Abstract
The long-held concept that rabies infection is lethal in humans once the causative rabies virus has reached the CNS has been called into question by the recent survival of a number of patients with clinical rabies. Studies in animal models provide insight into why survival from a rabies virus infection that has spread to the CNS is possible and the immune mechanisms involved. In the CNS, both innate mechanisms capable of inhibiting virus replication and the activity of infiltrating rabies virus-specific T and B cells with the capacity to clear the virus are required. Deficiencies in the induction of either aspect of rabies immunity can lead to lethal consequences but may be overcome by novel approaches to active and passive immunization.
Keywords: neuroimmunity, rabies, rabies virus, vaccination, virus clearance
Highly lethal for humans in the absence of treatment, rabies viruses (RABV) are endemic in wildlife as a variety of antigenically related but phenotypically distinct variants, each associated with a particular reservoir species [1]. A disease of antiquity, rabies has persisted owing to the inability to eliminate the reservoirs of the virus that exist in such diverse animals as dogs, foxes, raccoons, skunks and bats. With appropriate organizational commitment and resources, terrestrial reservoirs of RABV can be controlled by vaccination. However, although there have been regional successes in the elimination of dog rabies, more than 50,000 people die worldwide each year after being infected with this RABV variant. In addition, prospects for the elimination of RABV reservoirs in bats are limited and these viruses have emerged as the leading cause of human rabies in certain geographical areas. Once patients have developed clinical rabies there is currently no treatment available owing to features of the infection that allow wild-type RABV to evade immune clearance from the CNS. As discussed in this article, better understanding of how laboratory-attenuated RABV are cleared from CNS tissues in animal models has led us to develop a strategy for the immune clearance of wild-type RABV from the CNS that may help expand the postexposure treatment window for human RABV infection.
Rationale for the development of new treatment strategies for human rabies
The paradigm that is still used for treatment of an individual ‘exposed’ to RABV was pioneered in 1885 by Louis Pasteur who used a vaccine being developed in collaboration with Emile Roux to vaccinate Joseph Meister, a 9-year-old boy who had been severely bitten in an attack by a rabid dog less than 3 days before [2]. The long incubation period of rabies was well known and Pasteur theorized that there was some time before the nervous system would become involved when vaccination could be effective. Meister was administered a series of preparations of spinal cord from rabbits that had died of rabies, beginning with tissues that had been desiccated for 15 days and ending, on the thirteenth inoculation, with virulent RABV. While the concept that postexposure rabies vaccination works by preventing central nervous system involvement cannot be formally proven in humans, it has been the theoretical basis of rabies postexposure prophylaxis (PEP) for over a century. Despite improved rabies virus vaccines and the addition of rabies virus neutralizing antibodies (VNA) to the PEP protocol, successful treatment remains limited to the first days after infection. Clearly, PEP with a conventional killed RABV vaccine and VNA is unsuccessful after clinical signs of rabies have developed, which supports the likelihood that the RABV-specific immune response elicited by this type of vaccine works by preventing spread of the virus to the CNS.
Most human rabies cases are in areas where dog rabies remains endemic and are a consequence of the fact that, in these areas, financial and logistical constraints in obtaining PEP outweigh the risk of developing rabies. Where dog rabies is controlled, RABV of bat origin have emerged as the leading causative agents of human rabies. Unlike the more obvious exposures to rabies virus via a wound caused by a dog or other large animal, contact with a bat that results in rabies virus infection often goes unnoticed and bat RABV are more commonly associated with human rabies cases without a recognized exposure than variants carried by terrestrial animals [3,4]. The in vitro growth characteristics of the silver-haired bat RABV (SHBRV), often associated with human rabies, suggest that these variants may more efficiently spread from peripheral sites of exposure than other RABV types [5]. Moreover, cases of human rabies associated with cave exploration support the likelihood that bat RABV may be infectious via the aerosol route [6]. Thus, there are two classes of patients who develop rabies: those with known exposure who know that they may have been infected with RABV but fail to receive prompt PEP due to choice or circumstance, and those who did not recognize contact with the virus. These people would clearly benefit from a treatment that is effective after the RABV has reached CNS tissues and early signs of the disease have appeared. The possibility that this can be accomplished is supported by work in animal models and by the survival of a handful of patients from clinical rabies regardless of whether or not PEP was administered. It should be noted here that while rabies encephalitis was naturally controlled in these patients, only two have recovered to regain independent lives, indicating that more advanced intervention strategies are a general requirement.
PEP & the immune response to natural rabies infection
Prospects for individuals infected with RABV who do not receive PEP prior to the development of the early, largely nonspecific signs of the disease are dismal. Natural RABV infections only rarely induce a protective immune response in humans [7] and, as noted above, PEP is ineffective after clinical signs of the disease have appeared. No survivors of infection with dog-associated RABV have been reported and, in the absence of PEP, only one individual has regained independent function after confirmed infection with bat-associated RABV [7,8]. However, the exceptional survivors of clinical rabies have proven that at least certain RABV can be cleared from the human nervous system, an outcome that has been well established in animal models for both bat- and dog-derived RABV [9,10]. To understand why the occasional person survives rabies while the vast majority of people with rabies die, regardless of whether or not they receive PEP, we need to examine the nature of the host–virus relationship and how this may be impacted by PEP. While it is expected from studies in animal models that both the virus type and host genotype would contribute to rabies pathogenesis, there is understandably little data to confirm this likelihood in humans. Regardless of whether the infecting virus is dog or bat-derived, rabies victims do not exhibit evidence of the natural development of antiviral immunity until relatively late in the course of the disease [11]. Thus, the active vaccination component of PEP is likely to be essential in promoting the development of protective RABV immunity. Rabies VNA are known to be required for clearance of the virus, and passively administered VNA should inactivate the virus in the periphery, limiting its entry into nerve endings. However, once the virus has reached CNS tissues, circulating rabies VNA should have a lesser effect due to their limited ability to cross the blood–brain barrier (BBB), a specialization of the neurovasculature that normally protects the vulnerable tissues of the CNS from potentially toxic cells and factors in the circulation. Antibody leakage across the choroid plexus would be insufficient to impact virus replication in CNS tissues, and greater amounts of antibody or VNA-producing B cells can only reach CNS tissues if the BBB becomes functionally altered or compromised. Active vaccination with a killed vaccine will elicit RABV-specific CD4 T cells, B cells and antibodies but is unlikely to induce all of the processes necessary for the clearance of the virus from infected CNS tissues [10]. Virus clearance from the CNS is not simply the result of activating the appropriate RABV-specific immune cells in peripheral lymphoid organs and inducing them to enter the circulation, as additional mechanisms initiated by CNS-resident cells are required to allow circulating immune cells to infiltrate CNS tissues [12]. Since peripheral vaccination with an inactivated RABV vaccine would not be expected to induce an innate response by CNS-resident cells, innate immunity in CNS tissues must be a consequence of the natural infection. While little is known about CNS innate immunity to RABV in humans, work in animal models suggests that infection with highly pathogenic RABV variants triggers an innate response in CNS tissues. However, there may be a deficit in this response, as immune cell infiltration into CNS tissues does not occur [9,10,12,13]. Consequently, the natural or PEP-induced development of RABV-specific immunity in an exposed individual is unlikely to lead to the clearance of wild-type RABV from the CNS. Thus, if the immune response to RABV develops too late to prevent the virus from reaching CNS tissues, the outcome is likely to be poor. Rarely, an occasional rabies patient mediates an appropriate response to the natural infection, clears the virus from the CNS and survives rabies [7]. However, either due to the failure to develop a RABV-specific immune response or deliver immune effectors into the infected CNS, naturally acquired rabies infection in humans is generally lethal in the absence of prompt PEP.
