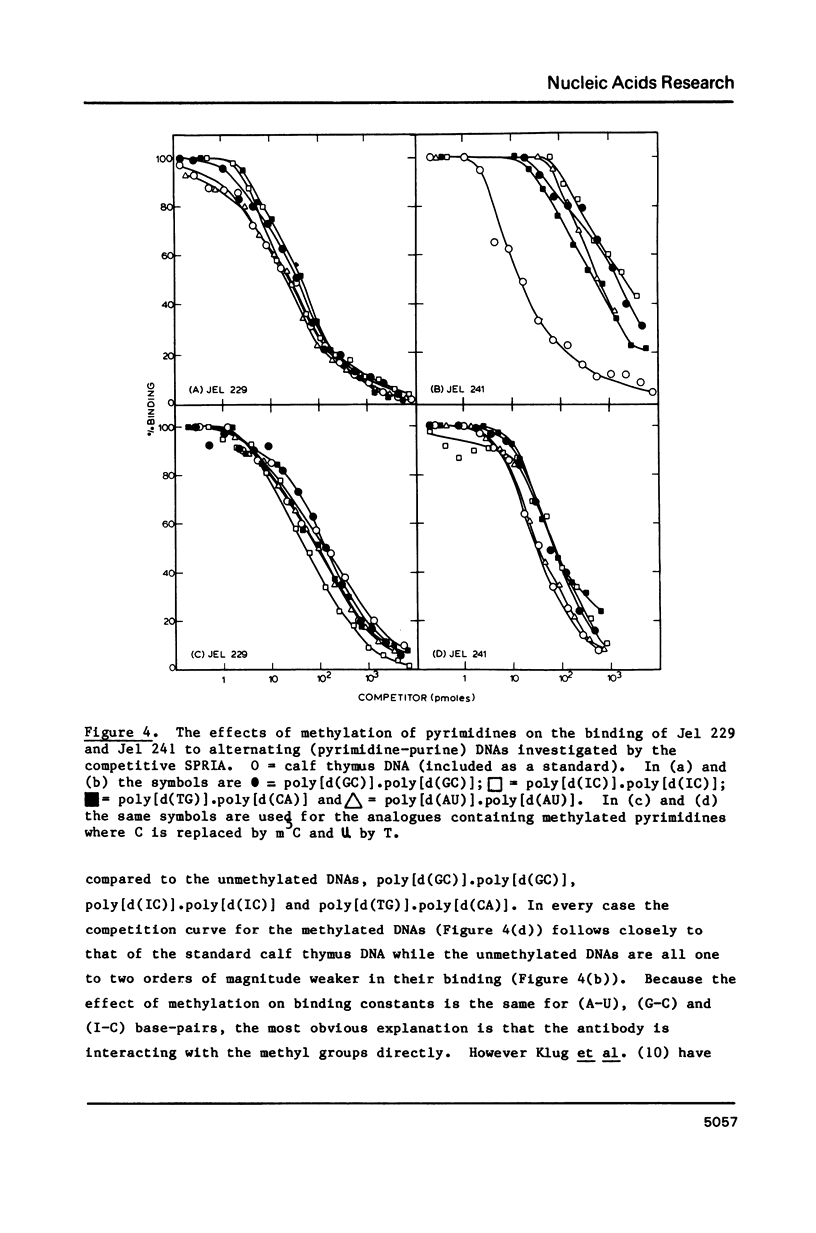
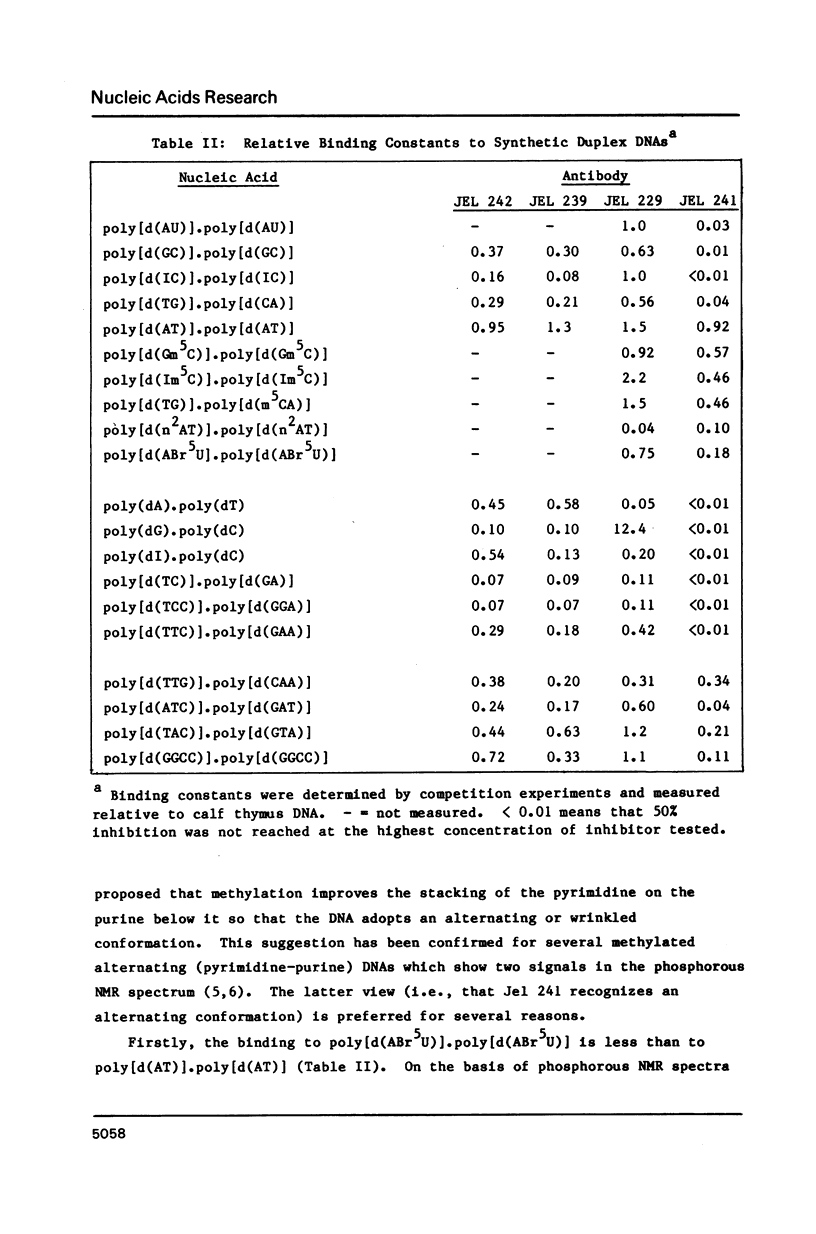
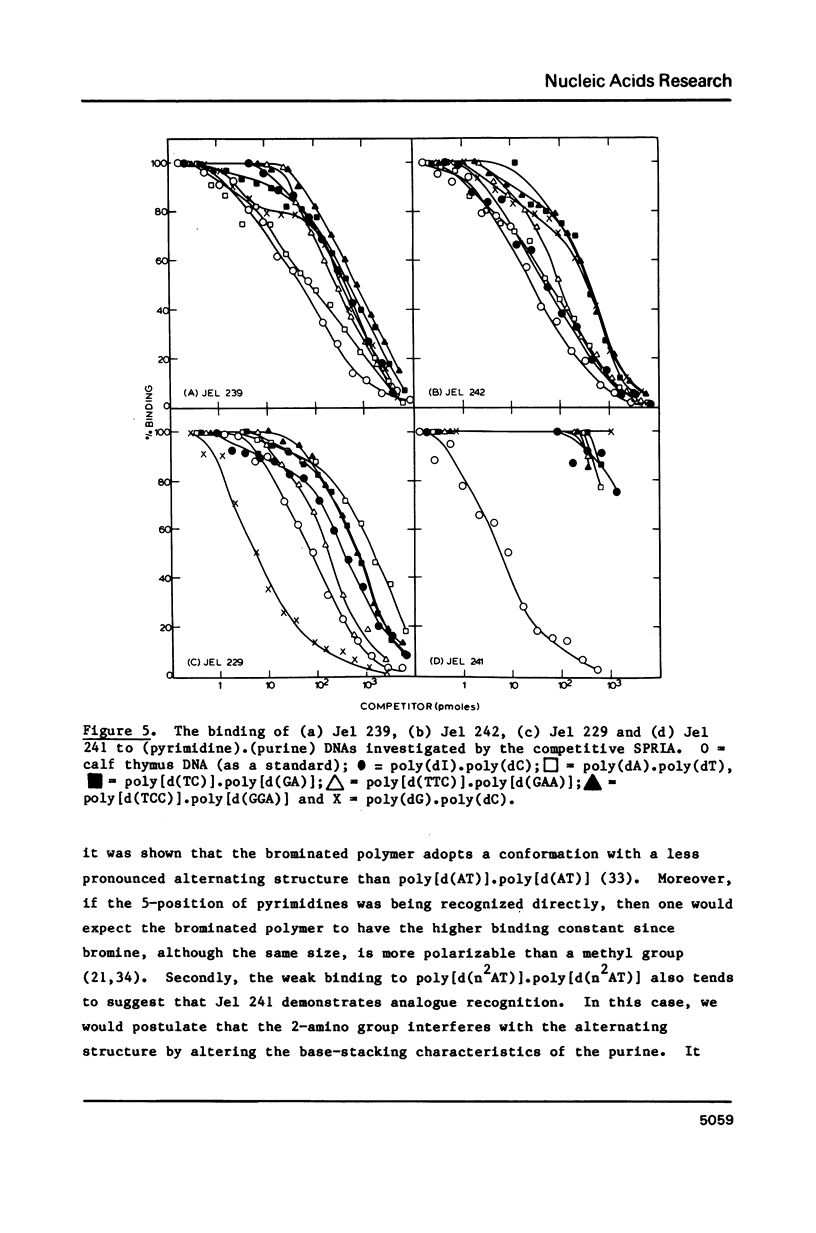
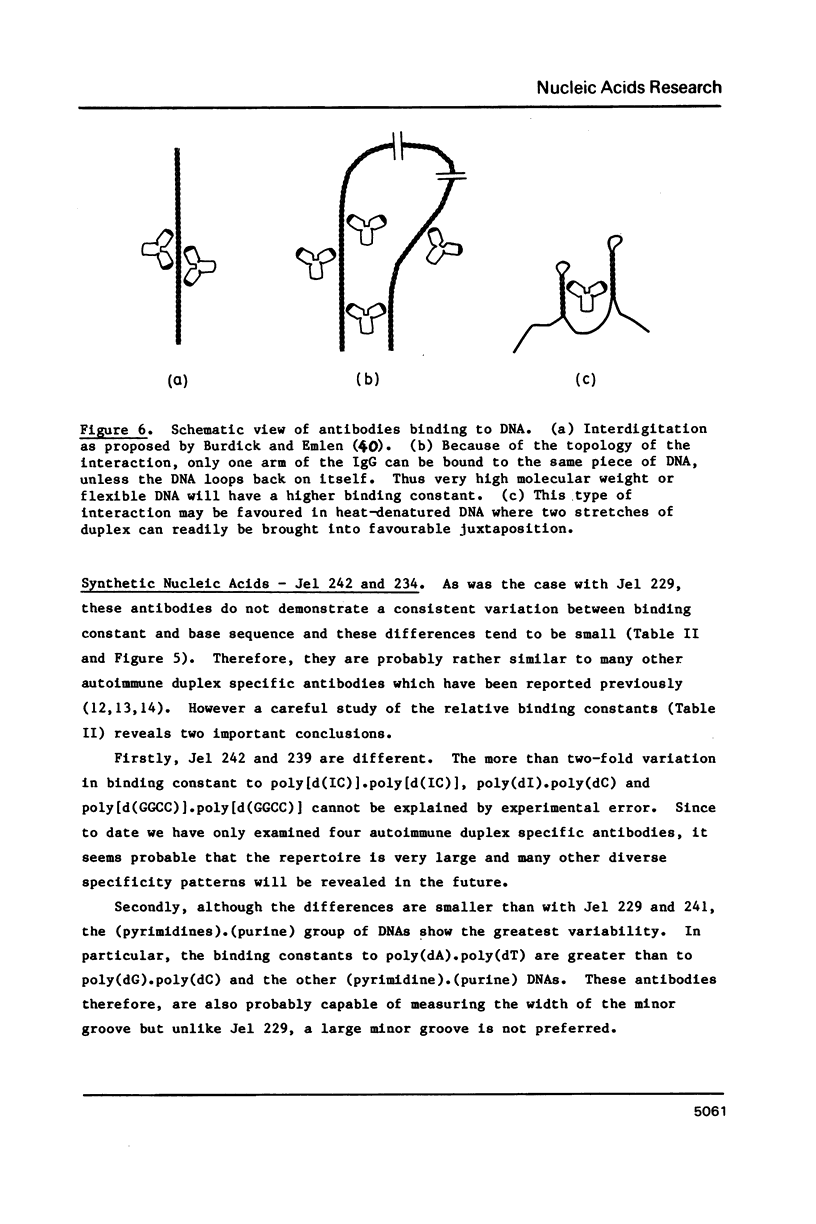
Abstract
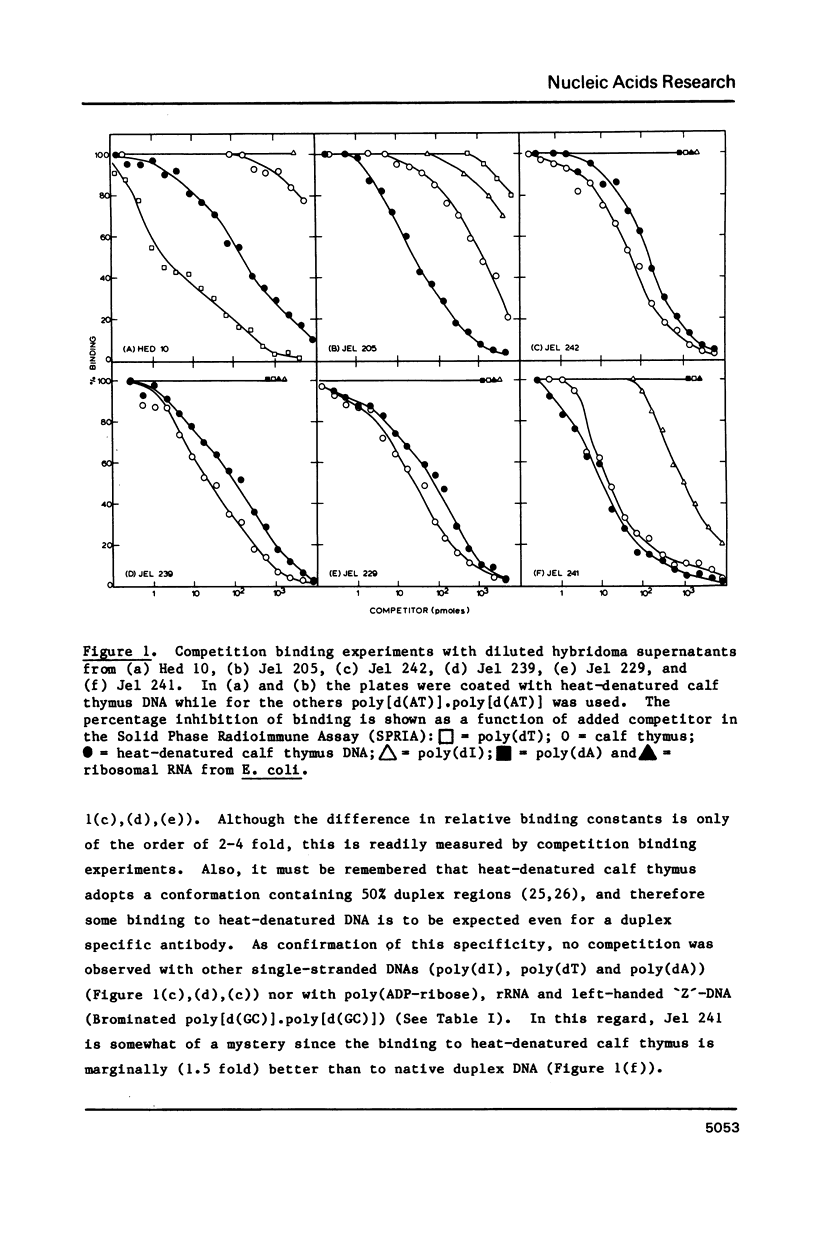
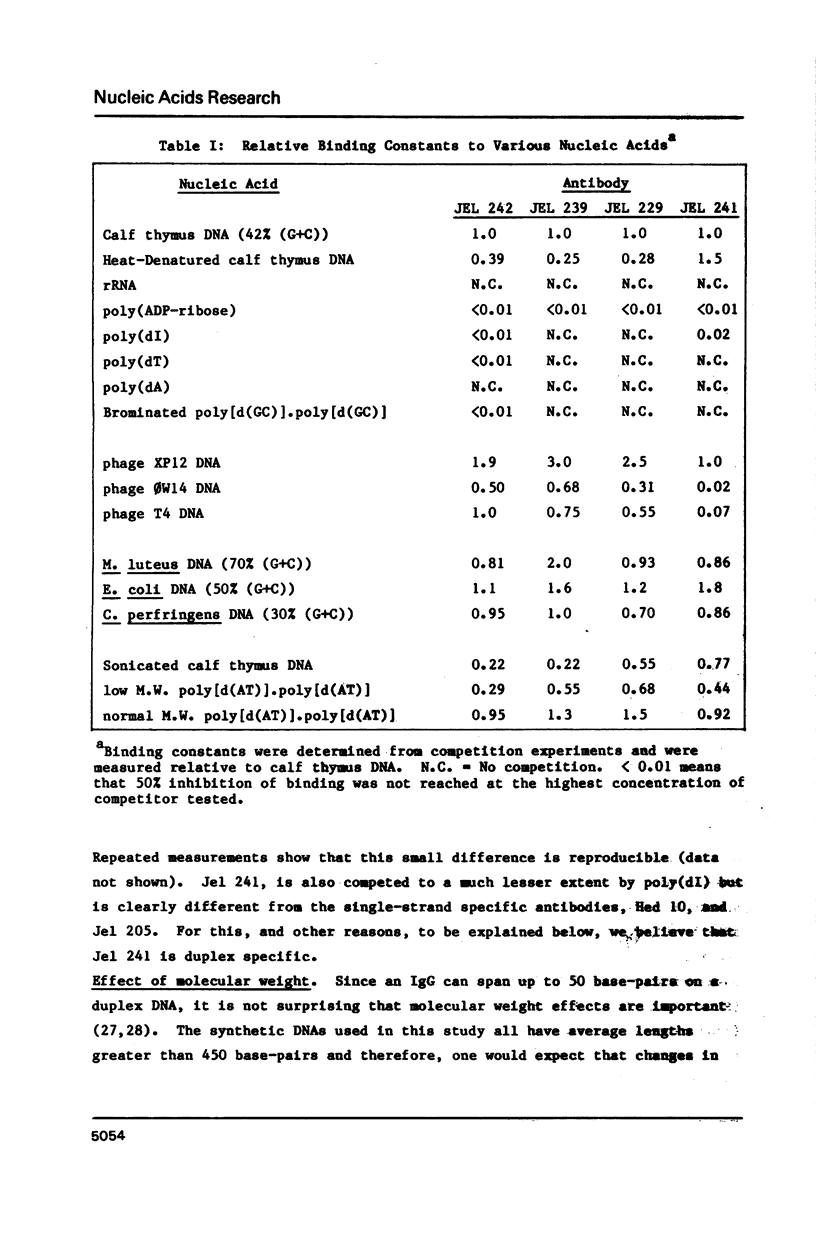
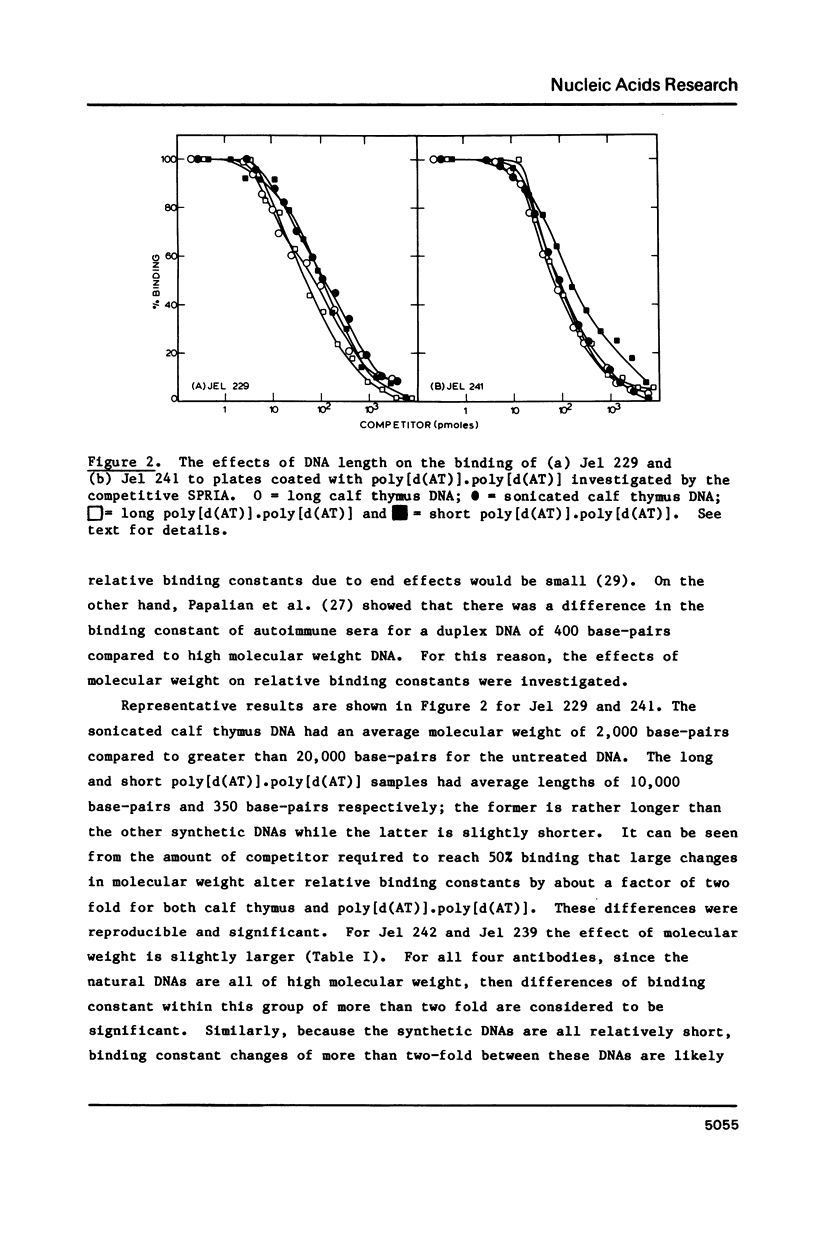
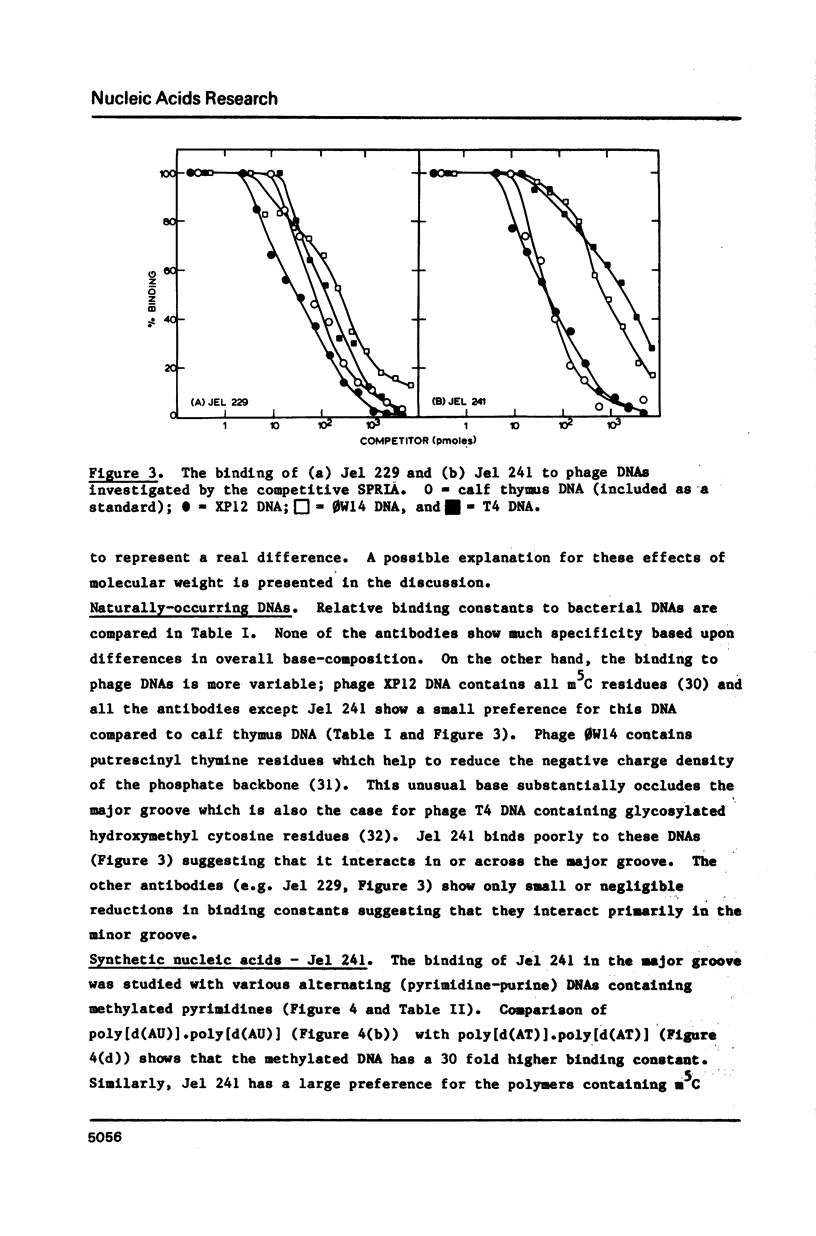
Four monoclonal antibodies (Jel 229, 239, 241, 242) which bound to duplex DNA were prepared from two autoimmune female NZB/NZW mice. Their binding to various nucleic acids was investigated by a competitive solid phase radioimmune assay which allows the estimation of relative binding constants. None of the antibodies showed any consistent variation of binding constant with base composition and thus they must recognize features of the DNA backbone. Jel 241 binds across the major groove but the interaction with poly(pyrimidine) X poly(purine) DNAs was barely detectable. This antibody appears to recognize the "alternating-B" conformation which is promoted by methylation of pyrimidines in alternating sequences. The other three antibodies bind in the minor groove. In particular, for Jel 229 the preferred antigen was poly(dG) X poly(dC) with only weak binding to poly(dA) X poly(dT). This suggests