Abstract
Epigenetic histone modifications are emerging as important mechanisms for conveyance of and maintenance of effects of the hormonal milieu to the developing brain. We hypothesized that alteration of histone acetylation status early in development by sex steroid hormones is important for sexual differentiation of the brain. It was found that during the critical period for sexual differentiation, histones associated with promoters of essential genes in masculinization of the brain (estrogen receptor α and aromatase) in the medial preoptic area, an area necessary for male sexual behavior, were differentially acetylated between the sexes. Consistent with these findings, binding of histone deacetylase (HDAC) 2 and 4 to the promoters was higher in males than in females. To examine the involvement of histone deacetylation on masculinization of the brain at the behavioral level, we inhibited HDAC in vivo by intracerebroventricular infusion of the HDAC inhibitor trichostatin A or antisense oligodeoxynucleotide directed against the mRNA for HDAC2 and -4 in newborn male rats. Aspects of male sexual behavior in adulthood were significantly reduced by administration of either trichostatin A or antisense oligodeoxynucleotide. These results demonstrate that HDAC activity during the early postnatal period plays a crucial role in the masculinization of the brain via modifications of histone acetylation status.
Although the involvement of genetic factors independent from testosterone has been identified (1), the sex of the brain is mostly determined by the effects of androgen and its primary metabolite, estradiol (2–4). In males, androgen transiently synthesized by and released from the testes during a critical perinatal period, the so-called androgen surge, organizes the developing brain into a masculinized phenotype. The female brain develops as the default feminine phenotype in the absence of an androgen surge. In the rodent, masculinization can be experimentally induced in females by administration of testosterone or estradiol during the critical period. This has led to the well-established view that testes-derived androgen does not affect the brain directly, but instead that masculinization is largely mediated by estradiol converted from testosterone by aromatase (Arom) in the brain (5, 6), although in recent studies, direct effects of androgens have been advocated (7, 8). Estrogen receptors (ER), mainly ERα, as well as Arom, are expressed in areas of the developing brain that participate in the regulation of sexually differentiated brain functions in adulthood (9–11), and activation of ER is necessary for the process of masculinization of sexual behavior (12, 13). Activation of ER during the perinatal critical period induces synthesis of prostaglandin E2, which is both necessary and sufficient for masculinization of the synaptic profile of the preoptic area (POA) and adult male sexual behavior (14, 15). However, the sexual differentiation of the brain is a permanent process established during the critical period, because administration of testosterone or estradiol after the critical period induces only partial masculinization (2–4). How the early effects of steroids on the developing brain are permanently maintained is one of the most fundamental issues in the study of sexual differentiation of the brain.
The sex-steroid hormone receptors, including ERα, are members of the nuclear receptor superfamily of ligand-dependent transcription factors, and their actions are transduced through the functions of coactivators (2, 16) that possess histone-directed enzymatic activities (histone acetyltransferase and histone methyltransferase) (17, 18). Histone acetylation/deacetylation is one of the main epigenetic mechanisms that control gene expression through modification of chromatin structure (17, 18) and is critically involved in the regulation of nuclear receptor-mediated transcription (19–21). In the presence of ligands, activated nuclear receptors bind with coactivators at the promoter region of target genes. These coactivators acetylate neighboring histones, activating transcription of the genes (18–21). Conversely, in the absence of ligands, corepressors associated with nuclear receptors recruit histone deacetylases (HDAC), resulting in histone deacetylation and down-regulation of transcription (18–21).
Taken together, these phenomena suggest that genes involved in maintenance of sexual differentiation of the brain may be expressed in a sex-specific manner due to differential histone acetylation levels of their promoters established during the perinatal period. In support of this view, ERα and Arom themselves exhibit sexually dimorphic expression patterns during the perinatal period in POA and hypothalamus, areas that are sexually dimorphic and important for regulating sexual behavior and reproductive function (9, 10, 22). Additionally, knockdown of steroid hormone receptor coactivator-1 (SRC-1), a coactivator with intrinsic histone acetyltransferase activity during the critical period, reduces sex-specific behavior in adulthood (23). Moreover, administration of the HDAC inhibitor valproic acid to male mice on postnatal days (PD) 1 and 2 eliminates development of the sex difference in the volume of the principal nucleus of the bed nucleus of the stria terminalis (BNSTp), which is larger in males than females (24). This suggests that HDAC activity in the early postnatal period is required for development of structural sexual dimorphism of the BNSTp.
In the present study, we sought to determine the involvement of histone deacetylation in functional sexual differentiation of the brain. Here, we report sex differences in histone acetylation in the medial POA (MPOA), a highly sexually dimorphic region within the POA that is necessary for male sex behavior. Specifically, histones H4 and H3 in promoters of ERα and Arom were differentially acetylated between the sexes. Male rats infused intracerebroventricularly with the HDAC inhibitor trichostatin A (TSA) during early postnatal development exhibited reduced male sexual behavior in adulthood. We identified HDAC2 and -4 as the HDAC subtypes maintaining masculinization of the brain. Chromatin immunoprecipitation (ChIP) experiments demonstrated that HDAC2 and -4 bound to ERα and Arom promoters at higher frequencies in males than in females, despite similar expression levels in the sexes. These data provide important insights into molecular mechanisms of sexual differentiation of the brain mediated by epigenetic histone modifications.