Immune clearance of attenuated RABV from the CNS
There is a long history of the use of laboratory-attenuated RABV variants for animal immunization and for studies of rabies immunology [14,15]. In normal mice, many of these attenuated RABV can spread to the CNS tissues from a peripheral site of inoculation but are cleared by the developing immune response in the absence of overt clinical signs of disease [16]. A transient weight loss is often the only evidence of the infection [16]. To clear RABV from the CNS, immune effectors must cross the BBB. The process of T-cell extravasation across the BBB into CNS tissues is generally studied in the context of an aggressive CNS inflammatory response, often using experimental allergic encephalomyelitis (EAE), an animal model of multiple sclerosis. EAE is associated with extensive BBB permeability that permits molecules of 150 kDa to leak from the circulation into CNS tissues, T cell and NOS-2-positive macrophage accumulation in the brain parenchyma and significant CNS pathology [17]. Lesions containing NOS-2-positive macrophages and nitrotyrosine, the footprint of peroxynitrite-dependent radical activity, are common to both spinal cord and brain tissues [17–19]. The fact that BBB integrity can be maintained by raising serum levels of peroxynitrite-dependent radical scavenger urate has led us to speculate that these radicals are involved in altering BBB function in EAE and in CNS inflammation caused by Borna disease virus infection [20]. In both situations, NOS-2-positive macrophages infiltrate CNS tissues and produce highly neurotoxic peroxynitrite-dependent radicals in the brain parenchyma [18,19,21]. This is very distinct from the nature of the limited immune cell accumulation associated with the clearance of attenuated RABV from CNS tissues. Attenuated RABV such as CVS-F3, which is a variant of the dog-derived challenge virus standard (CVS) RABV attenuated via the conversion of amino acid 333 of the glycoprotein from Arg to Glu [22], often spread to the CNS tissues of mice from a peripheral site of inoculation but are cleared without overt signs of disease apart from a transient reduction in bodyweight [16]. CD4 and CD8 T cells, as well as B cells producing VNA, accumulate in CNS tissues, but infiltration of NOS-2-positive macrophages is not seen and the elevation in BBB permeability is limited to fluid-phase markers [17]. The immune clearance of attenuated RABV is unique in avoiding the extensive changes in BBB integrity and NOS-2-positive cell accumulation associated with a more pathogenic CNS inflammatory response.
Adoptive transfer experiments have implicated CD4 T cells in the modulation of BBB integrity during RABV clearance, the extent of which is linearly correlated with the level of IFN-γ mRNA expressed in the tissues [23]. Moreover, treatment of monolayers of primary cells obtained from brain microvessels with IFN-γ but not TNF-α causes increased permeability to a fluid-phase marker in vitro through a mechanism that evidently involves peroxynitrite-dependent radicals [23]. Based on these and other studies, we speculate that peroxynitrite-dependent radicals contribute alterations in BBB integrity associated with CNS autoimmunity and RABV clearance [19]. However, while NOS-2 is found at high levels in the CNS during RABV clearance, its expression and nitrotyrosine formation are limited to the neurovasculature [19,23]. Consequently, peroxynitrite-based radicals with tissue-damaging potential are produced in the blood vessels rather than brain parenchyma as seen in EAE and Borna disease [19,21]. We theorize that the production of IFN-γ at the BBB by CD4 and CD8 T cells induces radical production by cells of the neurovascular unit leading to changes in the BBB that permit T and B cells to enter CNS tissues infected with attenuated RABV.
Virus neutralizing antibodies are well known to be the key mediators of RABV clearance; mice lacking CD8 T cells and other elements of immunity can clear CVS-F3 from the CNS but animals without CD4 T cells or B cells cannot [16]. During the natural immune response to attenuated RABV, fluid-phase BBB permeability is elevated prior to the development of high-titer VNA in the circulation, and virus clearance largely occurs after BBB integrity has normalized [16]. However, high levels of mRNAs specific for the B-cell phenotype marker CD19 and the κ antibody light chain appear in CNS tissues infected with attenuated RABV, and cells producing rabies-specific antibodies can be recovered from the tissues [24]. These data suggest that the production of RABV-specific antibody in the CNS tissues is critical to the clearance of the virus. However, while the non-specific infiltration of large molecules from the circulation into the CNS does not occur during RABV clearance, we have recently obtained evidence that under certain circumstances, passive administration of RABV-specific antibodies can impact RABV replication in the CNS tissues [Kean RB and Hooper DC, Unpublished Data]. This is illustrated by an experiment summarized in Figure 1 where CVS-F3 replication in the CNS tissues of B-cell-deficient mice was inhibited by the administration of RABV-specific antibodies at 5 days after infection. These effects are consistent with our observation of RABV-specific antibody binding to RABV-infected neurons following peripheral administration of the antibody to B-cell-deficient, CVS-F3-infected mice [24]. These findings indicate that, under certain conditions, passively administered RABV-specific antibody has access across the BBB and has therapeutic antiviral effects in the CNS.