a requirement for a wide minor groove. Thus autoimmune antibodies provide examples of "analogue" recognition and can be used to detect structural variations in the grooves of duplex DNA.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali R., Dersimonian H., Stollar B. D. Binding of monoclonal anti-native DNA autoantibodies to DNA of varying size and conformation. Mol Immunol. 1985 Dec;22(12):1415–1422. doi: 10.1016/0161-5890(85)90065-3. [DOI] [PubMed] [Google Scholar]
- Andrzejewski C., Jr, Rauch J., Lafer E., Stollar B. D., Schwartz R. S. Antigen-binding diversity and idiotypic cross-reactions among hybridoma autoantibodies to DNA. J Immunol. 1981 Jan;126(1):226–231. [PubMed] [Google Scholar]
- Arnott S., Chandrasekaran R., Hall I. H., Puigjaner L. C. Heteronomous DNA. Nucleic Acids Res. 1983 Jun 25;11(12):4141–4155. doi: 10.1093/nar/11.12.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard D. W., Voss E. W., Jr Base specificity and idiotypy of anti-DNA autoantibodies reactive with synthetic nucleic acids. J Immunol. 1985 Nov;135(5):3372–3380. [PubMed] [Google Scholar]
- Ballard D. W., Voss E. W., Jr Monoclonal murine anti-nucleic acid antibody with double-stranded specificity. Mol Immunol. 1982 Jun;19(6):793–799. doi: 10.1016/0161-5890(82)90005-0. [DOI] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick G., Emlen W. Effect of antibody excess on the size, stoichiometry, and DNAse resistance of DNA anti-DNA immune complexes. J Immunol. 1985 Oct;135(4):2593–2597. [PubMed] [Google Scholar]
- Cantor C. R., Efstratiadis A. Possible structures of homopurine-homopyrimidine S1-hypersensitive sites. Nucleic Acids Res. 1984 Nov 12;12(21):8059–8072. doi: 10.1093/nar/12.21.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll P., Stafford D., Schwartz R. S., Stollar B. D. Murine monoclonal anti-DNA autoantibodies bind to endogenous bacteria. J Immunol. 1985 Aug;135(2):1086–1090. [PubMed] [Google Scholar]
- Christophe D., Cabrer B., Bacolla A., Targovnik H., Pohl V., Vassart G. An unusually long poly(purine)-poly(pyrimidine) sequence is located upstream from the human thyroglobulin gene. Nucleic Acids Res. 1985 Jul 25;13(14):5127–5144. doi: 10.1093/nar/13.14.5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. S., Wooten J. B., Chatterjee C. L. Characterization of alternating deoxyribonucleic acid conformations in solution by phosphorus-31 nuclear magnetic resonance spectroscopy. Biochemistry. 