Abstract
Much of the heart, including the atria, right ventricle and outflow tract (OFT) is derived from a progenitor cell population termed the second heart field (SHF) that contributes progressively to the embryonic heart during cardiac looping. Several studies have revealed anterior-posterior patterning of the SHF, since the anterior region (anterior heart field) contributes to right ventricular and OFT myocardium whereas the posterior region gives rise to the atria. We have previously shown that Retinoic Acid (RA) signal participates to this patterning. We now show that Hoxb1, Hoxa1, and Hoxa3, as downstream RA targets, are expressed in distinct sub-domains within the SHF. Our genetic lineage tracing analysis revealed that Hoxb1, Hoxa1 and Hoxa3-expressing cardiac progenitor cells contribute to both atria and the inferior wall of the OFT, which subsequently gives rise to myocardium at the base of pulmonary trunk. By contrast to Hoxb1Cre, the contribution of Hoxa1-enhIII-Cre and Hoxa3Cre-labeled cells is restricted to the distal regions of the OFT suggesting that proximo-distal patterning of the OFT is related to SHF sub-domains characterized by combinatorial Hox genes expression. Manipulation of RA signaling pathways showed that RA is required for the correct deployment of Hox-expressing SHF cells. This report provides new insights into the regulatory gene network in SHF cells contributing to the atria and sub-pulmonary myocardium.
Keywords: Retinoic Acid, Heart development, Mouse, Hox genes, Cardiac progenitor cells
INTRODUCTION
The four-chambered mammalian heart forms from a heterogeneous population of progenitor cells in anterior lateral mesoderm. Studies in mouse and chick have established that the heart forms from two sources of progenitor cells (Buckingham et al., 2005; Vincent and Buckingham, 2010). As the embryo grows, cells of the cardiac crescent fuse at the midline to form the primitive heart tube. The primitive heart tube initially functions to support the embryonic circulation and provides a scaffold into which the cells from the second heart field (SHF) migrate prior to chamber morphogenesis. SHF cells are first located medially to the cardiac crescent, and subsequently reside in mesoderm underlying the pharynx before they accrue to the heart. The contribution of this population of cardiac progenitors to the heart was revealed by studies of the LIM transcription factor Islet1 (Isl1), which is a pan-marker of the SHF (Cai et al., 2003). The rostral part of the SHF, the anterior heart field (AHF), which is marked by Fgf10 expression (Kelly et al., 2001) contributes to the formation of right ventricular and outflow tract (OFT) myocardium (Zaffran et al., 2004), whereas cells in the posterior SHF (Cai et al., 2003) expressing Isl1, but not AHF markers, contribute to atrial myocardium (Galli et al., 2008). These data indicate that the SHF is patterned along the anterior-posterior (AP) axis of the mouse embryo, however, a detailed understanding of the molecular regulatory pathways governing this process is lacking.
We have recently shown that the retinoic acid (RA) signaling pathway plays a potent role in limiting cardiac specification. Mouse embryos lacking the RA synthesis enzyme Raldh2 have an expanded SHF, resulting in morphogenetic defects at both the arterial and venous poles (Ryckebusch et al., 2008). Consistent with this, zebrafish embryos lacking RA signaling exhibit an excess of cardiac progenitor cells in the lateral mesoderm (Keegan et al., 2005). In the avian model, RA signaling promotes atrial cell identity within the heart field (Xavier-Neto et al., 1999; Xavier-Neto et al., 2001; Hochgreb et al., 2003). It remains unknown whether the functions of RA signaling on SHF development and cardiac identity are distinct or overlapping. Identifying RA-target genes in cardiac progenitor cells will help to elucidate the mechanisms downstream of RA signaling that delimit the SHF. Studies in zebrafish embryos demonstrated that Hoxb5b, expressed in the forelimb field, acts downstream of RA signaling to restrict the number of cardiac progenitor cells (Waxman and Yelon, 2009). Thus, we hypothesized that some of the homeobox (Hox) genes may be functional targets of RA in cardiac lineages in the mouse.
Hox genes are a large family of related genes that encode homeodomain transcription factors. Mammalian Hox genes are clustered in four chromosomal loci (the Hox clusters), and play an important role in regulating the specification of positional identities along the AP axis during development (Alexander et al., 2009; Wellik, 2009). Within each cluster, the genes are arranged in a sequence that reflects their sequential activation during development (temporal collinearity) (Izpisua-Belmonte et al., 1990) and the position of the anterior boundary of their expression domains along the AP body axis (spatial collinearity) (Duboule and Dolle, 1989; Graham et al., 1989). Initial Hox transcription and rostral expansion of Hox expression domains are regulated in part by events that are connected to the emergence and extension of the primitive streak (Forlani et al., 2003; Iimura and Pourquie, 2006). A contribution of RA signaling to the initial activation of Hox expression has been suggested, since at early developmental stages, embryos with impaired RA synthesis (Raldh2−/− mutants) exhibit abnormal initial 3′ Hox gene expression domains (Niederreither et al., 1999). Moreover, RA was shown to regulate embryonic AP patterning, in particular by controlling the expression of specific Hox genes (Niederreither and Dolle, 2008; Alexander et al., 2009).
In this study, we show that the anterior Hox genes, Hoxb1, Hoxa1 and Hoxa3, are expressed in the SHF as early as embryonic day (E) 7.5 and define distinct sub-domains in the splanchnic mesoderm. Genetic (cre-mediated) lineage tracing reveals that Hoxb1, Hoxa1 and Hoxa3-expressing cardiac progenitor cells give rise to the atria and the inferior wall of the OFT, which subsequently yields the myocardium at the base of the pulmonary trunk. Furthermore, Hoxb1IRES-Cre, Hoxa1-enhIII-Cre and Hoxa3IRES-Cre marked cells shows differential contributions to the proximal and distal regions of the OFT. Manipulation of the RA signaling pathway using Raldh2−/− embryos or injection of all-trans-RA demonstrates that expression of these Hox genes in the SHF and their cardiac contribution to the heart are sensitive to RA dosage. Comparison of transgenes expression in Raldh2 mutant embryos reveals that RA signaling is required for these Hox-expressing cardiac progenitor cell populations to contribute to the heart.
MATERIALS AND METHODS
Mouse lines and breeding
All mouse lines used in this study have been previously described: Raldh2-null (Niederreither et al., 1999), Hoxa3IRES-Cre (Macatee et al., 2003), Hoxb1IRES-Cre (Arenkiel et al., 2003), alleles and Mlc1v-nlacZ-24/Fgf10lacZ (Kelly et al., 2001), RARE-hsp68-lacZ (Rossant et al., 1991), y96-Myf5-nlacZ-16 (96-16), A17-Myf5-nlacZ-T55 (T55) (Bajolle et al., 2008) and R26R-lacZ (Soriano, 1999) transgenes. Mice were genotyped by PCR as described in the original reports. Embryos were staged taking embryonic day (E) 0.5 as the morning of the vaginal plug. Cre-induced recombination was analyzed by breeding Cre mice with R26R-lacZ reporter mice and analyzing embryos with the genotype Cre; R26R-lacZ by X-gal staining. Animal care was in accordance with national and institutional guidelines.
Generation of novel transgenic line
The Hoxa1 enhancer III-Cre (Hoxa1-enhIII-Cre) construct DNA was previously described by Li and Lufkin (2000) (Li and Lufkin, 2000). Transgenic mice were generated by microinjection of purified plasmid DNA into fertilized (C57BL/6XDBA/2) F2 eggs at a concentration of approx. 1ng/ul using standard techniques. Injected eggs were re-implanted the day after the injection into pseudo-pregnant (C57BL/6), foster mothers.
X-gal staining, histology and RNA in situ hybridizations
To visualize β-galactosidase activity, embryos or hearts were isolated, fixed in 4% paraformaldehyde for 20min and moved into X-gal solution, according to standard procedures. Embryos or hearts were photographed (Zeiss Lumar V12 stereomicroscope) as whole-mount specimens and then embedded in O.C.T. and cut into 12μM histological section before being counterstained with eosin.
Whole-mount in situ hybridization (ISH) was performed as previously described (Ryckebusch et al., 2008). Double whole-mount ISH with digoxigenin (DIG)- and FITC-labeled riboprobes were performed according to the Stern laboratory protocol (http://www.ucl.ac.uk/cdb/research/stern/stern_lab/insitu, Protocols section).
The following riboprobes used in this study were Bmp4, Hoxa1, Hoxb1, Hoxa2, Hoxa3, islet1, Tbx5, Raldh2, and Wnt11. For single ISH, hybridization signals were then detected by alkalin phosphatase (AP)-conjugated anti-DIG antibodies (1/2000; Roche), which were followed by color development with NBT/BCIP (magenta) substrate (Promega). For double ISH hybridization signals we used an anti-FITC antibody coupled to AP (1/2000; Roche), and the NBT-BCIP (magenta) (promega) for the first detection, and an anti-DIG antibody coupled to AP (1/2000; Roche) and the INT-BCIP (brick red) (Roche) for the second detection. After staining, the samples were washed in PBS and post-fixed. Embryos were photographed using a Zeiss Lumar stereomicroscope coupled to an Axiocam digital camera (AxioVision 4.4, Zeiss). The number of embryos examined was at least 3 for each stage.
Immunostaining
Embryos were fixed at 4°C for 20min in 4% paraformaldehyde, rinsed in PBS, equilibrated to 15% sucrose and embedded in O.C.T. Cryosections were cut at 12μm, washed in PBS and pre-incubated in blocking solution (1%BSA, 1% Serum, 0.2% Tween20 in PBS). Primary antibodies were applied overnight at 4°C, followed by secondary detection using Alexa Fluor conjugated (Molecular Probes) secondary antibodies. Sections were photographed using Leica DM 5000B microscope.
