Abstract
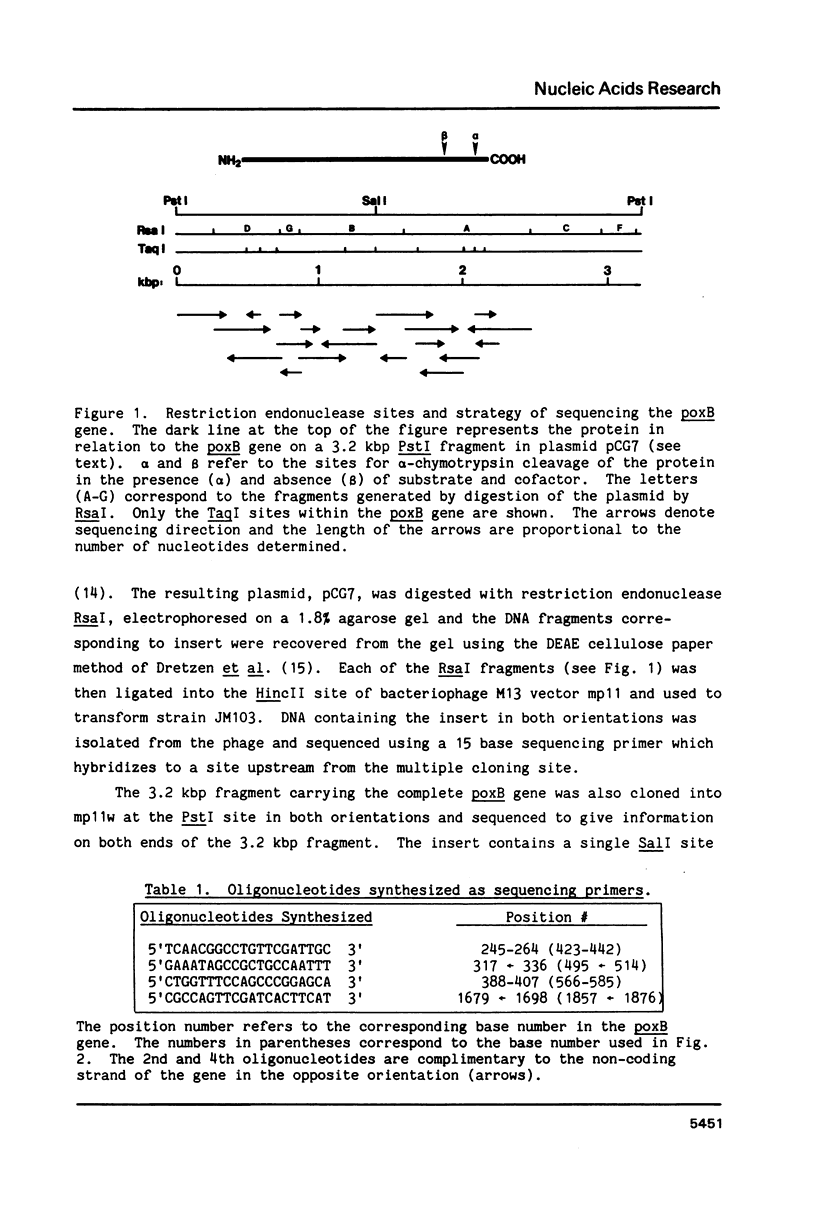
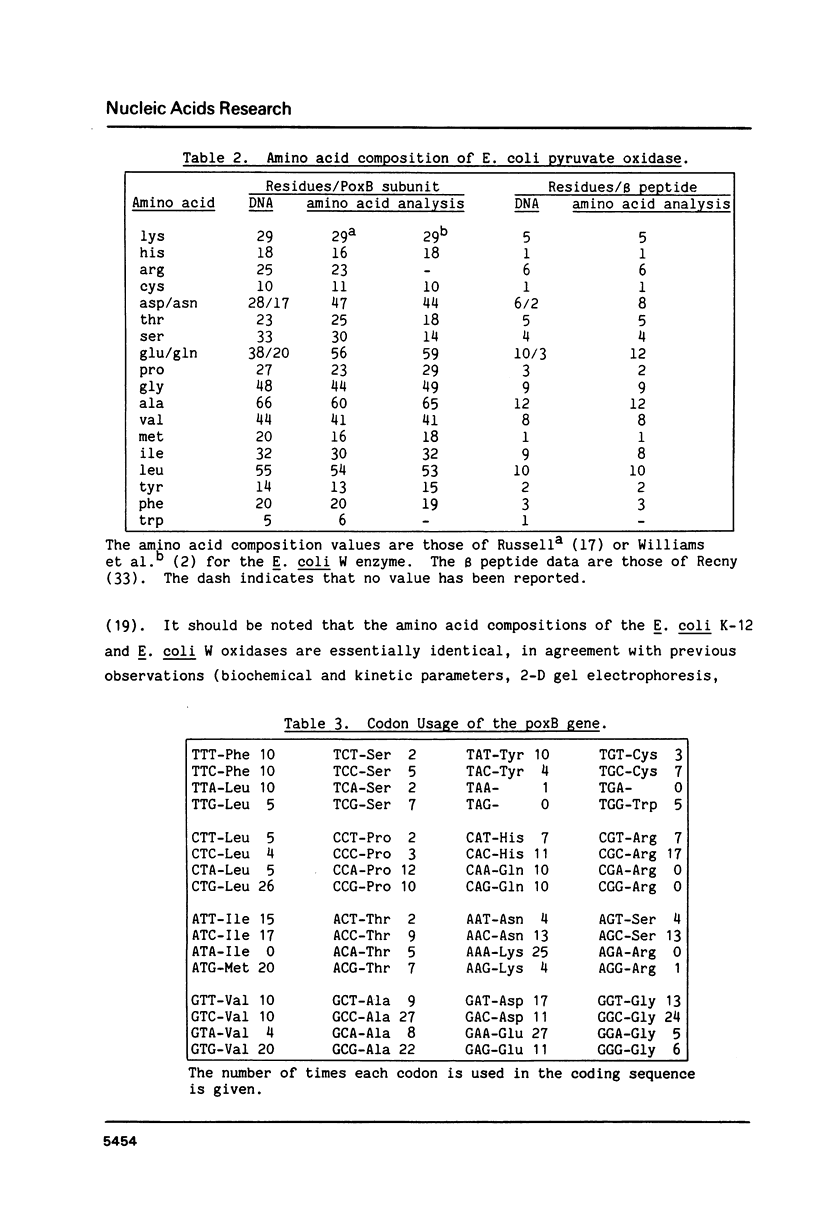
The entire nucleotide sequence of the poxB (pyruvate oxidase) gene of Escherichia coli K-12 has been determined by the dideoxynucleotide (Sanger) sequencing of fragments of the gene cloned into a phage M13 vector. The gene is 1716 nucleotides in length and has an open reading frame which encodes a protein of Mr 62,018. This open reading frame was shown to encode pyruvate oxidase by alignment of the amino acid sequences deduced for the amino and carboxy termini and several internal segments of the mature protein with sequences obtained by amino acid sequence analysis. The deduced amino acid sequence of the oxidase was not unusually rich in hydrophobic sequences despite the peripheral membrane location and lipid binding properties of the protein. The codon usage of the oxidase gene was typical of a moderately expressed protein. The deduced amino acid sequence shares homology with the large subunits of the acetohydroxy acid synthase isozymes I, II, and III, encoded by the ilvB, ilvG, and ilvI genes of E. coli.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake R., Hager L. P. Activation of pyruvate oxidase by monomeric and micellar amphiphiles. J Biol Chem. 1978 Mar 25;253(6):1963–1971. [PubMed] [Google Scholar]
- Chang Y. Y., Cronan J. E. An Escherichia coli mutant deficient in pyruvate oxidase activity due to altered phospholipid activation of the enzyme. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4348–4352. doi: 10.1073/pnas.81.14.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. Y., Cronan J. E., Jr Genetic and biochemical analyses of Escherichia coli strains having a mutation in the structural gene (poxB) for pyruvate oxidase. J Bacteriol. 1983 May;154(2):756–762. doi: 10.1128/jb.154.2.756-762.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Cunningham C. C., Hager L. P. Crystalline pyruvate oxidase from Escherichia coli. 3. Phospholipid as an allosteric effector for the enzyme. J Biol Chem. 1971 Mar 25;246(6):1583–1589. [PubMed] [Google Scholar]