1981 May 26;20(11):3049–3055. doi: 10.1021/bi00514a010. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E., Drew H. R. Structure of a B-DNA dodecamer. II. Influence of base sequence on helix structure. J Mol Biol. 1981 Jul 15;149(4):761–786. doi: 10.1016/0022-2836(81)90357-0. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Travers A. A. DNA structural variations in the E. coli tyrT promoter. Cell. 1984 Jun;37(2):491–502. doi: 10.1016/0092-8674(84)90379-9. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Travers A. A. Structural junctions in DNA: the influence of flanking sequence on nuclease digestion specificities. Nucleic Acids Res. 1985 Jun 25;13(12):4445–4467. doi: 10.1093/nar/13.12.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M., Ehrlich K., Mayo J. A. Unusual properties of the DNA from Xanthomonas phage XP-12 in which 5-methylcytosine completely replaces cytosine. Biochim Biophys Acta. 1975 Jun 16;395(2):109–119. doi: 10.1016/0005-2787(75)90149-5. [DOI] [PubMed] [Google Scholar]
- Eilat D. Monoclonal autoantibodies: an approach to studying autoimmune disease. Mol Immunol. 1982 Jul;19(7):943–955. doi: 10.1016/0161-5890(82)90360-1. [DOI] [PubMed] [Google Scholar]
