Abstract
We examined whether men with restless legs syndrome (RLS) have a higher prevalence of Parkinson’s disease (PD) among 23,119 US participants of the Health Professional Follow-up Study who were free of diabetes and arthritis. RLS was assessed using a set of standardized questions recommended by the International RLS Study Group. PD cases were identified by self-reported questionnaires and confirmed by review of medical records. Compared to men without RLS, multivariate-adjusted odds ratios for PD were 1.1 (95% confidence interval: 0.4, 3.0) for men with RLS symptoms 5-14 times per month and 3.09 (95% confidence interval: 1.5, 6.2; P trend=0.003) for those with symptoms 15 times or more per month, after adjusting for age, smoking, use of antidepressant, and other covariates. In conclusion, men with RLS are more likely to have concurrent PD. Prospective studies are warranted to clarify the temporal relationship between RLS and PD.
Restless legs syndrome (RLS) is the most common movement disorder, affecting 5-15% adults. 1, 2 Because dopaminergic hypofunction in the central nervous system is involved in the disease pathophysiology of both RLS and Parkinson’s disease (PD),3 it has been suggested that RLS is a possible pre-clinical marker of PD.4 However, previous epidemiologic studies of RLS and PD generated inconsistent results. 5-7 We, therefore, conducted a cross-sectional analysis to examine whether men with RLS have a higher likelihood of having PD in the Health Professional Follow-up Study (HPFS), a large ongoing cohort of men.
Materials and methods
Study populations
The HPFS was established in 1986, when 51,529 male US health professionals (dentists, optometrists, osteopaths, podiatrists, pharmacists, and veterinarians) aged 40-75 years completed a mailed questionnaire about their medical history and lifestyle. Follow-up questionnaires have been mailed to participants every 2 years to update information on potential risk factors and to ascertain newly diagnosed diseases in both cohorts. The institutional review board at Brigham and Women’s Hospital reviewed and approved this study, and receipt of each questionnaire implies participant’s consent.
Assessment of RLS
We asked questions in 2002 about RLS symptoms and severity based on the International RLS Study Group criteria (n=37,431, mean age 68.7 ± 9 y) among participants who were still actively participating in the study.8, 9 The following question was asked: “Do you have unpleasant leg sensations (like crawling, paraesthesia, or pain) combined with motor restlessness and an urge to move? ” with the possible responses of: no; less than once/month; 2-4 times/month; 5-14 times/month; and 15 or more times per month. Those who answered that they had these feelings were asked the following two questions: 1) “Do these symptoms occur only at rest and does moving improve them?”; and 2) “Are these symptoms worse in the evening/night compared with the morning?” A participant who had symptoms 5-14 times per month and answered yes to the subsequent questions was considered to have RLS for these analyses.
The questions on RLS were completed by 31,729 (85%) men. Men who did not complete the RLS questions had a similar mean age to those who did (69.0 vs. 68.6 years), and a non-significant slightly higher prevalence of PD (0.95 vs. 0.62%). To reduce possible misclassification of RLS, we excluded participants with diabetes and arthritis, leaving 23,119 men in primary analyses. In a secondary analysis, we further examined the association between RLS and PD with including all participants with RLS information.
Assessment of PD and covariates
Assessment of PD has been described elsewhere. 10-13 Briefly, we identified new PD cases by biennial self-reported questionnaires. We then asked the treating neurologists to complete a questionnaire to confirm the diagnosis of PD or to send a copy of the medical records. A case was confirmed if a diagnosis of PD was considered definite or probable by the treating neurologist or internist, or if the medical record included either a final diagnosis of PD made by a neurologist, or evidence of at least two of the three cardinal signs (rest tremor, rigidity, bradykinesia) in the absence of features suggesting other diagnoses. Overall, the diagnosis was confirmed by the neurologist in >80% of the cases. PD cases included only confirmed definite and probable cases up to 2004.
