Abstract
The plant organelles, chloroplast and nucleus, change their position in response to light. In Arabidopsis thaliana leaf cells, chloroplasts and nuclei are distributed along the inner periclinal wall in darkness. In strong blue light, they become positioned along the anticlinal wall, while in weak blue light, only chloroplasts are accumulated along the inner and outer periclinal walls. Blue-light dependent positioning of both organelles is mediated by the blue-light receptor phototropin and controlled by the actin cytoskeleton. Interestingly, however, it seems that chloroplast movement requires short, fine actin filaments organized at the chloroplast edge, whereas nuclear movement does cytoplasmic, thick actin bundles intimately associated with the nucleus. Although there are many similarities between photo-relocation movements of chloroplasts and nuclei, plant cells appear to have evolved distinct mechanisms to regulate actin organization required for driving the movements of these organelles.
Key words: actin, Arabidopsis, blue light, chloroplast positioning, phototropin, nuclear positioning
Intracellular Distribution of Chloroplasts and Nuclei in Darkness and Light
In epidermal and mesophyll cells of Arabidopsis thaliana leaves, chloroplasts and nuclei take specific positions in darkness and blue light.1,2 In darkness, chloroplasts are distributed along the inner periclinal walls and a lower half of anticlinal walls, and nuclei are also along the inner periclinal walls (called the dark position). In strong blue light, they become positioned along the anticlinal walls (called the avoidance response), whereas in weak blue light, only chloroplasts gather along the inner and outer periclinal walls (called the accumulation response). It has been shown that light-dependent positioning of chloroplasts and nuclei is mediated by the blue-light receptor phototropin. A. thaliana has two phototropins, phototropin1 and 2,3–5 and phototropin2 solely mediates the avoidance response of chloroplasts6 and nuclei,2,7 whereas phototropin1 and 2 do the accumulation response of chloroplasts.6 Intriguingly, phototropin2 is involved in regulating the dark position of chloroplasts8 and nuclei in mesophyll cells, but not that of nuclei in epidermal cells.2,7 On the other hand, it appears that both dark and light positions of chloroplasts and nuclei are unaffected by gravity.
In a recent study, we reported that the residing site of nuclei in darkness and in strong blue light is precisely regulated in leaf epidermal cells of A. thaliana. In darkness, nuclei are confined near the cell center, and in strong blue light, they become positioned preferably to the concave region of the anticlinal wall, most of which are nearest the cell center.7 The reason why nuclei migrate within such a confined area may be that nuclei need to move quickly in epidermal cells of relatively large size to avoid excess light that causes DNA damage.9 In the case of chloroplasts, on the other hand, the dark position is strictly determined to the inner periclinal wall, but the light position is not; namely, chloroplasts can freely change their positions corresponding to the direction of incident light.10
Different Types of Actin-Based System Involved in Movements of Chloroplasts and Nuclei
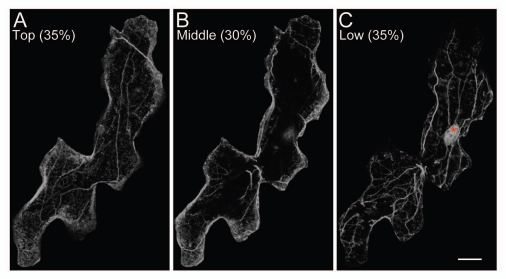
We also demonstrated that nuclear movement in A. thaliana leaf cells is dependent on the actin cytoskeleton, and nuclei in epidermal cells are always associated with cytoplasmic, thick actin bundles reorganized in a blue light-dependent manner.7 Figure 1 shows the representative arrangement of actin filaments in an epidermal cell of the 16-h dark-adapted A. thaliana matured leaf. Actin was visualized by immunofluorescence microscopy, and cells were scanned along z-axis from the top to bottom at 0.25-µm optical intervals with a confocal microscope. Here we divide an epidermal cell into three parts along z-axis; the top 35%, middle 30%, and bottom 35% of the maximal projection, to clearly show that nuclei are associated with thick actin bundles arranged along the inner periclinal walls in darkness. At the top part facing the outer periclinal wall, relatively thin actin filaments are arranged in the longitudinal direction of the cell (Fig. 1A). At the middle part, which includes the anticlinal regions, actin filaments are evident only near the anticlinal walls but scarce in the central area, probably where central vacuole occupies (Fig. 1B). At the bottom part including the inner periclinal wall, the nucleus residing at the cell center (an asterisk in Fig. 1C) is apparently associated with the thick actin bundles, roughly parallel to the longest axis of the cell (Fig. 1C). In strong blue light, the actin bundles, especially in the vicinity of the nuclei, become rearranged close to the anticlinal walls under the control of phototropin2.7 On the basis of these results, we proposed that phototropin-mediated reorganization of the actin cytoskeleton plays a crucial role in light-dependent nuclear positioning in A. thaliana.7
Figure 1.
