Abstract
Ultrasonic vocalizations in the 50 kHz range (50 kHz USVs) are emitted by rodents upon activation of positive affective states and appear to be a direct measure of internal emotional and motivational urges to seek rewarding stimuli such as drugs of abuse. Since these behavioral responses do not rely on training for expression, they can be viewed as a “spontaneous” measure of affective state. The goal of the present study was to monitor spontaneous USVs throughout a widely-used cocaine self-administration and reinstatement model of addiction and relapse. To gain insight into the changes in affective state across the different phases of a standard self-administration experiment, we measured 50 kHz USVs in rats during cocaine self-administration and reinstatement, and compared these to sucrose self-administration and reinstatement. During cocaine self-administration, the number of 50 kHz USVs increased over acquisition of self-administration and decreased during extinction. Furthermore, the number of USVs on the first day of acquisition in the cocaine experiment was positively correlated with how rapidly cocaine self-administration was acquired. These findings suggest that the initial affective response to cocaine may be a sensitive predictor of the motivational efficacy of rewarding stimuli and therefore the susceptibility to acquire self-administration of cocaine. Cue-and cocaine-induced reinstatement elevated 50 kHz USVs above extinction levels. Rats trained for sucrose self-administration showed no elevation in USVs during acquisition when USVs were considered over the entire 2 hr session, but they did show an elevation in USVs during acquisition when considered over only the first 5 min of the session. As with cocaine-induced reinstatement, sucrose-induced reinstatement produced significantly more USVs compared to the prior extinction day. Taken together, USVs may serve as a sensitive and dynamic noninvasive measure that spontaneously (i.e. without any formal reinforcement contingencies) quantifies the extent to which positive affect is elicited by rewards such as drugs of abuse.
Keywords: Affective State, Cocaine, Reinstatement, Self-Administration, Sucrose, Ultrasonic Vocalization
1. Introduction
The drug self-administration reinstatement model in rodents has high face validity for the study of human drug addiction and relapse (Shaham et al., 2003) and relies on motor output in the form of lever presses or nose-pokes. However, it is unclear how these motor responses are linked to drug-elicited emotional and motivational states, which lie at the core of drug addiction. In human drug addicts, affective state plays a role in the addiction process itself and in relapse (Baker et al., 2004; Hodgins et al., 1995; Kassel, 2010; Koob, 2009; Sinha, 2008; Tiffany and Drobes, 1990). Such processes have been difficult to quantify in pre-clinical models. Thus, a measure of spontaneous affective changes in addiction paradigms without the requirement for any formal conditioning would be useful for gauging the underlying neuropsychological processes that mediate addiction.
Juvenile and adult rats emit two general frequencies of ultrasonic vocalizations, henceforth referred to as USVs: those in the 22 kHz range (22 kHz USVs) and those in the 50 kHz range (50 kHz USVs) (Knutson et al., 2002; Miczek et al., 2002). Studies over the past 30 years under a multitude of conditions have helped to define the significance of these USVs and have shown that they serve a communicatory role and are independent of general arousal and locomotor activity (Knutson et al., 2002). The 22 kHz USVs are considered distress calls because they are emitted in response to aversive and conditioned aversive experiences (Blanchard et al., 1991; Brudzynski, 2001; Burgdorf et al., 2001b; Francis, 1977; Thomas et al., 1983; Tonoue et al., 1986; Vivian and Miczek, 1998), while 50 kHz USVs are considered to reflect positive affective states that are closely related to brain dopamine-modulated reward substrates that mediate addiction.
Ultrasonic vocalizations can be used as an index of positive affective states in pre-clinical rat models of addiction (Kassel, 2010; Knutson et al., 1999; Panksepp et al., 2004). For example, repeated systemic injections of cocaine or amphetamine elicit USVs (Ahrens et al., 2009; Mu et al., 2009; Williams and Undieh, 2010), and injection of amphetamine into the nucleus accumbens unconditionally increases USVs (Burgdorf et al., 2001a; Thompson et al., 2006). In the conditioned place preference task, more USVs are observed in a chamber paired with either amphetamine or morphine than in a chamber paired with vehicle (Knutson et al., 1999). Playback of USVs in the 50 kHz range promotes approach behavior and elicits USVs from recipient male rats (Wohr and Schwarting, 2007). Both social and non-social rewards elicit approach behaviors and increase USVs (Bialy et al., 2000; Burgdorf et al., 2008). Electrical brain stimulation in the ventral tegmental area and the lateral hypothalamus leads to increases in USVs, and all electrode sites that evoke such USVs also sustain self-stimulation reward (Burgdorf et al., 2007). Using 50 kHz USVs in rats, we monitored positive affective states across different phases of a standard cocaine self-administration study, including acquisition, extinction, and cue- and cocaine-induced reinstatement.
Importantly, unlike operantly trained behaviors typically studied in drug addiction paradigms, USVs arise spontaneously, without conditioning, which allow for the assessment of changes in affective state in situations in which internal positive and negative affective states cannot be inferred by changes in conditioned behaviors (including behavioral responses to initial drug exposure as well as changes during phases of learning such as extinction). Recently, two studies have analyzed 50 kHz USV production during cocaine self-administration in rats. Barker et al. (2010) found dose-dependent differences in the duration, frequency, and frequency modulation of USVs in animals self-administering cocaine on a variable interval schedule. Maier et al. (2010b) reported a method for assessing USVs during cocaine self-administration in which USVs were time-locked to cocaine delivery. In the present study, we assessed changes in 50 kHz USVs utilizing the widely-used, fixed-ratio schedule of reinforcement in which rats self-administered a typical dose of cocaine (0.5 mg/kg/infusion) intravenously during daily 2 hr training sessions. We report how 50 kHz USVs change across time during the major stages of cocaine self-administration, including during cue-and cocaine-induced reinstatement. To contrast types of reward, an additional group working for a conventional sucrose reward was concurrently studied. In addition to monitoring 50 kHz USVs, we also assessed 22 kHz USVs and found that they constituted a very small fraction (less than 1%) of all USVs emitted over the course of cocaine and sucrose self-administration.
2. Methods and Materials
2.1 Animals
A total of 21 male Sprague Dawley rats (Simonsen Laboratories, Gilroy, CA) weighing 250–300g were individually housed under a 12:12 hr reversed light/dark cycle in a temperature- and humidity-controlled colony room at 21 ± 1°C and were handled daily. Care of the animals was in accordance with the National Institute of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978), and all procedures were approved by the Institutional Animal Care and Use Committee of Washington State University. All efforts were made to minimize animal suffering and to reduce the number of animals used.