The following primary antibodies were used in this study: rabbit anti-Hoxb1 (Covance; 1/200), rabbit anti-GFP (Invitrogen; 1/500), rabbit anti-βGal (sigma; 1/500) and mouse anti-Islet1 (DSHB: 1/100).
Retinoic acid treatment of embryos
All-trans-RA (Sigma) was dissolved in DMSO and diluted at 20mg/ml. At E7.75, the mice were given a single intra-peritoneal injection of RA (70mg/kg or 85mg/kg) or control DMSO. Embryos were later dissected at E8.5 or E9.5.
RESULTS
Anterior-posterior patterning of the second heart field
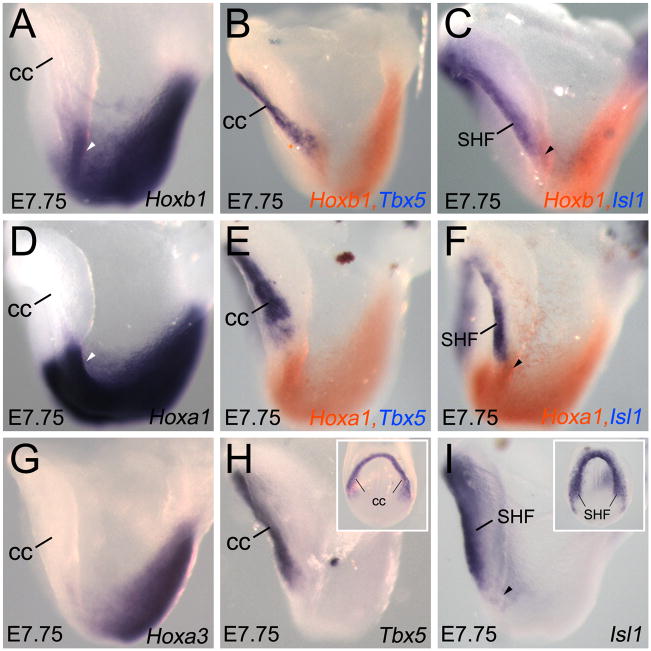
We previously reported that RA signaling is required to establish the posterior limit of the second heart field (SHF) in splanchnic mesoderm of mouse embryos (Ryckebusch et al., 2008). However, this study did not identify the molecules downstream of RA signaling that are responsible for the restriction of cardiac progenitors. Hox genes have been suggested to be among the key downstream effectors of RA signaling during cardiac patterning (Searcy and Yutzey, 1998; Waxman et al., 2008). Therefore, we examined the expression pattern of several Hox genes within the lateral mesoderm and compared their expression to cardiac markers. We selected Hoxb1, Hoxa1 and Hoxa3 because they are among the first Hox genes to be activated at the primitive streak and consequently display anterior limits of expression close to the cardiac field. At E7.25, the expression domains of Hoxb1, Hoxa1 and Hoxa3 overlapped with those of the RA-synthesizing enzyme Raldh2, and the RARE-lacZ transgene, a reporter for RA activity (Supplemental Fig. S1). During extension of the primitive streak, transcription of Hoxb1 initiates earlier than Hoxa1 and Hoxa3 (Supplemental Fig. S1), suggesting that sequential temporal activation of these Hox genes is important to establish anterior-posterior (AP) patterning of the lateral mesoderm. At E7.75, the anterior border of Hoxb1 expression is more rostral than those of Hoxa1 and Hoxa3 (Fig. 1A,D,G).
Figure 1.
Relation between Hoxb1 and Hoxa1 expression and the heart fields. (A–F) Hoxb1 and Hoxa1 expression analysis by single and double in situ hybridizations (ISH) on E7.75 embryos. (G) Hoxa3 expression analysis by ISH. (H,I) Whole-mount ISH with Tbx5 and Islet1 (Isl1) probes, which mark the cardiac crescent (cc) and the second heart field (SHF) respectively. Insets display a ventral view of same stained embryo. (A,D,G) At E7.75, Hoxb1, Hoxa1 and Hoxa3 reach their most anterior border of expression near the cardiac crescent (cc). (B,C) Whole-mount ISH analysis showing that the anterior border of Hoxb1 expression overlaps with Isl1 (arrowhead in C), but not with Tbx5. (E,F) Whole-mount ISH analysis showing Hoxa1 expression in an adjacent domain of Tbx5 and Isl1 (arrowhead in F). (G) Anterior border of Hoxa3 expression is posterior to Tbx5 and Isl1 regions. cc, cardiac crescent; SHF, second heart field.
Despite reported expression in early mesodermal cells (Frohman et al., 1990; Murphy and Hill, 1991), Hox gene expression relative to the heart field has never been explored in the mouse. To assess the expression of Hox genes in the heart field, we performed double in situ hybridization with Tbx5 and Islet1 (Isl1), which label the cardiac crescent and the SHF respectively (Fig. 1H,I) (Buckingham et al., 2005). At the early cardiac crescent stage, Hoxb1 (orange) and Isl1 (purple) exhibit an overlap of their expression domains (compare Fig. 1A,C and I; arrowheads), suggesting that Hoxb1 is expressed in cardiac progenitor cells. Embryos double-stained for Hoxb1 and Tbx5 (purple) display no overlap, indicating that Hoxb1 is not expressed in Tbx5-positive cells (compare Fig. 1A,B and H). Consistent with our previous observations (Ryckebusch et al., 2008), double labeling confirms that the Hoxa1 expression domain is adjacent to the cardiogenic region marked by Tbx5 at E7.75 (Fig. 1E). However, we detected a small overlap between Hoxa1 and Isl1 in the splanchnic mesoderm (compare Fig. 1D,F and I, arrowheads). As the heart tube forms, Hoxb1 and Hoxa1 are expressed in both the splanchnic mesoderm and the ventral and lateral foregut endoderm, but not in the heart tube (Fig. 2A,C), as confirmed by double in situ hybridization with Isl1 (Supplemental Fig. S2). Double immunohistochemistry for Hoxb1 and Isl1 revealed that Hoxb1 and Isl1-positive nuclei co-localize in the caudal region of the SHF (Supplemental Fig. S3), suggesting that Hoxb1 expression characterizes a sub-domain of the Isl1+ splanchnic mesodermal population.
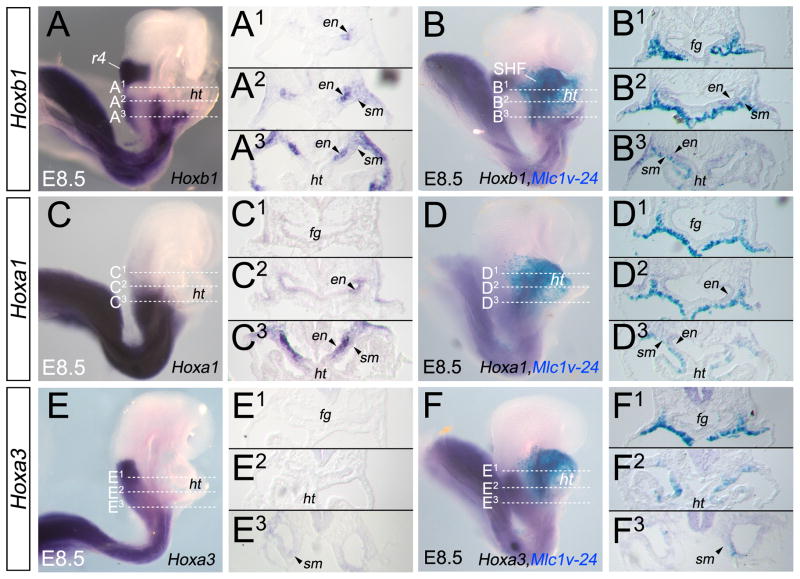
Figure 2.
Hoxb1 and Hoxa1 expression patterns define distinct sub-domains within the second heart field. (A–F) Lateral views of embryos at E8.5. (A,C,E) Whole-mount in situ hybridization (ISH) analysis of Hoxb1 (A), Hoxa1 (C) and Hoxa3 (E) mRNAs. (B,D,F) Whole-mount ISH analysis of Hoxb1 (B), Hoxa1 (D) and Hoxa3 (F) genes combined with X-gal staining for the Mlc1v-nlacZ-24 transgene, which marks the anterior heart field (AHF). Dotted lines in A–F indicate the plane of sections in A1–F2. (A1–A3) Sections showing expression of Hoxb1 in the medial (A2, arrowhead) and posterior (A3) domains of the second heart field (SHF), and the absence of expression in the anterior domain of the SHF (A1). Note the expression of Hoxb1 in the anterior foregut endoderm. (B1–B3) Sections showing co-localization of Hoxb1 and X-gal staining in the caudal region of the AHF (B2,B3) but not in the anterior region of the AHF (B1). (C1–C3) Expression of Hoxa1 is only detected in the posterior region of the SHF (C3, arrowheads). Note the expression of Hoxa1 in the anterior foregut endoderm (C2, C1). (D1–D3) Sections showing the co-localization of Hoxa1 and X-gal labeled cells only in the posterior region of the AHF (D3, arrowhead). (E1–E3) Hoxa3 expression is observed in the splanchnic mesoderm (E3, arrowhead) located posteriorly to the heart tube. (F1–F3) Sections showing that Hoxa3 is not detected in the AHF. cc, cardiac crescent; en, endoderm; fg, foregut; ht, heart tube; SHF, second heart field; sm, splanchnic mesoderm.
