Abstract
During vascular development, endothelial cells are exposed to a variety of rapidly changing factors, including fluctuating oxygen levels. We have previously shown that Ankyrin Repeat and SOCS Box Protein 4 (ASB4) is the most highly differentially expressed gene in the vascular lineage during early differentiation and is expressed in the embryonic vasculature at a time when oxygen tension is rising due to the onset of placental blood flow. In order to further our understanding of the regulation of ASB4 expression in endothelial cells, we tested the effect of various stressors for their ability to alter ASB4 expression in the immortalized murine endothelial cell lines MS1 and SVR. ASB4 expression is decreased during hypoxic insult and shear stress whereas it is increased in response to TNF-α. Further investigation indicated that Nuclear Factor kappa B (NF-κB) is the responsible transcription factor involved in the TNF-α-induced upregulation of ASB4, placing ASB4 downstream of NF-κB in the TNF-α signaling cascade and identifying it as a potential regulator for TNF-α’s numerous functions associated with inflammation, angiogenesis and apoptosis.
INTRODUCTION
Ankyrin repeat (AR) and SOCS (suppressor of cytokine signaling) box proteins (ASBs) are characterized by two functional domains: a variable number of N-terminal ARs and a C-terminal SOCS box1, 2. The N-terminal ARs recruit and bind substrate proteins to mediate substrate polyubiquitination and proteasome-mediated degradation. The C-terminal SOCS box mediates interactions with an elongin B/elongin C/cullin 5/Roc protein complex to form an E3 ubiquitin ligase complex3. So far, 18 ASB family members have been identified in mammals. They are involved in numerous processes including ubiquitination of a broad range of target proteins, such as tumor necrosis factor receptor II (ASB3)4, adaptor protein with PH and SH2 domains (ASB6)5 and creatine kinase B (ASB9) but also regulatory functions like the inhibition of mitochondrial function (ASB9)6, spermatogenesis (ASB9)7, alteration of myoblast differentiation (ASB15)8 and stimulation of angiogenesis (ASB5)9.
Our previous studies have demonstrated that ASB4 is highly differentially expressed in cells of the vascular lineage (84-h Flk1+ cells) during development at a time when oxygen tensions are rapidly changing in the embryo10, suggesting that ASB4 may act as a cellular “oxygen sensor” in the developing vasculature. Oxygen tension is important for vasculogenesis as it is one of the major stimuli for the growth of new vessels. Failure of vasculogenesis would be catastrophic for the organism because passive diffusion is not sufficient to supply all cells with oxygen and nutrients. In vitro studies have demonstrated that ASB4 interacts with FIH (Factor Inhibiting HIF1α) and promotes differentiation of embryonic stem cells (ES) into the vascular lineage in an oxygen-dependent manner11, 12. These studies suggest that ASB4 may function to modulate an endothelium-specific response to changing oxygen tension during embryonic development.
In addition to ASB4’s putative role as an oxygen sensor during vasculogenesis, it also plays a role in energy homeostasis. ASB4 reduces insulin receptor substrate 1 (IRS-1) phosphorylation when co-expressed with G-protein pathway suppressor 1 (GPS1)13. In the paraventricular nucleus of the rat brain, insulin and leptin both cause an increase in ASB4 expression, with leptin also causing an increase in ASB4 expressionin the arcuate nucleus of the thalamus14. Although there is some knowledge of the regulation of ASB4 in neuronal systems, nothing has been reported about the factors that affect ASB4 expression in endothelial cells. It is still unclear how ASB4 expression is regulated in response to changes in oxygen tension or what transcription factors are involved in this regulation. These are critical holes in our understanding, not only in terms of ASB4’s involvement in vasculogenesis during development, but also with respect to the role that ASB4 might play in pathogenic processes in the adult.
In this report we attempt to fill in these gaps by identifying several conditions under which ASB4 expression is altered in the SVR and MS1 endothelial cell lines, including changes in oxygen tension, shear stress and TNF-α expression. In addition, we demonstrate that NF-κB is a potential transcription factor for ASB4 expression. Our results suggest that ASB4 functions downstream of NF-κB and acts as a potential regulator of numerous pathways involved in inflammation, angiogenesis and apoptosis.
MATERIALS AND METHODS
Cell culture and transfection conditions
MS1 and SVR murine endothelial pancreatic islet cells were obtained from ATCC 15. Cells were grown at 37°C in Dulbecco’s modified Eagle’s medium (DMEM) with10% heat-inactivated fetal bovine serum, 10 U/mL penicillin, and 10 μg/mL streptomycin (Gibco). Normoxic cultures were performed under atmospheric oxygen tension (~21%) and 5% CO2. Cells from passages 4–17 were used for all experiments and all cells were grown to confluency. Hypoxic cultures were performed at 1% O2 and 5% CO2. Cells were serum starved for 16 hours before treatment and incubated for 24 h after treatment before lysis unless otherwise noted.