2.2 Surgery
Rats in the cocaine self-administration experiment underwent jugular catheter implantation as described (Neisewander et al., 2000). Briefly, rats were anesthetized with an intraperitoneal (IP) injection (1 mL/kg) of a solution containing ketamine hydrochloride (87 mg/mL) and xylazine (150 mg/mL). Catheters were implanted into the jugular vein and mounted onto the skull with screws and dental acrylic. Following surgery, animals received an intramuscular (IM) injection of ketoprofen (10mg/mL) as an analgesic. Animals were allowed at least 5 days recovery before cocaine self-administration training began. Animals were handled, weighed, and their catheters were flushed daily with 0.1 mL intravenous (IV) injection of a solution containing gentamicin sulfate (5 mg/mL) and heparin (1,000 USP Units/mL) during recovery from surgery and during acquisition training. Catheters were flushed following self-administration sessions or at the same time of day when animals were not run in self-administration (8–10 hours after “lights off” in the colony room).
2.3 Drugs
Cocaine hydrochloride (Sigma-Aldrich, St. Louis, MO) was dissolved at room temperature in 0.9% sodium chloride and filtered through a 0.2 µm filter.
2.4 Cocaine Self-Administration Experiment
2.4.1 Acquisition training
Rats (n=7) began cocaine self–administration acquisition training 5–7 days post-surgery. All training and testing occurred 8–10 hours after “lights off” in the colony room. Animals were initially food restricted to 16 g of standard food chow per day. Self-administration sessions were carried out 2 hr per day, 6 days per week. During training sessions, animals were placed in 40.5 × 40.5 × 30.5 cm acrylic self-administration chambers. Chambers were equipped with active and inactive nose-poke holes (diameter of 3.2 cm) in which nose insertion resulted in interruption of a photocell beam. The active and inactive nose-poke holes were in opposite corners of the self-administration apparatus and were not counterbalanced for drug delivery. The active nose-poke contained a red light that served as a cue light. Each chamber also contained a house light 25.4 cm from the floor of the chamber. Rats were allowed to self-administer cocaine (0.5 mg/kg/0.1 mL, IV) via nose-pokes into the active hole, resulting in activation of an infusion pump. A response on the active nose-poke simultaneously activated the house light and cue light, with an infusion occurring 1 sec after response and lasting for 5.1 sec. The house light remained on for a total of 20 sec, at which time cocaine was unavailable (time out period). Responses on the inactive nose-poke were recorded but had no consequences. Rats were initially trained to self-administer on a fixed ratio1 (FR1) schedule of reinforcement (1 infusion of cocaine per nose-poke response). Once animals had reached the criteria of at least 10 infusions per session for 5 days, they were moved to an FR5 schedule. Animals required 11 to 16 days to transition to an FR5 schedule. One of the 7 animals had not transitioned to an FR5 schedule by Day 14 (Middle Acquisition). Ad libitum feeding began on the first day of training on the FR5 schedule of reinforcement. All animals used in the study reached the criteria of 10 infusions per day for 5 days on an FR5 schedule with less than 10% variability in the number of cocaine rewards over the last 3 days. Responses on the last three days of acquisition training were averaged for each animal and used as the acquisition (maintenance) baseline. Acquisition training lasted a total of 4–5 weeks. USVs and locomotor activity were analyzed at three points during acquisition: the first day of acquisition (Beginning Acquisition), day 14 of acquisition (Middle Acquisition), and the last day of acquisition (End Acquisition).
2.4.2 Extinction training
Extinction began the day after the last day of acquisition training for each animal. During extinction the animals were placed in the self-administration chambers for 2 hr per day. The IV catheter was attached to the tether, but no cocaine infusion occurred upon active nose-poke responses. Neither the house light nor cue light was present during extinction. Extinction proceeded until each animal achieved ≥ 90% reduction in number of active nose-poke responses compared with its own acquisition baseline (McFarland and Kalivas, 2001). Animals received a total of 1–2 weeks of extinction. Active nose-poke responses, 50 kHz USVs, and locomotor activity on the last day of extinction (End Extinction) were used as an extinction baseline for comparison with cue-induced reinstatement. Ultrasonic vocalizations and locomotor activity were analyzed the first day (Beginning Extinction) and last day (End Extinction) of extinction.
2.4.3 Reinstatement testing
Rats were tested for both cue- and cocaine-induced reinstatement. Cue-induced reinstatement occurred the day following the last extinction day. Animals were placed in the self-administration chambers and given one non-contingent cue presentation (the red cue light, house light and sound of the infusion pump). Animals were then allowed to respond for cues in the absence of cocaine administration for 2 hr. Following cue-induced reinstatement, beginning the next day, animals underwent a second round of extinction before being given cocaine-induced reinstatement. For these extinction sessions, rats were given IP saline injections just prior to extinction to control for stressful effects of injection. Four days of extinction were required for animals to reach the extinction criteria of ≥ 90% reduction in active nose-poke responses from the acquisition baseline (as above for Extinction Training). Active nose-poke responses, 50 kHz USVs, and locomotor activity on the last day of extinction (Second Extinction) were used as an extinction baseline for comparison with cocaine-induced reinstatement. Cocaine-induced reinstatement was carried out the following day. Animals received a 10mg/kg IP injection of cocaine and were immediately placed into the self-administration chambers for 2 hr. Active and inactive nose-poke responses were recorded but had no consequences.
2.5 Sucrose Self-Administration Experiment
2.5.1 Sucrose acquisition training
A separate group of animals (n=6) was trained for sucrose self-administration and reinstatement. Rats in the sucrose experiment were not given a surgical procedure. As with the cocaine experiment, all training and testing occurred 8–10 hours after “lights off” in the colony room and self-administration sessions were carried out 2 hr per day, 6 days per week. Acquisition of sucrose self-administration was carried out in the same chambers used for cocaine self-administration. Animals were fed ad libitum throughout the sucrose self-administration experiment. Active nose-poke responses resulted in the dispensing of a grape-flavored sucrose pellet (Bio-Serv, 45 mg) via activation of food hoppers (Med Associates). Animals were trained and maintained on an FR1 schedule of reinforcement (one pellet per active nose-poke response), and responding was accompanied by the same cue and house lights as for the cocaine experiment. The inactive nose-poke responses were recorded but had no consequences. USVs and locomotor activity were analyzed as in the cocaine experiment.