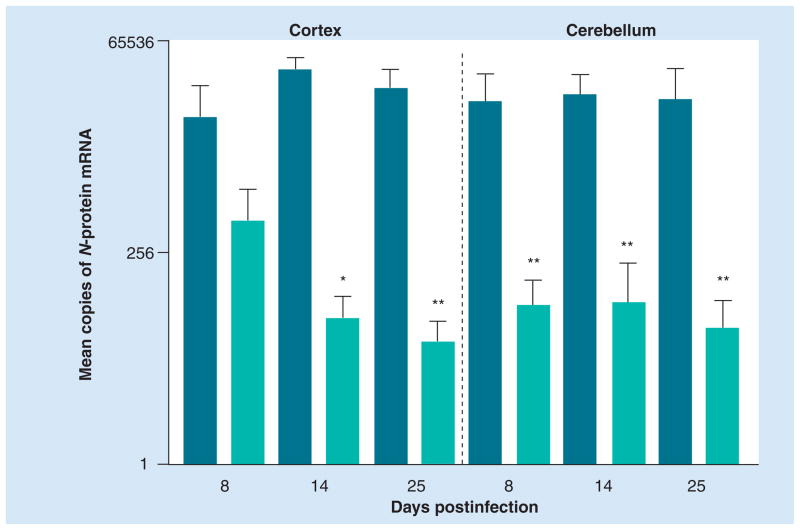
Figure 1. Passive administration of rabies virus immune sera from normal mice inhibits CVS-F3 replication in the CNS tissues of B-cell-deficient mice.
Groups of five JHD−/− mice were infected with 105 focus-forming units of CVS-F3 intranasally. A total of 5 days later, mice were injected intraperitoneally with 0.5 ml of either normal or rabies virus immune serum. At the time points indicated, cortex and cerebellar tissues were collected and virus replication was assessed by real-time quantitative RT-PCR. Virus replication is expressed as the mean plus the standard error of the mean copies of rabies virus nucleoprotein mRNA in 50 ng of cDNA isolated from CNS tissues. Statistically significant differences in gene expression between tissues from mice receiving normal and rabies virus immune serum, determined by the Mann–Whitney test, are denoted by *(p < 0.05) and **(p < 0.01).
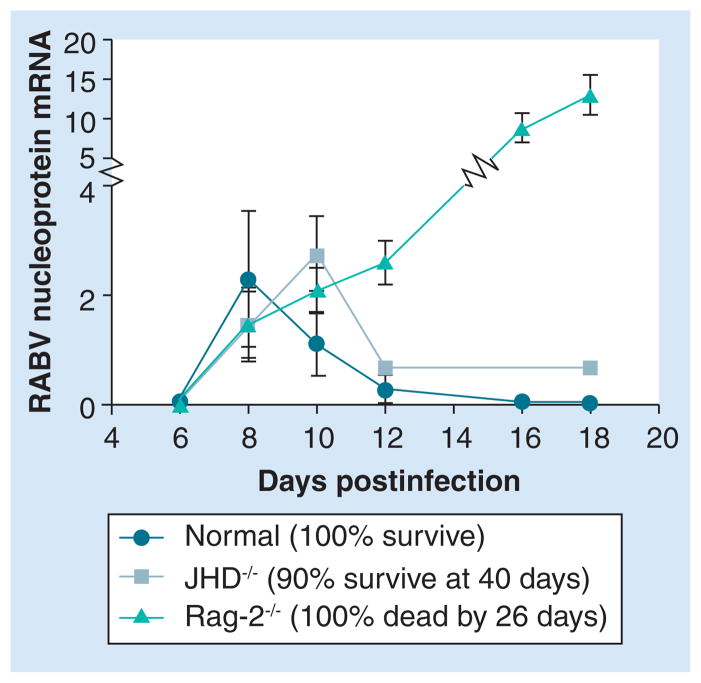
Although RABV clearance is dependent upon antibodies, T-cell-driven mechanisms evidently contribute to the control of virus replication. Replication of the attenuated RABV CVS-F3 is unchecked in the CNS tissues of mice lacking T and B cells, resulting in the death of these animals approximately 20 days after infection (Figure 2). However, like their normal counterparts, mice that are deficient in B cells but have intact T-cell function exhibit a strong reduction in replication of the virus in the CNS approximately 2 weeks after infection and, despite failing to clear the virus, survive for extended periods of time (Figure 2). We speculate that the T-cell-mediated control of RABV replication in the CNS tissues is mediated, at least in part, through the production of IFN-γ by infiltrating T cells.
Figure 2. Antibody-independent control of CVS-F3 replication in the CNS.
Groups of normal B6.129, JHD−/− and Rag-2−/− mice on a B6.129 background were infected intranasally with 105 focus-forming units of CVS-F3. At the indicated times after infection, rabies virus replication in the CNS was estimated by quantifying the amount of viral nucleoprotein mRNA in CNS tissues using real-time quantitative RT-PCR as detailed previously [26]. Additional groups of ten mice each were followed for survival.
RABV: Rabies viruses.
Evasion of RABV from immune clearance Innate immunity
In mice, wildlife RABV infection can be compared and contrasted with infection with a number of attenuated RABV strains that cause varying disease severity [25]. Many of these attenuated RABV spread from peripheral sites of inoculation into the CNS tissues of normal mice and are cleared by immune mechanisms without consequence [25]. In contrast to infection with wildlife RABV, substantial infiltration of immune cells into CNS tissues occurs during infection with attenuated RABV [12,26]. Differences in the capacity of RABV variants to induce or control innate immune mechanisms could explain these divergent outcomes. Innate immune mechanisms undoubtedly play a major role in the early containment of RABV infection, particularly through the production of type 1 interferons, and are central to the development of an appropriate adaptive immune response. The RABV phosphoprotein interacts with STAT1 and STAT2 after their activation by interferon, thereby interfering with the innate control of virus replication [27,28]. Inhibition of interferon signaling could also have the potential to alter the nature of the protective RABV-specific immune response from its conventional T-helper 1 (Th1) bias [16] to a less neuroinvasive T-helper 2 (Th2) phenotype. While differences in phosphoprotein production levels or phosphoprotein structure relevant to the STAT interaction may help to explain certain differences in host responses to attenuated and highly pathogenic RABV variants, normal mice invariably develop a Th1 response to RABV regardless of the nature of the infecting RABV and the outcome of the infection [Hooper et al., Unpublished Data]. Altering the RABV immune response towards Th2 reactivity would likely require that sufficient phosphoprotein is accessible for binding STAT1/2 in dendritic cells presenting RABV antigens early in the infection, which is probably not the case.
