940 | 1.68 |
| 14 | 996 | 1140 | 980 | 1.1 |
| 15 | 453 | 1190 | 906 | 2.6 |
| 16 | 778 | 1090 | 1000 | 1.4 |
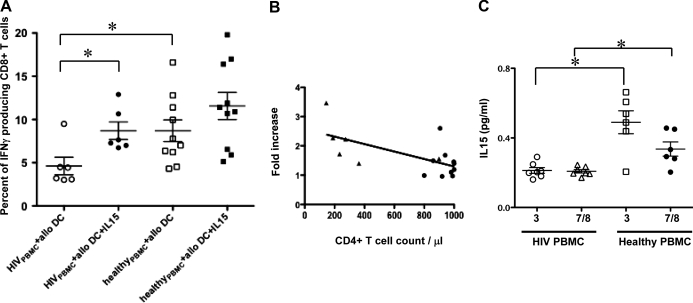
As shown in Fig. 5A, CD8+ T cell responses of PBMCs from HIV-1-infected individuals to alloantigen were significantly lower than those of PBMCs from healthy donors (P<0.05). Importantly, when IL-15 was added, CD8+ T cell responses to alloantigen increased to the level of healthy donor responses in every case (P<0.05). By contrast, the enhancement of CD8+ T cell responses to allogeneic MDDCs in PBMCs of healthy donors (without CD4 deficiency) by IL-15 was not significant. Among the six patients examined, the four patients who had CD4+ T cell counts below 300 had 1.7- to 3.5-fold increases of CD8+ T responses with addition of IL-15 not seen in those with higher CD4 counts or normal donors (Table 1). When we analyzed the relationship between the fold increase of CD8+ T responses and CD4+ T cell counts in HIV-1-infected individuals and healthy donors, we found that the fold increase of CD8+ T responses in the presence of IL-15 was inversely correlated with the CD4+ T cell counts (Fig. 5B). These findings suggested that IL-15 could enhance primary CD8+ T cell responses from HIV-1-infected individuals to alloantigen, especially when responses were diminished as a result of a deficiency in CD4+ T cell help.
Figure 5. IL-15 enhances CD8+ T cell responses to allogeneic MDDCs in HIV-1-infected individuals.
(A) The CD8+ T cell responses 6 of HIV-1-infected patients to allogeneic MDDCs were evaluated in our coculture system in the presence or absence of IL-15 as compared with the 10 healthy donors. Significantly lower CD8+ T cell responses were observed in HIVPBMC + allo DC as compared with healthy donors. The responses of HIV PBMC could be fully restored by addition of IL-15 (*P<0.05). (B) The fold increase of CD8+ T responses with or without IL-15 was correlated with the CD4+ T cell counts. Each dot represents data from a single donor from HIV-1-infected patients (▴) and healthy donors (●). The diagonal line represents the linear regression line for the effect of CD4+ T cell counts on the respective CD8+ T responses (R2=0.4; P<0.05). (C) IL-15 levels in the culture supernatants of PBMC. PBMCs from HIV-1-infected patients and healthy donors were stimulated in vitro for 36 h with TLR3 (indicated as 3) and TLR7/8 ligands (indicated as 7/8), and IL-15 in the culture supernatants was measured by using a commercial ELISA kit. *P < 0.05.
Interestingly, a previous study showed that IL-15 levels are decreased significantly in the sera of HIV-infected/AIDS patients compared with healthy control sera [20]. In agreement with this previous study, upon stimulation by the TLR3 ligand and TLR7/8 ligands, significantly less up-regulation of IL-15 levels in the culture supernatants of PBMC from HIV-1-infected patients compared with healthy donors was observed in our present study (Fig. 5C). Taken together with the results above, these data suggested that IL-15 administration might help to restore the immune function of HIV-1-infected patients, especially when delivered under the cover of cART to prevent increases in viral load and when given locally in conjunction with a vaccine to act at the same draining LNs as the vaccine rather than delivered systemically.