Shear stress
Shear stress experiments were performed as described previously16. MS1 cells were maintained in DMEM with 10% fetal bovine serum and 1% penicillin/streptomycin. All cell cultures were kept in a humidified 5% CO2/95% air incubator at 37°C. MS1 cells cultured on 38 × 76 mm slides to confluence were either kept as static controls or subjected to shear stress in a parallel plate flow chamber so that multiple slides could be sheared simultaneously. A surface area of 14 cm2 on the MS1-seeded slide was exposed to fluid shear stress generated by perfusing culture medium over the cells. The pH of the system was kept constant by gassing with 5% CO2/95% air and the temperature was maintained at 37°C. Shear stress at 12 dyn/cm2 was used for all experiments, which is correspondent with the physiological range in human arteries.
Reverse transcriptase PCR (RT-PCR) analysis
Total RNA was isolated with the RNeasy system (QIAGEN). RNA was reverse transcribed with the iScript system (Bio-Rad) using the following specific primers: ASB4 PCR, forward primer 5′TGCCCAGTTATAAGCTGAAGTCTTC and reverse primer 5′GTGTCTCTTCATCCTGGTTGTTG; glyceraldehydes-3-phosphate dehydrogenase (GAPDH), forward primer 5′ACCCAGAAGACTGTGGATGG and reverse primer TGTGAGGAGGGGAGATTCAG. For real-time PCR analysis, total RNA was reverse transcribed with the iScript system (Bio-Rad). Multiplex real-time PCR was performed with the ABI PRISM 7900 sequence detection system, software and reagents. The following primers were used together with probes from the Universal Probe Library (Roche): ASB4 forward primer 5′TTTGCACTTCTGCACCACAC, reverse primer 5′CCTGGTTGTTGGTTTTCATGT, probe #58 5′CTCCATCC. RNA samples, dissolved in water, were always prepared in triplicate. The ASB4 primers, DIG-labeled probe #58 as well as VIC-labeled Human 18S rRNA from applied Biosystems (Cat. # 4319413E – 0810041) were added to each sample as well as to the control samples and to wells containing only water. The absorptions of the fluorophore-labeled RNA probes in these samples were measured and the absorptions normalized with those wells that contained only water. RNA input was calibrated with 18S expression levels. Relative ASB4 mRNA levels were normalized to ASB4 RNA expression in the control samples.
Statistical analysis
An unpaired two-tailed Student t-test was used for specific comparisons between endpoints in the experiments. Data are expressed as mean plus standard error of the mean. Statistical significance was accepted at the 95% confidence interval.
RESULTS
ASB4 is expressed in the immortalized murine endothelial cell lines MS1 and SVR
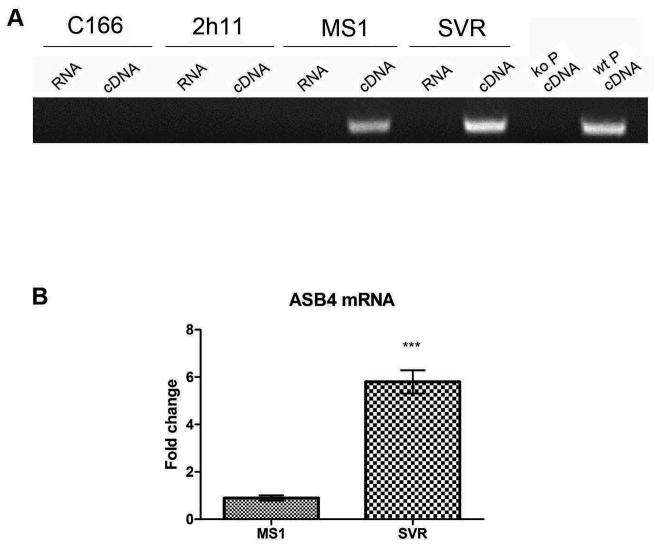
Despite the fact that ASB4 is known to be the most highly differentially expressed gene in the vascular lineage during early differentiation11, very little is known about the cellular triggers or signaling pathways involved in the induction of ASB4 expression in endothelial cells during vasculogenesis. We started our exploration of this gap in knowledge by examining the basal expression level of ASB4 in endothelial cell lines. RNA from a variety of endothelial cell lines was tested for ASB4 expression by RT-PCR analysis. C166, 2h11, myocardial endothelial cells (MEC) and human umbilical vein endothelial cells (HUVEC) did not express detectable amounts of ASB4 (Figure 1A, data for MEC and HUVEC are not shown), despite the fact that all of these cell types are considered to be endothelial in nature17, 18. However, MS1 and SVR cell lines displayed high levels of ASB4 expression (Figure 1A). Both MSI and SVR cells are derived from murine endothelial cells located in the pancreatic islets by sequential introduction of simian virus 40 (SV40) large T (tumor) antigen (for MS1) and additionally H-ras (for SVR).15 Endothelial cells expressing the temperature-sensitive large T antigen are immortalized in vitro. Upon implantation into mice, MS1 cells form dormant hemangiomas. SVR cells, due to the additional expression of H-ras, form rapidly proliferating angiosarcomas15. Each cell line retains properties of endothelial cells including uptake of acetylated LDL and expression of VEGF receptor 1 and 219. When expression of ASB4 mRNA was compared between MS1 and SVR cell lines by real time PCR, SVR cells demonstrated up to 6 fold higher expression levels of ASB4 (Figure 1B) possibly owing to the fact that H-ras is overexpressed in SVR cells. H-ras causes endothelial cells to be capable of forming rapidly proliferating angiosarcomas in vivo15, suggesting the possibility that ASB4 may participate in or be activated by one of the pathways downstream of H-ras responsible for this modulation of endothelial phenotype.