Materials and Methods
Animals
For experiments conducted at Kyoto Prefectural University of Medicine, Sprague Dawley rats purchased from Shimizu Laboratory Supplies Co. (Kyoto, Japan) were kept under a 12-h reverse light/dark cycle and allowed free access to food and water. The day of birth was defined as PD0. Animal care, maintenance, and surgery were performed in accordance with the Rules and Regulations of Animal Research, Kyoto Prefectural University of Medicine. For experiments conducted at the University of Maryland School of Medicine, Sprague Dawley rats purchase from Harlan Laboratories were housed under the same conditions and all procedures were approved by the University of Maryland Institutional Animal Care and Use Committee.
ChIP assay
To cross-link histones to DNA, embryos [embryonic day (ED) 21] and pups (PD1 and PD3) were cryoanesthetized and then perfused from the left ventricle with 4% paraformaldehyde in physiological saline (10 ml) using a 27-gauge needle with a 10-ml syringe (25). After fixation, brains were removed and postfixed in the same fixation solution (overnight, 4 C) and then transferred to 0.1 m phosphate buffer (pH 7.4) with 20% sucrose for 48 h (25). Brain slices at the chiasmatic level were cut at a thickness of 400 μm using a microslicer (Dosaka EM, Kyoto, Japan), and then tissue fragments of the MPOA were bilaterally isolated using a stainless steel pipe (inner diameter, 0.65 mm) as previously described (26). MPOA fragments from five brains (total 10 fragments) were gathered as one sample. ChIP assays were performed using a ChIP assay kit (17-295; Millipore, Billerica, MA) according to the manufacturer's instructions. The MPOA fragments were lysed and sonicated (UR-20P; Tomy, Tokyo, Japan) to shear the chromatin. The optimal condition of sonication (output 2, duty cycle 40, 10 sec × 12) was determined by agarose electrophoresis after reverse cross-linking and purification of DNA. The resulting chromatin solution was immunoprecipitated using anti-acetylated histone H3 (06-599; Millipore), histone H4 (06-866; Millipore), HDAC2 (ab12169; Abcam, Cambridge, MA), or HDAC4 (ab12171; Abcam). To confirm specific immunoprecipitation, the same procedure was carried out without adding antibody [Ab(−)]. One tenth of the lysate was kept to quantify the amount of DNA present in samples before immunoprecipitation (input). Reverse cross-linked DNA purified from ChIP, Ab(−), and input samples were subjected to PCR amplification. Primers used for detection of the ERα 1b promoter were as reported by Champagne et al. (25), and those for detection of Arom promoters were as follows: If promoter forward, AAC CTG TCT GGG ATA CCA CA; If promoter reverse, GGC ACT CTT TGT GTG CCA TC; II promoter forward, GCC TCA GCA AAT GCT GCT GA; and II promoter reverse, TAG TTT GGC TGT GGC TCC TG. PCR was performed using Taq polymerase (Toyobo, Osaka, Japan) under the following conditions: 95 C for 5 min; 95 C for 1 min, 60 C for 1 min, 72 C for 1 min; and 72 C for 10 min (25). The cycle number was determined to be in the log-linear amplification range (see Supplemental Methods, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). PCR products were separated on a 2% agarose gel. The fluorescence intensity of ethidium bromide was quantified by densitometric analysis using ImageJ software. After correction of the intensity of ChIP samples using the input amount, the ratio of intensity in males to that in females was calculated. Sample collection was carried out three times independently, and experiments were repeated three times for each set of collected samples.
Intracerebroventricular injections of TSA and antisense ODN
Male pups were treated on PD0 and PD1 under cryoanesthesia (prechilled on ice and placed on an ice pack during injection). Bilateral intracerebroventricular injections were carried out at 1 mm caudal to the bregma and 1 mm lateral to the midline over each hemisphere (27). A 26-gauge 1-μl beveled Hamilton syringe attached to a stereotaxic manipulator was lowered 3.0 mm into the brain and then backed out 1 mm before infusion of 1 μl TSA (0.5 ng/μl; Wako Pure Chemicals, Osaka, Japan) (see Supplemental Methods) (28), antisense ODN (0.5 μg/μl; Exiqon, Woburn, MA), or vehicle (0.9% saline) over 60 sec (23). ODN sequences were generated from GenBank accession numbers for mRNA sequences for HDAC2 (NM_053447.1) and HDAC4 (XM_343629.3). A random sequence (SCRM) served as a control for ODN infusion. The synthetic ODN sequences were as follows: SCRM, CAG TCG AGC CGA TGG GTG CCG A; HDAC2, GAC TGT ACG CCA TGG GCT CC; and HDAC4, CGC CGT CGG CCC ACC AGC CAT. Sequences were queried in the BLAST database and showed significant homology only to their specific target sequences within the rat genome. The SCRM sequence had no significant homology to any sequence in the genome.
Male sexual behavior
Beginning 10 wk after neonatal treatment, rats were tested for male sexual behavior as previously described (27, 29). In brief, animals received three weekly 30-min tests after a 5-min acclimation period to the testing arena (60 cm long × 30 cm wide × 30 cm high) during the dark phase of the light cycle and under red-light illumination. Testing began with the addition of a hormonally primed receptive female (5 μg estradiol benzoate in 0.1 ml sesame oil 2 d before testing and 0.5 mg progesterone in 0.1 ml sesame oil 4 h before testing) to the arena. Latencies to first mount with thrust (mount latency), intromission (intromission latency), and ejaculation (ejaculation latency) were quantified. The ratio of intromission number to the sum of mount number and intromission number (intromission ratio) was calculated (30).