With RABV replication occurring primarily in the CNS, the production by the innate response of cytokines and chemokines that attract immune cells from the circulation into the infected tissues is an essential element of virus clearance. There is evidence of subtle differences between different RABV variants in the capacity to induce innate cytokine and chemokine production by CNS-resident cells in mice [12,13]. Nevertheless, a variety of factors that should contribute to the establishment of RABV-specific CNS immunity are expressed at high levels in CNS tissues, regardless of the nature of the infecting RABV [12,13,26]. These include chemokines that attract immune cells and the important proinflammatory cytokine TNF-α [12,26]. In addition, elevated levels of the TNF-α-inducible adhesion molecule ICAM-1 are readily detectable in the neurovasculature of mice infected with either attenuated or pathogenic RABV strains [12]. These observations indicate that diverse aspects of the innate immune response are active in the CNS tissues of mice infected with either attenuated or pathogenic wild-type RABV, but, as later discussed, do not exclude the possibility that differing outcomes of RABV infection have their genesis in an element of innate immunity that is unique to the CNS.
Adaptive immunity
Most rabies patients fail to develop a substantial antigen-specific immune response to a natural RABV infection until symptoms of the disease have appeared [11]. However, there is no inherent defect in the capacity to mediate a RABV-specific immune response, as RABV vaccination in the first few days following exposure generally protects against the development of rabies. More likely, there is insufficient rabies glycoprotein associated with the initial infection to be recognized by the immune system. As the disease progresses, a subset of infected, but untreated patients develop detectable cell mediated immune response to RABV [29]. There is no evidence of the suppression of cellular immunity in these patients [29], as previously reported at late stages in RABV infection of mice [30]. Our finding that experimental infection of mice with wild-type RABV induces a strong peripheral immune response that develops several days prior to death of the animals is consistent with the observations in human rabies victims that immune suppression does not occur. In fact, we do not detect any difference between the activity of RABV-specific CD4 T and B cells in the periphery of mice infected with a representative highly pathogenic wildlife RABV derived from a human rabies case, SHBRV-17, and the attenuated CVS-F3 virus [12]. This is best illustrated by the fact that immune cells adoptively transferred from mice lethally infected with SHBRV-17 can readily mediate the clearance of CVS-F3 from immunocompromised recipients [12]. Nevertheless, it is evident that conventional PEP is less successful when its administration is delayed [31] and that the natural development of RABV-specific immunity in humans has little effect on the early stages of RABV replication in the CNS. The development of RABV immunity after the virus has reached the human CNS appears to be generally without consequence, with only relatively mild histopathological changes limited to perivascular cuffing of mononuclear cells [32]. This is similar to the outcome of infection of mice with wildlife-derived RABV, where immune cell infiltration of CNS tissues is minimal [12]. Thus, observations in humans and mouse models suggest that infection with wild-type RABV is unlikely to be generally immunosuppressive but may be associated with the failure of peripheral immune effectors to reach infected CNS tissues at a stage when the infection may be contained by immune mechanisms. In mice, where comparative studies are feasible, immune cell infiltration into the CNS tissues of animals infected with pathogenic RABV is greatly reduced by comparison with mice clearing attenuated RABV from CNS tissues [12]. In addition, the few T cells that enter the CNS tissues of mice infected with highly pathogenic RABV undergo apoptosis, thereby preventing any further contribution to protective immunity [33].
Maintenance of BBB integrity
In addition to the development of RABV-specific immunity in the periphery and innate immunity in the infected CNS, RABV clearance has been associated with functional changes in the neurovasculature that promote contact between the CNS tissues, the chemoattractants they produce, and circulating immune effectors [26]. The neurovasculature exhibits elevated fluid-phase permeability, enhanced adhesion molecule expression and other alterations in association with the infiltration of circulating T and B cells into CNS tissues infected with attenuated RABV [26]. However, while certain types of these changes occur in mice infected with wildlife RABV, elevated fluid-phase BBB permeability and immune cell invasion does not [12]. The BBB evidently remains relatively intact until the end stages of rabies in humans, as MRI studies did not reveal gadolinium enhancement until patients became comatose [34]. In support of these findings, VNA are generally not detected in the CNS of dog rabies patients [29]. Relatively low levels of VNA have been detected in the cerebrospinal fluid of at least some human bat rabies patients [7]. While the genesis of these antibodies is unknown, enhancement on MRI does not appear to be a prerequisite [35]. MRI enhancement was also not detected in the CNS of dogs experimentally infected with RABV [36]. The absence of neurovascular permeability in mice infected with wild-type RABV is consistent with these observations [12]. However, it should be noted that the infiltration of VNA and B cells into mouse CNS tissues infected with attenuated RABV is only associated with neurovascular leakage of fluid-phase markers [24], which is unlikely to be sufficient to permit the accumulation of gadolinium-based MRI contrast agents.