- Evans D. H., Lee J. S., Morgan A. R., Olsen R. K. A method for the specific inhibition of poly[d(A-T)] synthesis using the A-T specific quinoxaline antibiotic TANDEM. Can J Biochem. 1982 Feb;60(2):131–136. doi: 10.1139/o82-018. [DOI] [PubMed] [Google Scholar]
- Gerhard B., Warren R. A. Reactivity of the alpha-putrescinylthymine amino groups in phi W-14 deoxyribonucleic acid. Biochemistry. 1982 Oct 26;21(22):5458–5462. doi: 10.1021/bi00265a012. [DOI] [PubMed] [Google Scholar]
- Gralla J., DeLisi C. mRNA is expected to form stable secondary structures. Nature. 1974 Mar 22;248(446):330–332. doi: 10.1038/248330a0. [DOI] [PubMed] [Google Scholar]
- Jacob L., Tron F. Monoclonal anti-deoxyribonucleic antibodies. I. Isotype and specificity studies. J Immunol. 1982 Feb;128(2):895–898. [PubMed] [Google Scholar]
- Klug A., Jack A., Viswamitra M. A., Kennard O., Shakked Z., Steitz T. A. A hypothesis on a specific sequence-dependent conformation of DNA and its relation to the binding of the lac-repressor protein. J Mol Biol. 1979 Jul 15;131(4):669–680. doi: 10.1016/0022-2836(79)90196-7. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Dombroski D. F., Mosmann T. R. Specificity of autoimmune monoclonal Fab fragments binding to single-stranded deoxyribonucleic acid. Biochemistry. 