Actin organization in leaf epidermal cells of A. thaliana. (A–C) Confocal micrographs of actin organization. Leaves were dark-adapted for 16 h. Actin (gray) was visualized by immunofluorescence microscopy. Epidermal cells were scanned from the upper to lower surfaces at 0.25-µm optical intervals, and the maximum projection was divided into three parts along the z-axis: the top 35% (A), middle 30% (B), and bottom 35% (C). Note that we manipulated images by blackening neighboring cells using image-processing software (Adobe Photoshop) to highlight the representative cell. An asterisk in (C) shows the nucleus. Bar = 20 µm.
Like nuclear movement, chloroplast movement requires the actin cytoskeleton.11,12 However, the type of the actin filament used may totally differ between these movements. Here, we propose two hypothetical models for nuclear movement, comparing them with that for chloroplasts (Fig. 2). One model is nuclear movement along an actin bundle. In darkness, nuclei are inner-periclinally positioned at the cell center in association with thick, longitudinally arranged actin bundles. When irradiated with strong blue light, nuclei start to migrate along the actin bundles and eventually reach the anticlinal wall (Fig. 2A). The other model is bundle-dependent nuclear movement. Upon strong blue-light irradiation, actin bundles associated with the nucleus move laterally from the inner periclinal wall toward anticlinal wall, thereby bringing the nucleus toward the anticlinal wall (Fig. 2B). On the other hand, chloroplast movement has been revealed to use unique actin filaments.12 In darkness, chloroplasts are anchored close to the plasma membrane via short, thin actin filaments, called the chloroplast actin (cp-actin) filaments, evenly distributed at the edge of each chloroplast (Fig. 2C). Upon accumulation and avoidance responses, the cp-actin filaments become reorganized to increase at the leading edge of each chloroplast (Fig. 2C). There is a good correlation between uneven distribution of the cp-actin filaments and the speed of chloroplast movement.12 The organization of the cp-actin filaments dose not appear to require the actin related protein 2/3 (Arp2/3) complex, which is known to nucleate actin filaments with complex arrays and generate the motive force to push organelle.13 Furthermore, chloroplast movement might not need myosin because normal chloroplast movement is observed in several lines of myosin-deficient mutants.14 However, this point is still controversial because plants, as well as animals and yeast, have multiple myosins, e.g., 17 molecular species of myosin in A. thaliana,15,16 which may function redundantly, and because myosin inhibitors inhibit at least the accumulation response of chloroplasts.17 In any case, chloroplast movement may be controlled by the mechanism different from that utilized for moving other organelles including the nucleus. Although the details of actin-based motile mechanisms for chloroplasts and nuclei are still unknown, plant cells may have evolved distinct mechanisms to drive movements of these organelles.
Figure 2.