Figure 1.
Expression of ASB4 in endothelial cells. (A) C166, 2h11, MS1 and SVR cells were cultured to confluency, RNA was purified and RT-PCR with primers for ASB4 performed. RNA that had not been reverse transcribed was used as a control for genomic contamination. Placental cells from ASB4 knockout (ko P) and wild-type (wt P) mice were used as negative and positive controls for ASB4 expression. (B) Real time PCR analysis was performed on untreated MS1 and SVR cells and ASB4 expression normalized to MS1 cells. Results are shown as mean ± SD of three independent experiments. ***p < 0.001.
Identifying regulators of ASB4 expression
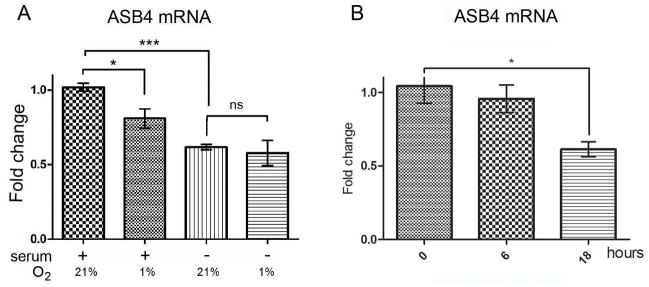
To date, no reports of ASB4 regulation within endothelial cells exists, in spite of its exquisite spatial and temporal regulation within the endothelial lineage during mouse development11. To explore the question of ASB4 regulation in endothelial cells, we challenged MS1 cells with hypoxia and shear stress, stress factors known to affect endothelial cells at a time during development when ASB4 is also expressed. MS1 cells were serum-starved overnight (16 h) as part of the general culture conditions to synchronize the cell cycle and afterwards were subjected to hypoxia (1% O2) for 16 hours, either with or without the addition of 10% fetal bovine serum (FBS) to the culture media in order to investigate the response to hypoxia under different levels of nutrition. mRNA samples from the treated cells were then analyzed by real time PCR. Culturing MS1 cells under hypoxic conditions with 10% FBS resulted in a 40% decrease in ASB4 expression (Figure 2A). This downregulation of ASB4 expression was also observed in SVR cells following treatment with hypoxia (Figure 2B). Interestingly, serum starvation was also a strong inducer of decreased ASB4 expression (Figure 2A). However, this decrease was not additive with that of hypoxic stress to the culture conditions, suggesting that ASB4 mRNA levels in cells not treated with FBS are already relatively low so that a further reduction due to hypoxia is not possible.
Figure 2.
ASB4 mRNA expression following hypoxia treatment. (A) Real time PCR analysis of ASB4 expression levels normalized to the first sample (+serum, 21% O2). MS1 cells were grown to confluency and treated for 16 hours with either serum containing medium or medium without serum. Afterwards, cells were grown for 24 hours under hypoxic or normoxic conditions. Results are shown as mean ± SD of three independent experiments. *p < 0.05, ***p < 0.001, ns = non-significant. (B) Real time PCR analysis of ASB4 expression levels. SVR cells were grown to confluency and treated with hypoxia for 0, 6 or 18 hours. Results are shown as mean ± SD of three independent experiments. Data are normalized to expression at 0 h; *p < 0.05.
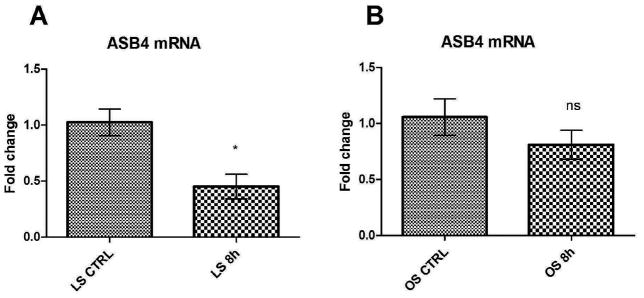
During vasculogenesis in the embryo, endothelial cells undergo shear stress as the circulatory system starts to develop and blood begins to flow within the new vessels. Shear stress of endothelial cells occurs at the same time in vascular development as ASB4 expression in endothelial cells, suggesting that this type of cellular stress may be an inducing factor in ASB4 expression10. To test this theory, confluent MS1 cells were serum starved and afterwards treated with 8 hours of laminar or oscillatory shear stress (both at 12 dyn/cm2), after which mRNA was isolated from the cells and analyzed with real-time PCR. Laminar shear stress simulates the conditions in a smooth, straight vessel in which endothelial cells lining the vessel are exposed to a constant, unidirectional flow. In contrast, oscillatory shear stress occurs to endothelial cells when they are positioned at a bifurcation in the vessel or, in mature vessels, when atherosclerotic plaques line the vessel wall. The resultant flow of blood is intermittent and turbulent. In culture conditions, oscillatory shear stress is achieved by alternating the flow of media over the cells. In cells exposed to laminar flow, ASB4 expression was decreased 50% compared to starved cells at rest (Figure 3A). In contrast, oscillatory shear stress resulted in no significant change in ASB4 expression in cultured MS1 cells (Figure 3B). Steady laminar shear stress is thought to be atheroprotective or anti-inflammatory20–24. However, oscillatory shear is inflammatory, causing neointimal hyperplasia and expression of pro-inflammatory markers, although endothelial responses vary depending on the severity of the shear stress25–29. The fact that laminar flow, which is atheroprotective, downregulates ASB4 suggests an involvement of ASB4 in inflammatory and angiogenic processes. The importance of shear stress on ASB4 expression was further emphasized by the fact that, while starved cells under hypoxic conditions did not show a further decrease in ASB4 expression, laminar flow stress did cause a reduction in ASB4 expression over that seen with serum starvation alone. These results demonstrate that blood flow conditions (more so than hypoxia) and the resultant shear stress experienced by endothelial cells could be a powerful regulator of ASB4 expression during early vascular development.