In order to examine the anterior boundaries of the expression of these Hox genes within the splanchnic mesoderm, we used Mlc1v-nlacZ-24 (Mlc1v-24) transgenic mice, in which a transgene integration at the Fgf10 locus leads to β-galactosidase expression in the anterior domain of the SHF, referred to as the anterior heart field (AHF) (Kelly et al., 2001). We thus found that Hoxb1 transcripts and β-galactosidase activity co-localize in the posterior half region of the AHF (Fig. 2A,B). Co-localization of Hoxa1 expression and Mlc1-nlacZ-v24 staining is more limited, since it is observed only in the most caudal margin of the AHF (Fig. 2C,D), suggesting that the anterior limit of the expression domain of Hoxa1 is within the posterior region of the AHF. In contrast, Hoxa3 expression is not detected in the AHF but in splanchnic mesoderm located posteriorly to the heart tube region (Fig. 2E,F). Taken together, these results show that Hoxb1 and Hoxa1 are expressed in the SHF with different anterior limits of expression within the caudal AHF, while Hoxa3 is essentially expressed in the most caudal region of the posterior SHF. Importantly, Hoxb1, Hoxa1 and Hoxa3 transcripts were not detected in differentiated cardiomyocytes.
Hoxb1-expressing cardiac progenitor cells contribute to the inferior wall of the outflow tract
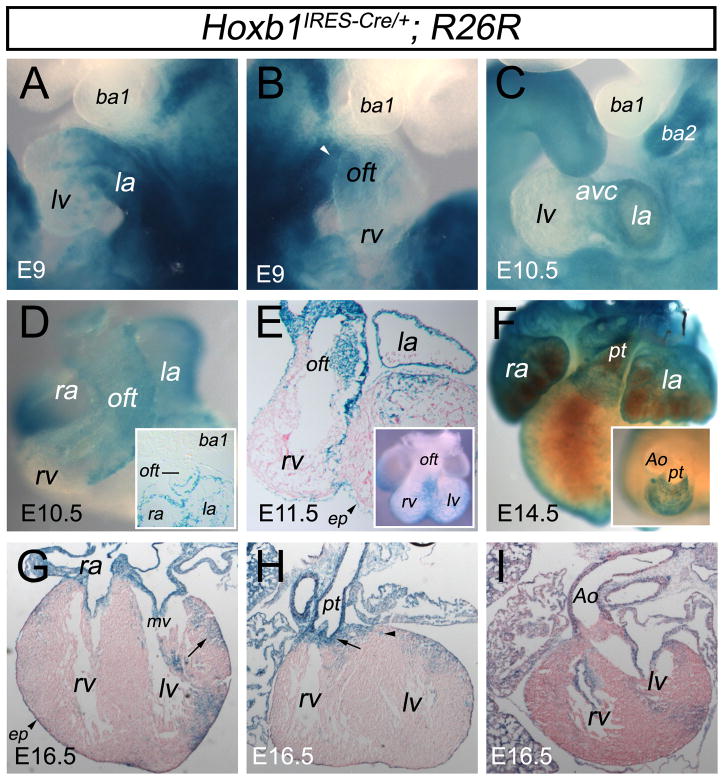
Recent evidence suggests that posterior SHF contributes to the atria in mice (Cai et al., 2003; Galli et al., 2008), whereas the AHF gives rise to the OFT and the right ventricle (Kelly et al., 2001; Zaffran et al., 2004). Our in situ hybridization analysis suggests that Hoxb1+ cardiac progenitor cells might thereby contribute to both the arterial and venous poles of the heart. To investigate this question, we performed genetic lineage tracing analysis of Hoxb1-expressing cells by crossing a Hoxb1IRES-Cre allele (Arenkiel et al., 2003) with the R26R-lacZ reporter line (Soriano, 1999), which expresses β-galactosidase upon Cre recombination. Until E7.75, the recombination pattern in lateral mesoderm of Hoxb1IRES-Cre; R26R-lacZ embryos was highly similar to the expression pattern of Hoxb1, including their anterior boundary (Supplemental Fig. S4). At E8.5, however, the pattern of recombination in Hoxb1IRES-Cre; R26R-lacZ embryos was discordant with that of Hoxb1 expression. Hoxb1, or Cre, transcripts were confined to the SHF, whereas β-galactosidase activity was found in the venous pole of the heart (Supplemental Fig. S4). Between E9 and E16.5, Hoxb1 expression was not detected in differentiated cardiomyocytes (data not shown), whereas β-galactosidase activity was found in the majority of the atrial cells and the atrioventricular canal (AVC) myocardium and its derivatives, including the atrioventricular valves (Fig. 3A,C,G). X-gal stained cells were found in the working myocardium of the left ventricular free wall contiguous with AVC derivatives (Fig. 3G), confirming that the AVC lineage provides a contribution to this region of the left ventricle (Aanhaanen et al., 2009). However, this contribution is less important than the one observed for Tbx2+ progeny by Aanhaanen et al. β-galactosidase activity was also detected in the epicardium (Fig. 3E–H) and subsequently in the walls of the main coronary vessels (Fig. 3H; arrowhead). Because Raldh2 is strongly expressed in the proepicardium (Moss et al., 1998; Xavier-Neto et al., 1999) and RA signaling has an established function in the fetal epicardium (Merki et al., 2005; Lin et al., 2010), we cannot exclude later activation of Hoxb1 in this tissue. This contribution is also observed in postnatal heart (data not shown).
Figure 3.
Genetic lineage analysis reveals a contribution of Hoxb1+ cardiac progenitors to the atria and the myocardium at the base of the pulmonary trunk. (A–I) Hoxb1-lineage visualized by X-gal staining of Hoxb1IRES-Cre; R26R-lacZ embryos. (A–C) Lateral views of X-gal stained embryos at E9 (A,B) and E10.5 (C). (D–F) Ventral views of X-gal stained hearts at E10.5 (D), E11.5 (E) and E14.5 (F). (G,I) Transverse sections of X-gal stained hearts at E16.5. (A,B) X-gal staining showing a contribution of Hoxb1-positive cells to the venous pole (left atrium) and arterial pole (white arrowhead) of Hoxb1IRES-Cre; R26R-lacZ hearts. (C) Lateral view of an E10.5 X-gal stained embryo, showing β-galactosidase activity in the left atrium and the atrioventricular canal. Note that neural crest derivatives populate the second branchial arch (ba2). (D) Ventral view of X-gal stained heart from Hoxb1IRES-Cre; R26R-lacZ embryo at E10.5. β-galactosidase activity is detected in the left and right atria but also in the outflow tract (OFT). Inset is a frontal section through stage E10.5 embryo, showing β-galactosidase activity only in the inferior wall of the OFT. (E) Transverse section of the heart from an E11.5 Hoxb1IRES-Cre; R26R-lacZ embryo. β-galactosidase activity is detected in the atria, the epicardium (arrowhead) and the left side of the OFT. Inset shows a ventral view of the same X-gal stained heart. (F) Ventral view of an X-gal stained heart at E14.5, showing that labeled cells are detected in right and left atria and at the base of the pulmonary trunk. Inset is a cranial view of the same heart. X-gal stained cells are concentrated at the base of the pulmonary trunk. (G–I) Transverse sections of an E16.5 heart. β-galactosidase activity is detected in the epicardium (arrowhead), the atrioventricular valves and in the myocardium at the base of the pulmonary trunk (arrow in H) but not at the base of the aorta (I). Arrow in G indicates X-gal stained cells in the left ventricular myocardium. Ao, aorta; avc, atrioventricular canal; ba, branchial arch; ep, epicardium; ht, heart tube; la, left atrium; lb, limb bud; lv, left ventricle; mv; mitral valve; oft, outflow tract; pt, pulmonary trunk; ra, right atrium; rv; right ventricle.
At E9 and E10.5, descendants of cells expressing Hoxb1 are also observed in the arterial pole (Fig. 3B) and in the OFT of Hoxb1IRES-Cre; R26R-lacZ embryos (Fig. 3D). X-gal labeled cells are detected exclusively in the inferior wall of the OFT (Fig. 3D). Between E11.5 and E16.5, β-galactosidase activity is found in the left side of the OFT wall and then in the myocardium at the base of the pulmonary trunk (Fig. 3E,F,H), but not at the base of the aorta (Fig. 3I). This confirms that the myocardial wall of the OFT rotates as previously suggested (Bajolle et al., 2006). Together, these findings indicate that precursors of the inferior wall of the OFT, which contribute to the base of the pulmonary trunk, segregate early in the SHF as proposed from a previous study of regionalized transgene expression and retrospective clonal analysis (Bajolle et al., 2008).