Western blotting
On PD2, POA of brains injected with antisense ODN were isolated and tissue homogenates were prepared (27). The homogenates (7.2 μg protein for HDAC2 and 25 μg protein for HDAC4) were electrophoresed on a precast 8–16% SDS-polyacrylamide gel (Invitrogen, Carlsbad, CA) and transferred onto a polyvinyl difluoride membrane (Bio-Rad, Hercules, CA). Membranes were blocked with 5% nonfat milk in 0.1% Tween-supplemented Tris-buffered saline. Antibodies against HDAC2 (1:1000, ab12169; Abcam) or HDAC4 (1:200, ab12171; Abcam) were applied, followed by antimouse secondary antibody (1:10,000). A Phototope chemiluminescence system (New England Biolabs, Beverly, MA) was used to detect the immunoblot by exposing the membrane to Hyperfilm-ECL (GE Healthcare, Piscataway, NJ). The integrative gray-scale pixel area was quantified with ImageJ software.
Quantitative real-time PCR
Pups (PD1) were cryoanesthetized, and brains were removed immediately after decapitation. Bilateral MPOA fragments from individual pups were isolated using the procedures described in the ChIP analysis and lysed. Total RNA was extracted using a RNeasy Micro Kit (QIAGEN, Hilden, Germany) and reverse transcribed using ReverTra Ace (Toyobo, Osaka, Japan) and random hexamers, according to the manufacturer's guidelines. Gene expression was assessed by quantitative real-time PCR using the Light Cycler 480 II with Universal Probe Library assays (Roche Diagnostics, Mannheim, Germany). Reactions were performed in duplicate in 96-well plates for 40 amplification cycles (95 C for 10 sec, 60 C for 10 sec, 72 C for 10 sec) in a 20-μl reaction volume. Primers were designed using the Roche Universal Probe Library assay design center (www.universalprobelibrary.com) (HDAC2 forward primer, TGG GCT GCT TCA ACC TAA CT; HDAC2 reverse primer, TCC AAC ATC GAG CAA CAT TC; HDAC4 forward primer, GAG ACC CAA TGC CAA TGC; HDAC4 reverse primer, AGG CAG CGC CAG TAC TCA; GAPDH forward primer, ACA ACT TTG GCA TCG TGG A; GAPDH reverse primer, CTT CTG AGT GGC AGT GAT GG). The carboxyfluorescein-labeled probe (Universal Probe Library, Roche Diagnostics) numbers were 10 for HDAC2, 105 for HDAC4, and 114 for GAPDH. For quantification of changes in gene expression, the comparative Cp (threshold cycle number) method was applied to calculate the relative fold changes normalized against GAPDH (percent GAPDH).
Statistics
A t test was used for comparisons between the sexes in the ChIP assay and quantitative real-time PCR analysis and for comparisons between groups in Western blotting analysis. For male sexual behavior, data were analyzed by two-way ANOVA with repeated measures across the three trials. In the event of a significant interaction between the treatment and trials, a t test for TSA treatment and a post hoc Fisher's protected least significant difference test for antisense ODN treatment were used within each trial to determine significant differences.
Results
Sex differences in the histone acetylation status of ERα and Arom gene promoters in the MPOA in the perinatal period
To examine the differential histone acetylation status of gene promoters involved in sexual differentiation of the brain during the perinatal sensitive period, a ChIP assay was performed using antibodies against acetylated histone H4 and H3. Male and female rats on ED21 and PD3 were compared because estradiol levels in the hypothalamus are higher in males than in females at ED21 but reduced gradually in both males and females during the early postnatal period (31). The MPOA was selectively collected (Fig. 1A), and chromatin with acetylated H4 or H3 was immunoprecipitated. Although there are multiple ERα gene promoters, the ERα 1b promoter is considered to be the most abundant in rat neuronal tissue (25). The rat Arom gene has two promoters: the Arom If promoter is used in the brain, and the Arom II promoter is predominantly used in the gonad but is also functional in the brain (22). Promoters of ERα 1b and Arom If and II in the precipitates were analyzed by semiquantitative PCR using specific primers (Fig. 1B). Acetylation levels were compared between the sexes after correction for the amount of input chromatin. Data are presented as relative acetylation levels of H4 (Fig. 1C) and H3 (Fig. 1D) in males compared with females.
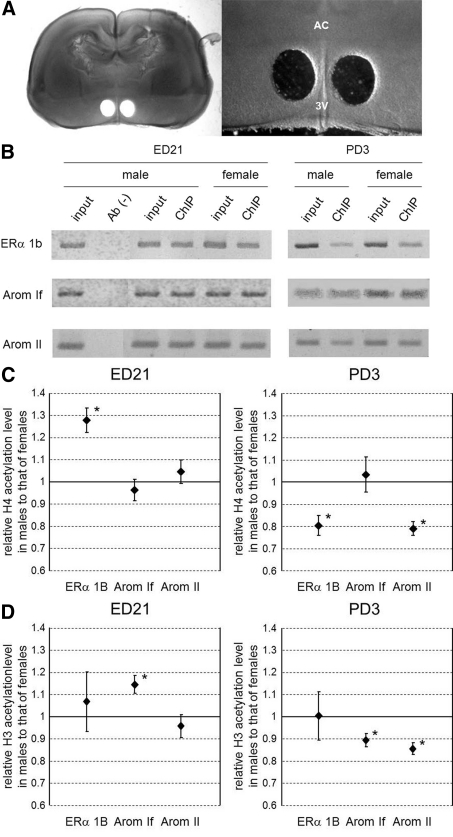
Fig. 1.