2.5.2 Sucrose extinction training
Extinction began the day after the last day of acquisition training for each animal. The same extinction protocol was used as in cocaine self-administration. Animals received 1–2 weeks of extinction training. Ultrasonic vocalizations and locomotor activity were analyzed the first day (Beginning Extinction) and last day (End Extinction) of extinction.
2.5.3 Sucrose reinstatement testing
Animals were tested for both cue- and sucrose-induced reinstatement. Cue-induced reinstatement occurred the day following the last extinction day. Animals were placed in the self-administration chambers and were given one non-contingent cue presentation (the red cue light, house light and sound of the food hopper). Animals were allowed to respond for the cues in the absence of sucrose pellets for the 2 hr test session. Following cue-induced reinstatement, beginning the next day, animals received a second round of extinction before being given sucrose-induced reinstatement. One day of extinction was all that was required for animals to meet the extinction criteria of ≥ 90% reduction in active nose-poke responses from the acquisition baseline. Active nose-poke responses, 50 kHz USVs, and locomotor activity on the last day of extinction (Second Extinction) were used as an extinction baseline for comparison with sucrose-induced reinstatement. Sucrose-induced reinstatement was carried out the following day. Animals received one non-contingent sucrose pellet and then were allowed to respond for sucrose in the absence of the cues for the duration of the 2 hr test session.
2.6 Non-treated (Naive) Controls
A separate group of non-treated control animals was tested (n=8) to assess the level of spontaneous USVs produced by animals placed into the self-administration chambers as a test for effects of cage novelty and to determine basal levels of vocalization. Sessions were carried out 8–10 hr after “lights off” in the vivarium. The rats were placed in the same self-administration chambers that were used for cocaine and sucrose self-administration for 2 hr per day for a total of 5 days. The responses on both the active and the inactive nose-pokes were recorded but had no consequences. Nose-poke responses, USVs and locomotor activity were analyzed during each of the 5 sessions.
2.7 Locomotor Activity and Ultrasonic Vocalization Recordings
Locomotor activity (total distance traveled in cm) and USVs were recorded in the self-administration chambers during training and test sessions. Locomotion was recorded with photocell beams using Digiscan Activity Monitors (AccuScan Instruments, Inc., USA) and analyzed with Digipro Version 1.4 software. USVs were recorded using the Avisoft Bioacoustic UltraSoundGate, system 116–200 (Berlin, Germany) and analyzed with SEApro 2.0h Real-Time Spectograph Standard Version software (AEST). USVs were identified through visual inspection by three trained experimenters who varied <10% on analyses of USVs. 50 kHz USVs were defined as all USVs in the range of 35–90 kHz. 22 kHz USVs were defined as flat frequency USVs in the range of 18–32 kHz having duration of ≥ 0.3 sec (Brudzynski, 2010; Knutson et al., 2002). 22 kHz USVs were not statistically analyzed due to the low number observed (< 1% of total calls) and thus only 50 kHz USVs are summarized below.
2.8 Data Analysis
The cumulative number of 50 kHz USVs, locomotor activity, and active nose-poke responses on Beginning Acquisition, Middle Acquisition, End Acquisition, and Beginning Extinction were analyzed using one-way, repeated-measures ANOVAs, followed by LSD post hoc tests to compare phases in the case of a significant interaction (P≤0.05). Behaviors during cue- and cocaine- or sucrose-induced reinstatement tests were compared to prior extinction days using paired t-tests. Time course data were analyzed using two-way, repeated measures ANOVAs with both time and phase as within-subject factors, followed by LSD post hoc analyses in the case of a significant interaction (P≤0.05). Because the main differences in USVs occurred during the first 30 min of the sessions, we statistically report differences in the first 30 min. Pearson correlation analyses were performed to assess days required for acquisition of stable self-administration with various behaviors on the first day of acquisition (significant correlation indicated by P≤0.05). USVs in Naïve animals across the five daily sessions were analyzed using one-way repeated measures ANOVA. Comparison of USVs on Day 1 across the three experiments was analyzed using one-way repeated measures ANOVA. Two-way repeated measures ANOVAs were analyzed using SPSS (IBM Corporation, Somers, NY). All other statistical analyses were performed using Prism 5 (Graphpad Software Inc., La Jolla, CA).
3 Results
3.1 USVs throughout cocaine acquisition, extinction, and reinstatement
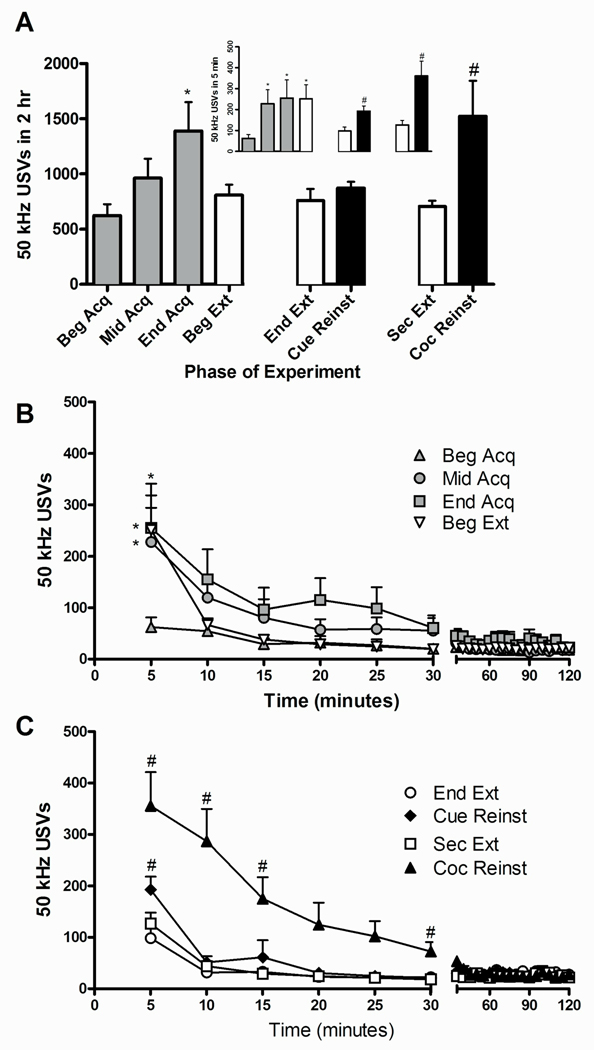
Figure 1 summarizes the findings of the cocaine self-administration experiment. Figure 1A shows the mean number of 50 kHz USVs throughout the 2 hr sessions during the various phases of the experiment (acquisition, extinction, and cue- and cocaine-induced reinstatement). We found a significant effect of phase on the number of 50 kHz USVs [F(3,18)=4.379, P≤0.05]. Post-hoc comparisons indicated that there was an increase in the number of 50 kHz USVs emitted during the last day of acquisition (End Acquisition) compared with the first day of acquisition (Beginning Acquisition). Furthermore, cocaine-induced reinstatement, but not cue-induced reinstatement, led to a higher number of USVs compared with the prior extinction day [t(6)=2.80, P≤0.05]. The inset in Figure 1A shows the number of 50 kHz USVs emitted within the first 5 min of each test session. There was a significant effect of phase on the number of 50 kHz USVs emitted within the first 5 min of the test sessions [F(3,18)=5.451, P≤0.01], with an increase in the number of USVs emitted during Middle Acquisition, End Acquisition, and Beginning Extinction compared with Beginning Acquisition. Within the first 5 min of the test, both cue-induced reinstatement [t(6)=3.650, P≤0.05] and cocaine-induced reinstatement [t(6)=3.814, P≤0.01] increased 50 kHz USVs compared to prior extinction session.