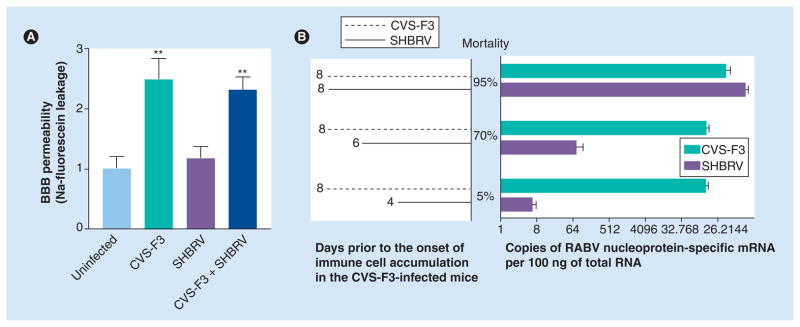
Immune cells from SHBRV-infected mice can clear CVS-F3 from immunocompetent recipients and adoptive transfer of cells from mice clearing CVS-F3 has no impact on survival from SHBRV infection [12]. Therefore, we concluded that the primary deficit in clearing SHBRV is not in the development of a RABV-specific immune response in the peripheral lymphoid organs but in the capacity of wild-type virus-infected CNS tissues to promote immune effector invasion [12]. ‘Opening’ the BBB by inducing a CNS inflammatory response [9] allows immune effectors to reach the CNS tissues and animals infected with wild-type RABV to survive, suggesting that the deficit is manifested in the neurovasculature. To determine whether infection with wild-type RABV inhibits or, alternatively, fails to induce the fluid-phase BBB permeability associated with CVS-F3 clearance, we infected groups of mice with CVS-F3, SHBRV or both viruses and assessed Na-fluorescein leakage from the circulation into CNS tissues 8 days later (Figure 3A). The results indicate that SHBRV infection has neither a stimulatory nor a strong inhibitory effect on this aspect of BBB function, suggesting that superinfection with an attenuated RABV may be a viable means of treating rabies. However, to promote survival of mice from SHBRV infection, CVS-F3 must be administered several days prior to SHBRV (Figure 3B), as we have previously demonstrated for the combination of CVS-F3 and the cloned, dog-derived RABV CVS-N2c [24]. Otherwise, SHBRV infection of the CNS tissues is evidently too extensive to be contained by the developing CNS immune response elicited by CVS-F3. As discussed later, the next-generation, genetically engineered live–attenuated vaccine RABV TriGAS is effective even when administered postinfection with wild-type RABV [10].
Figure 3. Elevated fluid-phase blood–brain barrier permeability is the dominant outcome of mixed CVS-F3 and silver-haired bat rabies virus infection but occurs too slowly following CVS-F3 infection to have practical therapeutic value.
(A) Groups of five to six 129SvEv mice were either left uninfected or were infected with 105 focus-forming units of CVS-F3 intranasally, 104 focus-forming units of SHBRV intradermally in the ear, as previously described [12], or infected using both regimens. The extent of fluid-phase BBB permeability was assessed by measuring leakage of Na-fluorescein from circulation into the cerebellar tissues at 8 days postinfection as detailed previously [18]. Results are expressed as the mean ± S.E.M. (B) Groups of ten 129SvEv mice were infected with 105 focus-forming units of CVS-F3 intranasally and with 104 focus-forming units of SHBRV intradermally in the ear at the indicated intervals. RABV nucleoprotein levels for each virus in brain tissues were determined by real-time quantitative RT-PCR at 8 days after CVS-F3 infection using primers and probes specific for the two viruses. Synthetic nucleoprotein cDNAs were used to quantify the copy numbers of each nucleoprotein mRNA in 100 ng of total brain RNA.
**Statistical significance of the differences between uninfected and infected groups was tested using the Mann–Whitney test (p < 0.01). BBB: Blood–brain barrier; RABV: Rabies virus; S.E.M.: Standard error of the mean; SHBRV: Silver-haired bat rabies virus.
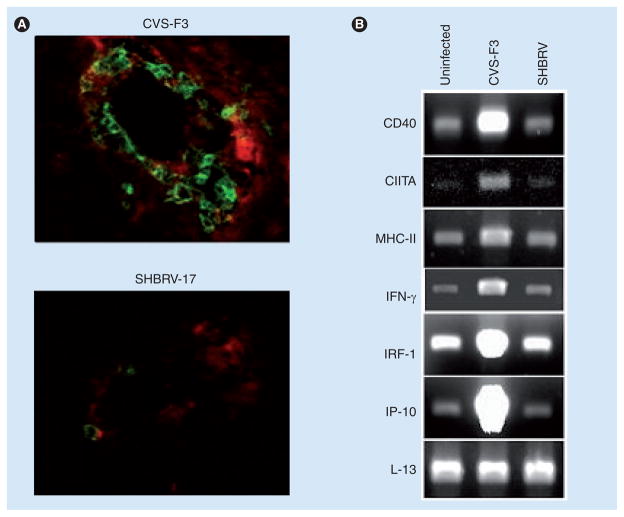
Based on these and other findings, we speculated that a key element of RABV immune evasion is manifested at the BBB [9,12,25], and have examined brain microvessels for changes that occur in CVS-F3 but not in SHBRV -infected mice. As demonstrated in Figure 4A, blood vessels in the CNS tissues of CVS-F3-infected mice are strongly MHC class II-positive and have substantial numbers of associated CD4 cells, unlike the neurovasculature of SHBRV-infected mice. The expression of mRNAs specific for IFN-γ and markers relevant to IFN-γ signaling pathways are elevated in microvessels isolated from CVS-F3-infected brain but not in a similar preparation from SHBRV-infected animals (Figure 4B). Particularly noteworthy is that MHC class II, CIITA and CD40 mRNA levels are differentially expressed. This leads us to hypothesize that an interaction between IFN-γ-producing T cells and MHC class II-positive cells in the neurovascular unit is central to the induction of lymphocyte invasion into the CNS tissue of RABV-infected mice. In this regard, it is important to note that CD8 T-cell infiltration into the CNS has been associated with antigen recognition in the neurovasculature in a different model [37]. Furthermore, this may conceivably be the case for CD4 T cells in RABV infection.
Figure 4. An interaction between CD4 T cells and MHC class II-positive cells in the neurovasculature is deficient in mice infected with wild-type rabies virus.
(A) Frozen brain sections from mice infected with either CVS-F3 or SHBRV 8 days previously, as described in the legend to Figure 3, were stained with fluorophore-conjugated antibodies specific for MHC class II (red) and CD4 (green), as previously described [17]. (B) RNA isolated from brain microvessels of uninfected or RABV-infected mice was subjected to RT-PCR with primers specific for the gene products indicated, as described elsewhere [24]. The house-keeping gene L-13 was included as a control for the level of mRNA in the samples.