Histone acetylation level of ERα and Arom gene promoters in the MPOA during the perinatal period. A, Isolation of the MPOA in perinatal rats, with representative image of a section of PD3 rat brain including the POA after isolation of the MPOA (left) and higher magnification of the image including the MPOA (right). AC, Anterior commissure; 3V, third ventricle. B, Immunoprecipitated chromatin containing acetylated H4. The amounts of ERα 1b (top), Arom If (middle), and Arom II (bottom) promoter DNA in the precipitate were examined by PCR. Images of ethidium bromide-stained gels were captured in grayscale and inverted. ChIP was conducted with samples from ED21 (left) and PD3 (right) brains. Ab(−), No-antibody control for confirmation of specific immunoprecipitation; input, input chromatin control to quantify the amount of DNA present in samples before immunoprecipitation; ChIP, ChIP sample. C, Pixel intensities of grayscale images were measured, and ChIP/input ratios were compared between the sexes. The graphs show the relative ChIP/input ratios of males compared with females (mean ± sem). Data from ED21 (left) and PD3 (right) brains. *, P < 0.05 for male vs. female. D, Immunoprecipitated chromatin containing acetylated H3. The graphs show the relative acetylated H3 level of males compared with females (mean ± sem). Data from ED21 (left) and PD3 (right) brains. *, P < 0.05 for male vs. female.
At the ERα 1b promoter, H4 acetylation was significantly more frequent in males at ED21 (P < 0.05). However, males at PD3 had less acetylated H4 at the ERα 1b promoter compared with females (P < 0.05). H4 acetylation at the Arom II promoter was similar in the two sexes at ED21 but higher in females at PD3 (P < 0.05). Acetylation of the Arom If promoter did not show a significant difference at ED21 or PD3. H3 acetylation levels also exhibited significant sex differences at the Arom promoters but not at the ERα 1b promoter. At ED21, the level of acetylated H3 at the Arom If promoter was higher in males than in females (P < 0.05), but at PD3, the levels of H3 acetylation at both Arom promoters was higher in females (P < 0.05). These results indicate that gene promoters involved in sexual differentiation of the brain are differentially acetylated in males and females at ED21 and that these acetylation states are rearranged by PD3. In addition, the acetylation levels in males, except for H3 acetylation in the ERα 1b promoter, tend to be reduced during this early postnatal period (Supplemental Fig. 1), correlating with the reduction in estradiol level. In females, the acetylation levels were relatively unchanged, whereas H3 acetylation in ERα 1b promoter was significantly increased (Supplemental Fig. 1).
Effect of neonatal inhibition of HDAC activity on male sexual behavior in adulthood
The analysis of histone acetylation status of the ERα and Arom gene promoters suggests that histone deacetylation between ED21 and PD3 is involved in masculinization of the brain. To assess this possibility, we inhibited HDAC in vivo in newborn rats and examined the effects on sexual behavior. Male rats were given intracerebroventricular infusions of the HDAC inhibitor TSA on PD0 and PD1 and allowed to grow to adulthood. Male sexual behavior was tested three times: once every week from 10–12 wk old. The latency to first mount, intromission and ejaculation, and the ratio of intromission number to the sum of mount number and intromission number (intromission ratio) were quantified (27, 29, 30).
The intromission ratio was significantly lower in the neonatally TSA-treated males compared with control males in each of the three trials [F(5,66) = 6.30; P < 0.01] (Fig. 2). The mean latencies to first mount, intromission, and ejaculation were also significantly longer in the TSA-treated males compared with controls (Supplemental Fig. 2, A–C). To confirm that other brain functions, such as locomotor activity and anxiety-like behavior, were not affected by TSA treatment, behavior in an open field was observed in the same rats. No significant differences were observed in either the number of squares crossed or the amount of time spent in the center squares (Supplemental Fig. 3, A and B). There were also no significant differences in body weight, testis weight, epididymis weight, and serum testosterone levels after the behavioral analysis, indicating no overt changes in androgen production (Supplemental Fig. 3, C–F). Therefore, we conclude that inhibition of HDAC activity in the early postnatal period specifically attenuated male sexual behavior without impairing other brain functions.
Fig. 2.
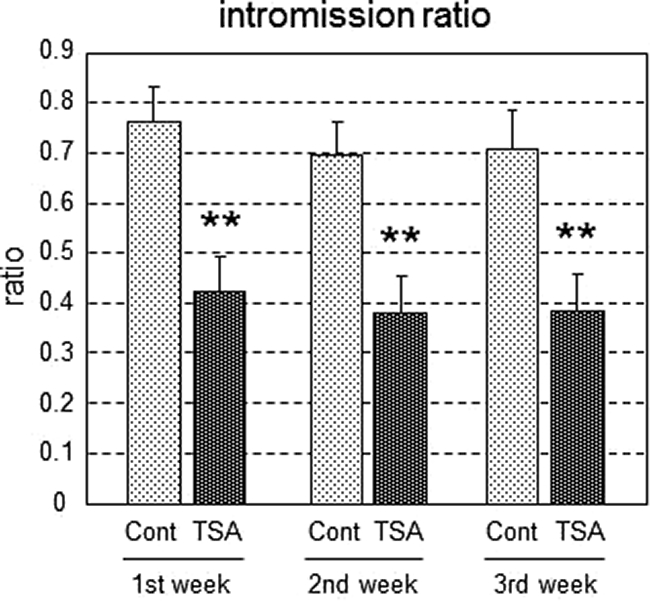
Intromission ratio in rats treated neonatally with TSA. Male sexual behavior was examined in TSA-infused (TSA, n = 12) and saline-infused control (Cont, n = 12) male rats. Values are means ± sem **, P < 0.01 vs. control in each trial.