Fig 1.
USVs during acquisition, extinction, and reinstatement of cocaine self-administration. (A) Total 50 kHz USVs are expressed as mean ± SEM. Gray bars represent acquisition training, white bars represent extinction training, and black bars represent reinstatement testing sessions. Inset: 50 kHz USVs during the first 5 min of each test session. *Represents significant difference from Beginning Acquisition (ANOVA followed by LSD test,*P≤0.05). # Represents significant difference from prior extinction day (paired t-test, # P≤0.05). (B) Time course of 50 kHz USVs during the first day of acquisition (Beg Acq), the 14th day of acquisition (Mid Acq), the last day of acquisition (End Acq), and the first day of extinction (Beg Ext) in the cocaine experiment. (C) Time course of 50 kHz USVs during reinstatement test sessions and prior extinction sessions. Cue-induced reinstatement (Cue Reinst) is compared to its prior extinction day (End Ext). Cocaine-induced reinstatement (Coc Reinst) is compared to its prior extinction day (Sec Ext). Behavior is expressed as the mean number of 50 kHz USVs ± SEM shown in 5 min bins. *Represents significant difference from Beginning Acquisition (ANOVA followed by LSD test,*P≤0.05). # Represents significant difference from prior extinction day (ANOVA followed by LSD test, # P≤0.05).
Figure 1B shows the time course of 50 kHz USVs emitted throughout the 2 hr sessions during phases cocaine acquisition and the first day of extinction shown in 5 min bins. There was a main effect of phase [F(3,18)=4.432, P≤0.05], time [F(5,30)=5.848,P≤0.001], and an interaction between phase and time [F(15,90)=1.957, P≤0.05]. Post-hoc analyses showed that significantly more USVs were emitted during the first 5 min of the Middle Acquisition, End Acquisition, and Beginning Extinction compared with the first 5 min of Beginning Acquisition. The time course of the 2 hr sessions during reinstatement and prior extinction phases is shown in Figure 1C. There was a main effect of phase [F(3,18)=7.511, P≤0.005], time [F(5,30)=27.209,P≤0.0001], and an interaction between phase and time [F(15,90)=5.568, P≤0.0001]. Post hoc analyses revealed that USVs were elevated during the first 15 min of cocaine-induced reinstatement compared with the prior extinction day in which animals received an injection of saline (Second Extinction). During cue-induced reinstatement, an increase in the number of USVs from the prior extinction day (End Extinction) was observed during the first 5 min of the session.
Once animals were on a stable schedule of reinforcement at Middle Acquisition (FR5 for cocaine self-administration and FR1 for sucrose self-administration), we assessed USVs per reward. We found a significant interaction between cocaine and sucrose self-administering rats and phase of acquisition (for cocaine group: Middle Acquisition = 32.2±9.0, End Acquisition = 62.2±16.7; for sucrose group: Middle Acquisition = 26.9±16.4, End Acquisition = 6.0±1.3; interaction P=0.036).
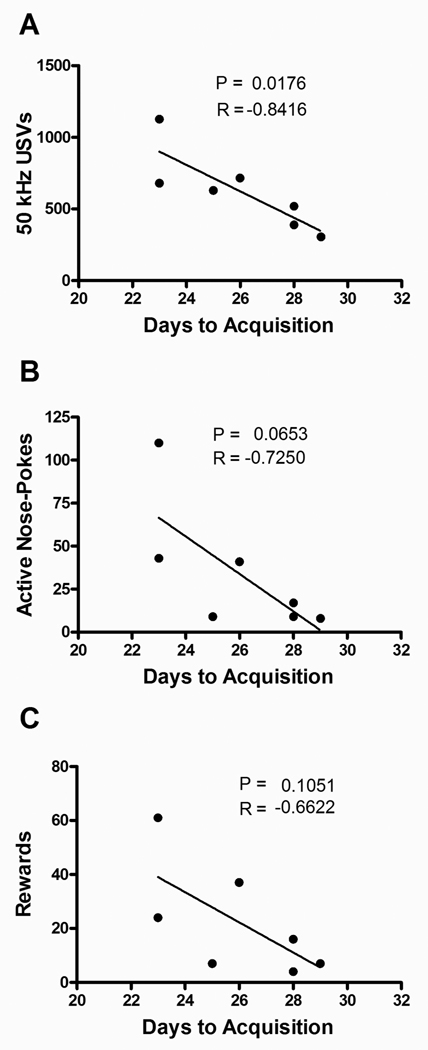
3.2 USVs on the first day of self-administration predict the number of days required for acquisition
Because 50 kHz USVs are thought to reflect positive affective-appetitive states, we determined whether the number of USVs in response to the first cocaine self-administration session was predictive of how rapidly the animals acquired self-administration (Fig. 2A). The number of “days to acquisition” was defined as the number of days required for an animal to reach a stable (≤10% variability in the number of rewards self-administered) acquisition baseline above the criteria of 10 rewards per day on the FR5 schedule. A negative correlation [R=−0.8416, P=0.0176] was observed between the number of calls emitted on the first day of acquisition and the number of days required for self-administration acquisition. Although there were trends toward negative correlations between the number of days required for acquisition of cocaine self-administration and both the active nose-poke responses on the first day of self-administration [R=−0.7250, P=0.0653] and the number of rewards on the first day of self-administration [R=−0.6622, P=0.1051] these were not significant (Figures 2B and 2C). In the sucrose experiment there was no correlation between USVs (P=0.1140), active nose-pokes (P=0.2919), or the number of rewards (P=0.2713) on the first day of training and the days required for acquisition (data not shown).
