Abstract
We recently identified a new component of flavonoid transport pathways in Arabidopsis. The MATE protein FFT (Flower Flavonoid Transporter) is primarily found in guard cells and seedling roots, and mutation of the transporter results in floral and growth phenotypes. The nature of FFT's substrate requires further exploration but our data suggest that it is a kaempferol diglucoside. Here we discuss potential partner H+-ATPases and possible redundancy among the close homologs within the large Arabidopsis MATE family.
Key words: auxin, flavonoid, guard cell, pollen, transporter
Plant flavonoids are becoming notorious for their wide and expanding range of possible functions. Beyond UV protection (itself not entirely without debate), further roles have been added in plant development; nodulation and interactions with pathogens; fertilization; and auxin transport. For such a well-described biochemical network, it interesting that few aspects of flavonoid function are clear-cut: perhaps it is the recently established link with auxin, so intimately involved in every aspect of plant development, that consigns them to multiple incompletely-known regulatory pathways. Knowledge is lacking, in particular, about the transport of flavonoids. Such transport is necessary1 and we now know that selective uptake of flavonoids and movement of flavonoids through the plant occur.2,3 When naringenin, dihydrokaempferol and dihydroquercetin were added to the Arabidopsis tt4 mutant [lacking the enzyme chalcone synthase (CHS) and thus all flavonoids] at root tip, mid-root or to cotyledons, they were converted to downstream products. Grafting on flavonoid-producing tissues to tt4 could also complement the mutation.3
Kitamura4 and Buer et al.5 speculate that MATE transporters are good candidates to enable flavonoid transport at the membrane, allowing the necessary movement from one membrane system to another. The link between MATE proteins and flavonoid transport is justified by work in tomato6 and confirmed by the discovery of TT12.7,8 Also conforming to this premise is our recent work on FFT (Flower Flavonoid Transporter), a MATE protein probably situated in the tonoplast membrane that has a role in flavonoid transport in specialised guard cells and anthers.9
Auxin and Flavonoids
The phenotype of the fft-1 mutant adds to the numerous recent descriptions of flavonoids influencing auxin transport. The mutant grew faster than wild type in all conditions we tested. Since Arabidopsis seedlings are very small the difference is subtle but was unvarying and statistically significant up to about 2 weeks post-germination, and culminated in fft-1 flowering earlier than wild type. The most notable effects of the fft-1 mutation are floral, namely lack of anther dehiscence and reduced pollen viability, but even these cannot be entirely dissociated from an auxin phenotype.10
We do not have exhaustive data on the substrate for FFT, but LC-MS comparing fft-1 mutant and Col-0 A. thaliana showed significant changes in the level of a kaempferol diglucoside in some floral tissues, among a range of perturbed flavonol glycosides. Although FFT promoter-GUS reporter plants showed strong, specific staining in root tissues, the faster root growth of seedlings remains unexplained, since we did not find differences in flavonoids outside floral tissues in our analyses. Both roots and floral tissues require careful dissection and visualization to confirm and quantify better the effects on the various kaempferol and quercitin compounds that were altered in fft-1. It would also be interesting to attempt to complement the mutation with a range of flavonoid compounds, and to visualize GUS expression following transformation of fft-1 plants with a dr5 auxin reporter construct.
Flavonoids and Pollen
As mentioned above, fft-1 plants recovered over time, appearing wild type in the mature vegetative phase. Early flowers are profoundly affected by the lesion, however, with anthers frequently nondehiscent and containing little or no viable pollen. Only low numbers of seeds are produced in siliques on the primary inflorescence. The importance of flavonoids for pollen development and fertility in maize and petunia is well known.11,12 In contrast, in a surprising paper in 1996, Ylstra et al. showed that the tt4 mutant was fertile, with normal pollen development.13 Arabidopsis has been seen since as a rare exception among the flowering plants, in being able to produce viable pollen in the apparent absence of flavonoids. Reduced fertility in fft-1 contradicts the received wisdom on flavonoids in Arabidopsis to some degree, in suggesting that perturbation of flavonoid metabolism is certainly capable of interfering with the plant's fertility. It is possible that the solution lies in the detail, with Arabidopsis having mechanisms to compensate for the absence of flavonoids, but lacking the mechanisms to adjust to alterations in the types and proportions of flavonoids present.