DISCUSSION
Antigen-specific CD8+ T cells have a central role in the host response to viral infections and cancers. Generation of effective CTL responses to new antigens is the goal of many vaccination protocols. The generation of long-lived, functional memory CD8+ T cells requires CD4+ T cell help [2, 3, 21, 22]. CD4+ T cell-dependent priming of the CTL responses requires the presence of APCs, often DCs. CD4+ T cells first ″condition″ the DCs, which then become empowered to stimulate the response of naïve CD8+ T cells [23–25]. CD4+ T cell helper deficiency could be found in cancer patients, some chemotherapy patients, and those with chronic viral infection, especially HIV infection [5, 6, 26, 27]. Discovering approaches to overcome CD4+ T cell helper deficiency is helpful in developing effective vaccines and optimal treatment of these diseases. Numerous studies have shown that CD40 agonists could replace CD4+ T cell help to elicit CD8+ T cell response [18, 23, 24].
In our present study, an alternative substitute for CD4 help, using IL-15 in induction of CD8+ T cell responses, was investigated. IL-15, as a vaccine adjuvant in murine models, has been shown to enhance CTL responses and compensate for lack of CD4+ T cells, even in the face of CD4+ T cell deficiency [10]. When IL-15 was used in combination with TLR ligands as adjuvant in nonhuman primates, enhanced antiviral polyfunctional CTL responses were observed in the immunized group [28].
In agreement with our study in murine models [10], here, we found that IL-15 could replace CD4+ T cell help for promoting primary (HCV core antigen and alloantigen) and recall (influenza matrix epitope) antigen-specific CD8+ T cell responses of humans. In other murine studies, IL-15 was also found to enable the induction of antigen-specific CD8+ T cells and humoral immunity, independently of CD4+ T cells [11, 18, 29, 30]. Whereas previous studies were all in experimental animals, which do not always translate to humans, here, we found that IL-15 could replace CD4+ T cell help for inducing the primary and recall antigen-specific CD8+ T cell responses in vitro in human cell cultures. Moreover, in a human disease characterized by deficient CD4+ T cell help, namely HIV infection, IL-15 could compensate for reduced help to induce primary T cell responses in those individuals with low CD4 counts, whereas it was not as necessary and thus, has less impact in those with higher CD4 counts or in healthy blood donors with normal levels of help.
IL-2 and IL-15, as members of the common γc cytokine family, share many functional activities, such as stimulating the proliferation of T cells and NK cells and facilitating the induction of CTLs [31–34]. In addition to these similarities, these cytokines have distinct functions [9]. IL-2 is required to eliminate self-reactive T cells and avoid autoimmunity, whereas IL-15 is dedicated to the prolonged maintenance of CTL responses. In the present study, we found that IL-15, more effectively than IL-2 over a range of concentrations, could substitute for CD4+ T cell help to stimulate primary CD8+ T cell responses, although IL-2 was nearly as effective in recall responses. The difference is consistent with other more stringent requirements for primary rather than secondary responses. For vaccines to induce responses to new antigens, primary responses are the main goal. These results also suggest that IL-15 may be more important in the initial priming dose of vaccine than in subsequent booster doses, as will need to be tested in vivo in clinical trials.
When IL-15 replaces CD4+ T cell help, the profile of antigen-specific CD8+ T cell response is similar to that in the presence of CD4+ T cell help (Fig. 3). As shown in this study, IL-15 substitutes for CD4+ T cell help to recall a similar quality of antigen-specific CD8+ T cell responses in producing multiple cytokines and effector molecules such as IL-2, TNF-α, IFN-γ, and perforin. Such polyfunctional CD8+ T cell responses have been found to correlate with protection [5].
In addition to IL-15 and IL-2, another γc-sharing cytokine, IL-21, plays a crucial role in inducing potent cytotoxic function and promotes antiviral activity in human CD8+ T cells [35]. It is interesting to mention that the synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function was observed [36]. It suggested that these γc-sharing cytokines not only share many functional activities but also manifest their own distinct functions.