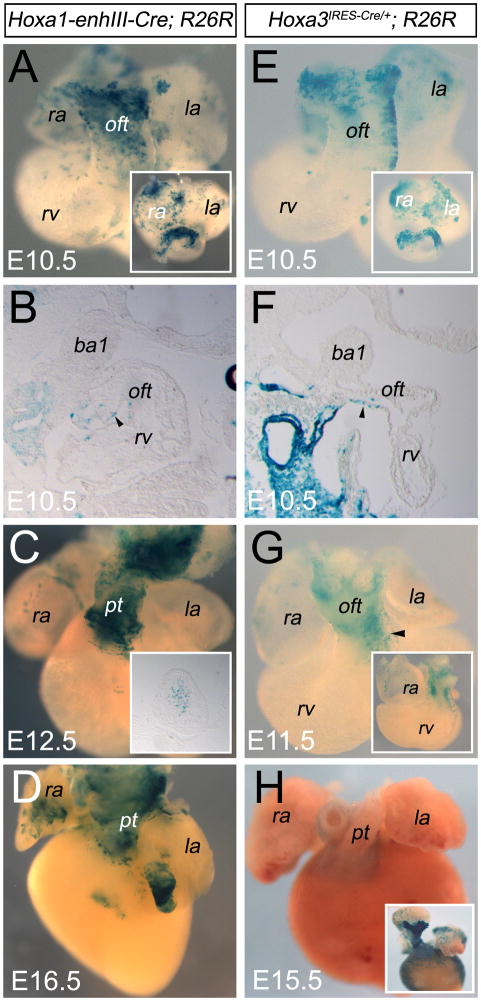
Our in situ hybridization analysis revealed a spatial difference between Hoxb1, Hoxa1 and Hoxa3 transcripts in the SHF (Fig. 2). To compare the contribution of Hoxa1- and Hoxa3-expressing cells with the Hoxb1-lineage, we performed genetic lineage tracing using a Hoxa1-enhIII-Cre transgene (Li and Lufkin, 2000) and a Hoxa3IRES-Cre allele (Macatee et al., 2003). We used the 0.5kb Hoxa1 enhancer III because it was reported to recapitulate a significant portion of the Hoxa1 expression domain and to contain a functional RA regulatory element (RARE) (Frasch et al., 1995). Until E8 to E9.5, the pattern of recombination in Hoxa1-enhIII-Cre; R26R-lacZ embryos was highly similar to the expression pattern of Hoxa1 (Supplemental Fig. S5). At E9.5, X-gal staining revealed minimal difference to that seen with Hoxa1IRES-Cre; R26R-lacZ embryos at the same stage (Supplemental Fig. S5), as recently described (Makki and Capecchi, 2010). This supports the use of the Hoxa1-enhIII-Cre transgene for our lineage analyses. Between E10.5 and E16.5, descendants of cells expressing Hoxa1 were observed in a small number of atrial cells in Hoxa1-enhIII-Cre; R26R-lacZ embryos (Fig. 4A–D). Of note, X-gal-labeled cells were never found in the AVC in these embryos (data not shown), suggesting that AVC myocytes are derived from the Hoxb1-lineage but not the Hoxa1-lineage. As in Hoxb1IRES-Cre; R26R-lacZ hearts, X-gal-labeled cells in the Hoxa1-enhIII-lineage were located in the inferior wall of the OFT (Fig. 4A,B). In contrast, β-galactosidase activity was only detected in the distal OFT of Hoxa1-enhIII-Cre; R26R-lacZ embryos (Fig. 4A,B), consistent with the small number of X-gal-labeled cells found later in the myocardium at the base of the pulmonary trunk (Fig. 4C,D). At E12.5, the Hoxa1-enhIII labeled cells were seen in the cushions of the OFT (Fig. 4C), which probably corresponds to recombination in neural crest cells.
Figure 4.
Cardiac contribution of the Hoxa1-enhIII-Cre and Hoxa3IRES-Cre progeny. (A–D) Hoxa1-lineage visualized by X-gal staining of Hoxa1-enhIII-Cre; R26R-lacZ embryos. (E–H) Hoxa3-lineage visualized by X-gal staining of Hoxa3IRES-Cre; R26R-lacZ embryos. Ventral views of X-gal stained hearts at E10.5 (A,E), E11.5 (G), E12.5 (C), E15.5 (H) and E16.5 (D). (A,E) β-galactosidase activity is detected in small number of left and right atrial cells. X-gal staining is observed in the distal outflow tract (OFT) in Hoxa1-enhIII-Cre; R26R-lacZ and Hoxa3IRES-Cre; R26R-lacZ embryos. Insets show cranial views of the same hearts confirming β-galactosidase activity in the inferior wall of the OFT. (B,F) Sagittal sections of embryos at the same stage showing X-gal labeled cells in the inferior wall of the OFT. (C,D) Ventral views of X-gal stained hearts at E12.5 and E16.5. β-galactosidase activity is detected in both atria and in the myocardium at the base of the pulmonary trunk. Inset in C displays X-gal labeled cells in the OFT cushions. (G,H) Ventral views of X-gal stained hearts at E11.5 and E15.5, showing that few labeled cells are detected in the left side of the OFT (arrowhead) and later in the myocardium at the base of the pulmonary trunk. Inset in G reveals X-gal labeled cells in OFT cushions. Inset in H shows a ventral view of stronger X-gal stained heart at the same stage. Ao, aorta; b, branchial arch; g, gut epithelium; ht, heart tube; la, left atrium; lb, limb bud; oft, outflow tract; pt, pulmonary trunk; ra, right atrium; rv; right ventricle.
Our Hoxa3+ lineage analysis confirms that Hoxa3IRES-Cre recombination in the pharyngeal region begins at E8 in surface ectoderm (Supplemental Fig. S5), and then extends into pharyngeal endoderm and mesoderm (Macatee et al., 2003; Zhang et al., 2005). The recombination pattern in Hoxa3IRES-Cre; R26R-lacZ embryos recapitulated the caudal to rostral progression of Hoxa3 expression (Supplemental Fig. S1 and Fig. S5). Consistent with the other Hox lineages, β-galactosidase activity was detected in the inferior myocardial wall of the distal OFT of Hoxa3IRES-Cre; R26R-lacZ embryos (Fig. 4E,F). Subsequently, X-gal staining was observed in myocardium at the base of the pulmonary trunk (Fig. 4H). We also found X-gal labeled cells in the OFT cushions (Fig. 4G). Together our findings suggest that spatial differences between Hoxb1, Hoxa1 and Hoxa3 observed in the splanchnic mesoderm at E8.5 (Fig. 2) identify overlapping populations of cardiac progenitor cells that contribute differentially to the proximal and distal regions of the OFT (Fig. 3 and 4).
Reduction or excess of RA signaling alter the Hoxa1- and Hoxb1-lineages
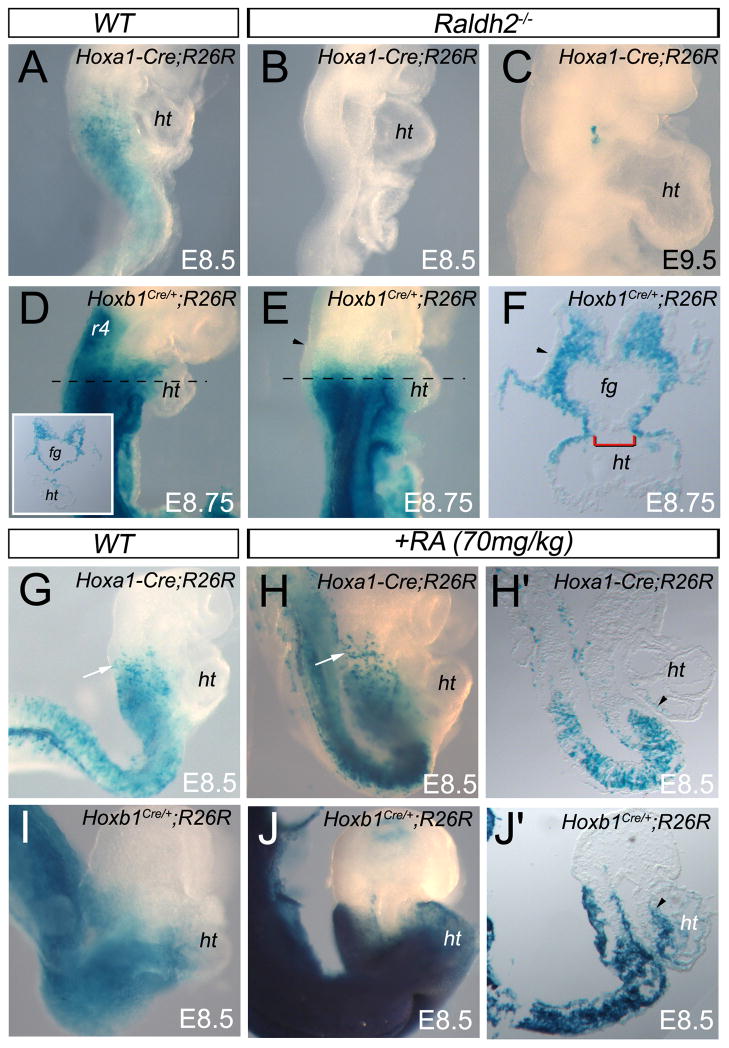
The homeobox genes Hoxa1 and Hoxb1 are known to be RA-regulated both in vitro and in vivo via RA-response elements (RAREs) present in their regulatory regions (Marshall et al., 1996; Langston et al., 1997; Studer et al., 1998; Niederreither et al., 1999; Huang et al., 2002; Sirbu et al., 2005). Our Hoxa1 and Hoxb1 genetic lineage analysis also shows similarities with the RA-activated cell lineages recently described by Dollé et al. (Dolle et al., 2010) and Li et al. (Li et al., 2010). This raises the possibility that a deficiency in RA biosynthesis might affect the Hox lineages. To address this question, we examined Hoxa1 and Hoxb1 expression patterns in embryos deficient in RA synthesis. We found that expression of Hoxa1 and Hoxb1, as well as Hoxa3, are downregulated in the splanchnic mesoderm at E8.5 in Raldh2−/− embryos (Supplemental Fig. S6). We next examined Hoxb1IRES-Cre and Hoxa1-enhIII-Cre lineages in Raldh2−/− embryos. At E8.5 and E9.5, there is no X-gal staining in Hoxa1-enhIII-Cre; R26R-lacZ; Raldh2−/− embryos (Fig. 5A-C). This observation supports the importance of signaling through the RA regulatory element (RARE) present in Hoxa1 enhancer III (Frasch et al., 1995; Li and Lufkin, 2000). In contrast to the Hoxa1-enhIII-Cre lineage, β-galactosidase positive cells were still detected in Hoxb1IRES-Cre; R26R-lacZ; Raldh2−/− embryos (Fig. 5D–F), suggesting that early expression of Hoxb1 in cardiac progenitor cells may not be activated by RA signaling. However, sections show that β-galactosidase activity was also not visible in the ectoderm of Raldh2−/− embryos (Fig. 5E,F), revealing tissue-specific sensitivity to RA signaling. Although the Hoxb1IRES-Cre lineage was detectable in Raldh2−/− mutant hearts, we believe that incorporation of X-gal labeled cells into the heart tube may be a consequence of the lack of the dorsal closure of the heart tube rather than a normal addition process at both the arterial and venous poles (Fig. 5F).
