Abstract
The gene encoding a puromycin N-acetyl transferase from Streptomyces alboniger has been cloned next to the SV40 early promoter in a mammalian cells-Escherichia coli shuttle vector. When this construction was introduced into VERO cells it expressed the relevant enzymic activity. Moreover, the puromycin N-acetyl transferase gene has been used as a dominant marker for the selection of transformed mammalian cells able to grow in the presence of the antibiotic.
Full text
PDF


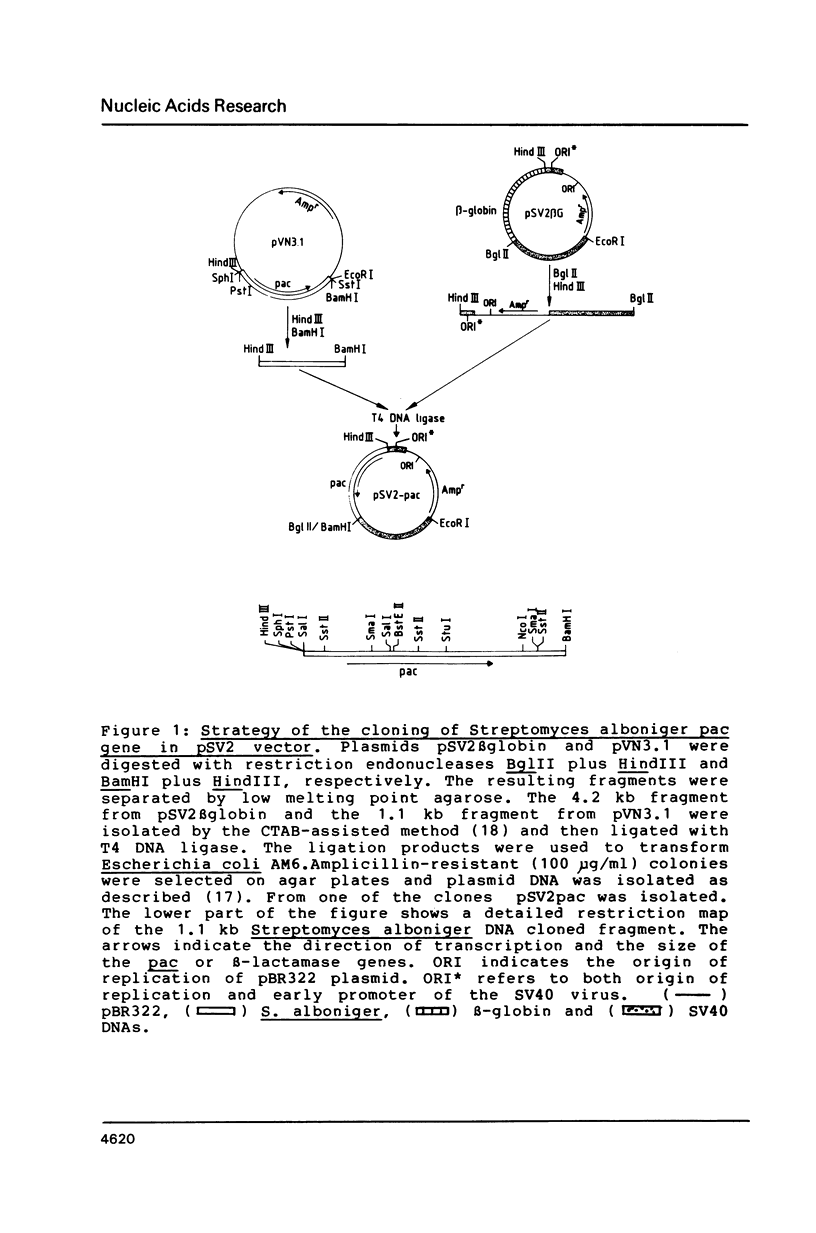
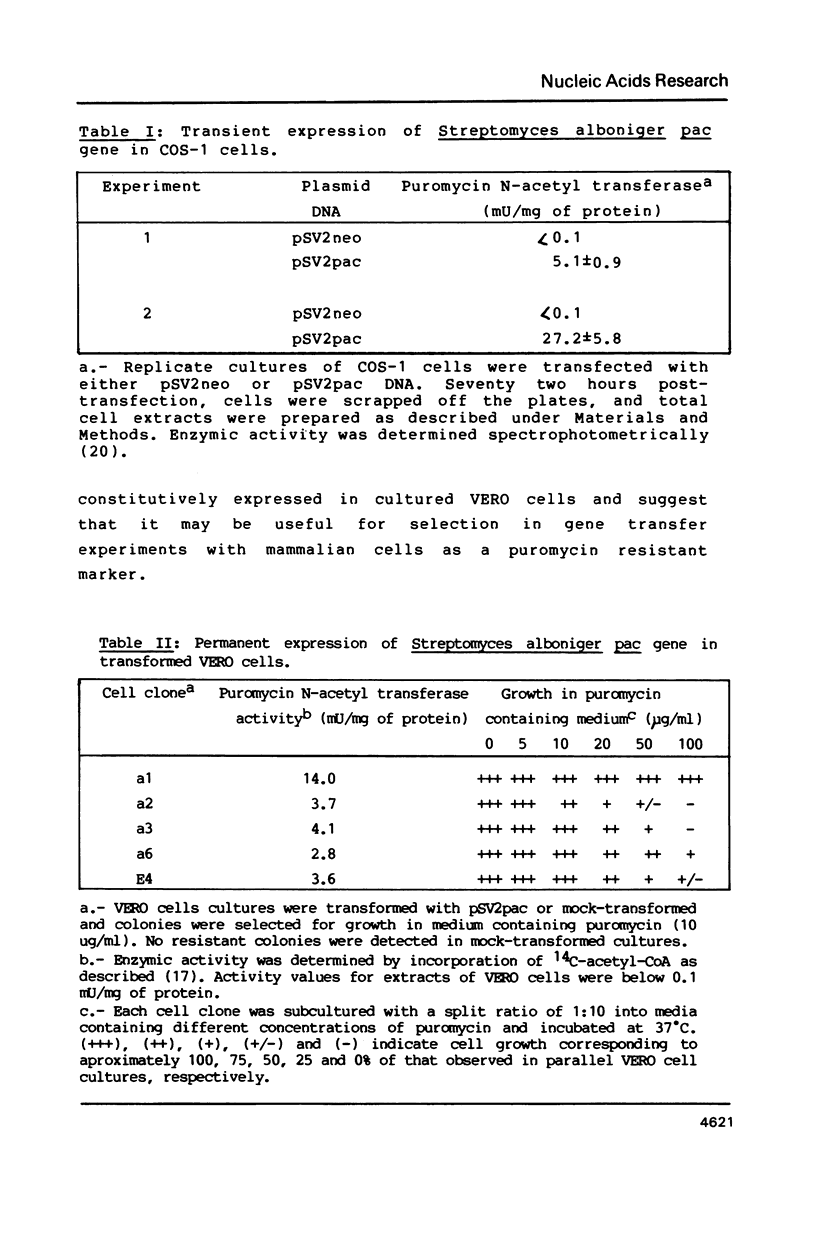

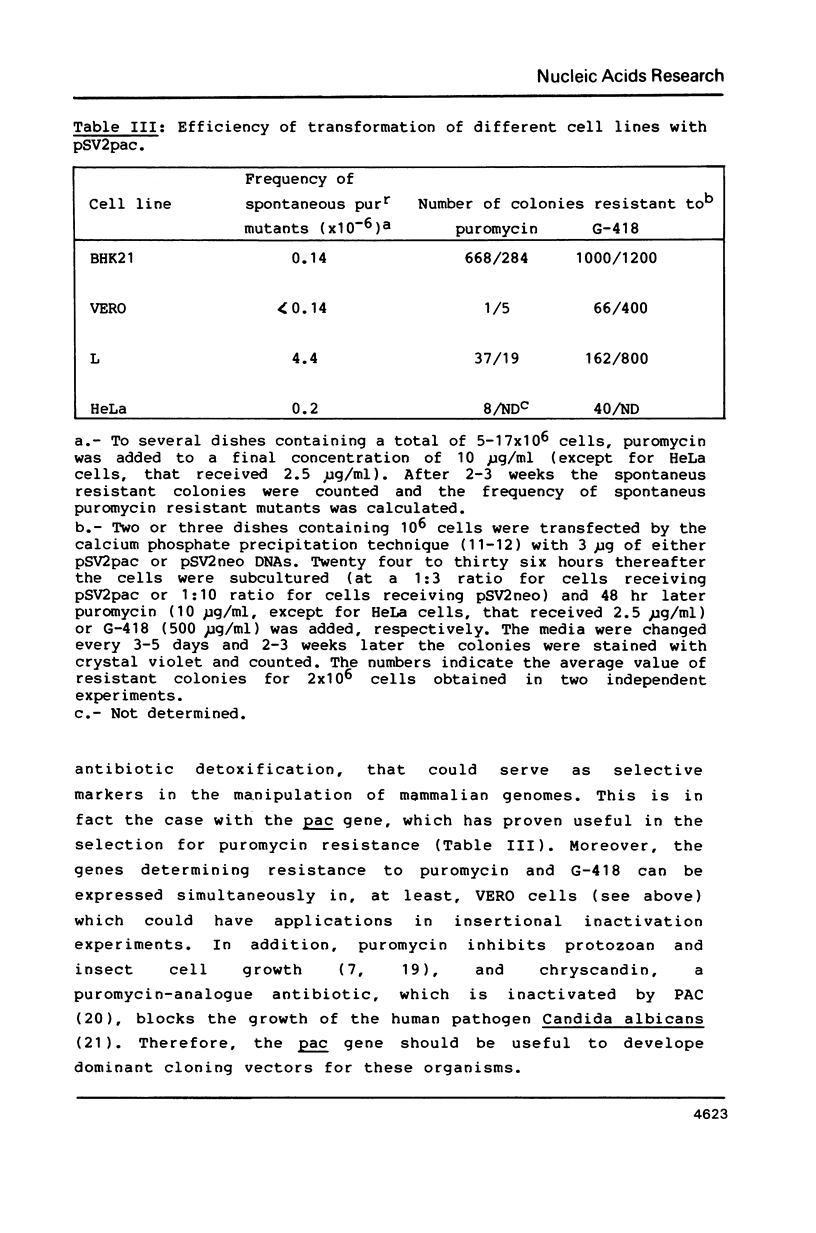

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bibb M. J., Bibb M. J., Ward J. M., Cohen S. N. Nucleotide sequences encoding and promoting expression of three antibiotic resistance genes indigenous to Streptomyces. Mol Gen Genet. 1985;199(1):26–36. doi: 10.1007/BF00327505. [DOI] [PubMed] [Google Scholar]
- Chater K. F., Hopwood D. A., Kieser T., Thompson C. J. Gene cloning in Streptomyces. Curr Top Microbiol Immunol. 1982;96:69–95. doi: 10.1007/978-3-642-68315-2_5. [DOI] [PubMed] [Google Scholar]
- Fallon A. M., Stollar V. Isolation and characterization of puromycin-resistant clones from cultured mosquito cells. Somatic Cell Genet. 1982 Jul;8(4):521–532. doi: 10.1007/BF01538712. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Kieser T. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid. 1984 Jul;12(1):19–36. doi: 10.1016/0147-619x(84)90063-5. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. Mapping temperature-sensitive mutants of simian virus 40: rescue of mutants by fragments of viral DNA. Virology. 1974 Aug;60(2):466–475. doi: 10.1016/0042-6822(74)90340-7. [DOI] [PubMed] [Google Scholar]
- Langridge J., Langridge P., Bergquist P. L. Extraction of nucleic acids from agarose gels. Anal Biochem. 1980 Apr;103(2):264–271. doi: 10.1016/0003-2697(80)90266-3. [DOI] [PubMed] [Google Scholar]
- Ortín J., Nájera R., López C., Dávila M., Domingo E. Genetic variability of Hong Kong (H3N2) influenza viruses: spontaneous mutations and their location in the viral genome. Gene. 1980 Nov;11(3-4):319–331. doi: 10.1016/0378-1119(80)90072-4. [DOI] [PubMed] [Google Scholar]
- Portela A., Melero J. A., Martínez C., Domingo E., Ortín J. A primer vector system that allows temperature dependent gene amplification and expression in mammalian cells: regulation of the influenza virus NS1 gene expression. Nucleic Acids Res. 1985 Nov 25;13(22):7959–7977. doi: 10.1093/nar/13.22.7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portela A., de la Luna S., Melero J. A., Vara J., Jiménez A., Ortín J. Regulation of gene amplification and expression in cells that constitutively express a temperature sensitive SV40 T-antigen. Nucleic Acids Res. 1985 Nov 25;13(22):7913–7927. doi: 10.1093/nar/13.22.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Thompson C. J., Gray G. S. Nucleotide sequence of a streptomycete aminoglycoside phosphotransferase gene and its relationship to phosphotransferases encoded by resistance plasmids. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5190–5194. doi: 10.1073/pnas.80.17.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vara J., Malpartida F., Hopwood D. A., Jiménez A. Cloning and expression of a puromycin N-acetyl transferase gene from Streptomyces alboniger in Streptomyces lividans and Escherichia coli. Gene. 1985;33(2):197–206. doi: 10.1016/0378-1119(85)90094-0. [DOI] [PubMed] [Google Scholar]
- Vara J., Perez-Gonzalez J. A., Jimenez A. Biosynthesis of puromycin by Streptomyces alboniger: characterization of puromycin N-acetyltransferase. Biochemistry. 1985 Dec 31;24(27):8074–8081. doi: 10.1021/bi00348a036. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M., Tsurumi Y., Hosoda J., Komori T., Kohsaka M., Imanaka H. Chryscandin, a novel peptidyl nucleoside antibiotic. I. Taxonomy, fermentation, isolation and characterization. J Antibiot (Tokyo) 1984 Nov;37(11):1279–1283. doi: 10.7164/antibiotics.37.1279. [DOI] [PubMed] [Google Scholar]