CTL responses are critical to control HIV-1 infection and slow disease progression [37–41]. Loss of CTL activity and AIDS progression is associated with declining CD4+ T cell numbers and function [21]. When we cocultured the PBMC with allogeneic MDDCs (labeled allo DC), significantly lower CD8+ T cell responses were observed from HIV-infected patients as compared with those of healthy donors. Importantly, addition of IL-15 to allogeneic DC and PBMC cocultures from HIV-1-infected individuals with low CD4+ T cell counts significantly enhanced CD8+ T cell responses to alloantigen in every case (Fig. 5). IL-15 has been shown to enhance the survival and function of murine HIV and tumor-specific CD8+ T cells [11, 42–44]. However, in contrast to these previous studies, our study examined the primary human CD8+ T cell response to new antigens, to which the person was naïve, and the ability to substitute for CD4+ T cell help. Moreover, the same was true when highly enriched, naïve CD8+ T cells were cultured with an antigen not previously encountered, demonstrating that the response is by truly naïve T cells and not T cells primed by some cross-reactive antigen (Supplemental Fig. 3).
The possible mechanism by which IL-15 substitutes for CD4+ T cell help might be related to prevention of TRAIL-mediated apoptosis [10, 45, 46]. More importantly, the interaction between CD40 on DC and CD40 ligand on CD4+ T cells induces IL-15 production [10, 18], which is important for CD4+ T cell function in the provision of help for CD8+ T cells in mice [10, 24, 25], so it is likely that IL-15 is one of the important mediators of T cell help for CD8+ T cells in humans as well, accounting for its ability to replace CD4+ T cell help in our studies.
A recent study showed that IL-15 treatment during acute SIV infection increased viral setpoint and accelerated the development of simian AIDS [47, 48]. IL-15 has been reported to be increased during acute HIV infection. Peak viremia is accompanied by immune activation and induces an intense proinflammatory cytokine production [49]. In another study, systemic therapy with IL-15 induced massive CD4+ T cell proliferation and countered the protective effect of a vaccine on setpoint viral load [50]. On the other hand, in a prophylactic vaccine when coinoculated with IL-15, SIV/CMVδ vif proviral plasmids showed significantly improved SIV-specific CD8+ T cell immunity characterized by increased IFN-γ ELISPOT and polyfunctional CD8+ T cell responses. Furthermore, these animals demonstrated a sustained suppression of plasma virus loads after multiple low-dose vaginal challenges with pathogenic SIVmac251 [51]. Even in a therapeutic setting, whether IL-15 affects viral load may depend on whether IL-15 is used as a systemic therapy and affecting CD4+ T cells globally or whether it is delivered to act locally on the immune response to a vaccine. Also, the timing may be critical, as early during acute infection, the immune response to the virus may not have been established yet. Moreover, earlier studies observed that IL-15 played a role in anti-HIV responses by stimulating CD8+ T cells and NK cells [52]. Moreover, IL-15 levels are decreased significantly in the sera of HIV-infected/AIDS patients, as well as on the stimulation of TLR ligands compared with healthy sera. An increased level of IL-15 was detected in response to effective cART [53]. Interestingly, high concentrations of IL- 15 in breast milk are associated with protection against postnatal HIV transmission [54]. The patients whose blood we studied were all on cART and thus, had suppressed viral loads. However, cART does not fully restore immune function, so IL-15 may provide help. For HIV patients on cART, the cART should prevent IL-15 from stimulating virus replication. So the combination may be beneficial without deleterious effects on viral load.