1982 Sep 28;21(20):4940–4945. doi: 10.1021/bi00263a017. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Lewis J. R., Morgan A. R., Mosmann T. R., Singh B. Monoclonal antibodies showing sequence specificity in their interaction with single-stranded DNAs. Nucleic Acids Res. 1981 Apr 10;9(7):1707–1721. doi: 10.1093/nar/9.7.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Woodsworth M. L., Latimer L. J. Monoclonal antibodies specific for poly(dG) X poly(dC) and poly(dG) X poly(dm5C). Biochemistry. 1984 Jul 3;23(14):3277–3281. doi: 10.1021/bi00309a024. [DOI] [PubMed] [Google Scholar]
- Leslie A. G., Arnott S., Chandrasekaran R., Ratliff R. L. Polymorphism of DNA double helices. J Mol Biol. 1980 Oct 15;143(1):49–72. doi: 10.1016/0022-2836(80)90124-2. [DOI] [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. Lac operator analogues: bromodeoxyuridine substitution in the lac operator affects the rate of dissociation of the lac repressor. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2574–2576. doi: 10.1073/pnas.69.9.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonossoff G. P., Butler P. J., Klug A. Sequence-dependent variation in the conformation of DNA. J Mol Biol. 1981 Jul 15;149(4):745–760. doi: 10.1016/0022-2836(81)90356-9. [DOI] [PubMed] [Google Scholar]