Figure 5.
Reduction or excess of RA signaling causes abnormalities of Hoxa1+ and Hoxb1+ cardiac progenitors contribution. (A–J) Lateral views of E8.5 (A,B,G–J), E8.75 (D–F) and E9.5 (C) embryos. (A–C) β-galactosidase activity is detected in Hoxa1-enhIII-Cre; R26R embryo, whereas no X-gal labeled cells are observed in Hoxa1-enhIII-Cre; R26R; Raldh2−/− mutant embryos, which reveals the requirement of retinoic acid (RA) for the induction of this transgene. (D,E) Lateral view of X-gal stained Hoxb1IRES-Cre; R26R-lacZ embryos at E8.75. (F) Transverse section of the embryo shown in E at the heart tube level. Inset in D shows a similar transverse section in the control embryo. X-gal labeled cells are yet detected in Raldh2−/− (E,F) mutant embryo. (F) Sections confirm that dorsal mesocardium is not closed in mutant embryos (brackets). Note the absence of X-gal staining in the surface ectoderm (arrowhead), suggesting differential response to deficiency in RA signaling. (G,H) X-gal staining showing intensify activity of Hoxa1-enhIII-Cre transgene (arrows) in the anterior domain of RA-treated embryos. (H′) Sagittal section of the embryo in H showing X-gal labeled cells in the heart tube (arrowhead). (I,J) X-gal staining showing increase of Hoxb1IRES-Cre in Hoxb1IRES-Cre; R26R-lacZ embryos treated with all-trans-RA. (J′) Sagittal section exhibits β-galactosidase activity in the heart tube (arrowhead) of the embryo shown in J. ht, heart tube.
To determine whether Hox-lineages are sensitive to increased RA signaling, we treated the mothers of Hoxa1-enhIII-Cre; R26R-lacZ and Hoxb1IRES-Cre; R26R-lacZ embryos with a teratogenic dose of RA to disrupt the normal boundary of RA activity (Niederreither et al., 1999; Sirbu et al., 2005). When we administrated a 70mg/kg dose of RA at E7.75, we confirmed the anterior shift of the rostral border of Hoxa1, Hoxb1 and Hoxa3 expression domains, including in the pharyngeal mesoderm (Supplemental Fig. S6). Consistently, comparison of control and RA-treated Hoxb1IRES-Cre; R26R-lacZ embryos demonstrated that the Hoxb1IRES-Cre lineage is responsive to RA (Fig. 5I,J). Importantly, X-gal labeled cells were found in the forming heart tube (Fig. 5J,J′), suggesting that the anterior boundary of RA activity defines the location of the Hoxb1IRES-Cre lineage boundary. Effect of RA-treatment on Hoxa1-enhIII-Cre; R26R-lacZ embryos was weaker (Fig. 5G,H), suggesting that the RARE within enhancer III-Cre transgene is less sensitive than in the context of the endogenous Hoxa1 promoter. When a 85mg/kg dose of RA was injected at E8.5 in Hoxa3IRES-Cre; R26R-lacZ embryos, only few X-gal labeled cells were found anterior to the otic vesicle and in the first branchial arch (Supplemental Fig. S7), suggesting that RA has a restricted effect on the activation of the Hoxa3-lineage after E8.
Our previous and present results showed that RA is required for correct deployment of the SHF (Ryckebusch et al., 2008). In addition, a recent study suggested that formation of the OFT is disrupted in RA receptor mutant embryos, resulting in a short, misaligned OFT (Li et al., 2010). To further explore the role of RA signaling on OFT formation, we compared the expression of y96-Myf5-nlacZ-16 (96-16) and A17-Myf5-nlacZ-T55 (T55) transgenes (Bajolle et al., 2006; Bajolle et al., 2008), which have complementary patterns in the OFT, in Raldh2−/− mutant embryos. Interestingly, the domain of expression of the 96-16 transgene corresponded to the β-galactosidase activity observed in the OFT in Hoxb1IRES-Cre; R26R-lacZ embryos (Fig. 6A and Fig. 3D). Thus, we found that the sub-domain of 96-16 transgene expressing cells is missing in the RA deficient embryos (Fig. 6A,B), whereas cells expressing the T55 transgene were still present in the OFT of Raldh2−/− mutant hearts (Fig. 6C,D). These results suggest that only a sub-domain of the OFT is affected in Raldh2−/− mutant embryos. We presume that perturbation of RA signaling causes a failure in the deployment of the Hoxb1-expressing cardiac progenitor cell subpopulation during the formation of the OFT.
Figure 6.
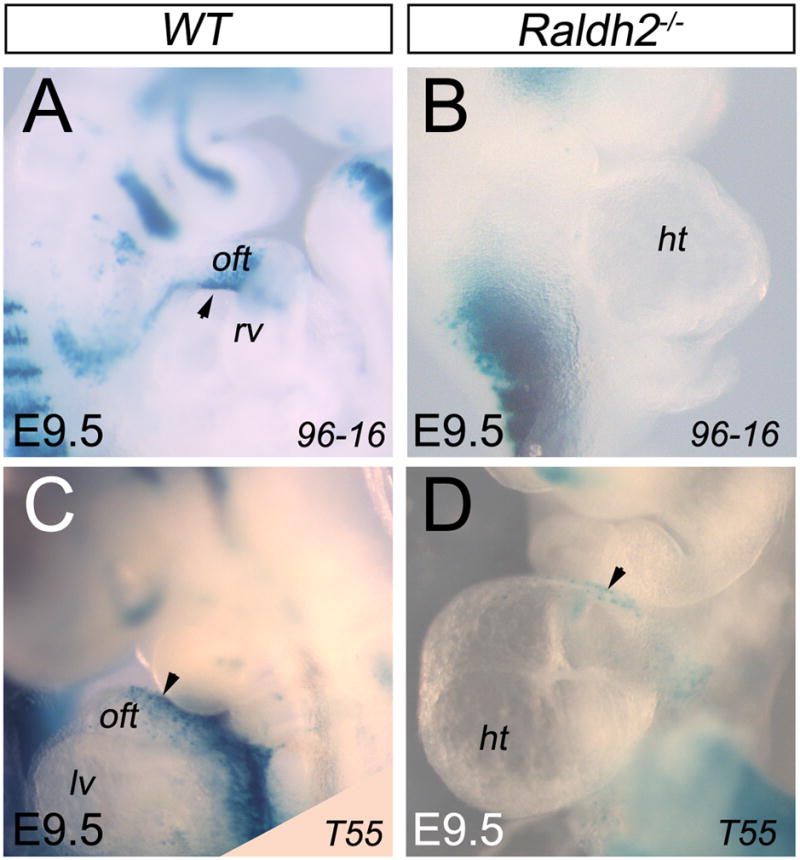
Reduction of RA signaling induces defect of the inferior wall of the outflow tract. (A,B) X-gal staining showing absence of y96-Myf5-nlacZ-16 (96-16) transgene expression in Raldh2−/− (B) embryos at E9.5. (C,D) At E9.5, β-galactosidase activity is detected in the superior wall of the heart tube of A17-Myf5-nlacZ-T55 (T55); Raldh2−/− (D) embryos. ht, heart tube; lv, left ventricle; oft, outflow tract; rv, right ventricle.
DISCUSSION
Anterior-posterior patterning of the SHF in the mouse
Previous expression and genetic lineage studies indicated that the majority of cardiac components (outflow tract [OFT], right ventricular and atrial myocardium) are derived from an Isl1+ splanchnic mesodermal cell population, called the second heart field (SHF) (Cai et al., 2003; Ma et al., 2008). The question of the early spatial segregation of cardiac progenitor cells is crucial to understand their specification in precardiac mesoderm and subsequent fate in the heart. There is increasing evidence for the existence of cardiac progenitor sub-populations in the SHF, such as the anterior region of the SHF (also called AHF and marked by expression of Fgf10) (Kelly et al., 2001), which contributes to right ventricular and OFT myocardium (Zaffran et al., 2004), whereas the posterior SHF, which expresses Isl1 but not Fgf10, contributes to atrial myocardium (Cai et al., 2003; Galli et al., 2008). These observations indicate that at least two sub-compartments are positioned along the AP axis within the SHF. Our data reveal that expression of Hox genes characterizes distinct progenitor cell sub-domains within the SHF. These results suggest that regionalized expression of Hoxb1 and Hoxa1 in splanchnic mesoderm may be required to establish AP patterning of the SHF. Furthermore, our genetic lineage tracing analysis shows that Hoxb1, Hoxa1 and Hoxa3 lineages contribute to both the arterial and venous poles of the heart (Fig. 7). Contribution of Hoxa1 and Hoxa3 lineages to the OFT supports the idea that posterior SHF cells might contribute the arterial pole of the forming heart tube. Interestingly, we observed a difference in the contribution of Hox lineages to the proximo-distal region of the OFT and atria; descendants of cells expressing Hoxb1 are found in the proximal OFT and atria, while descendants of cells expressing Hoxa1 and Hoxa3 are observed only in the distal OFT and in discrete regions of the atria. These results indicate that segregation among cardiac progenitor cells occurs before their incorporation to the arterial and venous poles of the heart. Interestingly, retrospective clonal analysis in the heart at E8.5 has identified clones that can contribute to regions of both the future atria and OFT (Meilhac et al., 2004). Thus, we believe that these clones are born from Hoxb1+ progenitor cells.