Effect of neonatal inhibition of HDAC2 and -4 expression on male sexual behavior in adulthood
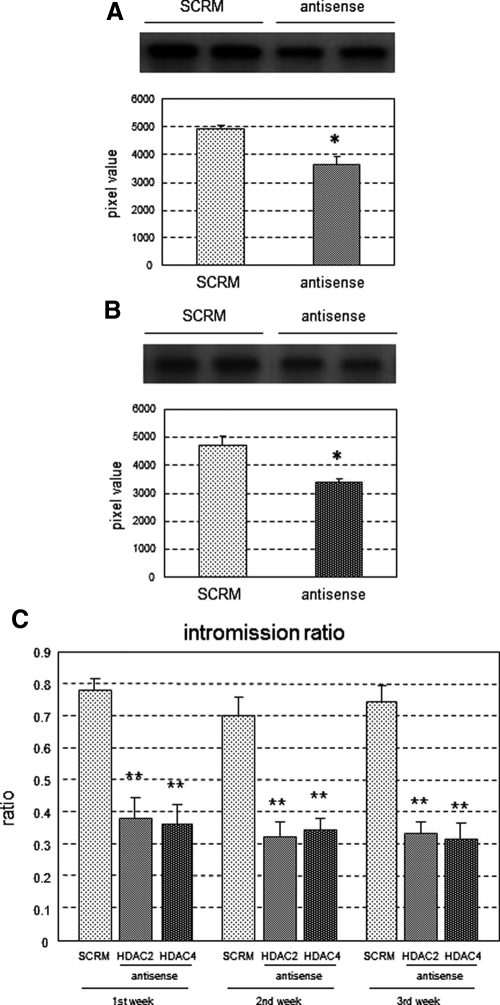
Next, we examined which subtype of HDAC is involved in masculinization of the rat brain. HDAC are classified into multiple subtypes within four classes (I–IV) (18). We investigated HDAC2 and -4, which are members of classes I and IIa, respectively, because these proteins are expressed in the developing brain (32, 33) and are related to steroid hormone signaling in other tissues (34, 35). Antisense ODN (27) against mRNA for HDAC2 and -4 were infused intracerebroventricularly using the same procedure as that for TSA treatment. Down-regulation of HDAC2 and -4 expression by the antisense ODN was confirmed by Western blot analyses of HDAC2 and -4 protein levels in the POA. The levels of both proteins were significantly lower in animals infused with an antisense ODN compared with an ODN with a scrambled sequence (P < 0.01) (Fig. 3, A and B). In a separate set of animals, male sexual behavior in adulthood was tested after developmental treatment with an antisense or scrambled ODN. Similar to the effect of TSA treatment, the intromission ratio was significantly lower in males that had been infused with the HDAC2 or -4 antisense ODN compared with controls in all three trials [F(8,63) = 16.62; P < 0.01] (Fig. 3C). There were also significant increases in the latency to first mount, intromission, and ejaculation in the HDAC2 or -4 antisense-treated males across all three trials (Supplemental Fig. 4, A–C).
Fig. 3.
Intromission ratio in rats treated neonatally with HDAC2/4 antisense ODN. A and B, Down-regulation of HDAC2/4 expression by infusion of antisense ODN. Lysates of HDAC2/4 antisense ODN-infused POA (antisense, n = 6) and scrambled ODN-infused POA (SCRM, n = 6) were subjected to Western blotting using antibodies against HDAC2 (A) or HDAC4 (B) with representative image of Western blot (top), and values (mean ± sem) of grayscale pixels were plotted (bottom). *, P < 0.05 vs. SCRM. C, Intromission ratios were determined for male rats infused with HDAC2 antisense ODN (HDAC2, n = 9), HDAC4 antisense ODN (HDAC4, n = 8), and scrambled ODN (SCRM, n = 8). Values are means ± sem. **, P < 0.01 vs. SCRM in each trial.
Expression of HDAC2 and -4 mRNA in the MPOA at PD1
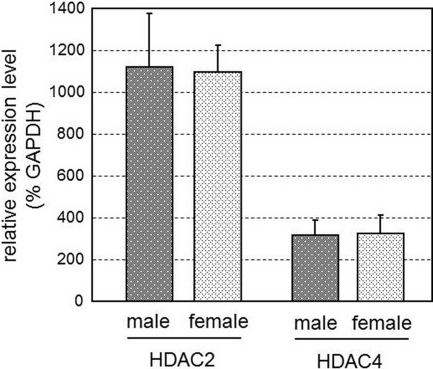
The results obtained above raise the question of how HDAC generate sex differences in histone acetylation status. Sex differences in HDAC expression are the simplest mechanism that could account for this observation. Thus, expression levels of mRNA for HDAC2 and -4 in the MPOA of PD1 rats were examined by quantitative real-time PCR. However, there was no significant sex difference in the HDAC2 or HDAC4 mRNA level (P > 0.05) (Fig. 4).
Fig. 4.
Quantitative expression level of HDAC2 and -4 mRNA in the MPOA of PD1 rats. cDNA synthesized from total RNA of MPOA of male (n = 4) and female (n = 4) rats on PD1 was subjected to quantitative real-time PCR. Expression levels of HDAC2 and -4 mRNA were normalized to GAPDH mRNA (percent GAPDH). There were no significant sex differences; values represent mean ± sem.