- Marion T. N., Lawton A. R., 3rd, Kearney J. F., Briles D. E. Anti-DNA autoantibodies in (NZB X NZW)F1 mice are clonally heterogeneous, but the majority share a common idiotype. J Immunol. 1982 Feb;128(2):668–674. [PubMed] [Google Scholar]
- McCall M., Brown T., Kennard O. The crystal structure of d(G-G-G-G-C-C-C-C). A model for poly(dG).poly(dC). J Mol Biol. 1985 Jun 5;183(3):385–396. doi: 10.1016/0022-2836(85)90009-9. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J Mol Biol. 1974 Jun 25;86(2):469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- Morgan A. R., Lee J. S., Pulleyblank D. E., Murray N. L., Evans D. H. Review: ethidium fluorescence assays. Part 1. Physicochemical studies. Nucleic Acids Res. 1979 Oct 10;7(3):547–569. doi: 10.1093/nar/7.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papalian M., Lafer E., Wong R., Stollar B. D. Reaction of systemic lupus erythematosus antinative DNA antibodies with native DNA fragments from 20 to 1,200 base pairs. J Clin Invest. 1980 Feb;65(2):469–477. doi: 10.1172/JCI109690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Schwartz R. S., Stollar B. D. Origins of anti-DNA autoantibodies. J Clin Invest. 