Information on potential confounders, including age, ethnicity, smoking status, weight, height, physical activity, use of medicines, phobic anxiety scale, and history of major chronic diseases, was collected via biennial questionnaires throughout the follow-up period. Body mass index (BMI) was calculated as weight (kg) / height (m) 2. The phobic anxiety scale was assessed by the Crown-Crisp phobia index. 14-16
Statistical analyses
Statistical analyses were completed with SAS version 9.1 (SAS Institute, Inc, Cary, NC). We categorized participants into three groups: no RLS, RLS with symptoms 5-14 times per month, and RLS with symptoms 15 or more times per month. Logistic regression was used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) and to test differences in prevalence of PD across categories of RLS status. Analyses were adjusted for age (y), ethnicity (Caucasian, African-American, and Asian and others), BMI (<23, 23-24.9, 25-26.9, 27-29.9, or ≥30 kg/m2), smoking (never smoked, former smoker, or current smoker: cigarettes/d, 1-14 or ≥ 15), physical activity (quintiles), use of antidepressants (yes/no), the Crown-Crisp phobic anxiety index (0-1, 2, 3, or ≥4), and presence of stroke, hypertension, or myocardial infraction (each of them, yes/no). We examined potential effect modification of the association between RLS and PD by age (< or ≥ 70 years, approximate median value), obesity (yes/no, based on BMI ≥30 kg/m2), and smoking status (never versus ever), by including multiplicative terms in the logistic regression models, with adjustment for other potential confounders.
Results
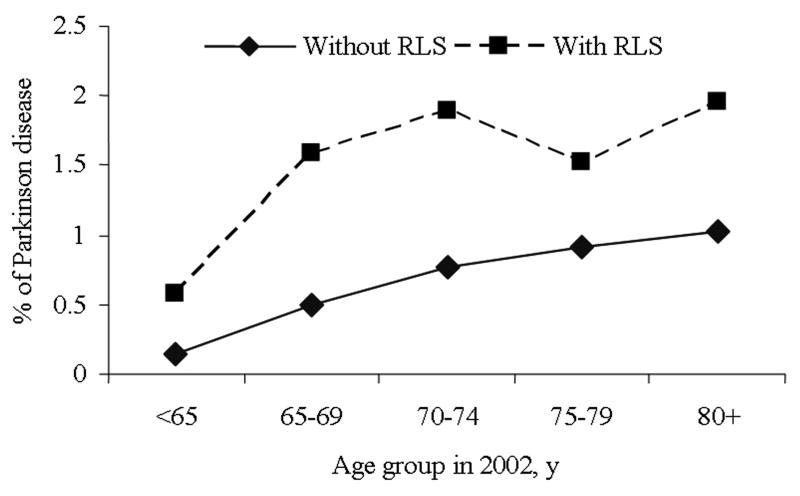
Men with RLS were older and more likely to be whites and current smokers, to use antidepressants, score higher on the anxiety test, have been diagnosed with hypertension and stroke, and have high BMI and low exercise levels than participants without RLS (Table 1). Men with RLS had a higher prevalence of PD relative to those without RLS in each age group (Figure). Compared to men without RLS, the OR for PD was 1.99 (95% CI: 1.1 to 3.6; P=0.02) for those with RLS symptoms, after adjusting for age, smoking, and other covariates. Higher frequency of RLS symptoms, a marker for the disease severity, was associated with increased prevalence of PD (Table 2). The multivariable-adjusted ORs for PD were 1.10 and 3.09 (95% CI: 1.5, 6.2; P for trend=0.003) for men with RLS symptoms 5-14 times per month, and 15 or more times per month, respectively. Among participants with PD, there was a non-significant difference in disease duration comparing those with RLS to those without RLS (9.0 ± 4.8 vs. 7.5 ± 4.2 y; P=0.7).
Table 1.
Basic characteristics according to restless legs syndrome status in 2002 in the Health Professionals Follow-up Study*
| Restless legs syndrome status in 2002
|
|||
|---|---|---|---|
| No RLS | RLS 5-14 times/mo | RLS 15+ times/mo | |
| n | 22175 | 549 | 395 |
| Age, y | 67.6 | 69.2 | 70.0 |
| Current smokers, % | 3.6 | 3.7 | 6.1 |
| Past smokers, % | 52.5 | 57.4 | 55.1 |
| African Americans, % | 0.6 | 0.5 | 0.9 |
| Asian & other ethnicity, % | 3.0 | 1.1 | 1.5 |
| BMI, kg/m2 | 25.9 | 26.4 | 26.2 |
| Physical activity, Mets/wk | 36.7 | 35.2 | 31.7 |
| Phobic anxiety index | 1.9 | 2.3 | 2.5 |
| Use of antidepressant, % | 4.4 | 8.0 | 11.3 |
| Presence of stroke in or prior to 2002, % | 1.3 | 2.4 | 2.9 |
| Presence of hypertension in or prior to 2002, % | 41.8 | 44.6 | 43.4 |
| Presence of myocardial infarction in or prior to 2002, % | 3.7 | 3.7 | 3.7 |
Values were standardized to the age distribution of the overall cohort.