Figure 3.
ASB4 mRNA expression following shear stress. MS1 cells were serum-starved for 16 hours and treated with 0 hours or 8 hours of (A) laminar (LS) or (B) oscillatory (OS) flow (12 dynes/cm2). Results are shown as mean ± SD of three independent experiments. Data are normalized to expression at 0 h. *p < 0.05, ns = non-significant.
TNF-α upregulates ASB4 expression
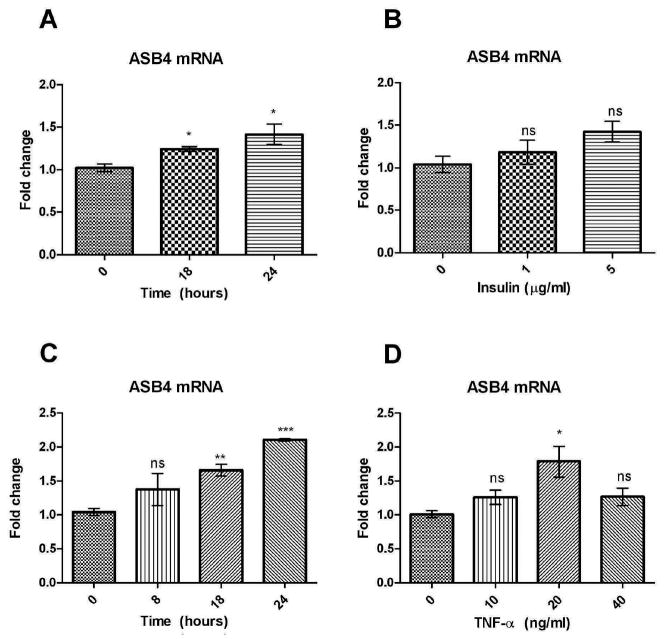
Nutrition rapidly improves in the embryo when the vasculature develops and blood begins to flow. Therefore, one explanation for the upregulation of ASB4 expression during the period of embryonic vascularization11 could be the increase in nutrition and certain cytokines that become available to the embryo due to increased blood flow. MS1 cells cultured in the presence of FBS exhibit a 2-fold higher expression of ASB4 than cells that do not received FBS (Figure 2A). FBS is composed of a variety of nutrients and cytokines. Of these ingredients, TNF-α and insulin were identified as potential factors that could cause an increase in ASB4 expression. The effect of TNF-α on ASB4 expression was investigated due to its ability to stimulate NF-κB30, which plays an important role in balancing angiogenesis by several mechanisms including chromatin remodeling31, induction of endothelial cell survival and secretion of angiogenic factors32–34 and could therefore also be a transcription factor controlling the expression of ASB4. Insulin was tested due to the fact that MS1 cells are derived from pancreatic islet endothelial cells which are tightly involved in the development35 and regulation of beta-cells36 and also play a role in regulating insulin secretion37 and hence it is possible that several of their functions are also regulated by insulin. MS1 cells were once again serum-starved overnight (16 h) and treated with 10 μg/ml insulin for 18 or 24 hours (Figure 4A) as well as 1 or 5 μg/ml insulin for 18 hours (Figure 4B). In a similar fashion, starved MS1 cells were treated with 20 ng/ml TNF-α for 8, 18 or 24 hours (Figure 4C) as well as 10, 20 or 40 ng/ml TNF-α for 24 hours (Figure 4D). Real-time PCR was then performed to analyze levels of ASB4 mRNA. Treatment of MS1 cells with insulin caused a slight increase in ASB4 expression whereas MS1 cells exhibited a dose- and time-dependent increase in ASB4 expression of up to 2-fold following treatment with TNF-α. Doubling the treatment dose of TNF-α (to 40 ng/ml) caused only a slight increase in ASB4 expression, possibly due to cell toxicity. These results suggest that TNF-α might be a key factor within FBS that contributes to stimulation of ASB4 expression.
Figure 4.
ASB4 mRNA expression levels in MS1 cells following TNF-α or insulin treatment. Cells were serum starved for 16 hours before treatment. After treatment, real-time PCR analysis was performed. Data were normalized and significance calculated to expression at 0 hours. (A) MS1 cells were treated with 20 ng/ml TNF-α for 0, 8, 18 or 24 hours. (B) MS1 cells were treated with 0, 10, 20 or 40 ng/ml TNF-α for 24 hours. (C) MS1 cells were treated with 10 μg/ml insulin for 0, 18 or 24 hours. (D) MS1 cells (P. 13) were treated with 0, 1 or 5 μg/ml insulin for 18 hours. Results are shown as mean ± SD of three independent experiments. ***p < 0.0001, **p < 0.005, *p < 0.05, ns = non-significant.