1985 Feb;75(2):321–327. doi: 10.1172/JCI111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollar B. D. Doubls-helical polynucleotides: immunochemical recognition of differing conformations. Science. 1970 Aug 7;169(3945):609–611. doi: 10.1126/science.169.3945.609. [DOI] [PubMed] [Google Scholar]
- Stollar B. D. The experimental induction of antibodies to nucleic acids. Methods Enzymol. 1980;70(A):70–85. doi: 10.1016/s0076-6879(80)70042-3. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Larson J. E., Grant R. C., Shortle B. E., Cantor C. R. Physicochemical studies on polydeoxyribonucleotides containing defined repeating nucleotide sequences. J Mol Biol. 1970 Dec 28;54(3):465–497. doi: 10.1016/0022-2836(70)90121-x. [DOI] [PubMed] [Google Scholar]
- Wolfes H., Fliess A., Pingoud A. A comparison of the structural requirements for DNA cleavage by the isoschizomers HaeIII, BspRI and BsuRI. Eur J Biochem. 1985 Jul 1;150(1):105–110. doi: 10.1111/j.1432-1033.1985.tb08994.x. [DOI] [PubMed] [Google Scholar]
- Wu H. Y., Behe M. J. Methylated pyrimidines stabilize an alternating conformation of poly(dA-dU).poly(dA-dU). Biochemistry. 1985 Sep 24;24(20):5499–5502. doi: 10.1021/bi00341a033. [DOI] [PubMed] [Google Scholar]