Sex differences in binding of HDAC2 and -4 to ERα and Arom gene promoters in the MPOA on PD1
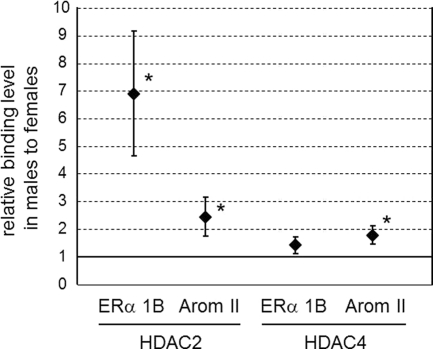
We next addressed whether the amount of HDAC2 and -4 binding to the ERα and Arom promoters differed between the sexes in the MPOA on PD1, using antibodies against HDAC2 and -4 in a ChIP assay. HDAC2 and -4 showed binding to the ERα 1b and Arom II promoters but not to the Arom If promoter. Intriguingly, the amount of HDAC2 binding to the ERα 1b promoter was about seven times higher in males than in females (P < 0.01) (Fig. 5), and HDAC2 and -4 binding to the Arom II promoter was also significantly higher in males (P < 0.05). Thus, although males and females have similar expression levels of HDAC2 and -4 (Fig. 4), there is greater binding of HDAC2 and -4 to the promoters of genes regulating sexual differentiation of the brain in males.
Fig. 5.
Binding of HDAC2 and -4 to ERα and Arom gene promoters in the MPOA of rats on PD1. The MPOA was isolated on PD1, chromatin with HDAC2 and HDAC4 was immunoprecipitated, and the amounts of DNA fragments of ERα 1b and Arom II promoters in the precipitates were examined by PCR. The graphs show the relative HDAC2 (left) and HDAC4 (right) binding levels in males to that in females (mean ± sem). *, P < 0.05 for male vs. female.
Discussion
Recent investigations of the development and function of the brain have revealed a remarkable role of epigenetic control of gene expression (16, 36, 37). In sexual differentiation of the brain, epigenetic changes are emerging as important mediators of hormonal and environmental effects (38). Histone acetylation is a well-characterized epigenetic modification that contributes to a wide range of transcriptional regulation (17, 18, 39), including sex steroid hormone receptor signaling (15, 19, 20). In response to the hormonal milieu, coactivator or corepressor complexes that associate with sex steroid hormone receptors control the histone acetylation status via intrinsic histone acetyltransferase activity or recruitment of HDAC, resulting in transcriptional activation or repression, respectively. In the present study, we showed the functional significance of histone deacetylation on sexual differentiation of the brain during the early postnatal period, which has enduring consequences for adult behavior.
Acetylation levels of H4 and H3 at the ERα and Arom promoters in the MPOA differed significantly in males and females at ED21, suggesting that gene promoters involved in sexual differentiation of the brain are differentially acetylated in specific brain areas related to sexual function. The acetylation differences observed at ED21 were rearranged by PD3, at which time acetylation levels in males declined in correspondence with the developmental decrease in testosterone (31). These results lead us to hypothesize that acetylation levels are adjusted to an appropriate level to organize masculinization of the brain within days after birth. This hypothesis was confirmed by experiments using HDAC inhibition. Murray et al. (24) demonstrated the requirement of HDAC activity during the early postnatal period for the development of structural sexual dimorphism of BNSTp, which is involved in the control of male sexual behavior (24). In addition, the current study revealed that male sexual behavior was reduced by administration of TSA at PD0 and PD1, suggesting a critical role for modification of the histone acetylation status during the early postnatal period in masculinization of the brain.
We propose that TSA inhibits modification of the histone acetylation status that normally occurs in the early postnatal period, resulting in dysregulation of male-specific gene expression patterns involved in sexual differentiation of the brain. This disturbance of sex-specific gene expression results in impaired masculinization of the brain. It is intriguing to consider the mechanisms mediating the enduring consequences of neonatal TSA treatment on adult masculine sexual behavior. Because structural sex dimorphism of reproduction-related brain areas are mostly created during the postnatal developmental period, TSA treatment may alter critical developmental processes at that time and thereby have enduring effects that are not manifest as long-term changes in the epigenome. Alternatively, it is possible that early postnatal TSA treatment leads to a permanent modification of sex-specific gene expression by inducing permanent alterations in acetylation status of the promoter of key genes regulating adult sexual behavior.
We identified HDAC2 and -4 as HDAC subtypes that contributed to induction of masculinization of the brain. Antisense ODN-induced down-regulation of HDAC2 and -4 on the first 2 d of postnatal life resulted in decreased male sexual behavior in adulthood. Moreover, although neonatal levels of mRNA for HDAC2 and -4 in the MPOA did not differ between the sexes, the amount of HDAC2 and -4 binding to the ERα and Arom promoters was higher in males than in females. These findings may provide insights into the molecular mechanism of HDAC-mediated masculinization of the brain. During the prenatal androgen surge and subsequent activation of ERα, the acetylation status of histones in the promoter region of genes involved in sexual differentiation of the brain is increased in males. After the androgen surge, inactivation of ERα due to the decline of ligand levels leads to recruitment of HDAC2 and -4 to the promoters, and the acetylation status of the promoter is reduced to an appropriate level to organize masculinization of the brain. This process is less robust in females, despite similar expression of HDAC in the female brain. The reduction in HDAC activity may occur because there is no androgen surge in females, and therefore, there are fewer circulating hormones available to trigger the process.