- Cunningham C. C., Hager L. P. Crystalline pyruvate oxidase from Escherichia coli. II. Activation by phospholipids. J Biol Chem. 1971 Mar 25;246(6):1575–1582. [PubMed] [Google Scholar]
- De Felice M., Levinthal M., Iaccarino M., Guardiola J. Growth inhibition as a consequence of antagonism between related amino acids: effect of valine in Escherichia coli K-12. Microbiol Rev. 1979 Mar;43(1):42–58. doi: 10.1128/mr.43.1.42-58.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Friden P., Donegan J., Mullen J., Tsui P., Freundlich M., Eoyang L., Weber R., Silverman P. M. The ilvB locus of Escherichia coli K-12 is an operon encoding both subunits of acetohydroxyacid synthase I. Nucleic Acids Res. 1985 Jun 11;13(11):3979–3993. doi: 10.1093/nar/13.11.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabau C., Cronan J. E., Jr Molecular cloning of the gene (poxB) encoding the pyruvate oxidase of Escherichia coli, a lipid-activated enzyme. J Bacteriol. 1984 Dec;160(3):1088–1092. doi: 10.1128/jb.160.3.1088-1092.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribskov M., Devereux J., Burgess R. R. The codon preference plot: graphic analysis of protein coding sequences and prediction of gene expression. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):539–549. doi: 10.1093/nar/12.1part2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- HAGER L. P. Trypsin activation of a ferricyanide-linked pyruvic acid oxidation. J Biol Chem. 1957 Nov;229(1):251–263. [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koland J. G., Miller M. J., Gennis R. B. Reconstitution of the membrane-bound, ubiquinone-dependent pyruvate oxidase respiratory chain of Escherichia coli with the cytochrome d terminal oxidase. Biochemistry. 1984 Jan 31;23(3):445–453. doi: 10.1021/bi00298a008. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- O'Brien T. A., Shelton E., Mather M., Gennis R. B. Conformational studies of Escherichia coli pyruvate oxidase. Biochim Biophys Acta. 1982 Aug 10;705(3):321–329. doi: 10.1016/0167-4838(82)90254-0. [DOI] [PubMed] [Google Scholar]