Fig 2.
Comparison between the numbers of days required for acquisition of cocaine self-administration and (A) total number of 50 kHz USVs; (B) active nose-poke responses; and (C) number of cocaine rewards on the first day of acquisition training. P and R values were derived from Pearson correlations.
3.3 USVs throughout sucrose acquisition, extinction, and reinstatement
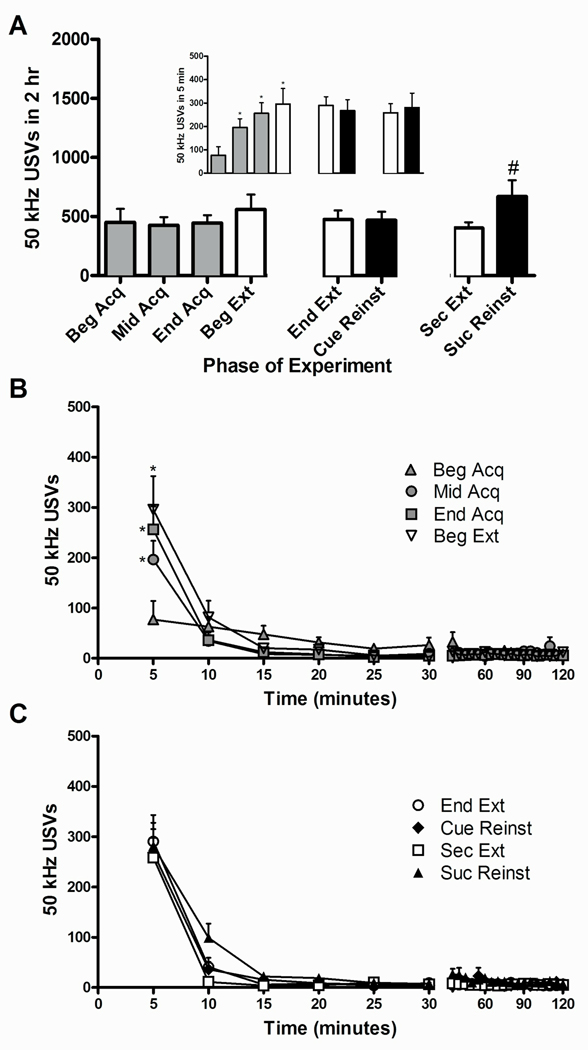
Unlike for the cocaine experiment, there was no effect of phase on the total number of 50 kHz USVs produced during the 2 hr sessions in the sucrose experiment [F(3,15)=1.57, P=0.24] (Fig. 3A). While the number of USVs during Beginning Acquisition was similar to that of rats self-administering cocaine, USVs did not increase over days across sucrose self-administration acquisition. Similar to the cocaine experiment, sucrose-induced, but not the cue-induced, reinstatement elevated USVs compared to the prior extinction day (Second Extinction) [t(5)=2.63, P≤0.05]. However, there was a significant effect of phase on the number of 50 kHz USVs emitted within the first 5 min of the test sessions [F(3,15)=16.335, P≤0.0001] (see inset). Furthermore, post-hoc analyses show an increase in USVs emitted by Middle Acquisition, End Acquisition, and Beginning Extinction compared to the Beginning Acquisition. No reinstatement of 50 kHz USVs by cue or sucrose pellets was observed when compared to prior extinction during the first 5 min of the test session in the sucrose experiment.
Fig 3.
Ultrasonic vocalizations during acquisition, extinction, and reinstatement of sucrose self-administration. (A) Total 50 kHz USVs are expressed as mean ± SEM. Gray bars represent acquisition training, white bars represent extinction training, and black bars represent reinstatement testing sessions. Inset: 50 kHz USVs during the first 5 min of each test session. *Represents significant difference from Beginning Acquisition (ANOVA followed by LSD test,*P≤0.05). # Represents significant difference from prior extinction day (Paired t-test, # P≤0.05). (B) Time course of 50 kHz USVs during the first day of acquisition (Beg Acq), the 14th day of acquisition (Mid Acq), the last day of acquisition (End Acq), and the first day of extinction (Beg Ext) in the sucrose experiment. (C) Time course of 50 kHz USVs during reinstatement test sessions and prior extinction sessions. Cue-induced reinstatement (Cue Reinst) is compared to its prior extinction day (End Ext). Sucrose-induced reinstatement (Suc Reinst) is compared to its prior extinction day (Sec Ext). Behavior is expressed as the mean number of 50 kHz USVs ± SEM shown in 5 min bins. *Represents significant difference from Beginning Acquisition (ANOVA followed by LSD test,*P≤0.05). # Represents significant difference from prior extinction day (ANOVA followed by LSD test, # P≤0.05).
In the time course analysis of sucrose acquisition and the first extinction phases (Figs. 3B), there was a main effect of phase [F(3,15)=4.463, P≤0.05], time [F(5,25)=22.546, P≤0.0001] and an interaction between phase and time [F(15,75)=14.202, P≤0.0001]. Post-hoc analyses show an increase in USVs emitted by Middle Acquisition, End Acquisition, and Beginning Extinction compared to the Beginning Acquisition. Analysis of the time course during prior extinction and reinstatement phases in the sucrose experiment revealed only a main effect of time [F(5,25)=55.468, P≤0.0001], however, there was no effect of phase [F(3,15)=1.061, P=.395], and no interaction between phase and time [F(15,75)=1.224, P=.273].