In conclusion, IL-15 can overcome CD4+ Th cell deficiency to induce recall and primary high-quality human CD8+ T cell responses ex vivo. Moreover, IL-15 enhances primary CD8+ T cell responses from HIV-1-infected individuals to alloantigen, especially in those for whom endogenous CD4+ T cell help is deficient. These findings suggest that IL-15 may prove useful in developing therapeutic and prophylactic vaccines for patients defective in CD4+ T cell help to enhance CD8+ T cell responses against cancers and viral infections.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the Center for Cancer Research, NCI, NIH. We are grateful to Dr. Thomas A. Waldmann (Metabolism Branch, NCI, NIH) and Dr. Giorgio Trinchieri (Laboratory of Experimental Immunology, NCI, NIH) for critical review of this manuscript and helpful discussion.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- γc
- commonγ chain
- cART
- combination antiretroviral therapy
- DTM
- Department of Transfusion Medicine
- FMP
- influenza (flu) matrix peptide
- HCV
- hepatitis C virus
- imDC
- immature DC
- mDC
- mature DC
- MDDC
- monocyte-derived dendritic cell
AUTHORSHIP
H.Y. designed and performed research, analyzed data, and wrote the paper; J.A.B, Y.S., M.T., and R.Y. designed research, analyzed data, and wrote the paper; A.T-A. and A.D. designed and performed research and analyzed data; and M.S. and K.A. performed research and analyzed data.
DISCLOSURE
The authors declare no competing financial interests.
REFERENCES
- 1. Kaech S. M., Wherry E. J., Ahmed R. (2002) Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2, 251–262 [DOI] [PubMed] [Google Scholar]
- 2. Shedlock D. J., Shen H. (2003) Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300, 337–339 [DOI] [PubMed] [Google Scholar]
- 3. Sun J. C., Bevan M. J. (2003) Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300, 339–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Janssen E. M., Lemmens E. E., Wolfe T., Christen U., von Herrath M. G., Schoenberger S. P. (2003) CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421, 852–856 [DOI] [PubMed] [Google Scholar]
- 5. Betts M. R., Nason M. C., West S. M., De Rosa S. C., Migueles S. A., Abraham J., Lederman M. M., Benito J. M., Goepfert P. A., Connors M., Roederer M., Koup R. A. (2006) HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107, 4781–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Altfeld M., Rosenberg E. S. (2000) The role of CD4(+) T helper cells in the cytotoxic T lymphocyte response to HIV-1. Curr. Opin. Immunol. 12, 375–380 [DOI] [PubMed] [Google Scholar]
- 7. Rosenberg E. S., Billingsley J. M., Caliendo A. M., Boswell S. L., Sax P. E., Kalams S. A., Walker B. D. (1997) Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278, 1447–1450 [DOI] [PubMed] [Google Scholar]
- 8. Rochman Y., Spolski R., Leonard W. J. (2009) New insights into the regulation of T cells by γ(c) family cytokines. Nat. Rev. Immunol. 9, 480–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Waldmann T. A. (2006) The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 6, 595–601 [DOI] [PubMed] [Google Scholar]
- 10. Oh S., Perera L. P., Terabe M., Ni L., Waldmann T. A., Berzofsky J. A. (2008) IL-15 as a mediator of CD4+ help for CD8+ T cell longevity and avoidance of TRAIL-mediated apoptosis. Proc. Natl. Acad. Sci. USA 105, 5201–5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kutzler M. A., Robinson T. M., Chattergoon M. A., Choo D. K., Choo A. Y., Choe P. Y., Ramanathan M. P., Parkinson R., Kudchodkar S., Tamura Y., Sidhu M., Roopchand V., Kim J. J., Pavlakis G. N., Felber B. K., Waldmann T. A., Boyer J. D., Weiner D. B. (2005) Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially independent of CD4 T cell help. J. Immunol. 175, 112–123 [DOI] [PubMed] [Google Scholar]