Figure.
Prevalence of Parkinson’s disease (PD) according to restless legs syndrome status.
Table 2.
Odds ratios (ORs) and 95% confidence interval (CI ) of Parkinson’s disease according to restless legs syndrome status in the Health Professional Follow-up Study
| No RLS (n=22175) | RLS 5-14 times/mo (n=549) | RLS 15+ times/mo (n=395) | P trend | |
|---|---|---|---|---|
| # cases | 132 | 4 | 9 | |
| Age adjusted OR | 1(ref.) | 1.10 (0.40, 2.98) | 3.24 (1.63,6.44) | 0.002 |
| Multivariate adjusted OR1 | 1(ref.) | 1.10 (0.41, 3.03) | 3.09 (1.54,6.19) | 0.003 |
Logistic regression models were used to calculate ORs, adjusted for age (in years), smoking status (never smoker, former smoker, or current smoker: cigarettes/d, 1-14 or ≥15), BMI (<23, 23-24.9, 25-26.9, 27-29.9, or ≥30 kg/m2), use of antidepressant drugs (yes/no), physical activity (quintiles), the Crown-Crisp phobic anxiety index (0-1, 2, 3, or ≥4) and presence of stroke, hypertension, or myocardial infraction (each of them, yes/no)
Similar significant results were observed in several sensitivity analyses. Multivariable-adjusted ORs did not materially change after excluding participants with the highest level of phobic anxiety, with MI, stroke, PD or hypertension, or those who used antidepressant (data not shown). Further inclusion of participants with diabetes or arthritis did not change the association between RLS and PD (OR=2.16; 95% CI: 1.2, 3.9). We did not find significant interaction between presence of RLS and age, obesity, and smoking status (P interaction >0.2 for all), in relation to prevalence of PD.
Discussion
In this large cohort of men, we observed that men with RLS had a higher prevalence of PD than those without RLS, across all age groups. Compared to men without RLS, those who reported having RLS symptoms 15 or more times per month had approximately three-fold higher prevalence of PD. Strengths of the current study include a large sample size, which enabled us to obtain a relatively stable estimate for the associations, and use of standardized questionnaire to assess RLS. As we did not collect information on several RLS-like syndrome (e.g., peripheral neuropathy, leg cramps, positional discomfort, radiculopathy), some misclassification in RLS assessment is possible. However, results were similar when we included or excluded men with diabetes, the most common cause of peripheral neuropathy, in our analyses. Another limitation is that we included only men and therefore our results cannot be generalized to women. Further, because of the cross-sectional design of our study, we are not able to know whether RLS occured before onset of PD or vice versa.
Associations between RLS and PD have been noticed for long time; 17, 18 both conditions are associated with dopamine hypofunction in CNS. Our findings are consistent with the results of some previous epidemiological studies,5, 6, 19 but not others.7 In a sample of 125 PD patients in Singapore, Tan et al reported that none of them met IRLSSG diagnostic criteria of RLS.7 However, recently, Loo and Tan found a marginally significant higher prevalence of RLS among PD cases (n=400) than controls (3% vs. 0.5%; P=0.07) in Singapore.19 In a cross-sectional study by Ondo et al, 20.8% of 303 PD patients had RLS symptoms.20 In a study examining prevalence of PD among RLS patients,21 Walters et al. found that 4 out of 85 RLS cases (4.7%) had PD, compared to ~1% PD prevalence expected among the general population over age 60. A recent report showed that in a family with a high prevalence of RLS, two (6.7%) out of 30 family member with RLS also had PD.22 However, none of these three studies included control groups. Interestingly, a recent genome-wide association study found that MEIS1, a gene involved in embryonic development of substantia nigra, was associated with RLS risk.23 The relation between MEIS1 and PD risk has only been reported in one case-control study, and was not significant. 24
In conclusion, we found a concurrence between RLS and PD in men. Further prospective studies are warranted to clarify whether the presence of RLS precedes onset of classic motor symptom of PD; if so, screening for RLS could help to identify individuals at high risk for PD.
Acknowledgments
AUTHOR ROLES:
1. Research project: A. Conception, B. Organization, C. Execution;
2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique;
3. Manuscript: A. Writing of the first draft, B. Review and Critique
Gao: 1A, 1B, 1C, 2A, 2B, 3A
Schwarzschild, 1A, 2C, 3B
O’Reilly: 1C, 2C, 3B
Wang: 1C, 2C, 3B
Ascherio: 1A, 1B, 2A, 2C, 3B
Finding/Support: The study was supported by NIH/NINDS grant R01 NS048517 and NHI/NINDS grant R01 NS062879-01A2. None of the sponsors participated in the design of study or in the collection, analysis, or interpretation of the data.