IKK-inhibitor inhibits TNF-α upregulation of ASB4
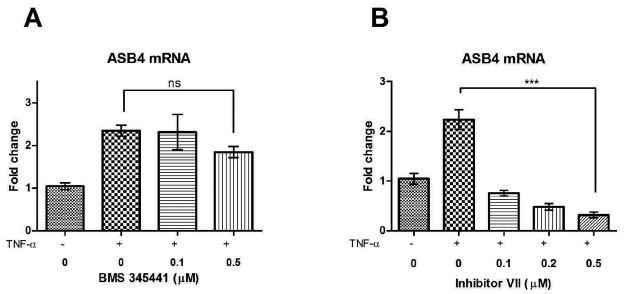
TNF-α activates several pathways including NF-κB and MAPK and it has also been associated with a weak induction of death cell signaling by activating caspase-838. We were particularly interested in the activation of NF-κB because of its known role in stimulation of angiogenesis33 and its potential effect on ASB4, which is highly expressed during vascular development. To determine if the upregulation of ASB4 expression following TNF-α treatment is due to an activation of the NF-κB pathway, we used IKK (inhibitory kappa-B kinase) inhibitors to inhibit NF-κB activity. Activation of IKK results in phosphorylation of IkBα with its subsequent polyubiquitination and degradation by the 26S proteasome39. This releases the p65/p50 heterodimer, which translocates to the nucleus and transcribes target genes, including cytokines and cell adhesion molecules. Inhibition of IKK reverses this effect and the NF-κB heterodimer is not released. We used BMS-345441 and IKK inhibitor VII as inhibitors of IKK-β40, 41. MS1 cells were treated with different concentrations of BMS-345541 or IKK Inhibitor VII one hour prior to the beginning of the 24 hour incubation of cells with TNF-α. ASB4 expression levels were determined with real-time PCR (Figure 5). Both BMS-345441 and Inhibitor VII were able to inhibit the TNF-α-induced ASB4 expression in MS1 cells, however Inhibitor VII was much more effective. The concentration of BMS-345441 was increased to determine if the inhibition of TNF-α-induced ASB4 expression could be raised to the effectiveness of Inhibitor VII. However, increased concentrations of BMS-345441 proved to be toxic to MS1 cells. These results indicate that NF-κB is a transcription factor capable of inducing expression of ASB4 in MS1 cells. The regulation of ASB4 by NF-κB makes sense as ASB4 is expressed during vascular growth, a cellular process in which NF-κB plays an essential role by balancing pro- and anti-angiogenic effects31 and by being part of the vascular endothelial growth factor signaling42, 43. However, it also suggests a potential role for ASB4 in inflammatory vascular processes like atherosclerosis, which are also known to be mediated in part by NF-κB-regulated pathways.
Figure 5.
Effect of IKK-inhibitors on ASB4 mRNA expression levels. MS1 cells were treated with different concentrations of (A) BMS-345541 or (B) IKK Inhibitor VII one hour before TNF-α (20 ng/ml) was added for 24 hours. ASB4 expression levels were determined with real-time PCR and data normalized to ASB4 expression without stimulation by TNF-α. Results are shown as mean ± SD of three independent experiments. ***p < 0.001, ns = non-significant.
DISCUSSION
To date, only a limited number of studies have been reported identifying putative regulators of ASB4 expression. Previous studies have shown that ASB4 can be influenced at the protein level by certain factors like oxygen to modify its interactions with other proteins. However, it is still not clear how the expression of the ASB4 gene can be modulated and which pathways are involved. Therefore, the present study was undertaken to determine the factors that affect ASB4 expression in endothelial cells. Our observations indicate that ASB4 is regulated by oxygen and nutrient levels, shear stress and TNF-α stimulation. Together, these observations shed new light on the regulation of ASB4 in vivo and highlight the complexity of the regulatory pathways involved in modulating the effects of this protein.
Previously we have demonstrated that ASB4 expression in embryonic stem cells influences the differentiation of these cells into the vascular lineage in an oxygen-dependent manner11. In addition, we discovered that, during embryonic development, the peak of ASB4 expression in the embryonic vasculature coincides with the time that oxygen tension is dramatically increasing owing to the initiation of placental blood flow11. In contrast, when the embryonic vessels mature and oxygen levels stabilize, ASB4 expression is quickly downregulated. We concluded from these results that ASB4 may be important in regulating endothelial-specific responses to increasing oxygen tension; however, a direct relationship between ASB4 expression and oxygen concentration had not been shown. In the present study, we observed that hypoxia induces a decrease in ASB4 mRNA levels in cultured MS1 cells, supporting our hypothesis that oxygen tension plays an important role in the regulation of ASB4. It is now apparent that oxygen levels affect ASB4 on multiple levels. Our previous studies demonstrated that ASB4 interacts with the factor inhibiting HIF1alpha (FIH) and is a substrate for FIH-mediated hydroxylation via an oxygen-dependent mechanism. This, along with our current findings suggests that oxygen regulates ASB4 both through hydroxylation of the ASB4 protein moiety and by transcriptional regulation of the gene.