Homologs
The MATE family of proteins clearly transport diverse compounds, from the organic cation tetraethylammonium by a human family member, antibiotics by prokaryotic forms, through to citrate secretion by FRD3 in Arabidopsis.14 Now more whole-genome sequences are being completed, we can see homologues of FFT in many other plants. The most closely related proteins currently seen in plants are strikingly similar, the most similar of which are a 75% identical Ricinus communis putative multidrug resistance protein (EEF49069); the 72% identical Solanum lycopersicum putative anthocyanin permease discussed by Matthews et al. (2003)6 and in our paper;9 and a 70% identical Vitis vinifera hypothetical protein. Others are now seen in Populis trichocarpa and Oryza sativa. The most similar A. thaliana proteins to FFT (DTX35) are 67% identical (At4g00350; DTX34), followed by 51% (At1g47530, DTX33; tagged as a “ripening responsive protein”), 50% (At5g38030, DTX30), and 48% identical (At3g26590, DTX29) family members, but these are part of a large family of 50+ putative proteins, as often seen in this species (for review, see Yazaki, 2005).15
Redundancy
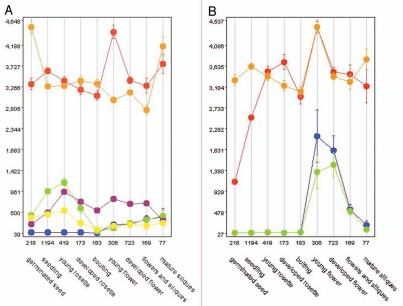
At1g47530/DTX33 and FFT transcription profiles are similar in collected microarrays. The microarray data showing widespread transcription for FFT in the profiling application, Genevestigator,16 correspond with our RT-PCR, namely in the lateral root and root elongation zone; senescent and mature leaves; and floral tissues especially sepal, petal and siliques (Fig. 1). Although alike in many microarrays and throughout development, where FFT has a peak in the young flower, At1g47530/DTX33 peaks in the developing seedling and appears, in this computer-based analysis, to be more highly expressed in roots. In the Arabidopsis gene expression database (www.arexdb.org17) At1g47530/DTX33 indeed shows very strong expression in the root cortex (Fig. 2).
Figure 1.
(A) Genevestigator developmental expression profiles of FFT (red), At4g00350/DTX34 (blue), At5g38030, DTX30 (green), At1g47530/DTX33 (orange), At3g26590/DTX29 ORFs (purple and yellow). (B) FFT (orange) with AT Pases At5g57350/ATPase 3 (red), At2g07560/AHA6 (blue) and At1g80660/AHA9 (green).
Figure 2.
Arexdb expression profiles in roots of FFT (At4g25640), At4g00350/DTX34, At5g38030/DTX30, At1g47530/DTX33, At3g26590/DTX29 MATE proteins with AT Pases At5g57350/ATPase 3, At2g07560/AHA6 and At1g80660/AHA9.
Interestingly, the most similar Arabidopsis MATE to FFT at sequence level, At4g00350/DTX34, has a large peak of expression in pollen, contrasting both with the FFT bioinformatics and our experimental data, neither of which show FFT transcript in pollen. At4g00350/DTX34 is described in TAIR (www.arabidopsis.org/index.jsp) as being found in mature and germinated pollen, petal differentiation and expansion stage, anthesis, and the globular stage embryo. In general, bioinformatics data suggest that At4g00350/DTX34 expression resembles that of the other close homologue At3g26590/DTX29, although the latter is missing the peak in pollen. These possible transcriptional similarities are interesting when thinking of the potential redundancy seen in the fft-1 phenotype and might guide future work. Notably, the expression patterns of many of this group of similar proteins seem to feature in anthesis, corresponding with the phenotype of fft-1.
Flavonoids in Guard Cells and the Anther
A GUS-promoter construct showed that guard cells are the likely principal location of the FFT transcript in aerial tissues. This is not surprising since much of a plant's phenylpropanoid and flavonoid content is found in the vacuoles of guard cells, which are central to UV protection and environmental regulation.18,19 The presence of FFT transcript in the hydathode, anther and nectaries is notable—are special roles required of flavonoids in these specialised guard cells? Of interest here too is the proposal that a partner for the TT12 MATE is the proton pumping-ATPase AHA10 (Arabidopsis H+-ATPase), which creates the gradient for active transport by TT12.20 Notably, there is a similar H+-ATPase, AHA9, that is expressed exclusively in anther tissues.21 Whether this forms an equivalent system in anthers with FFT, like that of TT12/AHA10 in seedcoats, requires investigation.
We therefore paired FFT with AHA transcripts in Genevestigator, to find the most similar profile to that of FFT (Fig. 1). At1g80660/AHA9 and FFT both display peaks of expression that correspond with development of the young flower, also matched by At5g57350/ATPase 3 and At2g07560/AHA6. Arexdb shows a different picture in root transcription (Fig. 2).
It would be of interest to further analyse the temporal expression of FFT in the floral organs to discover any synchronisation with anthesis. FFT transcription may also be affected by transcription factors such as CRABS CLAW which are required for anther development.22 More technically challenging would be finding which flavonoids occur in the anther endothecium (thus concerned with dehiscence), or the tapetum (i.e., compounds needed for microsporogenesis and pollen development). Any overlap in expression between different MATE proteins may allow redundancy in some cell types but not in others; for example, fft-1's most profound phenotype is in the anther although its transcript is also found in guard cells in the vegetative tissue of young seedlings, where the mutation did not have any obvious effect (besides the subtle changes in germination and growth at this stage).