SHBRV: Silver-haired bat rabies virus.
Circumventing RABV immune evasion strategies: approaches to the clearance of wild-type RABV from the CNS
If the primary deficit in the immune response to wild-type RABV infection is in the delivery of immune effectors to the CNS, it may be possible to treat the early stages of rabies by overcoming this block. It is conceivable that direct injection of RABV-specific immune effectors across the BBB into the infected CNS could have therapeutic value, but this would depend on the ability of the effectors to spread through the tissues and clear the virus. Is the nonspecific induction of BBB permeability a more viable option? As demonstrated in the past, this can be accomplished by the administration of the hyperosmotic agent, mannitol [38]. More recently, it has been demonstrated that downregulation of tight junction protein expression by siRNA can cause transiently increased BBB permeability for low molecular-weight tracer molecules [39]. Another technique that is gaining popularity is the use of molecular ‘Trojan horses’ to deliver therapeutic agents across the BBB [40]. Whether or not any of these approaches could safely promote RABV-specific immune effector infiltration into CNS tissues remains to be determined, but the nonspecific nature of their induction may prove problematic. As noted above, the induction of CNS autoimmune inflammation can deliver RABV-specific immune effectors into CNS tissues and rescue mice from lethal SHBRV infection [9], but the inherent pathogenicity of this approach precludes its use.
Unlike mice, humans often do not mediate a strong immune response to infecting wild-type RABV until relatively late in the disease [11], and vaccination is necessary to ensure that RABV-specific immunity develops. Thus, from the perspective of the development of a treatment for CNS RABV infections, super-infection with a live–attenuated RABV would have the advantage of inducing RABV-specific immune effectors and targeting them to CNS tissues. A novel live–attenuated RABV vaccine strain, TriGAS, has recently been developed that is the first reagent to have success in the PEP of mice [10]. Like CVS-F3, TriGAS induces elevated fluid-phase BBB permeability and immune effector delivery into the CNS [10]. However, unlike CVS-F3, superinfection with TriGAS can save mice infected with a lethal dose of wild-type virus several days previously [10]. The importance of rapidly targeting immune effectors to the CNS tissues is illustrated by differences in the outcome of peripheral versus intracranial administration of TriGAS. Postexposure superinfection of mice via the intracranial route remains effective for several days after any protective effect of peripheral superinfection has been lost [10]. In addition, postexposure administration of killed RABV vaccine has little protective effect [10]. While killed vaccine may alter peripheral immunity, there is little possibility that it would have any direct effect on the neurovasculature or CNS. We conclude that rapid spread of an attenuated RABV to the CNS is critical for the induction of the processes that provide specific immune effectors access to RABV-infected CNS tissues.
The current PEP regimen in humans, consisting of the administration of killed vaccine and VNA, may not be expected to clear wild-type RABV from the CNS. As attested by the fact that RABV infection of the CNS is usually lethal in humans, natural RABV infection is unlikely to induce immune effector infiltration across the BBB. Until we better understand how to therapeutically modulate BBB function, treatment with live–attenuated RABV followed by the administration of VNA capable of infiltrating CNS tissues may be a way forward for the late-stage prophylaxis of RABV infection and, possibly, for early-stage immune therapy of rabies. The approach of using a live RABV to treat rabies infection is not new, since the initial postexposure vaccination regimen of Pasteur and Roux involved administration of virulent RABV [2]. More defined experimental studies using intrathecal injection of attenuated RABV in rabid dogs and monkeys were performed a number of years ago by Baer and his colleagues [41]. Notably, dogs that received intrathecal administration of live, but not inactivated, vaccine at the first signs of rabies recovered, but similar treatment was unsuccessful in rhesus macaques [41]. Based on a better understanding of host RABV interactions and the ability to engineer RABV to express exogenous proteins, it is now possible to construct attenuated RABV tailored to target CNS immune clearance mechanisms. At worst, superinfection with these viruses could induce immune-mediated tissue damage that precipitates the expected lethal outcome. However, if the spread of the naturally infecting RABV has not reached a threshold where survival is impossible, targeting the appropriate immune response to the infected CNS tissues would be expected to be beneficial. Our recent observation that attenuated RABV infection promotes RABV-specific antibody entry into CNS tissues also has relevance for future rabies therapy. The passive establishment of useful levels of this important effector molecule more rapidly than is possible by active vaccination should limit the spread of both the disease-causing wild-type RABV and the live–attenuated vaccine virus, thereby providing an additional element of safety for the approach.
Conclusion
Understandably, R ABV spreads rapidly through the mouse nervous system, and infections of these animals with wild-type RABV are generally lethal within 10 days. The discovery that a live–attenuated RABV engineered to over-express glycoprotein (TriGAS) has utility in the postexposure treatment of RABV-infected mice [10] has profound implications for the treatment of humans, where the spread of RABV through the nervous system takes considerably longer. We speculate that the appropriate use of such reagents and passive VNA administration will enable us to extend the successful window for rabies PEP, possibly to the point when early, nonspecific signs of the disease have already appeared.
Future perspective
The rapid induction of innate control of RABV spread in CNS tissues coupled with the delivery of immune effectors into CNS tissues, both achievable with live–attenuated RABV vaccines and passive VNA administration, can contain and eliminate a RABV infection from the CNS. As we continue to better understand the processes involved, we expect that reagents specifically developed for these purposes will become available. However, while we can manipulate the immune response to the virus to minimize immunopathogenesis and promote virus clearance, we have only limited insight into RABV neuronal pathogenesis and do not know if infected neurons can survive virus clearance. Thus, the prospect for the successful treatment of patients with clinical rabies will remain limited by two important, related and poorly understood elements of rabies pathogenesis: the inherent pathogenicity of different RABV strains for infected neurons; and the extent of the infection that is survivable when antiviral mechanisms become operative. Nevertheless, in opposition to the current dogma, the spread of RABV to the CNS will no longer represent an untreatable stage of the infection.
Executive summary.
Rabies viruses (RABV) remain endemic in a variety of animal species throughout much of the world, and can cause significant human mortality when there is a failure to administer postexposure prophylaxis in a timely manner.