One limitation of our study is that, despite looking in numerous endothelial cell lines as well as primary endothelial cells such as HUVECs, we were only able to detect significant levels of ASB4 in MS1 and SVR endothelial cells, both of which are immortalized murine cell lines. It is possible that the apparent lack of ASB4 expression in the other cells could be due to the fact that ASB4 is not expressed in these cells under standard conditions. It remains to be determined if induction of ASB4 expression can be achieved in these cells under certain conditions, such as TNF-α treatment, shear stress, hyper-oxygenation or insulin treatment. It is also plausible that the lack of detectable ASB4 expression in these other cell types could be due to the use of nonfunctional primers or unsuitable PCR temperature settings. Finally it is possible that either the murine origin or immortalization of MS1 and SVR cells altered them in such a way that promoted ASB4 expression. Further investigation of ASB4 expression in these different endothelial cell types is clearly warranted.
Despite the fact that we were not able to detect ASB4 in primary endothelial cells, our data obtained in MSI and SVR cells are still physiologically relevant. MS1 and SVR cell lines are both derived from murine pancreatic islet endothelial cells and are primarily used in tumor angiogenesis studies. When implanted into mice, MS1and SVR cells form dormant hemangiomas and rapidly growing angiosarcomas, respectively. They were originally used to demonstrate the capability of H-ras to function as an angiogenic switch.15 The fact that ASB4 is highly expressed in these cells suggests the possibility that there is a link between aggressive angiogenic activity and ASB4 expression, although the significance of this relationship remains to be determined.
Two aspects of our findings in this report suggest that ASB4 may play a role in pro-inflammatory responses. Firstly, we found ASB4 to be down regulated in response to laminar shear stress, the form of shear stress that is thought to be atheroprotective or anti-inflammatory24. Further support of the notion that ASB4 may be pro-inflammatory comes from our observation that ASB4 is upregulated by TNF-α and most likely transcriptionally activated by NF-κB. The cytokine TNF-α induces systemic inflammation via activation of the NF-κB and MAPK pathways. NF-κB is a transcription factor that is known for its pro-inflammatory effects but that also stimulates angiogenesis, in part, by increasing VEGF transcription33, 44. The link between ASB4 expression and the NF-κB-regulated pathways could suggest a potential role for ASB4 in inflammatory vascular processes like atherosclerosis, although no known association of ASB4 and atherosclerotic plaques has been reported, and nor has ASB4 been shown to be expressed in endothelial cells of adult organisms to date.
The upregulation of ASB4 in MS1 endothelial cells in response to insulin indicates that ASB4 may not only be important for pro-inflammatory responses and vascular development but also for energy homeostasis and vascular response to nutrition levels. Glucose homeostasis in particular appears to be a target of ASB4. ASB4 interacts with IRS-1, which plays a key role in transmitting signals from the insulin receptor to intracellular pathways13. In the paraventricular nucleus of the rat brain, insulin and leptin both cause an increase in ASB4 expression, with leptin also causing an increase in ASB4 expressionin the arcuate nucleus of the thalamus14. In our study of endothelial cells, ASB4 was upregulated by insulin which is consistent with the previously published data. Interestingly, although we did not find ASB4 to be expressed in all endothelial cell lines, we did find it to be highly expressed in MS1 cells, which are also the cells in which ASB4 was induced by insulin. MS1 cells are derived from the endothelial cells within the islets of the pancreas. Given the ability of these cells to upregulate ASB4 expression an insulin-dependent manner, it is possible that ASB4 plays a role in regulating blood flow within the pancreatic islets, perhaps in response to changing nutritional demands that alter the circulating level of insulin that could then alter the level of ASB4 either via a paracrine action or through regulation of the blood supply within the pancreatic islets.
The data presented in this study offer insight into the subtle yet complex effects of ASB4 in endothelial cells and may help us to better understand ASB4’s role in managing the cells’ response to various stimuli during angiogenesis. Our discovery of the involvement of NF-κB in the regulation of ASB4 expression is particularly exciting as this suggests a potential role for ASB4 in inflammatory responses in the embryonic period. It will be important, however, to also investigate ASB4 protein expression to confirm that the observed changes in ASB4 RNA levels translate into translational changes. If this turns out to be a phenomenon at both the RNA and protein levels, and if ASB4’s influence is demonstrated to extend beyond the embryonic period and the MS1/SVR cell lines, it could suggest that ASB4 is potentially instrumental in angiogenic and inflammatory processes later in life, such as those associated with tumors, wound healing or bypassing of a thrombosis. Clearly though, further studies will be needed in order to better understand the role of ASB4 not only in embryogenesis but also in adult life.
Acknowledgments
This work was supported by NIH GRANT R01 HL61656 (CP).