Full Financial Disclosures of all Authors for the Past Year
| Gao: | |
| Stock Ownership in medically-related fields | None |
| Intellectual Property Rights | None |
| Consultancies | None |
| Expert Testimony | None |
| Advisory Boards | Dr. Gao serves on the Monitoring Committee of the Parkinson Study Group |
| Partnerships | None |
| Contracts | None |
| Employment | Instructor in Medicine at Harvard Medical School; Research Scientist at Harvard School of Public Health; Associate Epidemiologist, Brigham and Women’s Hospital |
| Honoraria | None |
| Royalties | None |
| Grants | PI for NHI/NINDS grant “Prospective study of restless legs syndrome” (R01 NS062879-01A2) |
| Schwarzschild: | |
| Stock Ownership in medically-related fields | None |
| Intellectual Property Rights | None |
| Consultancies | None |
| Expert Testimony | None |
| Advisory Boards | None |
| Partnerships | None |
| Contracts | None |
| Employment | Associate Professor in Medicine at Harvard Medical School and Mass General Hospital |
| Honoraria | Non-industry-sponsored speaker honoraria |
| Royalties | None |
| Grants | PI for NHI/NINDS grant (K24 NS060991, R01 NS 054978, R21 NS058324), the US Department of Defense (W81XHW-04-1-0881) and the Michael J Fox Foundation, the Parkinson Disease Foundation, the RJG Parkinson’s disease Foundation, and the American Federation for Aging Research. |
| O’Reilly: | |
| Stock Ownership in medically-related fields | None |
| Intellectual Property Rights | None |
| Consultancies | None |
| Expert Testimony | None |
| Advisory Boards | None |
| Partnerships | None |
| Contracts | None |
| Employment | Research fellow at Harvard School of Public Health |
| Honoraria | None |
| Royalties | None |
| Grants | None |
| Wang: | |
| Stock Ownership in medically-related fields | None |
| Intellectual Property Rights | None |
| Consultancies | None |
| Expert Testimony | None |
| Advisory Boards | None |
| Partnerships | None |
| Contracts | None |
| Employment | Research fellow at Harvard School of Public Health |
| Honoraria | None |
| Royalties | None |
| Grants | None |
| Ascherio: | |
| Stock Ownership in medically-related fields | None |
| Intellectual Property Rights | None |
| Consultancies | None |
| Expert Testimony | None |
| Advisory Boards | Dr. Ascherio serves on the scientific advisory board of the Michael J Fox Foundation |
| Partnerships | None |
| Contracts | None |
| Employment | Professor in Nutrition and Epidemiology at Harvard School of Public Health and Professor in Medicine at Harvard Medical School |
| Honoraria | None |
| Royalties | None |
| Grants | PI for NIH (R01 NS045893, R01 NS047467, R01NS48517, R01 NS042194, and R01 NS046635) and the US department of Defense (W81XWH-05-1-0117) |
Footnotes
Financial Disclosure/Competing Interests: The authors have no financial disclosures to make, and no competing interests.