A number of features that allow pathogenic RABV to interfere with immune mechanisms and evade immune clearance from CNS tissues have been identified. Comparison of host responses to wild-type and laboratory-attenuated strains of RABV have led us to understand why current rabies postexposure prophylaxis is likely to fail once wild-type RABV has reached the CNS and to develop reagents and a strategy for RABV clearance from the CNS.
The expectation is that the window for intervention following rabies exposure can be extended.
Footnotes
Financial & competing interests disclosure
This publication was made possible by studies supported by Grant Numbers AI077033 and AI083046 (DCH) from NIAID and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Baer GM, Bellini WJ, Fishbein DB. Rhabdoviruses. In: Fields BN, editor. Virology. 2. Raven press; NY, USA: 1990. pp. 883–930. [Google Scholar]
- 2▪▪.Pasteur L. Bulletin de l’Académie de médecine, séance du 27 octobre, 2e sér. 1885;XIV:1431–1439. Report of first treatment of a rabies virus (RABV)-exposed individual using vaccination. [Google Scholar]
- 3.Messenger SL, Smith JS, Orciari LA, Yager PA, Rupprecht CE. Emerging pattern of rabies deaths and increased viral infectivity. Emerg Infect Dis. 2003;9(2):151–154. doi: 10.3201/eid0902.020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson AC. Human rabies and bat bites. Lancet. 2001;357:1714. doi: 10.1016/S0140-6736(00)04852-2. [DOI] [PubMed] [Google Scholar]
- 5.Morimoto K, Patel M, Corisdeo S, et al. Characterization of a unique variant of bat rabies virus responsible for newly emerging human cases in North America. Proc Natl Acad Sci USA. 1996;93:5653–5658. doi: 10.1073/pnas.93.11.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibbons RV. Cryptogenic rabies, bats, and the question of aerosol transmission. Ann Emerg Med. 2002;39:528–536. doi: 10.1067/mem.2002.121521. [DOI] [PubMed] [Google Scholar]
- 7▪▪.Willoughby RE, Jr, Tieves KS, Hoffman GM, et al. Survival after treatment of rabies with induction of coma. N Engl J Med. 2005;352(24):2508–2514. doi: 10.1056/NEJMoa050382. Report of the first human recovery from clinical rabies in the absence of postexposure prophylaxis. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Recovery of a patient from clinical rabies – Wisconsin, 2004. MMWR Morb Mortal Wkly Rep. 2004;57(17):460–462. [Google Scholar]
- 9.Roy A, Hooper DC. Lethal silver-haired bat rabies virus infection can be prevented by opening the blood-brain barrier. J Virol. 2007;81:7993–7998. doi: 10.1128/JVI.00710-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10▪.Faber M, Li J, Kean RB, Hooper DC, Alugupalli KR, Dietzschold B. Effective preexposure and postexposure prophylaxis of rabies with a highly attenuated recombinant rabies virus. Proc Natl Acad Sci USA. 2009;106(27):11300–11305. doi: 10.1073/pnas.0905640106. First report of successful postexposure treatment of mice with a recombinant-engineered live–attenuated RABV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson N, Cunningham AF, Fooks AR. The immune response to rabies virus infection and vaccination. Vaccine. 2010;28(23):3896–3901. doi: 10.1016/j.vaccine.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 12▪▪.Roy A, Phares TW, Koprowski H, Hooper DC. Failure to open the blood-brain barrier and deliver immune effectors to the CNS tissues leads to the lethal outcome of Silver-haired bat rabies virus infection. J Virol. 2007;81(3):1110–1118. doi: 10.1128/JVI.01964-06. Evidence from an experimental model that blood–brain barrier (BBB) integrity is maintained during a lethal RABV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang ZW, Sarmento L, Wang Y, et al. Attenuated rabies virus activates, while pathogenic rabies virus evades, the host innate immune responses in the central nervous system. J Virol. 2005;79:12554–12565. doi: 10.1128/JVI.79.19.12554-12565.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneider LG, Cox JH, Müller WW, Hohnsbeen KP. Current oral rabies vaccination in Europe: an interim balance. Rev Infect Dis. 1988;10(Suppl 4):S654–S659. doi: 10.1093/clinids/10.supplement_4.s654. [DOI] [PubMed] [Google Scholar]
- 15.Rupprecht CE, Dietzschold B, Cox JH, Schneider LG. Oral vaccination of raccoons (Procyon lotor) with an attenuated (SAD-B19) rabies virus vaccine. J Wildl Dis. 1989;25(4):548–554. doi: 10.7589/0090-3558-25.4.548. [DOI] [PubMed] [Google Scholar]
- 16.Hooper DC, Morimoto K, Bette M, Weihe E, Koprowski H, Dietzschold B. Collaboration of antibody and inflammation in clearance of rabies virus from the central nervous system. J Virol. 1998;72(5):3711–3719. doi: 10.1128/jvi.72.5.3711-3719.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabis MJ, Phares TW, Kean RB, Koprowski H, Hooper DC. Blood–brain barrier changes and cell invasion differ between therapeutic immune clearance of neurotrophic virus and CNS autoimmunity. Proc Natl Acad Sci USA. 2008;105(40):15511–15516. doi: 10.1073/pnas.0807656105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hooper DC, Bagasra O, Marini JC, et al. Prevention of experimental allergic encephalomyelitis by targeting nitric oxide and peroxynitrite: implications for the treatment of multiple sclerosis. Proc Natl Acad Sci USA. 1997;94(6):2528–2533. doi: 10.1073/pnas.94.6.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooper DC, Scott GS, Zborek A, et al. Uric acid, a peroxynitrite scavenger, inhibits CNS inflammation, blood–CNS barrier permeability changes, and tissue damage in a mouse model of multiple sclerosis. FASEB J. 2000;14(5):691–698. doi: 10.1096/fasebj.14.5.691. [DOI] [PubMed] [Google Scholar]
- 20.Scott GS, Hooper DC. The role of uric acid in protection against peroxynitrite-mediated pathology. Med Hypotheses. 2001;56(1):95–100. doi: 10.1054/mehy.2000.1118. [DOI] [PubMed] [Google Scholar]