Although the single epigenetic change presented here may not explain all aspects of the maintenance of sexual differentiation of the brain, the results strongly suggest that modification of histone acetylation status by HDAC during early postnatal development is critical to enduring masculinization of the brain and expression of adult male sexual behavior. In the present study, we evaluated the functional contribution of HDAC2 and -4 subtypes on development of male sexual behavior. However, there are many HDAC subtypes (18), and it is possible that other subtypes participate in the sexual differentiation of the brain, with functional diversity and/or complementarity among the subtypes.
Epigenetic control of gene expression also occurs via mechanisms such as histone methylation, histone phosphorylation, and DNA methylation (20, 36, 39). Some of these processes have been implicated in neuroendocrine and behavioral responses as well as sex steroid hormone signaling (24, 25, 28, 38, 40, 41), and synergistic or hierarchical interactions between these epigenetic modifications are important topics for future investigation. Here, we evaluated the effect of HDAC after the developmental androgen surge on masculinization of the brain. Epigenetic events occurring during other developmental periods, especially before and after the onset of the androgen surge, would also be intriguing to examine.
Acknowledgments
We thank Drs. T. Inoue-Nishida (Tottorio University), Y. Sugimoto (Kumamoto University), Tsukahara (Saitama University), Y. Kondo (Teikyo University of Science and Technology), and Y. Sakuma (Nippon Medical School) for their valuable advice.
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, Culture, and Technology, Japan (KAKENHI20700292 to K.I.M. and KAKENHI20240036 to M.K.) and National Institutes of Health Grant (RO1MH52716-010 to M.M.M.).
Disclosure Summary: The authors declare no conflict of interest.
Footnotes
- Arom
- Aromatase
- BNSTp
- principal nucleus of the bed nucleus of the stria terminalis
- ChIP
- chromatin immunoprecipitation
- ED
- embryonic day
- ER
- estrogen receptor
- HDAC
- histone deacetylase
- MPOA
- medial POA
- PD
- postnatal day
- POA
- preoptic area
- TSA
- trichostatin A.
References
- 1. Arnold AP, Burgoyne PS. 2004. Are XX and XY brain cells intrinsically different? Trends Endocrinol Metab 15:6–11 [DOI] [PubMed] [Google Scholar]
- 2. Kawata M. 1995. Roles of steroid hormones and their receptors in structural organization in the nervous system. Neurosci Res 24:1–46 [DOI] [PubMed] [Google Scholar]
- 3. McCarthy MM. 2008. Estradiol and the developing brain. Physiol Rev 88:91–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matsuda K, Sakamoto H, Kawata M. 2008. Androgen action in the brain and spinal cord for the regulation of male sexual behaviors. Curr Opin Pharmacol 8:747–751 [DOI] [PubMed] [Google Scholar]
- 5. Matsumoto T, Honda S, Harada N. 2003. Alteration in sex-specific behaviors in male mice lacking the aromatase gene. Neuroendocrinology 77:416–424 [DOI] [PubMed] [Google Scholar]
- 6. McEwen BS, Lieberburg I, Chaptal C, Krey LC. 1977. Aromatization: important for sexual differentiation of neonatal rat brain. Horm Behav 9:249–263 [DOI] [PubMed] [Google Scholar]
- 7. Sato T, Matsumoto T, Kawano H, Watanabe T, Uematsu Y, Sekine K, Fukuda T, Aihara K, Krust A, Yamada T, Nakamichi Y, Yamamoto Y, Nakamura T, Yoshimura K, Yoshizawa T, Metzger D, Chambon P, Kato S. 2004. Brain masculinization requires androgen receptor function. Proc Natl Acad Sci USA 101:1673–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zuloaga DG, Puts DA, Jordan CL, Breedlove SM. 2008. The role of androgen receptors in the masculinization of brain and behavior: what we've learned from the testicular feminization mutation. Horm Behav 53:613–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. DonCarlos LL, Handa RJ. 1994. Developmental profile of estrogen receptor mRNA in the preoptic area of male and female neonatal rats. Brain Res Dev Brain Res 79:283–289 [DOI] [PubMed] [Google Scholar]
- 10. DonCarlos LL. 1996. Developmental profile and regulation of estrogen receptor (ER) mRNA expression in the preoptic area of prenatal rats. Brain Res Dev Brain Res 94:224–233 [DOI] [PubMed] [Google Scholar]
- 11. McAbee MD, DonCarlos LL. 1998. Ontogeny of region-specific sex differences in androgen receptor messenger ribonucleic acid expression in the rat forebrain. Endocrinology 139:1738–1745 [DOI] [PubMed] [Google Scholar]
- 12. Ogawa S, Lubahn DB, Korach KS, Pfaff DW. 1997. Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci USA 94:1476–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ogawa S, Chester AE, Hewitt SC, Walker VR, Gustafsson JA, Smithies O, Korach KS, Pfaff DW. 2000. Abolition of male sexual behaviors in mice lacking estrogen receptors α and β (αβERKO). Proc Natl Acad Sci USA 97:14737–14741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amateau SK, McCarthy MM. 2004. Induction of PGE2 by estrogen mediates developmental masculinization of sex behavior. Nat Neurosci 7:643–650 [DOI] [PubMed] [Google Scholar]