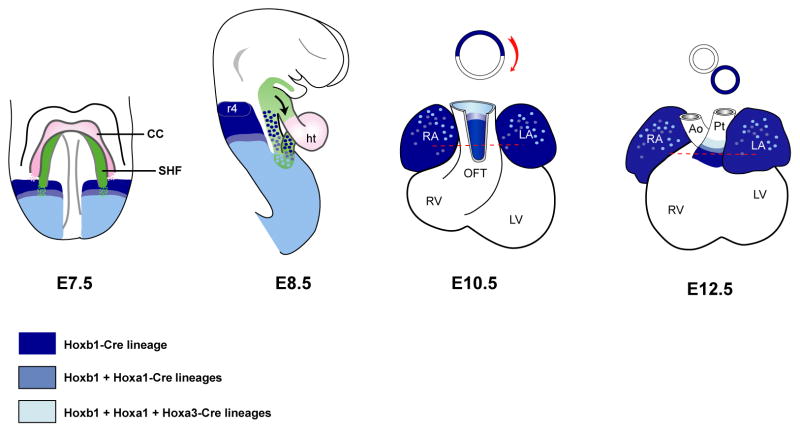
Figure 7.
Model for cardiac contributions of progenitor cells expressing Hox genes in the second heart field. Genetic lineage analysis was made with Hoxb1Cre, Hoxa1-EnhIII-Cre, Hoxa3Cre and R26R-lacZ lines. X-gal stained cells are represented by blue colors. The location of the second heart field (SHF) is shown in green. Frontal view is shown for embryonic day 7.5 (E7.5) and lateral view for E8.5. Early Hoxb1/a1/a3 expressing cells characterize distinct subdomains along the antero-posterior axis in the SHF. Later, these cardiac progenitor cells contribute to both atria and the inferior wall of the OFT, which subsequently gives rise to myocardium at the base of pulmonary trunk. Ao, aorta; CC, cardiac crescent; ep, epicardium; ht, heart tube; LA, left atria; Pt, pulmonary trunk; RA, right atria, r4, rhombomere 4.
Hox genes have been proposed to be the key downstream effectors of RA signaling during cardiac development (reviewed in (Rosenthal and Xavier-Neto, 2000)). This is supported by recent studies in zebrafish, which identified Hoxb5b as a downstream target of RA signaling that restricts the numbers of both atrial and ventricular cells that emerge from the heart field (Waxman et al., 2008), and showed that injection of Hoxb5b mRNA phenocopies RA treatment, which consists in the loss of both atrial and ventricular cardiomyocytes (Waxman and Yelon, 2009). Waxman et al. have shown that reduction of RA signaling does not lead to an increase in ventricular cells at the expense of the atrial lineage (Waxman et al., 2008). These observations are not consistent with a role for RA signaling in partitioning the heart tube, as suggested by previous studies in amniotes (Yutzey et al., 1994; Hochgreb et al., 2003; Ryckebusch et al., 2008; Sirbu et al., 2008). Indeed, treatment of chick embryos with a RA pan-antagonist produces a heart with reduction of the atrial compartment and an oversized ventricle (Hochgreb et al., 2003), while RA deficiency mouse embryos have impaired atrial and sinus venosus development (Niederreither et al., 2001), as well as loss of the inferior wall of the OFT (this study). We have shown that manipulation of RA signaling affects the rostral boundaries of Hoxa1, Hoxb1 and Hoxa3 expression in the SHF. Therefore, RA may control AP patterning of the SHF through the tight regulation of the expression of these Hox genes in splanchnic mesoderm. The question of the role of anterior Hox genes in the SHF can only be determined by inactivating and overexpressing these genes in their respective precursor populations. Of note, no abnormality in the heart of Hoxa1 and Hoxb1 mutant mice have been reported (Lufkin et al., 1991; Carpenter et al., 1993; Goddard et al., 1996; Studer et al., 1996), but non-lethal cardiovascular morphological defects may not have been examined in detail. Moreover, Hoxa1 and Hoxb1 have been shown to function together in several structures including the hindbrain, cranial nerves and second pharyngeal arch (Gavalas et al., 1998; Studer et al., 1998; Rossel and Capecchi, 1999). Hence, characterization of cardiac defects in double Hoxa1;Hoxb1 homozygous mutant embryos will be essential.
Contribution of Hox lineages to the outflow tract of the heart
Diverse subpopulations of the SHF have been defined in the mouse and chick. These include the anterior heart field (AHF), giving rise to all OFT myocardium, and the “secondary” heart field, situated in the dorsal pericardial wall giving rise to myocardium of the distal OFT (Kelly et al., 2001; Mjaatvedt et al., 2001; Waldo et al., 2001). Our results indicate that the splanchnic mesodermal cells expressing Hoxa1 are part of the murine “secondary” heart field. Surprisingly, our findings show that cardiac progenitor cells expressing Hoxb1, Hoxa1 and Hoxa3 contribute to sub-pulmonary myocardium but not myocardial cells at the base of the aorta. This is consistent with observations on the clustering of clonally related cells in the OFT (Bajolle et al., 2008), which had suggested that future sub-pulmonary myocardium is pre-patterned in distinct sub-domains of progenitor cells. Future sub-aortic myocardial progenitor cells are positioned anterior to future sub-pulmonary progenitor cells in the SHF. Interestingly, the contribution of Hoxb1-expressing cardiac progenitor cells to the OFT is very similar to that of Tbx1Cre at E10.5 (Huynh et al., 2007). Deletion of Tbx1, the major candidate gene for DiGeorge syndrome, results in a persistent truncus arteriosus (PTA) (Baldini, 2005), and sub-pulmonary myocardium is specifically affected in Tbx1 mutant hearts (Theveniau-Ruissy et al., 2008). Thus, it will be of interest to explore the contribution of the Hoxb1-lineage in the absence of Tbx1.
Studies on RA receptors (RARα1/RARβ/RXRα) and Raldh2 mutant embryos have demonstrated the importance of RA signaling during OFT development (Lee et al., 1997; Niederreither et al., 2001; Jiang et al., 2002; Li et al., 2010). Interestingly, results from RAR mutants imply that proximal and distal domains of the OFT are pre-patterned in the splanchnic mesoderm (Li et al., 2010), consistent with the differential AP expression of Hoxb1, Hoxa1 and Hoxa3 in the SHF, and their different contributions to the OFT. Thus, we hypothesize that the SHF is patterned from anterior to posterior, as follows: cells giving rise to the right ventricle and superior wall of the OFT (Hox-negative cells), to the inferior wall of the proximal OFT (Hoxb1-positive cells), to the inferior wall of the distal OFT (Hoxb1 and Hoxa1-positive cells), and, most posteriorly, to atrial myocardium. Analysis of RA synthesis and RA response suggest that RA signaling acts predominantly in SHF cells characterized by Hoxb1 expression (Moss et al., 1998; Hochgreb et al., 2003; Ryckebusch et al., 2008; Sirbu et al., 2008; Dolle et al., 2010). This is supported by analysis of Raldh2 and RA receptor mutants in which derivatives of Hoxb1+ sub-domains as described above are lacking (Ryckebusch et al., 2008; Li et al., 2010 and this study). OFT defects observed in RA receptor mutants as well as in RA-rescued Raldh2 mutants, suggest that RA signaling acts from E7.5 to E10.5 to allow correct deployment of Hoxb1+ progenitor cells (Niederreither et al., 2001; Li et al., 2010). Works performed on Raldh2−/− mutants have shown that RA signaling is required first to determine the posterior limit of the SHF as posterior expansion of SHF markers is observed as soon as E7.5 (Ryckebusch et al., 2008; Sirbu et al., 2008). Maternal RA supplementation experiments reveal a further requirement of RA until E10.5 that we believe is associated with contribution of Hoxb1+ progenitor cells to the heart tube (Niederreither et al., 2001). RA could allow this contribution through controlling specification, differentiation and/or migration of those SHF cells. With respect to absent septation and misalignments of the OFT including PTA, double outlet right ventricle (DORV) and overriding aorta observed in RA receptor and RA-rescued Raldh2 mutant embryos (Niederreither et al., 2001; Li et al., 2010), we speculate that lack of the inferior wall of the OFT, a derivative of particular Hoxb1+ progenitor cells, may be sufficient to lead to these defects. Of course, the latter speculation does not exclude a potential role of RA signaling on cardiac NCC (Niederreither et al., 2001; Jiang et al., 2002).