- 21.Hooper DC, Kean RB, Scott GS. The central nervous system inflammatory response to neuro- tropic virus infection is peroxynitrite dependent. J Immunol. 2001;167(6):3470–3477. doi: 10.4049/jimmunol.167.6.3470. [DOI] [PubMed] [Google Scholar]
- 22.Dietzschold B, Wunner WH, Wiktor TJ, et al. Characterization of an antigenic determinant of the glycoprotein which correlates with pathogenicity of rabies virus. Proc Natl Acad Sci USA. 1983;80:70–74. doi: 10.1073/pnas.80.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23▪▪.Phares TW, Fabis MJ, Brimer CM, Kean RB, Hooper DC. A peroxynitrite-dependent pathway is responsible for blood–brain barrier permeability changes during a central nervous system inflammatory response: TNF-α is neither necessary nor sufficient. J Immunol. 2007;178(11):7334–7343. doi: 10.4049/jimmunol.178.11.7334. Evidence from an experimental model that the clearance of attenuated RABV from CNS tissues is associated with functional changes in the BBB likely induced by IFN-γ. [DOI] [PubMed] [Google Scholar]
- 24▪.Hooper DC, Phares TW, Fabis MJ, Roy A. The production of antibody by invading B cells is required for the clearance of rabies virus from the central nervous system. PLoS Negl Trop Dis. 2009;3(10):E535. doi: 10.1371/journal.pntd.0000535. Demonstration that RABV clearance from the CNS is associated with antibody production by B cells infiltrating CNS tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25▪.Roy A, Hooper DC. Immune evasion by rabies viruses through the maintenance of blood-brain barrier integrity. J Neurovirol. 2008;14(5):401–411. doi: 10.1080/13550280802235924. Demonstration that the pathogenicity of different RABV correlates with the capacity of the host to deliver immune effectors to the CNS. [DOI] [PubMed] [Google Scholar]
- 26▪▪.Phares TW, Kean RB, Mikheeva T, Hooper DC. Regional differences in blood-brain barrier permeability changes and inflammation in the apathogenic clearance of virus from the central nervous system. J Immunol. 2006;176(12):7666–7675. doi: 10.4049/jimmunol.176.12.7666. Evidence from an experimental model that the clearance of attenuated RABV from CNS tissues is associated with functional changes in the BBB. [DOI] [PubMed] [Google Scholar]
- 27.Brzózka K, Finke S, Conzelmann KK. Identification of the rabies virus α/β interferon antagonist: phosphoprotein P interferes with phosphorylation of interferon regulatory factor 3. J Virol. 2005;79(12):7673–7681. doi: 10.1128/JVI.79.12.7673-7681.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brzózka K, Finke S, Conzelmann KK. Inhibition of interferon signaling by rabies virus phosphoprotein P: activation-dependent binding of STAT1 and STAT2. J Virol. 2006;80(6):2675–2683. doi: 10.1128/JVI.80.6.2675-2683.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hemachudha T, Wacharapluesadee S, Mitrabhakdi E, Wilde H, Morimoto K, Lewis RA. Pathophysiology of human paralytic rabies. J Neurovirol. 2005;11(1):93–100. doi: 10.1080/13550280590900409. [DOI] [PubMed] [Google Scholar]
- 30.Wiktor TJ, Doherty PC, Koprowski H. Suppression of cell-mediated immunity by street rabies virus. J Exp Med. 1977;145(6):1617–1622. doi: 10.1084/jem.145.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson AC, Warrell MJ, Rupprecht CE, et al. Management of rabies in humans. Clin Inf Dis. 2003;36:60–63. doi: 10.1086/344905. [DOI] [PubMed] [Google Scholar]
- 32.Jackson AC, Randle E, Lawrance G, Rossite JP. Neuronal apoptosis does not play an important role in human rabies encephalitis. J Neurovirol. 2008;14(5):368–375. doi: 10.1080/13550280802216502. [DOI] [PubMed] [Google Scholar]
- 33.Baloul L, Lafon M. Apoptosis and rabies virus neuroinvasion. Biochimie. 2003;85(8):777–788. doi: 10.1016/s0300-9084(03)00137-8. [DOI] [PubMed] [Google Scholar]
- 34.Laothamatas J, Hemachuda T, Mitrabhajdi E, Wannakrairot P, Tulayadaechonont S. MR imaging in human rabies. AJNR Am J Neuroradiol. 2003;24(6):1102–1109. [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. Epidemiologic notes and reports human rabies -Minnesota. MMWR Morb Mortal Wkly Rep. 2007;57(17):460–462. [PubMed] [Google Scholar]
- 36.Laothamatas J, Wacharapluesadee S, Lumlertdacha B, et al. Furious and paralytic rabies of canine origin: neuroimaging with virological and cytokine studies. J Neurovirol. 2008;14(2):119–129. doi: 10.1080/13550280701883857. [DOI] [PubMed] [Google Scholar]
- 37.Galea I, Bernardes-Silva M, Forse PA, van Rooijen N, Liblau RS, Perry VH. An antigen-specific pathway for CD8 T cells across the blood-brain barrier. J Exp Med. 2007;204(9):2023–2030. doi: 10.1084/jem.20070064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neuwelt EA, Maravilla KR, Frenkel EP, Rapaport SI, Hill SA, Barnett PA. Osmotic blood–brain barrier disruption Computerized tomographic monitoring of chemotherapeutic agent delivery. J Clin Invest. 1979;64(2):684–688. doi: 10.1172/JCI109509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campbell M, Kiang AS, Kenna PF, et al. RNAi-mediated reversible opening of the blood–brain barrier. J Gene Med. 2008;10(8):930–947. doi: 10.1002/jgm.1211. [DOI] [PubMed] [Google Scholar]
- 40.Pardridge WM. Molecular Trojan horses for blood-brain barrier drug delivery. Discov Med. 2006;6(34):139–143. [PubMed] [Google Scholar]
- 41▪▪.Baer GM, Shaddock JH, Williams LW. Prolonging morbidity in rabid dogs by intrathecal injection of attenuated rabies vaccine. Infect Immun. 1975;12(1):98–103. doi: 10.1128/iai.12.1.98-103.1975. First evidence from an experimental model that superinfection with an attenuated RABV can lead to survival from a wild-type RABV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]