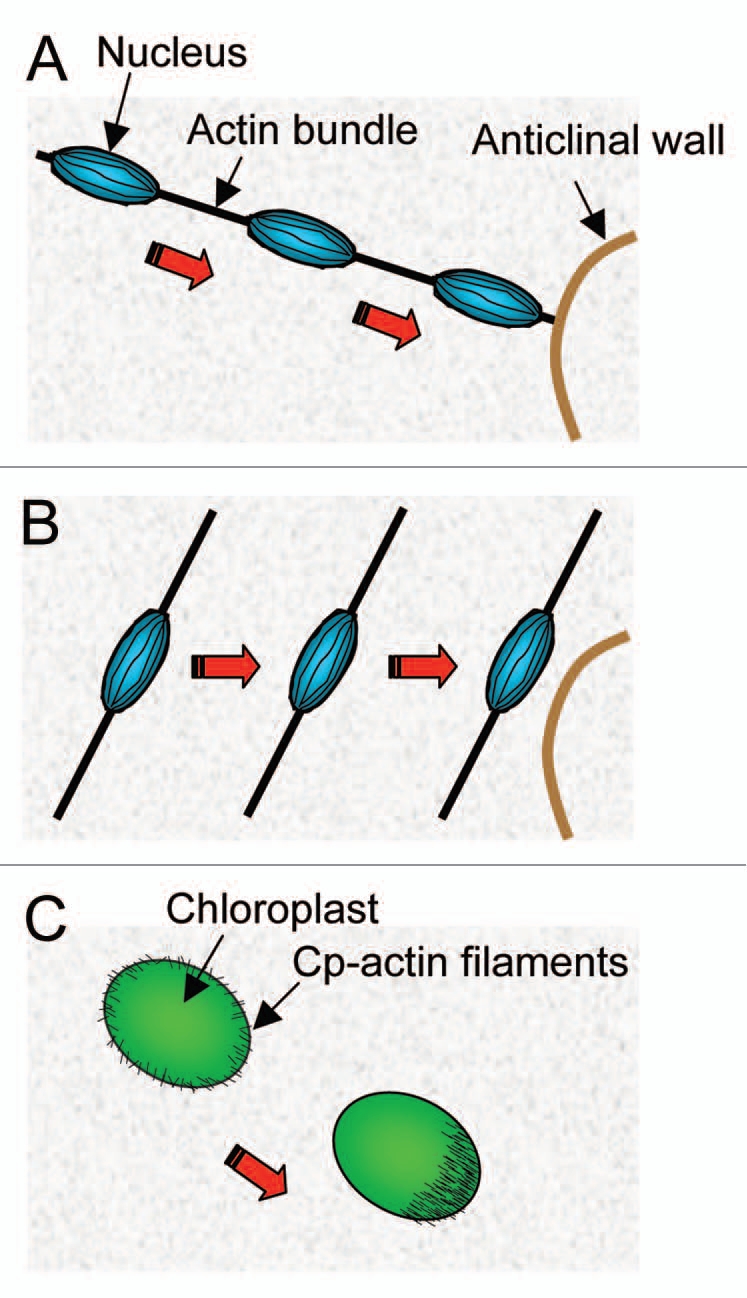
Models proposed for actin-based motility of nucleus and chloroplast. (A) Nuclear movement along an actin bundle. Upon strong blue-light irradiation, the nucleus (blue) starts to move along the actin bundle (black) toward the anticlinal wall (brown). (B) Nuclear movement following lateral movement of an actin bundle. The nucleus is anchored statically to the actin bundle, which laterally moves toward the anticlinal wall under strong blue light, enabling the nucleus to reach the anticlinal wall. (C) Chloroplast movement using the cp-actin filaments. In strong blue light, evenly distributed cp-actin filaments (black) become biased to increase at the leading edge of the chloroplast (green) with the onset of chloroplast movement (adopted from Kadota et al. 2009). Red arrows indicate the moving direction of the nucleus and chloroplast.