In human congenital heart disease, OFT alignment defects, such as tetralogy of Fallot, are frequent and may result from a deficit in sub-pulmonary myocardium (Van Praagh, 2009). It is interesting to note that recent studies in humans have identified homozygous HOXA1 coding mutations in patients with OFT defects, including tetralogy of Fallot (Tischfield et al., 2005; Bosley et al., 2008). Together with our present results, these observations should lead to further investigations into the causative role of HOX mutations in the pathogenesis of heart and great vessels malformations in humans.
Supplementary Material
Acknowledgments
We thank T. Lufkin for the Hoxa1 Enhancer III-Cre vector and members of the SEAT CNRS UPS44 mouse facility for microinjection. This manuscript was improved by helpful comments from M. Buckingham, H. Etchevers and R.G. Kelly. This work is supported by the “Agence Nationale de la Recherche” (ANR-07-MRAR-003), (to S.Z), the “Association Française contre les Myopathies” (AFM 13517 and 14134) (to S.Z.), and the National Institutes of Health (R01 HL070733) (to K.N.). L.R. and M.R. received fellowships from the “Ministère de l’Enseignement Supérieur et de la Recherche” and the “Université de la Méditerranée” (Monitorat).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aanhaanen WT, Brons JF, Dominguez JN, Rana MS, Norden J, Airik R, Wakker V, de Gier-de Vries C, Brown NA, Kispert A, Moorman AF, Christoffels VM. The Tbx2+ primary myocardium of the atrioventricular canal forms the atrioventricular node and the base of the left ventricle. Circ Res. 2009;104:1267–1274. doi: 10.1161/CIRCRESAHA.108.192450. [DOI] [PubMed] [Google Scholar]
- Alexander T, Nolte C, Krumlauf R. Hox genes and segmentation of the hindbrain and axial skeleton. Annu Rev Cell Dev Biol. 2009;25:431–456. doi: 10.1146/annurev.cellbio.042308.113423. [DOI] [PubMed] [Google Scholar]
- Arenkiel BR, Gaufo GO, Capecchi MR. Hoxb1 neural crest preferentially form glia of the PNS. Dev Dyn. 2003;227:379–386. doi: 10.1002/dvdy.10323. [DOI] [PubMed] [Google Scholar]
- Bajolle F, Zaffran S, Kelly RG, Hadchouel J, Bonnet D, Brown NA, Buckingham ME. Rotation of the myocardial wall of the outflow tract is implicated in the normal positioning of the great arteries. Circ Res. 2006;98:421–428. doi: 10.1161/01.RES.0000202800.85341.6e. [DOI] [PubMed] [Google Scholar]
- Bajolle F, Zaffran S, Meilhac SM, Dandonneau M, Chang T, Kelly RG, Buckingham ME. Myocardium at the base of the aorta and pulmonary trunk is prefigured in the outflow tract of the heart and in subdomains of the second heart field. Dev Biol. 2008;313:25–34. doi: 10.1016/j.ydbio.2007.09.023. [DOI] [PubMed] [Google Scholar]
- Baldini A. Dissecting contiguous gene defects: TBX1. Curr Opin Genet Dev. 2005;15:279–284. doi: 10.1016/j.gde.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Bosley TM, Alorainy IA, Salih MA, Aldhalaan HM, Abu-Amero KK, Oystreck DT, Tischfield MA, Engle EC, Erickson RP. The clinical spectrum of homozygous HOXA1 mutations. Am J Med Genet A. 2008;146A:1235–1240. doi: 10.1002/ajmg.a.32262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–835. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter EM, Goddard JM, Chisaka O, Manley NR, Capecchi MR. Loss of Hox-A1 (Hox-1.6) function results in the reorganization of the murine hindbrain. Development. 1993;118:1063–1075. doi: 10.1242/dev.118.4.1063. [DOI] [PubMed] [Google Scholar]
- Dolle P, Fraulob V, Gallego-Llamas J, Vermot J, Niederreither K. Fate of retinoic acid-activated embryonic cell lineages. Dev Dyn. 2010;239:3260–3274. doi: 10.1002/dvdy.22479. [DOI] [PubMed] [Google Scholar]
- Duboule D, Dolle P. The structural and functional organization of the murine HOX gene family resembles that of Drosophila homeotic genes. Embo J. 1989;8:1497–1505. doi: 10.1002/j.1460-2075.1989.tb03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlani S, Lawson KA, Deschamps J. Acquisition of Hox codes during gastrulation and axial elongation in the mouse embryo. Development. 2003;130:3807–3819. doi: 10.1242/dev.00573. [DOI] [PubMed] [Google Scholar]
- Frasch M, Chen X, Lufkin T. Evolutionary-conserved enhancers direct region-specific expression of the murine Hoxa-1 and Hoxa-2 loci in both mice and Drosophila. Development. 1995;121:957–974. doi: 10.1242/dev.121.4.957. [DOI] [PubMed] [Google Scholar]
- Frohman MA, Boyle M, Martin GR. Isolation of the mouse Hox-2.9 gene; analysis of embryonic expression suggests that positional information along the anterior-posterior axis is specified by mesoderm. Development. 1990;110:589–607. doi: 10.1242/dev.110.2.589. [DOI] [PubMed] [Google Scholar]
- Galli D, Dominguez JN, Zaffran S, Munk A, Brown NA, Buckingham ME. Atrial myocardium derives from the posterior region of the second heart field, which acquires left-right identity as Pitx2c is expressed. Development. 2008;135:1157–1167. doi: 10.1242/dev.014563. [DOI] [PubMed] [Google Scholar]
- Gaufo GO, Flodby P, Capecchi MR. Hoxb1 controls effectors of sonic hedgehog and Mash1 signaling pathways. Development. 2000;127:5343–5354. doi: 10.1242/dev.127.24.5343. [DOI] [PubMed] [Google Scholar]
- Gavalas A, Studer M, Lumsden A, Rijli FM, Krumlauf R, Chambon P. Hoxa1 and Hoxb1 synergize in patterning the hindbrain, cranial nerves and second pharyngeal arch. Development. 1998;125:1123–1136. doi: 10.1242/dev.125.6.1123. [DOI] [PubMed] [Google Scholar]
- Goddard JM, Rossel M, Manley NR, Capecchi MR. Mice with targeted disruption of Hoxb-1 fail to form the motor nucleus of the VIIth nerve. Development. 1996;122:3217–3228. doi: 10.1242/dev.122.10.3217. [DOI] [PubMed] [Google Scholar]
- Graham A, Papalopulu N, Krumlauf R. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell. 1989;57:367–378. doi: 10.1016/0092-8674(89)90912-4. [DOI] [PubMed] [Google Scholar]
- Hochgreb T, Linhares VL, Menezes DC, Sampaio AC, Yan CY, Cardoso WV, Rosenthal N, Xavier-Neto J. A caudorostral wave of RALDH2 conveys anteroposterior information to the cardiac field. Development. 2003;130:5363–5374. doi: 10.1242/dev.00750. [DOI] [PubMed] [Google Scholar]
- Huang D, Chen SW, Gudas LJ. Analysis of two distinct retinoic acid response elements in the homeobox gene Hoxb1 in transgenic mice. Dev Dyn. 2002;223:353–370. doi: 10.1002/dvdy.10057. [DOI] [PubMed] [Google Scholar]
- Huynh T, Chen L, Terrell P, Baldini A. A fate map of Tbx1 expressing cells reveals heterogeneity in the second cardiac field. Genesis. 2007;45:470–475. doi: 10.1002/dvg.20317. [DOI] [PubMed] [Google Scholar]
- Iimura T, Pourquie O. Collinear activation of Hoxb genes during gastrulation is linked to mesoderm cell ingression. Nature. 2006;442:568–571. doi: 10.1038/nature04838. [DOI] [PubMed] [Google Scholar]
- Izpisua-Belmonte JC, Dolle P, Renucci A, Zappavigna V, Falkenstein H, Duboule D. Primary structure and embryonic expression pattern of the mouse Hox-4.3 homeobox gene. Development. 1990;110:733–745. [PubMed] [Google Scholar]
- Jiang X, Choudhary B, Merki E, Chien KR, Maxson RE, Sucov HM. Normal fate and altered function of the cardiac neural crest cell lineage in retinoic acid receptor mutant embryos. Mech Dev. 2002;117:115–122. doi: 10.1016/s0925-4773(02)00206-x. [DOI] [PubMed] [Google Scholar]
- Keegan BR, Feldman JL, Begemann G, Ingham PW, Yelon D. Retinoic acid signaling restricts the cardiac progenitor pool. Science. 2005;307:247–249. doi: 10.1126/science.1101573. [DOI] [PubMed] [Google Scholar]
- Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1:435–440. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- Langston AW, Thompson JR, Gudas LJ. Retinoic acid-responsive enhancers located 3′ of the Hox A and Hox B homeobox gene clusters. Functional analysis. J Biol Chem. 1997;272:2167–2175. doi: 10.1074/jbc.272.4.2167. [DOI] [PubMed] [Google Scholar]
- Lee RY, Luo J, Evans RM, Giguere V, Sucov HM. Compartment-selective sensitivity of cardiovascular morphogenesis to combinations of retinoic acid receptor gene mutations. Circ Res. 1997;80:757–764. doi: 10.1161/01.res.80.6.757. [DOI] [PubMed] [Google Scholar]
- Li P, Pashmforoush M, Sucov HM. Retinoic acid regulates differentiation of the secondary heart field and TGFbeta-mediated outflow tract septation. Dev Cell. 2010;18:480–485. doi: 10.1016/j.devcel.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Lufkin T. Cre recombinase expression in the floorplate, notochord and gut epithelium in transgenic embryos driven by the Hoxa-1 enhancer III. Genesis. 2000;26:121–122. [PubMed] [Google Scholar]