3.4 USVs do not change with novelty of the self-administration chamber
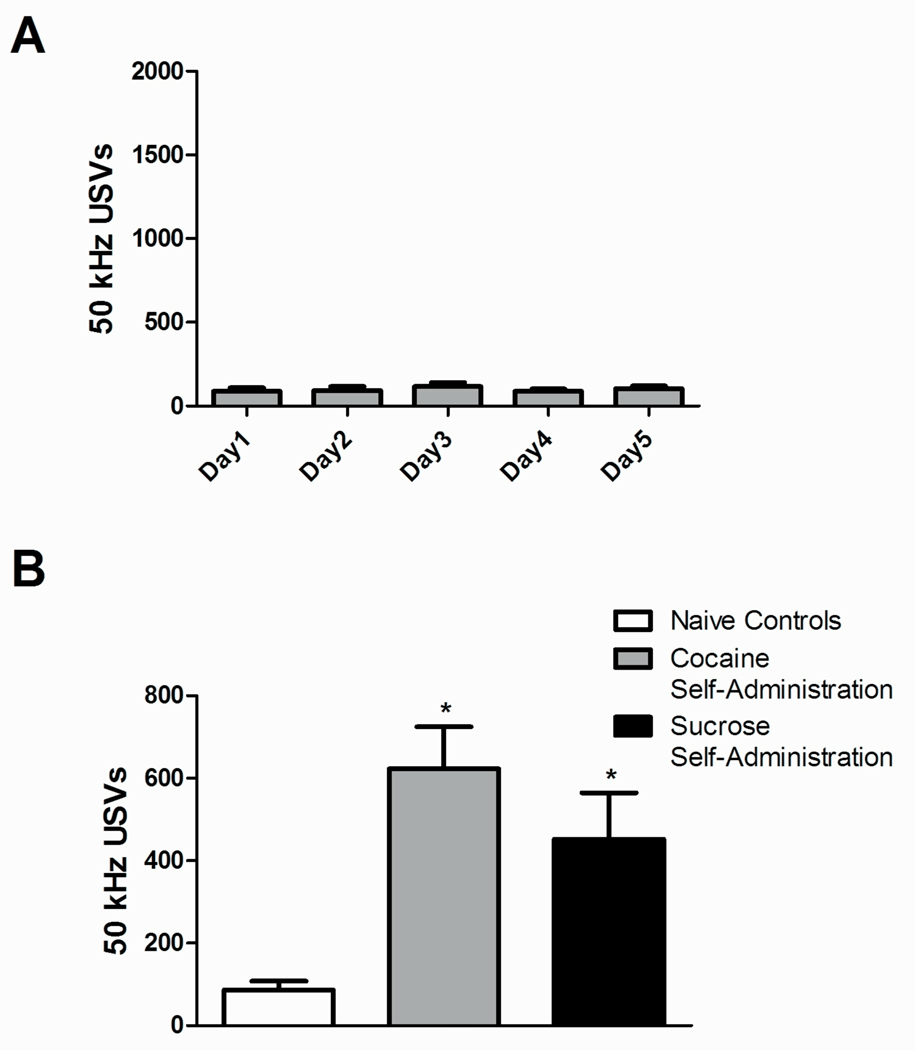
Non-treated naïve controls were placed in the self-administration chambers 2 hr per day for 5 consecutive days. The number of vocalizations in non-treated controls did not vary over the five days [F(4,28)=0.4415, P=0.7776], showing that spontaneous USVs in non-treated animals remained stable over this time (Fig. 4A). Furthermore, USVs in naïve controls on the first session were much lower than those observed for either cocaine self-administration or for sucrose self-administration [F(2,18)=12.15, P≤0.001], suggesting that the initial experience of both cocaine and sucrose self-administration produced positive affect above basal levels (Fig. 4B).
Fig 4.
Ultrasonic vocalizations in non-treated naïve animals and comparison of USVs emitted on the first day among naïve, cocaine, and sucrose self-administering animals. (A) 50 kHz USVs did not vary over five days in non-treated control animals during 2 hr sessions. (B) USVs on Day 1 of training were increased in the sucrose and cocaine treated animals compared to non-treated control animals. Total 50 kHz USVs are expressed as mean ± SEM. *Represents significant difference from non-treated control animals (ANOVA followed by LSD test,*P≤0.05).
3.4 Active and inactive nose-poke responses
Active nose-pokes increased from the beginning to the end of acquisition in both the cocaine and sucrose experiments (Supplemental Fig. 1A and 1B). Cue-induced reinstatement led to increased active nose-poke responding in the cocaine but not in the sucrose experiment compared to prior extinction. Cocaine-induced reinstatement did not result in significant increases in active nose-pokes, although there was a strong trend (P=0.0759). In contrast, sucrose-induced reinstatement led to a significant increase in active nose-poke responding.
Inactive nose-pokes decreased in the sucrose experiment from the beginning to the end of acquisition training, which was not observed in the cocaine experiment (Supplemental Fig. 1C and 1D). In addition, inactive nose-pokes were greater only during cue-primed reinstatement in the cocaine experiment compared to the prior extinction day.
4 Discussion
In the present study we report 50 kHz USVs emitted during the course of a typical cocaine self-administration study using a standard cocaine dose. In addition, we assessed comparable affective states associated with a non-drug reward (sucrose). Interestingly, we found that the number of 50 kHz USVs on the first day of cocaine self-administration was negatively correlated with the number of days required for acquisition of stable self-administration behavior. This demonstrates that spontaneous, untrained 50 kHz USVs may be a sensitive predictor of the rat’s vulnerability to attain sustained cocaine self-administration. While both the number of active nose-poke responses and the number of cocaine rewards on the first day of acquisition showed similar trends, these were not significant, suggesting that USV emission upon initial cocaine experience is a better predictor of the rate of self-administration acquisition. This finding supports those made in humans wherein individuals who rate their first experience with cocaine as a positive subjective experience are more likely to develop cocaine dependence and/or lifetime cocaine use (Lambert et al., 2006).