- 12. Shirai M., Okada H., Nishioka M., Akatsuka T., Wychowski C., Houghten R., Pendleton C. D., Feinstone S. M., Berzofsky J. A. (1994) An epitope in hepatitis C virus core region recognized by cytotoxic T cells in mice and humans. J. Virol. 68, 3334–3342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gotch F., Rothbard J., Howland K., Townsend A. R. M., McMichael A. J. (1987) Cytotoxic T lymphocytes recognize a fragment of influenza virus matrix protein in association with HLA-A2. Nature 326, 881–882 [DOI] [PubMed] [Google Scholar]
- 14. Tawab A., Fan Y., Read E. J., Kurlander R. J. (2009) Effect of ex vivo culture duration on phenotype and cytokine production by mature dendritic cells derived from peripheral blood monocytes. Transfusion 49, 536–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dubois S. P., Waldmann T. A., Muller J. R. (2005) Survival adjustment of mature dendritic cells by IL-15. Proc. Natl. Acad. Sci. USA 102, 8662–8667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rinaldo C. R. (2009) Dendritic cell-based human immunodeficiency virus vaccine. J. Intern. Med. 265, 138–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lapteva N., Seethammagari M. R., Hanks B. A., Jiang J., Levitt J. M., Slawin K. M., Spencer D. M. (2007) Enhanced activation of human dendritic cells by inducible CD40 and Toll-like receptor-4 ligation. Cancer Res. 67, 10528–10537 [DOI] [PubMed] [Google Scholar]
- 18. Ostrowski M. A., Justement S. J., Ehler L., Mizell S. B., Lui S., Mican J., Walker B. D., Thomas E. K., Seder R., Fauci A. S. (2000) The role of CD4(+) T cell help and CD40 ligand in the in vitro expansion of HIV-1-specific memory cytotoxic CD8(+) T cell responses. J. Immunol. 165, 6133–6141 [DOI] [PubMed] [Google Scholar]
- 19. Hersperger A. R., Makedonas G., Betts M. R. (2008) Flow cytometric detection of perforin upregulation in human CD8 T cells. Cytometry A 73, 1050–1057 [DOI] [PubMed] [Google Scholar]
- 20. Ahmad R., Sindhu S. T., Toma E., Morisset R., Ahmad A. (2003) Studies on the production of IL-15 in HIV-infected/AIDS patients. J. Clin. Immunol. 23, 81–90 [DOI] [PubMed] [Google Scholar]
- 21. Kalams S. A., Walker B. D. (1998) The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J. Exp. Med. 188, 2199–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bevan M. J. (2004) Helping the CD8(+) T-cell response. Nat. Rev. Immunol. 4, 595–602 [DOI] [PubMed] [Google Scholar]
- 23. Ridge J. P., Di Rosa F., Matzinger P. (1998) A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393, 474–478 [DOI] [PubMed] [Google Scholar]
- 24. Bennett S. R. M., Carbone F. R., Karamalis F., Flavell R. A., Miller J. F. A. P., Heath W. R. (1998) Help for cytotoxic-T-cell responses is mediated by CD40 signaling. Nature 393, 478–480 [DOI] [PubMed] [Google Scholar]
- 25. Schoenberger S. P., Toes R. E. M., van der Voort E. I. H., Offringa R., Melief C. J. M. (1998) T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 393, 480–483 [DOI] [PubMed] [Google Scholar]
- 26. Clerici M., Stocks N. I., Zajac R. A., Boswell R. N., Lucey D. R., Via C. S., Shearer G. M. (1989) Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients: independence of CD4 cell numbers and clinical staging. J. Clin. Invest. 