References
- 1.Kile BT, Schulman BA, Alexander WS, Nicola NA, Martin HM, Hilton DJ. The SOCS box: a tale of destruction and degradation. Trends Biochem Sci. 2002;27:235–241. doi: 10.1016/s0968-0004(02)02085-6. [DOI] [PubMed] [Google Scholar]
- 2.Kile BT, Viney EM, Willson TA, et al. Cloning and characterization of the genes encoding the ankyrin repeat and SOCS box-containing proteins Asb-1, Asb-2, Asb-3 and Asb-4. Gene. 2000;258:31–41. doi: 10.1016/s0378-1119(00)00402-9. [DOI] [PubMed] [Google Scholar]
- 3.Kamura T, Sato S, Haque D, et al. The Elongin BC complex interacts with the conserved SOCS-box motif present in members of the SOCS, ras, WD-40 repeat, and ankyrin repeat families. Genes Dev. 1998;12:3872–3881. doi: 10.1101/gad.12.24.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung AS, Guan YJ, Yuan ZL, Albina JE, Chin YE. Ankyrin repeat and SOCS box 3 (ASB3) mediates ubiquitination and degradation of tumor necrosis factor receptor II. Mol Cell Biol. 2005;25:4716–4726. doi: 10.1128/MCB.25.11.4716-4726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilcox A, Katsanakis KD, Bheda F, Pillay TS. Asb6, an adipocyte-specific ankyrin and SOCS box protein, interacts with APS to enable recruitment of elongins B and C to the insulin receptor signaling complex. J Biol Chem. 2004;279:38881–38888. doi: 10.1074/jbc.M406101200. [DOI] [PubMed] [Google Scholar]
- 6.Kwon S, Kim D, Rhee JW, et al. ASB9 interacts with ubiquitous mitochondrial creatine kinase and inhibits mitochondrial function. BMC Biol. 2010;8:23. doi: 10.1186/1741-7007-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee MR, Kim SK, Kim JS, Rhim SY, Kim KS. Expression of murine Asb-9 during mouse spermatogenesis. Mol Cells. 2008;26:621–624. [PubMed] [Google Scholar]
- 8.McDaneld TG, Spurlock DM. Ankyrin repeat and suppressor of cytokine signaling (SOCS) box-containing protein (ASB) 15 alters differentiation of mouse C2C12 myoblasts and phosphorylation of mitogen-activated protein kinase and Akt. J Anim Sci. 2008;86:2897–2902. doi: 10.2527/jas.2008-1076. [DOI] [PubMed] [Google Scholar]
- 9.Boengler K, Pipp F, Fernandez B, Richter A, Schaper W, Deindl E. The ankyrin repeat containing SOCS box protein 5: a novel protein associated with arteriogenesis. Biochem Biophys Res Commun. 2003;302:17–22. doi: 10.1016/s0006-291x(03)00095-0. [DOI] [PubMed] [Google Scholar]
- 10.Patterson C. Even flow: shear cues vascular development. Arterioscler Thromb Vasc Biol. 2005;25:1761–1762. doi: 10.1161/01.ATV.0000175755.93591.56. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson JE, 3rd, Wu Y, Smith K, et al. ASB4 is a hydroxylation substrate of FIH and promotes vascular differentiation via an oxygen-dependent mechanism. Mol Cell Biol. 2007;27:6407–6419. doi: 10.1128/MCB.00511-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metzen E, Ratcliffe PJ. HIF hydroxylation and cellular oxygen sensing. Biol Chem. 2004;385:223–230. doi: 10.1515/BC.2004.016. [DOI] [PubMed] [Google Scholar]
- 13.Li JY, Chai BX, Zhang W, Liu YQ, Ammori JB, Mulholland MW. Ankyrin repeat and SOCS box containing protein 4 (Asb-4) interacts with GPS1 (CSN1) and inhibits c-Jun NH2-terminal kinase activity. Cell Signal. 2007;19:1185–1192. doi: 10.1016/j.cellsig.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li JY, Chai BX, Zhang W, Wang H, Mulholland MW. Expression of ankyrin repeat and suppressor of cytokine signaling box protein 4 (Asb-4) in proopiomelanocortin neurons of the arcuate nucleus of mice produces a hyperphagic, lean phenotype. Endocrinology. 2010;151:134–142. doi: 10.1210/en.2009-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arbiser JL, Moses MA, Fernandez CA, et al. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci U S A. 1997;94:861–866. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 2001;20:4639–4647. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Connell KA, Edidin M. A mouse lymphoid endothelial cell line immortalized by simian virus 40 binds lymphocytes and retains functional characteristics of normal endothelial cells. J Immunol. 1990;144:521–525. [PubMed] [Google Scholar]
- 18.Wang SJ, Greer P, Auerbach R. Isolation and propagation of yolk-sac-derived endothelial cells from a hypervascular transgenic mouse expressing a gain-of-function fps/fes proto-oncogene. In Vitro Cell Dev Biol Anim. 1996;32:292–299. doi: 10.1007/BF02723062. [DOI] [PubMed] [Google Scholar]
- 19.Arbiser JL, Larsson H, Claesson-Welsh L, et al. Overexpression of VEGF 121 in immortalized endothelial cells causes conversion to slowly growing angiosarcoma and high level expression of the VEGF receptors VEGFR-1 and VEGFR-2 in vivo. Am J Pathol. 2000;156:1469–1476. doi: 10.1016/S0002-9440(10)65015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimmeler S, Haendeler J, Rippmann V, Nehls M, Zeiher AM. Shear stress inhibits apoptosis of human endothelial cells. FEBS Lett. 1996;399:71–74. doi: 10.1016/s0014-5793(96)01289-6. [DOI] [PubMed] [Google Scholar]