The number of 50 kHz USVs increased from the beginning to end of acquisition training in the cocaine self-administration experiment. USV production is dose-dependent (Barker et al., 2010); thus, USVs may have increased as the number of cocaine rewards increased from the beginning to the end of self-administration. Alternatively, USVs may have sensitized from the beginning to end of acquisition training. 50 kHz USVs sensitize with repeated administration of the same dose of non-contingent cocaine (Mu et al., 2009; Williams and Undieh, 2010), amphetamine (Ahrens et al., 2009), and methylphenidate (Panksepp et al., 2002). The increase in 50 kHz USVs may reflect an increase of the incentive motivational properties of cocaine since USVs have been characterized as motivational markers (Burgdorf et al., 2000; Knutson et al., 1998, 1999; Sales, 1972a, b). However, no increase in the total number of USVs was observed from the beginning to the end of sucrose self-administration acquisition despite an increase in sucrose rewards. These findings suggest that the increase in USVs observed over the 2 hr sessions may not generalize to non-drug rewards.
Very few 22 kHz USVs were observed in either cocaine- or sucrose-self-administering rats. 22 kHz USVs are emitted during withdrawal following cocaine binges, but this effect is not detectable unless additional mildly aversive stimuli such as air puffs are applied to the rat (Barros and Miczek, 1996; Mutschler et al., 2001; Mutschler and Miczek, 1998a, b). Furthermore, Barker et al. (2010) reported 22 kHz USV emissions during cocaine self-administration, but the vast majority were less than 0.3 sec. While both short (<0.3 sec) and long (>0.3 sec) 22 kHz USV have been reported (Brudzynski et al., 1993; Brudzynski et al., 1991; Sales, 1972a), only the long 22 kHz USVs have been associated with negative affect (Brudzynski, 2001; Knutson et al., 2002; Van der Poel and Miczek, 1991). Thus, only 22 kHz USVs over 0.3 sec may reflect negative affect. Based on this standard, 22 kHz USVs collectively accounted for less than 1% of the calls observed in all of our experiments. Indeed, such scarcity of 22 kHz USVs upon cocaine exposure is also reported in several other studies (Ahrens et al., 2009; Barker et al., 2010; Williams and Undieh, 2010).
During extinction, 50 kHz USVs decreased in rats exposed to cocaine self-administration but not in rats exposed to sucrose self-administration. During the first day of cocaine extinction, USV levels were similar to those found at the end of acquisition only during the first 5 min. However, after the first 5 min, the number of USVs dropped to the level observed during the first day of acquisition. This decrease in USVs may reflect a rapid decrease in positive affect during extinction. However, at no point did USVs in the cocaine or sucrose experiments drop to the level of non-treated naïve animals, suggesting that an environment paired with rewarding stimuli continues to elicit a modestly elevated positive affective state even in the absence of the reward.
Interestingly, although animals demonstrated a rapid decrease in 50 kHz USVs during the first extinction session, several more days were required for the animals’ nose-poke responses to decrease to extinction levels. This result suggests that rats continued to attempt to obtain cocaine by nose-poke responding even though they may no longer have maintained the previously-associated positive affective state. This is reasonable from the perspective that the USVs were not an operant for the receipt of cocaine, and hence the affective measure should have declined much more rapidly than the instrumental responding, which may have become a learned behavioral habit (Everitt et al., 2001).
50 kHz USVs also reflect anticipation of both natural and drug rewards (Burgdorf et al., 2000; Knutson et al., 1998; Ma et al., 2010). Panksepp & Moskal (2008) proposed that USV production demonstrates reward expectancy resulting from the activation of an ethologically-characterized expectancy/seeking system (Panksepp et al., 2002). Recently, Ma et al. (2010) reported that anticipatory 50 kHz USVs increased during a 10 min period prior to the start of each cocaine self-administration session between the beginning and end of acquisition training regardless of whether the drug was administered contingently or non-contingently, and this anticipatory USV production was greater after a 2-day forced abstinence period (Maier et al., 2010a). Indeed, in the present study, most USVs were emitted at the start of the self-administration sessions. This may signify that most of the USVs observed were in anticipation of the drug reward, rather than just in response to the cocaine itself, suggesting that USVs may reflect an anticipatory state. Another possibility is that the start of the training session elicits a greater level of positive affect. This may result from the alleviation of negative affect experienced by the separation of the animal from cocaine between training sessions. However, this possibility is mitigated by the fact that during the first 5 min of the first day of extinction, the number of USVs was roughly equal to that found at the end of acquisition, suggesting that USVs in the first 5 min of the first extinction day represent an anticipatory state based on prior experience. Likewise, in the sucrose experiment, there was no change in the total number of USVs throughout acquisition, and the increase in USVs was constrained largely to the first 5 min of testing. In other words, the majority of the USVs emitted appear to have shifted toward the beginning of the session as conditioning proceeded, perhaps in anticipation of reward.
Some investigators have proposed the theoretical possibility that USVs are an artifactual by-product of intense locomotor activity (Blumberg, 1992; Thiessen et al., 1980) while other studies do not support this conclusion (Burgdorf et al., 2000; Burgdorf et al., 2001a; Knutson et al., 1998; Williams and Undieh, 2010) (for review see Knutson (Knutson et al., 2002)). Also, in the present study, the changes in locomotor activity throughout the study could not fully account for changes in USV production (Supplemental Fig. 2). We found no correlation between locomotor activity and the number of USVs emitted during any part of the experiment.
The production of USVs is closely linked to dopamine availability in the nucleus accumbens. Amphetamine injection into the nucleus accumbens elicits 50 kHz USVs (Burgdorf et al., 2001a; Thompson et al., 2006). Dopamine depletion in the striatum decreases 50 kHz frequency-modulated USVs induced by female odor in males (Ciucci et al., 2009), and by play or rewarding electrical brain stimulation (Burgdorf et al., 2007). Furthermore, dopamine antagonists decrease 50 kHz USVs induced by cocaine, while apomorphine (a D1/D2 agonist) elicits USVs (Williams and Undieh, 2010). The increase in 50 kHz USVs during cue- and cocaine-induced reinstatement in the cocaine experiment may therefore reflect increased dopamine levels in the nucleus accumbens (Kiyatkin and Stein, 1996; Neisewander et al., 1996; Phillips et al., 2003; Weiss et al., 2000). This suggests that the 50-kHz USVs may index the amount of endogenous dopamine release during behavioral tasks.