84, 1892–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hung K., Hayashi R., Lafond-Walker A., Lowenstein C., Pardoll D., Levitsky H. (1998) The central role of CD4(+) T cells in the antitumor immune response. J. Exp. Med. 188, 2357–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sui Y., Zhu Q., Gagnon S., Dzutsev A., Terabe M., Vaccari M., Venzon D., Klinman D., Strober W., Kelsall B., Franchini G., Belyakov I. M., Berzofsky J. A. (2010) Innate and adaptive immune correlates of vaccine and adjuvant-induced control of mucosal transmission of SIV in macaques. Proc. Natl. Acad. Sci. USA 107, 9843–9848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Assudani D., Cho H. I., DeVito N., Bradley N., Celis E. (2008) In vivo expansion, persistence, and function of peptide vaccine-induced CD8 T cells occur independently of CD4 T cells. Cancer Res. 68, 9892–9899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Steel J. C., Ramlogan C. A., Yu P., Sakai Y., Forni G., Waldmann T. A., Morris J. C. (2010) Interleukin-15 and its receptor augment dendritic cell vaccination against the neu oncogene through the induction of antibodies partially independent of CD4 help. Cancer Res. 70, 1072–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Waldmann T. A., Dubois S., Tagaya Y. (2001) Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity 14, 105–110 [PubMed] [Google Scholar]
- 32. Waldmann T. A., Tagaya Y. (1999) The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu. Rev. Immunol. 17, 19–49 [DOI] [PubMed] [Google Scholar]
- 33. Fehniger T. A., Cooper M. A., Caligiuri M. A. (2002) Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine Growth Factor Rev. 13, 169–183 [DOI] [PubMed] [Google Scholar]
- 34. Fehniger T. A., Caligiuri M. A. (2001) Interleukin 15: biology and relevance to human disease. Blood 97, 14–32 [DOI] [PubMed] [Google Scholar]
- 35. Parmigiani A., Pallin M., Schmidtmayerova H., Lichtenheld M., Pahwa S. (2011) Interleukin-21 and cellular activation concurrently induce potent cytotoxic function and promote antivrial activity in human CD8 T cells. Hum. Immunol. 72, 115–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zeng R., Spolski R., Finkelstein S. E., Oh S., Kovanen P. E., Hinrichs C. S., Pise-Masison C. A., Radonovich M. F., Brady J. N., Restifo N. P., Berzofsky J. A., Leonard W. J. (2005) Synergy of IL-21 and IL-15 in regulating CD8+ T-cell expansion and function. J. Exp. Med. 201, 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cao H., Kanki P., Sankale J. L., Dieng-Sarr A., Mazzara G. P., Kalams S. A., Korber B., Mboup S., Walker B. D. (1997) Cytotoxic T-lymphocyte cross-reactivity among different human immunodeficiency virus type 1 clades: implications for vaccine development. J. Virol. 71, 8615–8623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koup R. A., Ho D. D. (1994) Shutting down HIV. Nature 370, 416 [DOI] [PubMed] [Google Scholar]
- 39. Rinaldo C., Huang X. L., Fan Z. F., Ding M., Beltz L., Logar A., Panicali D., Mazzara G., Liebmann J., Cottrill M., et al. (1995) High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J. Virol. 69, 5838–5842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schmitz J. E., Kuroda M. J., Santra S., Sasseville V. G., Simon M. A., Lifton M. A., Racz P., Tenner-Racz K., Dalesandro M., Scallon B. J., Ghrayeb J., Forman M. A., Montefiori D. C., Rieber E. P., Letvin N. L., Reimann K. A. (1999) Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283, 857–860 [DOI] [PubMed] [Google Scholar]
- 41. Jin X., Bauer D. E., Tuttleton S. E., Lewin S., Gettie A., Blanchard J., Irwin C. E., Safrit J. T., Mittler J., Weinberger L., Kostrikis L. G., Zhang L., Perelson A. S., Ho D. D. (1999) Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189, 991–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kanai T., Thomas E. K., Yasutomi Y., Letvin N. L. (1996) IL-15 stimulates the expansion of AIDS virus-specific CTL1. J. Immunol. 157, 3681–3687 [PubMed] [Google Scholar]
- 43. Mueller Y. M., Bojczuk P. M., Halstead E. S., Kim A. H., Witek J., Altman J. D., Katsikis P. D. (2003) IL-15 enhances survival and function of HIV-specific CD8+ T cells. Blood 101, 1024–1029 [DOI] [PubMed] [Google Scholar]