- 21.Lehoux S, Tedgui A. Signal transduction of mechanical stresses in the vascular wall. Hypertension. 1998;32:338–345. doi: 10.1161/01.hyp.32.2.338. [DOI] [PubMed] [Google Scholar]
- 22.Levesque MJ, Nerem RM, Sprague EA. Vascular endothelial cell proliferation in culture and the influence of flow. Biomaterials. 1990;11:702–707. doi: 10.1016/0142-9612(90)90031-k. [DOI] [PubMed] [Google Scholar]
- 23.Lin K, Hsu PP, Chen BP, et al. Molecular mechanism of endothelial growth arrest by laminar shear stress. Proc Natl Acad Sci U S A. 2000;97:9385–9389. doi: 10.1073/pnas.170282597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol. 1998;18:677–685. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- 25.Bakker SJ, Gans RO. About the role of shear stress in atherogenesis. Cardiovasc Res. 2000;45:270–272. doi: 10.1016/s0008-6363(99)00392-2. [DOI] [PubMed] [Google Scholar]
- 26.Dardik A, Chen L, Frattini J, et al. Differential effects of orbital and laminar shear stress on endothelial cells. J Vasc Surg. 2005;41:869–880. doi: 10.1016/j.jvs.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 27.Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985;5:293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- 28.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 29.Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983;53:502–514. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]
- 30.Osborn L, Kunkel S, Nabel GJ. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989;86:2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aurora AB, Biyashev D, Mirochnik Y, et al. NF-kappaB balances vascular regression and angiogenesis via chromatin remodeling and NFAT displacement. Blood. 2010;116:475–484. doi: 10.1182/blood-2009-07-232132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Martin R, Hoeth M, Hofer-Warbinek R, Schmid JA. The transcription factor NF-kappa B and the regulation of vascular cell function. Arterioscler Thromb Vasc Biol. 2000;20:E83–88. doi: 10.1161/01.atv.20.11.e83. [DOI] [PubMed] [Google Scholar]
- 33.Huang S, Robinson JB, Deguzman A, Bucana CD, Fidler IJ. Blockade of nuclear factor-kappaB signaling inhibits angiogenesis and tumorigenicity of human ovarian cancer cells by suppressing expression of vascular endothelial growth factor and interleukin 8. Cancer Res. 2000;60:5334–5339. [PubMed] [Google Scholar]
- 34.Klein S, de Fougerolles AR, Blaikie P, et al. Alpha 5 beta 1 integrin activates an NF-kappa B-dependent program of gene expression important for angiogenesis and inflammation. Mol Cell Biol. 2002;22:5912–5922. doi: 10.1128/MCB.22.16.5912-5922.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 36.Nikolova G, Jabs N, Konstantinova I, et al. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell. 2006;10:397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 37.Lammert E, Gu G, McLaughlin M, et al. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol. 2003;13:1070–1074. doi: 10.1016/s0960-9822(03)00378-6. [DOI] [PubMed] [Google Scholar]
- 38.Gaur U, Aggarwal BB. Regulation of proliferation, survival and apoptosis by members of the TNF superfamily. Biochem Pharmacol. 2003;66:1403–1408. doi: 10.1016/s0006-2952(03)00490-8. [DOI] [PubMed] [Google Scholar]
- 39.Valen G, Yan ZQ, Hansson GK. Nuclear factor kappa-B and the heart. J Am Coll Cardiol. 2001;38:307–314. doi: 10.1016/s0735-1097(01)01377-8. [DOI] [PubMed] [Google Scholar]
- 40.Burke JR, Pattoli MA, Gregor KR, et al. BMS-345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF-kappa B-dependent transcription in mice. J Biol Chem. 2003;278:1450–1456. doi: 10.1074/jbc.M209677200. [DOI] [PubMed] [Google Scholar]
- 41.Waelchli R, Bollbuck B, Bruns C, et al. Design and preparation of 2-benzamido-pyrimidines as inhibitors of IKK. Bioorg Med Chem Lett. 2006;16:108–112. doi: 10.1016/j.bmcl.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 42.Grosjean J, Kiriakidis S, Reilly K, Feldmann M, Paleolog E. Vascular endothelial growth factor signalling in endothelial cell survival: a role for NFkappaB. Biochem Biophys Res Commun. 2006;340:984–994. doi: 10.1016/j.bbrc.2005.12.095. [DOI] [PubMed] [Google Scholar]
- 43.Marumo T, Schini-Kerth VB, Busse R. Vascular endothelial growth factor activates nuclear factor-kappaB and induces monocyte chemoattractant protein-1 in bovine retinal endothelial cells. Diabetes. 1999;48:1131–1137. doi: 10.2337/diabetes.48.5.1131. [DOI] [PubMed] [Google Scholar]
- 44.Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J Biol Chem. 2001;276:7614–7620. doi: 10.1074/jbc.M009705200. [DOI] [PubMed] [Google Scholar]