References
- 1.Kushida CA. Clinical presentation, diagnosis, and quality of life issues in restless legs syndrome. Am J Med. 2007;120(1 Suppl 1):S4–S12. doi: 10.1016/j.amjmed.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4(2):101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 3.Allen RP. Controversies and challenges in defining the etiology and pathophysiology of restless legs syndrome. Am J Med. 2007;120(1 Suppl 1):S13–21. doi: 10.1016/j.amjmed.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhuri KR, Healy DG, Schapira AH. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5(3):235–245. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 5.Nomura T, Inoue Y, Miyake M, Yasui K, Nakashima K. Prevalence and clinical characteristics of restless legs syndrome in Japanese patients with Parkinson’s disease. Mov Disord. 2006;21(3):380–384. doi: 10.1002/mds.20734. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan PR, Bhatia M, Behari M. Restless legs syndrome in Parkinson’s disease: a case-controlled study. Mov Disord. 2003;18(2):181–185. doi: 10.1002/mds.10307. [DOI] [PubMed] [Google Scholar]
- 7.Tan EK, Lum SY, Wong MC. Restless legs syndrome in Parkinson’s disease. J Neurol Sci. 2002;196(1-2):33–36. doi: 10.1016/s0022-510x(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 8.Gao X, Schwarzschild MA, Wang H, Ascherio A. Obesity and restless legs syndrome in men and women. Neurology. 2009;72:1255–1261. doi: 10.1212/01.wnl.0000345673.35676.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao X, Schwarzschild MA, O’Reilly EJ, Wang H, Ascherio A. Restless legs syndrome and erectile dysfunction. Sleep. 2010;33(1):75–79. doi: 10.1093/sleep/33.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ascherio A, Zhang SM, Hernan MA, et al. Prospective study of caffeine consumption and risk of Parkinson’s disease in men and women. Ann Neurol. 2001;50(1):56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- 11.Gao X, Chen H, Fung TT, et al. Prospective study of dietary pattern and risk of Parkinson disease. Am J Clin Nutr. 2007;86(5):1486–1494. doi: 10.1093/ajcn/86.5.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao X, Chen H, Choi HK, Curhan G, Schwarzschild MA, Ascherio A. Diet, urate, and Parkinson’s disease risk in men. Am J Epidemiol. 2008;167(7):831–838. doi: 10.1093/aje/kwm385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao X, Chen H, Schwarzschild MA, Logroscino G, Ascherio A. Perceived imbalance and risk of Parkinson’s disease. Mov Disord. 2008;23(4):613–616. doi: 10.1002/mds.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawachi I, Colditz GA, Ascherio A, et al. Prospective study of phobic anxiety and risk of coronary heart disease in men. Circulation. 1994;89(5):1992–1997. doi: 10.1161/01.cir.89.5.1992. [DOI] [PubMed] [Google Scholar]
- 15.Albert CM, Chae CU, Rexrode KM, Manson JE, Kawachi I. Phobic anxiety and risk of coronary heart disease and sudden cardiac death among women. Circulation. 2005;111(4):480–487. doi: 10.1161/01.CIR.0000153813.64165.5D. [DOI] [PubMed] [Google Scholar]
- 16.Weisskopf MG, Chen H, Schwarzschild MA, Kawachi I, Ascherio A. Prospective study of phobic anxiety and risk of Parkinson’s disease. Mov Disord. 2003;18(6):646–651. doi: 10.1002/mds.10425. [DOI] [PubMed] [Google Scholar]
- 17.Sandyk R, Iacono RP, Bamford CR. Spinal cord mechanisms in amitriptyline responsive restless legs syndrome in Parkinson’s disease. Int J Neurosci. 1988;38(1-2):121–124. doi: 10.3109/00207458809000489. [DOI] [PubMed] [Google Scholar]
- 18.Lang AE. Restless legs syndrome and Parkinson’s disease: insights into pathophysiology. Clin Neuropharmacol. 1987;10(5):476–478. doi: 10.1097/00002826-198710000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Loo HV, Tan EK. Case-control study of restless legs syndrome and quality of sleep in Parkinson’s disease. J Neurol Sci. 2008;266(1-2):145–149. doi: 10.1016/j.jns.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 20.Urata Y, Yamaguchi M, Higashiyama Y, et al. Reactive oxygen species accelerate production of vascular endothelial growth factor by advanced glycation end products in RAW264.7 mouse macrophages. Free Radic Biol Med. 2002;32(8):688–701. doi: 10.1016/s0891-5849(01)00823-1. [DOI] [PubMed] [Google Scholar]
- 21.Walters AS, LeBrocq C, Passi V, et al. A preliminary look at the percentage of patients with Restless Legs Syndrome who also have Parkinson Disease, Essential Tremor or Tourette Syndrome in a single practice. J Sleep Res. 2003;12(4):343–345. doi: 10.1046/j.0962-1105.2003.00368.x. [DOI] [PubMed] [Google Scholar]
- 22.Young JE, Vilarino-Guell C, Lin SC, Wszolek ZK, Farrer MJ. Clinical and genetic description of a family with a high prevalence of autosomal dominant restless legs syndrome. Mayo Clin Proc. 2009;84(2):134–138. doi: 10.4065/84.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winkelmann J, Schormair B, Lichtner P, et al. Genome-wide association study of restless legs syndrome identifies common variants in three genomic regions. Nat Genet. 2007;39(8):1000–1006. doi: 10.1038/ng2099. [DOI] [PubMed] [Google Scholar]
- 24.Vilarino-Guell C, Soto AI, Young JE, et al. Susceptibility genes for restless legs syndrome are not associated with Parkinson disease. Neurology. 2008;71(3):222–223. doi: 10.1212/01.wnl.0000317101.67684.e3. [DOI] [PubMed] [Google Scholar]