- 44. Klebanoff C. A., Finkelstein S. E., Surman D. R., Lichtman M. K., Gattinoni L., Theoret M. R., Grewal N., Spiess P. J., Antony P. A., Palmer D. C., Tagaya Y., Rosenberg S. A., Waldmann T. A., Restifo N. P. (2004) IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc. Natl. Acad. Sci. USA 101, 1969–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Janssen E. M., Droin N. M., Lemmens E. E., Pinkoski M. J., Bensinger S. J., Ehst B. D., Griffith T. S., Green D. R., Schoenberger S. P. (2005) CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature 434, 88–93 [DOI] [PubMed] [Google Scholar]
- 46. Bulfone-PauS S., Bulanova E., Pohl T., Budagian V., Durkop H., Ruckert R., Kunzendorf U., Paus R., Krause H. (1999) Death deflected: IL-15 inhibits TNF-α-mediated apoptosis in fibroblasts by TRAF2 recruitment to the IL-15Rα chain. FASEB J. 13, 1575–1585 [DOI] [PubMed] [Google Scholar]
- 47. Mueller Y. M., Do D. H., Altork S. R., Artlett C. M., Gracely E. J., Katsetos C. D., Legido A., Villinger F., Altman J. D., Brown C. R., Lewis M. G., Katsikis P. D. (2008) IL-15 treatment during acute simian immunodeficiency virus (SIV) infection increases viral set point and accelerates disease progression despite the induction of stronger SIV-specific CD8+ T cell responses. J. Immunol. 180, 350–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Al-Harthi L., Roebuck K. A., Landay A. (1998) Induction of HIV-1 replication by type 1-like cytokines, interleukin (IL)-12 and IL-15: effect on viral transcriptional activation, cellular proliferation, and endogenous cytokine production. J. Clin. Immunol. 18, 124–131 [DOI] [PubMed] [Google Scholar]
- 49. Roberts L., Passmore J., Williamson C., Little F., Babell L., Mlisana K., Burgers W., van Loggerenberg F., Walzl G., Djoba Siawaya J., Karim Q., Karim S. (2010) Plasma cytokine levels during acute HIV-1 infection predict HIV disease progression. AIDS 24, 819–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hryniewicz A., Price D. A., Moniuszko M., Boasso A., Edghill-Spano Y., West S. M., Venzon D., Vaccari M., Tsai W. P., Tryniszewska E., Nacsa J., Villinger F., Ansari A. A., Trindade C. J., Morre M., Brooks D., Arlen P., Brown H. J., Kitchen C. M., Zack J. A., Douek D. C., Shearer G. M., Lewis M. G., Koup R. A., Franchini G. (2007) Interleukin-15 but not interleukin-7 abrogates vaccine-induced decrease in virus level in simian immunodeficiency virus mac251-infected macaques. J. Immunol. 178, 3492–3504 [DOI] [PubMed] [Google Scholar]
- 51. Dubie R. A., Maksaereekul S., Shacklett B. L., Lemongello D., Cole K. S., Villinger F., Blozis S. A., Luciw P. A., Sparger E. E. (2009) Co-immunization with IL-15 enhances cellular immune responses induced by a vif-deleted simian immunodeficiency virus proviral DNA vaccine and confers partial protection against vaginal challenge with SIVmac251. Virology 386, 109–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mastroianni C. M., d′Ettorre G., Forcina G., Vullo V. (2004) Teaching tired T cells to fight HIV: time to test IL-15 for immunotherapy? Trends Immunol. 25, 121–125 [DOI] [PubMed] [Google Scholar]
- 53. Forcina G., D′Ettorre G., Mastroianni C. M., Carnevalini M., Scorzolini L., Ceccarelli G., D′Agostino C., Lichtner M., Massetti A. P., Vullo V. (2004) Interleukin-15 modulates interferon-γ and β-chemokine production in patients with HIV infection: implications for immune-based therapy. Cytokine 25, 283–290 [DOI] [PubMed] [Google Scholar]
- 54. Walter J., Ghosh M. K., Kuhn L., Semrau K., Sinkala M., Kankasa C., Thea D. M., Aldrovandi G. M. (2009) High concentrations of interleukin 15 in breast milk are associated with protection against postnatal HIV transmission. J. Infect. Dis. 200, 1498–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.