- 15. Wright CL, Schwarz JS, Dean SL, McCarthy MM. 2010. Cellular mechanisms of estradiol-mediated sexual differentiation of the brain. Trends Endocrinol Metab 21:553–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu L, Glass CK, Rosenfeld MG. 1999. Coactivator and corepressor complexes in nuclear receptor function. Curr Opin Genet Dev 9:140–147 [DOI] [PubMed] [Google Scholar]
- 17. Keverne EB, Curley JP. 2008. Epigenetics, brain evolution and behaviour. Front Neuroendocrinol 29:398–412 [DOI] [PubMed] [Google Scholar]
- 18. Marks PA, Xu WS. 2009. Histone deacetylase inhibitors: potential in cancer therapy. J Cell Biochem 107:600–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fu M, Wang C, Zhang X, Pestell RG. 2004. Acetylation of nuclear receptors in cellular growth and apoptosis. Biochem Pharmacol 68:1199–1208 [DOI] [PubMed] [Google Scholar]
- 20. Leader JE, Wang C, Popov VM, Fu M, Pestell RG. 2006. Epigenetics and the estrogen receptor. Ann NY Acad Sci 1089:73–87 [DOI] [PubMed] [Google Scholar]
- 21. Green KA, Carroll JS. 2007. Oestrogen-receptor-mediated transcription and the influence of co-factors and chromatin state. Nat Rev Cancer 7:713–722 [DOI] [PubMed] [Google Scholar]
- 22. Colciago A, Celotti F, Pravettoni A, Mornati O, Martini L, Negri-Cesi P. 2005. Dimorphic expression of testosterone metabolizing enzymes in the hypothalamic area of developing rats. Brain Res Dev Brain Res 155:107–116 [DOI] [PubMed] [Google Scholar]
- 23. Auger AP, Tetel MJ, McCarthy MM. 2000. Steroid receptor coactivator-1 (SRC-1) mediates the development of sex-specific brain morphology and behavior. Proc Natl Acad Sci USA 97:7551–7555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murray EK, Hien A, de Vries GJ, Forger NG. 2009. Epigenetic control of sexual differentiation of the bed nucleus of the stria terminalis. Endocrinology 150:4241–4247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. 2006. Maternal care associated with methylation of the estrogen receptor-α1b promoter and estrogen receptor-α expression in the medial preoptic area of female offspring. Endocrinology 147:2909–2915 [DOI] [PubMed] [Google Scholar]
- 26. Tsukahara S, Kakeyama M, Toyofuku Y. 2006. Sex differences in the level of Bcl-2 family proteins and caspase-3 activation in the sexually dimorphic nuclei of the preoptic area in postnatal rats. J Neurobiol 66:1411–1419 [DOI] [PubMed] [Google Scholar]
- 27. Wright CL, Burks SR, McCarthy MM. 2008. Identification of prostaglandin E2 receptors mediating perinatal masculinization of adult sex behavior and neuroanatomical correlates. Dev Neurobiol 68:1406–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. 2004. Epigenetic programming by maternal behavior. Nat Neurosci 7:847–854 [DOI] [PubMed] [Google Scholar]
- 29. Kondo Y, Sudo T, Tomihara K, Sakuma Y. 2003. Activation of accessory olfactory bulb neurons during copulatory behavior after deprivation of vomeronasal inputs in male rats. Brain Res 962:232–236 [DOI] [PubMed] [Google Scholar]
- 30. Agmo A, Villalpando A, Picker Z, Fernández H. 1995. Lesions of the medial prefrontal cortex and sexual behavior in the male rat. Brain Res 696:177–186 [DOI] [PubMed] [Google Scholar]
- 31. Konkle AT, McCarthy MM. 2011. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology 152:223–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Montgomery RL, Hsieh J, Barbosa AC, Richardson JA, Olson EN. 2009. Histone deacetylases 1 and 2 control the progression of neural precursors to neurons during brain development. Proc Natl Acad Sci USA 106:7876–7881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bolger TA, Yao TP. 2005. Intracellular trafficking of histone deacetylase 4 regulates neuronal cell death. J Neurosci 25:9544–9553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Biçaku E, Marchion DC, Schmitt ML, Münster PN. 2008. Selective inhibition of histone deacetylase 2 silences progesterone receptor-mediated signaling. Cancer Res 68:1513–1519 [DOI] [PubMed] [Google Scholar]
- 35. Leong H, Sloan JR, Nash PD, Greene GL. 2005. Recruitment of histone deacetylase 4 to the N-terminal region of estrogen receptor α. Mol Endocrinol 19:2930–2942 [DOI] [PubMed] [Google Scholar]
- 36. MacDonald JL, Roskams AJ. 2009. Epigenetic regulation of nervous system development by DNA methylation and histone deacetylation. Prog Neurobiol 88:170–183 [DOI] [PubMed] [Google Scholar]
- 37. McCarthy MM, Crews D. 2008. Epigenetics: new frontiers in neuroendocrinology. Front Neuroendocrinol 29:341–343 [DOI] [PubMed] [Google Scholar]
- 38. McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, Murray EK, Nugent BM, Schwarz JM, Wilson ME. 2009. The epigenetics of sex differences in the brain. J Neurosci 29:12815–12823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kouzarides T. 2007. Chromatin modifications and their function. Cell 128:693–705 [DOI] [PubMed] [Google Scholar]
- 40. Kurian JR, Olesen KM, Auger AP. 2010. Sex differences in epigenetic regulation of the estrogen receptor-α promoter within the developing preoptic area. Endocrinology 151:2297–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schwarz JM, Nugent BM, McCarthy MM. 2010. Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology 151:4871–4881 [DOI] [PMC free article] [PubMed] [Google Scholar]