Overall, although much work remains to be done to establish the details of flavonoid transport in particular tissues, the transport of specific flavonoids by different transporters can be seen to add another level of control to the complex pathways through which plant metabolites influence developmental programmes.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/11894
References
- 1.Kubasek WL, Shirley BW, McKillop A, Goodman HM, Briggs W, Ausubel FM. Regulation of flavonoid biosynthetic genes in germinating Arabidopsis seedlings. Plant Cell. 1992;4:1229–1236. doi: 10.1105/tpc.4.10.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buer CS, Muday GK, Djordjevic MA. Flavonoids are differentially taken up and transported long distances in Arabidopsis. Plant Physiol. 2007;145:478–490. doi: 10.1104/pp.107.101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buer CS, Muday GK, Djordjevic MA. Implications of long-distance flavonoid movement in Arabidopsis thaliana. Plant Signal Behav. 2008;3:415–417. doi: 10.4161/psb.3.6.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitamura S. In: Transport of flavonoids. In The Science of Flavonoids. Grotewold E, editor. New York: Springer; 2006. pp. 123–146. [Google Scholar]
- 5.Buer CS, Imin N, Djordjevic MA. Flavonoids: new roles for old molecules. J Integr Plant Biol. 2010;52:98–111. doi: 10.1111/j.1744-7909.2010.00905.x. [DOI] [PubMed] [Google Scholar]
- 6.Mathews H, Clendennen SK, Caldwell CG, et al. Activation tagging in tomato identifies a transcriptional regulator of anthocyanin biosynthesis, modification and transport. Plant Cell. 2003;15:1689–1703. doi: 10.1105/tpc.012963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Debeaujon I, Peeters AJM, Léon-Kloosterziel KM, Koornneef M. The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell. 2001;13:853–871. doi: 10.1105/tpc.13.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marinova K, Pourcel L, Weder B, et al. The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+-antiporter active in proanthocyanidin-accumulating cells of the seed coat. Plant Cell. 2007;19:2023–2038. doi: 10.1105/tpc.106.046029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson E, Wilkins C, Demidchik V, Davies JM, Glover BJ. An Arabidopsis flavonoid transporter is required for anther dehiscence and pollen development. J Exp Bot. 2010;61:439–451. doi: 10.1093/jxb/erp312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cecchetti V, Altamura MM, Falasca G, Costantino P, Cardarelli M. Auxin regulates Arabidopsis anther dehiscence, pollen maturation and filament elongation. Plant Cell. 2008;20:1760–1774. doi: 10.1105/tpc.107.057570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mo Y, Nagel C, Taylor LP. Biochemical complementation of chalcone synthase mutants defines a role for flavonols in functional pollen. Proc Natl Acad Sci USA. 1992;89:7213–7217. doi: 10.1073/pnas.89.15.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ylstra B, Busscher J, Franken J, Hollman PCH, Mol JNM, van Tunen AJ. Flavonols and fertilization in Petunia hybrid—localization and mode of action during pollen tube growth. Plant J. 1994;6:201–212. [Google Scholar]
- 13.Ylstra B, Muskens M, van Tunen AJ. Flavonols are not essential for fertilization in Arabidopsis. Plant Mol Biol. 1996;32:1155–1158. doi: 10.1007/BF00041399. [DOI] [PubMed] [Google Scholar]
- 14.Durrett TP, Gassmann W, Rogers EE. The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiol. 2007;144:197–205. doi: 10.1104/pp.107.097162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yazaki K. Transporters of secondary metabolites. Curr Opin Plant Biol. 2005;8:301–307. doi: 10.1016/j.pbi.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Hruz T, Laule O, Szabo G, et al. Genevestigator V3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinformatics. 2008:420747. doi: 10.1155/2008/420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birnbaum K, Shasha DE, Wang JY, et al. A gene expression map of the Arabidopsis root. Science. 2003;302:1956–1960. doi: 10.1126/science.1090022. [DOI] [PubMed] [Google Scholar]
- 18.Gitz DC, Liu-Gitz L. How do UV photomorphogenic responses confer water stress tolerance? Photochem Photobiol. 2003;78:529–534. doi: 10.1562/0031-8655(2003)078<0529:hduprc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Hutzler P, Fischbach R, Heller W, et al. Tissue localization of phenolic compounds in plants by confocal laser scanning microscopy. J Exp Bot. 1998;49:953–965. [Google Scholar]
- 20.Baxter IR, Young JC, Armstrong G, et al. A plasma membrane H+-ATPase is required for the formation of proanthocyanidins in the seed coat endothelium of Arabidopsis thaliana. Proc Natl Acad Sci USA. 2005;102:2649–2654. doi: 10.1073/pnas.0406377102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houlné G, Boutry M. Identification of an Arabidopsis thaliana gene encoding a plasma membrane H+-ATPase whose expression is restricted to anther tissues. Plant J. 1994;5:311–317. doi: 10.1111/j.1365-313x.1994.00311.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee J-Y, Baum SF, Alvarez J, Patel A, Chitwood DH, Bowman JL. Activation of CRABS CLAW in the nectaries and carpels of Arabidopsis. Plant Cell. 2005;17:25–36. doi: 10.1105/tpc.104.026666. [DOI] [PMC free article] [PubMed] [Google Scholar]