- Lin SC, Dolle P, Ryckebusch L, Noseda M, Zaffran S, Schneider MD, Niederreither K. Endogenous retinoic acid regulates cardiac progenitor differentiation. Proc Natl Acad Sci U S A. 2010;107:9234–9239. doi: 10.1073/pnas.0910430107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lufkin T, Dierich A, LeMeur M, Mark M, Chambon P. Disruption of the Hox-1.6 homeobox gene results in defects in a region corresponding to its rostral domain of expression. Cell. 1991;66:1105–1119. doi: 10.1016/0092-8674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- Ma Q, Zhou B, Pu WT. Reassessment of Isl1 and Nkx2-5 cardiac fate maps using a Gata4-based reporter of Cre activity. Dev Biol. 2008;323:98–104. doi: 10.1016/j.ydbio.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatee TL, Hammond BP, Arenkiel BR, Francis L, Frank DU, Moon AM. Ablation of specific expression domains reveals discrete functions of ectoderm- and endoderm-derived FGF8 during cardiovascular and pharyngeal development. Development. 2003;130:6361–6374. doi: 10.1242/dev.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makki N, Capecchi MR. Hoxa1 lineage tracing indicates a direct role for Hoxa1 in the development of the inner ear, the heart, and the third rhombomere. Dev Biol. 2010;341:499–509. doi: 10.1016/j.ydbio.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall H, Morrison A, Studer M, Popperl H, Krumlauf R. Retinoids and Hox genes. Faseb J. 1996;10:969–978. [PubMed] [Google Scholar]
- Meilhac SM, Esner M, Kelly RG, Nicolas JF, Buckingham ME. The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Dev Cell. 2004;6:685–698. doi: 10.1016/s1534-5807(04)00133-9. [DOI] [PubMed] [Google Scholar]
- Merki E, Zamora M, Raya A, Kawakami Y, Wang J, Zhang X, Burch J, Kubalak SW, Kaliman P, Belmonte JC, Chien KR, Ruiz-Lozano P. Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proc Natl Acad Sci U S A. 2005;102:18455–18460. doi: 10.1073/pnas.0504343102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjaatvedt CH, Nakaoka T, Moreno-Rodriguez R, Norris RA, Kern MJ, Eisenberg CA, Turner D, Markwald RR. The outflow tract of the heart is recruited from a novel heart-forming field. Dev Biol. 2001;238:97–109. doi: 10.1006/dbio.2001.0409. [DOI] [PubMed] [Google Scholar]
- Moss JB, Xavier-Neto J, Shapiro MD, Nayeem SM, McCaffery P, Drager UC, Rosenthal N. Dynamic patterns of retinoic acid synthesis and response in the developing mammalian heart. Dev Biol. 1998;199:55–71. doi: 10.1006/dbio.1998.8911. [DOI] [PubMed] [Google Scholar]
- Murphy P, Hill RE. Expression of the mouse labial-like homeobox-containing genes, Hox 2.9 and Hox 1.6, during segmentation of the hindbrain. Development. 1991;111:61–74. doi: 10.1242/dev.111.1.61. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Dolle P. Retinoic acid in development: towards an integrated view. Nat Rev Genet. 2008;9:541–553. doi: 10.1038/nrg2340. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Subbarayan V, Dolle P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Messaddeq N, Schuhbaur B, Chambon P, Dolle P. Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development. 2001;128:1019–1031. doi: 10.1242/dev.128.7.1019. [DOI] [PubMed] [Google Scholar]
- Rosenthal N, Xavier-Neto J. From the bottom of the heart: anteroposterior decisions in cardiac muscle differentiation. Curr Opin Cell Biol. 2000;12:742–746. doi: 10.1016/s0955-0674(00)00162-9. [DOI] [PubMed] [Google Scholar]
- Rossant J, Zirngibl R, Cado D, Shago M, Giguere V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5:1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- Rossel M, Capecchi MR. Mice mutant for both Hoxa1 and Hoxb1 show extensive remodeling of the hindbrain and defects in craniofacial development. Development. 1999;126:5027–5040. doi: 10.1242/dev.126.22.5027. [DOI] [PubMed] [Google Scholar]
- Ryckebusch L, Wang Z, Bertrand N, Lin SC, Chi X, Schwartz R, Zaffran S, Niederreither K. Retinoic acid deficiency alters second heart field formation. Proc Natl Acad Sci U S A. 2008;105:2913–2918. doi: 10.1073/pnas.0712344105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searcy RD, Yutzey KE. Analysis of Hox gene expression during early avian heart development. Dev Dyn. 1998;213:82–91. doi: 10.1002/(SICI)1097-0177(199809)213:1<82::AID-AJA8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Sirbu IO, Gresh L, Barra J, Duester G. Shifting boundaries of retinoic acid activity control hindbrain segmental gene expression. Development. 2005;132:2611–2622. doi: 10.1242/dev.01845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbu IO, Zhao X, Duester G. Retinoic acid controls heart anteroposterior patterning by down-regulating Isl1 through the Fgf8 pathway. Dev Dyn. 2008;237:1627–1635. doi: 10.1002/dvdy.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Studer M, Gavalas A, Marshall H, Ariza-McNaughton L, Rijli FM, Chambon P, Krumlauf R. Genetic interactions between Hoxa1 and Hoxb1 reveal new roles in regulation of early hindbrain patterning. Development. 1998;125:1025–1036. doi: 10.1242/dev.125.6.1025. [DOI] [PubMed] [Google Scholar]
- Studer M, Lumsden A, Ariza-McNaughton L, Bradley A, Krumlauf R. Altered segmental identity and abnormal migration of motor neurons in mice lacking Hoxb-1. Nature. 1996;384:630–634. doi: 10.1038/384630a0. [DOI] [PubMed] [Google Scholar]
- Theveniau-Ruissy M, Dandonneau M, Mesbah K, Ghez O, Mattei MG, Miquerol L, Kelly RG. The del22q11.2 candidate gene Tbx1 controls regional outflow tract identity and coronary artery patterning. Circ Res. 2008;103:142–148. doi: 10.1161/CIRCRESAHA.108.172189. [DOI] [PubMed] [Google Scholar]
- Tischfield MA, Bosley TM, Salih MA, Alorainy IA, Sener EC, Nester MJ, Oystreck DT, Chan WM, Andrews C, Erickson RP, Engle EC. Homozygous HOXA1 mutations disrupt human brainstem, inner ear, cardiovascular and cognitive development. Nat Genet. 2005;37:1035–1037. doi: 10.1038/ng1636. [DOI] [PubMed] [Google Scholar]
- Van Praagh R. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2009. The first Stella van Praagh memorial lecture: the history and anatomy of tetralogy of Fallot; pp. 19–38. [DOI] [PubMed] [Google Scholar]
- Vincent SD, Buckingham ME. How to make a heart: the origin and regulation of cardiac progenitor cells. Curr Top Dev Biol. 2010;90:1–41. doi: 10.1016/S0070-2153(10)90001-X. [DOI] [PubMed] [Google Scholar]
- Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, Kirby ML. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128:3179–3188. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- Waxman JS, Keegan BR, Roberts RW, Poss KD, Yelon D. Hoxb5b acts downstream of retinoic acid signaling in the forelimb field to restrict heart field potential in zebrafish. Dev Cell. 2008;15:923–934. doi: 10.1016/j.devcel.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman JS, Yelon D. Increased Hox activity mimics the teratogenic effects of excess retinoic acid signaling. Dev Dyn. 2009;238:1207–1213. doi: 10.1002/dvdy.21951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellik DM. Hox genes and vertebrate axial pattern. Curr Top Dev Biol. 2009;88:257–278. doi: 10.1016/S0070-2153(09)88009-5. [DOI] [PubMed] [Google Scholar]
- Xavier-Neto J, Neville CM, Shapiro MD, Houghton L, Wang GF, Nikovits W, Jr, Stockdale FE, Rosenthal N. A retinoic acid-inducible transgenic marker of sino-atrial development in the mouse heart. Development. 1999;126:2677–2687. doi: 10.1242/dev.126.12.2677. [DOI] [PubMed] [Google Scholar]
- Xavier-Neto J, Rosenthal N, Silva FA, Matos TG, Hochgreb T, Linhares VL. Retinoid signaling and cardiac anteroposterior segmentation. Genesis. 2001;31:97–104. doi: 10.1002/gene.10009. [DOI] [PubMed] [Google Scholar]
- Yutzey KE, Rhee JT, Bader D. Expression of the atrial-specific myosin heavy chain AMHC1 and the establishment of anteroposterior polarity in the developing chicken heart. Development. 1994;120:871–883. doi: 10.1242/dev.120.4.871. [DOI] [PubMed] [Google Scholar]
- Zaffran S, Kelly RG, Meilhac SM, Buckingham ME, Brown NA. Right Ventricular Myocardium Derives From the Anterior Heart Field. Circ Res. 2004;95:261–268. doi: 10.1161/01.RES.0000136815.73623.BE. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Cerrato F, Xu H, Vitelli F, Morishima M, Vincentz J, Furuta Y, Ma L, Martin JF, Baldini A, Lindsay E. Tbx1 expression in pharyngeal epithelia is necessary for pharyngeal arch artery development. Development. 2005;132:5307–5315. doi: 10.1242/dev.02086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.