- Porter T. D., Kasper C. B. Coding nucleotide sequence of rat NADPH-cytochrome P-450 oxidoreductase cDNA and identification of flavin-binding domains. Proc Natl Acad Sci U S A. 1985 Feb;82(4):973–977. doi: 10.1073/pnas.82.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj T., Russell P., Flygare W. H., Gennis R. B. Quasi-elastic light scattering studies on pyruvate oxidase. Biochim Biophys Acta. 1977 Mar 15;481(1):42–49. doi: 10.1016/0005-2744(77)90135-8. [DOI] [PubMed] [Google Scholar]
- Recny M. A., Grabau C., Cronan J. E., Jr, Hager L. P. Characterization of the alpha-peptide released upon protease activation of pyruvate oxidase. J Biol Chem. 1985 Nov 15;260(26):14287–14291. [PubMed] [Google Scholar]
- Recny M. A., Hager L. P. Isolation and characterization of the protease-activated form of pyruvate oxidase. Evidence for a conformational change in the environment of the flavin prosthetic group. J Biol Chem. 1983 Apr 25;258(8):5189–5195. [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Russell P., Schrock H. L., Gennis R. B. Lipid activation and protease activation of pyruvate oxidase. Evidence suggesting a common site of interaction on the protein. J Biol Chem. 1977 Nov 10;252(21):7883–7887. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens D. J., Gennis R. B. Studies on the quaternary structure of Escherichia coli pyruvate oxidase. J Biol Chem. 1980 Jan 25;255(2):379–383. [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek R. C., Hauser C. A., Hatfield G. W. The nucleotide sequence of the ilvBN operon of Escherichia coli: sequence homologies of the acetohydroxy acid synthase isozymes. Nucleic Acids Res. 1985 Jun 11;13(11):3995–4010. doi: 10.1093/nar/13.11.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams F. R., Hager L. P. Crystalline flavin pyruvate oxidase from Escherichia coli. I. Isolation and properties of the flavoprotein. Arch Biochem Biophys. 1966 Sep 26;116(1):168–176. doi: 10.1016/0003-9861(66)90025-7. [DOI] [PubMed] [Google Scholar]