Future Perspectives
How do plant cells regulate movements of chloroplasts and nuclei differently? Several genes involved in the controlling mechanism of chloroplast movement have been reported. The J-domain protein required for chloroplast accumulation response1 (JAC1),8 plastid movement impaired1 (PMI1),18 and chloroplast unusual positioning1 (CHUP1)19 proteins are thought to regulate the accumulation response, whereas PMI1, 2,20 and 15,20 and CHUP1 do the avoidance response. As discussed in a previous paper,21 nuclear movement has many similarities with chloroplast avoidance response. Therefore, it is possible that PMI1, 2 and 15 are involved in nuclear movement as well. Among PMIs, PMI2 is especially worth investigation because PMI2 gene has putative nuclear localization signals.20 JAC1 is localized in the cytoplasm,8 which may raise the possibility that JAC1 proteins are also involved in movements of other organelles including the nucleus. These proteins may regulate movements not only of chloroplasts but also of other organelles. Nevertheless, the CHUP1 protein is thought to be involved only in chloroplast movement. The CHUP1 protein is specifically localized at the outer envelope of chloroplasts to play an important role in connecting chloroplasts to the actin cytoskeleton.19,22–24 We are considering that similar proteins may also present in nuclear envelope. The SUN (Sad1/UNC-84) proteins are the good candidates as the functional counterpart of CHUP1 protein. A. thaliana has two SUN proteins designated as AtSUN1 and AtSUN2.25 They are localized to the nuclear envelope, although their physiological roles are still unknown.25,26 Since in fungi and animals, SUN proteins are localized at the inner nuclear envelope to connect the cytoskeleton and the nucleus, functioning in positioning nuclei,27–30 it is worth testing whether AtSUN1 and AtSUN2 proteins are involved in positioning nuclei in plants. In addition, it is of our interest to test whether Arp2/3 and myosins are involved in nuclear movement. Thus, although regulatory genes involved in nuclear movement have not yet been identified, comparative molecular genetic studies on those genes will provide important information to understand how and why chloroplasts and nuclei move using different motile systems under blue light.
Acknowledgements
This work was supported by a grant from the Japan Society for the Promotion of Science for young research fellow to K.I. (grant no. 201712).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/12233
References
- 1.Trojan A, Gabrys H. Chloroplast distribution in Arabidopsis thaliana (L.) depends on light conditions during growth. Plant Physiol. 1996;111:419–425. doi: 10.1104/pp.111.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwabuchi K, Sakai T, Takagi S. Blue light-dependent nuclear positioning in Arabidopsis thaliana leaf cells. Plant Cell Physiol. 2007;48:1291–1298. doi: 10.1093/pcp/pcm095. [DOI] [PubMed] [Google Scholar]
- 3.Huala E, Oeller PW, Liscum E, Han I-S, Larsen E, Briggs WR. Arabidopsis NPH1: a protein kinase with a putative redox-sensing domain. Science. 1997;278:2120–2123. doi: 10.1126/science.278.5346.2120. [DOI] [PubMed] [Google Scholar]
- 4.Jarillo JA, Gabrys H, Capel J, Alonso JM, Ecker JR, Cashmore AR. Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature. 2001;410:952–954. doi: 10.1038/35073622. [DOI] [PubMed] [Google Scholar]
- 5.Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, et al. Arabidopsis NPL1: a phototropin homolog controlling the chloroplast high-light avoidance response. Science. 2001;291:2138–2141. doi: 10.1126/science.291.5511.2138. [DOI] [PubMed] [Google Scholar]
- 6.Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, et al. Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA. 2001;98:6969–6974. doi: 10.1073/pnas.101137598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwabuchi K, Minamino R, Takagi S. Actin reorganization underlies phototropin-dependent positioning of nuclei in Arabidopsis leaf cells. Plant Physiol. 2010;152:1309–1319. doi: 10.1104/pp.109.149526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suetsugu N, Kagawa T, Wada M. An auxilin-like J-domain protein, JAC1, regulates phototropin-mediated chloroplast movement in Arabidopsis. Plant Physiol. 2005;139:151–162. doi: 10.1104/pp.105.067371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng X-Q, Chow WS, Su L-J, Peng X-X, Peng C-L. Protective effect of supplemental anthocyanins on Arabidopsis leaves under high light. Physiol Plant. 2010;138:215–225. doi: 10.1111/j.1399-3054.2009.01316.x. [DOI] [PubMed] [Google Scholar]
- 10.Tsuboi H, Yamashita H, Wada M. Chloroplasts do not have a polarity for light-induced accumulation movement. J Plant Res. 2009;122:131–140. doi: 10.1007/s10265-008-0199-z. [DOI] [PubMed] [Google Scholar]
- 11.Kandasamy MK, Meagher RB. Actin-organelle interaction: association with chloroplast in Arabidopsis leaf mesophyll cells. Cell Motil Cytoskel. 1999;44:110–118. doi: 10.1002/(SICI)1097-0169(199910)44:2<110::AID-CM3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 12.Kadota A, Yamada N, Suetsugu N, Hirose M, Saito C, Shoda K, et al. Short actin-based mechanism for light-directed chloroplast movement in Arabidopsis. Proc Natl Acad Sci USA. 2009;106:13106–13111. doi: 10.1073/pnas.0906250106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goley ED, Welch MD. The ARP2/3 complex: an actin nucleation comes of age. Nat Rev Mol Cell Biol. 2006;7:713–726. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- 14.Avisar D, Prokhnevsky AI, Makarova KS, Koonin EV, Dolja VV. Myosin XI-K is required for rapid trafficking of Golgi stacks, peroxisomes, and mitochondria in leaf cells of Nicotiana benthamiana. Plant Physiol. 2008;146:1098–1108. doi: 10.1104/pp.107.113647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berg JS, Powell BC, Cheney RE. A millennial myosin census. Mol Biol Cell. 2001;12:780–794. doi: 10.1091/mbc.12.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy AS, Day IS. Analysis of the myosins encoded in the recently completed Arabidopsis thaliana genome sequence. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-7-research0024. RESEARCH0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paves H, Truve E. Myosin inhibitors block accumulation movement of chloroplasts in Arabidopsis thaliana leaf cells. Protoplasma. 2007;230:165–169. doi: 10.1007/s00709-006-0230-y. [DOI] [PubMed] [Google Scholar]
- 18.DeBlasio SL, Luesse DL, Hangarter RP. A plant-specific protein essential for blue-light-induced chloroplast movements. Plant Physiol. 2005;139:101–114. doi: 10.1104/pp.105.061887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oikawa K, Kasahara M, Kiyosue T, Kagawa T, Suetsugu N, Takahashi F, et al. CHLOROPLAST UNUSUAL POSITIONING1 is essential for proper chloroplast positioning. Plant Cell. 2003;15:2805–2815. doi: 10.1105/tpc.016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luesse DR, DeBlasio SL, Hangarter RP. Plastid movement impaired 2, a new gene involved in normal blue-light-induced chloroplast movements in Arabidopsis. Plant Physiol. 2006;141:1328–1337. doi: 10.1104/pp.106.080333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwabuchi K, Takagi S. How and why do plant nuclei move in response to light? Plant Signal Behav. 2008;3:266–268. doi: 10.4161/psb.3.4.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wada M, Suetsugu N. Plant organelle positioning. Curr Opin Plant Biol. 2004;7:626–631. doi: 10.1016/j.pbi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Oikawa K, Yamasato A, Kong S-G, Kasahara M, Nakai M, Takahashi F, et al. Chloroplast outer envelope protein CHUP1 is essential for chloroplast anchorage to the plasma membrane and chloroplast movement. Plant Physiol. 2008;148:829–842. doi: 10.1104/pp.108.123075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt von Braun S, Schleiff E. The chloroplast outer membrane protein CHUP1 interacts with actin and profiling. Planta. 2008;227:1151–1159. doi: 10.1007/s00425-007-0688-7. [DOI] [PubMed] [Google Scholar]
- 25.Graumann K, Runions J, Evans DE. Characterization of SUN-domain proteins at the higher plant nuclear envelope. Plant J. 2010;61:134–144. doi: 10.1111/j.1365-313X.2009.04038.x. [DOI] [PubMed] [Google Scholar]
- 26.Meier I, Brkljacic J. Curr Opin Plant Biol. 2009;12:752–759. doi: 10.1016/j.pbi.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Starr DA, Han M. ANChors away: an actin based mechanism of nuclear positioning. J Cell Sci. 2003;116:211–216. doi: 10.1242/jcs.00248. [DOI] [PubMed] [Google Scholar]
- 28.Wilhelmsen K, Ketema M, Truong H, Sonnenberg A. KASH-domain proteins in nuclear migration, anchorage and other processes. J Cell Sci. 2006;119:5021–5029. doi: 10.1242/jcs.03295. [DOI] [PubMed] [Google Scholar]
- 29.Starr DA. Communication between the cytoskeleton and the nuclear envelope to position the nucleus. Mol Biosyst. 2007;3:583–589. doi: 10.1039/b703878j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Starr DA. A nuclear-envelope bridge positions nuclei and moves chromosomes. J Cell Sci. 2009;122:577–586. doi: 10.1242/jcs.037622. [DOI] [PMC free article] [PubMed] [Google Scholar]