There are also some qualifications that need to be highlighted. First, the failure of the two types of rewards--cocaine and sucrose--to parallel each other in all behavioral measures suggests that the rewarding properties (presumably affective) of conventional rewards and drug rewards are somehow distinct for animals. In this context, it is especially interesting that with sucrose reward, no decrease in the number of USVs was observed in animals during extinction. It is possible that extinction from sucrose does not decrease the positive expectancy of reward as much as extinction from cocaine, perhaps because cocaine withdrawal induces a stronger negative affective state than does termination of access to sucrose. Alternatively, the two rewards may be qualitatively different at the affective level. Second, it should be noted that the methodology for the cocaine and sucrose experiments was not entirely parallel. We did not observe cue-induced reinstatement of active nose-poke responding in the sucrose experiment so it may be expected that USVs would not be elevated during this reinstatement, suggesting that the cue light did not become a salient stimulus when paired with sucrose in the present study. In addition, during the final reinstatement session, rats in the sucrose experiment were allowed to nose-poke for sucrose pellets while rats in the cocaine experiment were given a non-contingent cocaine injection but did not receive cocaine upon nose-poke responding. Therefore, we must constrain our interpretation to the idea that reinstatement by the unconditioned stimulus, whether contingent sucrose or non-contingent cocaine, elevated 50 kHz USVs. Finally, the response requirements for cocaine self-administration were increased (FR1→FR5) during the acquisition phases, while response requirements did not increase in the sucrose experiment (FR1 only). Although there is the potential for response requirements to alter USVs, we chose to balance the number of active nose-poke responses during the acquisition phases. Third, 50 kHz USVs have been classified into several distinct categories (Wright et al., 2010). Although these categories of USVs may represent specific affective states, that proposition remains to be tested. Clearly, additional studies are required to link these several different types of USVs with distinct behavioral or affective profiles. Here we focused on changes in the total number of 50 kHz USVs. Of note, repeated amphetamine injection leads to sensitization of “trill” USVs, but a similar pattern of sensitization is observed when assessing the total number of 50 kHz USVs (Ahrens et al., 2009). Still, further analyses of the different sub-types of 50 kHz USVs may allow for a greater understanding of affective states involved in self-administration and reinstatement.
In summary, changes in 50 kHz USVs may reflect the dynamic affective state of animals during self-administration of rewards including drugs of abuse. Such a direct non-invasive measure of affect in behaving animals may serve as a sensitive tool in bridging addiction-related behavioral outputs with their corresponding emotional/motivational states.
Research Highlights.
Browning et al.
Measured 50 kHz ultrasonic vocalizations (USVs) during cocaine self-administration
USVs are a spontaneous (untrained) index of affective responses to cocaine
50 kHz USVs are a dynamic index that quantifies reinforcing efficacy of cocaine
USVs may provide a gauge of underlying affective processes mediating addiction
Ability to measure affect in animals may link preclinical models to human addiction
Supplementary Material
Active and inactive nose-poke responding. Active nose-poke responding during (A) cocaine self-administration and (B) sucrose self-administration. Inactive nose-poke responding during (C) cocaine self-administration and (D) sucrose self-administration. Data are expressed as mean ± SEM. *Represents significant difference from Beginning Acquisition (ANOVA followed by LSD test,*P≤0.05). #Represents significant difference from prior extinction day (Paired t-test, # P≤0.05). $ Represents a trend towards significance (P=0.0759). Note: N=4 instead of 7 on the cocaine-reinstatement day due to equipment malfunction in which nose-pokes were not recorded.
Locomotor activity during cocaine and sucrose self-administration. Locomotor activity over 2 hr sessions in the (A) cocaine experiment and (B) sucrose experiment. Data are expressed as mean ± SEM of distance traveled (cm). *Represents significant difference from Beginning Acquisition (ANOVA followed by LSD test,*P≤0.05). # Represents significant difference from prior extinction day (Paired t-test, #P≤0.05). (C–F) Time course of locomotor activity during acquisition (C and D) and extinction and reinstatement (E and F) in the cocaine experiment (C and E) and the sucrose experiment (D and F). *Represents significant difference from Beginning Acquisition (ANOVA followed by LSD test,*P≤0.05). # Represents significant difference from prior extinction day (ANOVA followed by LSD test, #P≤0.05).
Acknowledgements
The authors thank Paolo Iacobucci, Gemaine Stark, Ryan P. Todd, and Stephen Houmes for technical assistance and Jenny Baylon for manuscript assistance. This work was supported by the Seattle Chapter of the Achievement Rewards for College Scientists (ARCS) Foundation (JRB) and NIH grant DA023202.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
There are no conflicts of interest to declare.
References
- Ahrens AM, Ma ST, Maier EY, Duvauchelle CL, Schallert T. Repeated intravenous amphetamine exposure: rapid and persistent sensitization of 50-kHz ultrasonic trill calls in rats. Behav Brain Res. 2009;197:205–209. doi: 10.1016/j.bbr.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Root DH, Ma S, Jha S, Megehee L, Pawlak AP, West MO. Dose-dependent differences in short ultrasonic vocalizations emitted by rats during cocaine self-administration. Psychopharmacology (Berl) 2010;211:435–442. doi: 10.1007/s00213-010-1913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros HM, Miczek KA. Withdrawal from oral cocaine in rate: ultrasonic vocalizations and tactile startle. Psychopharmacology (Berl) 1996;125:379–384. doi: 10.1007/BF02246021. [DOI] [PubMed] [Google Scholar]
- Bialy M, Rydz M, Kaczmarek L. Precontact 50-kHz vocalizations in male rats during acquisition of sexual experience. Behav Neurosci. 2000;114:983–990. doi: 10.1037//0735-7044.114.5.983. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Agullana R, Weiss SM. Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiol Behav. 1991;50:967–972. doi: 10.1016/0031-9384(91)90423-l. [DOI] [PubMed] [Google Scholar]
- Blumberg MS. Rodent ultrasonic short calls: locomotion, biomechanics, and communication. J Comp Psychol. 1992;106:360–365. doi: 10.1037/0735-7036.106.4.360. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM. Pharmacological and behavioral characteristics of 22 kHz alarm calls in rats. Neurosci Biobehav Rev. 2001;25:611–617. doi: 10.1016/s0149-7634(01)00058-6. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, editor. Handbook of Mammalian Vocalization: An Integrative Neuroscience Approach. Oxford: Academic Press; 2010. [Google Scholar]
- Brudzynski SM, Bihari F, Ociepa D, Fu XW. Analysis of 22 kHz ultrasonic vocalization in laboratory rats: long and short calls. Physiol Behav. 1993;54:215–221. doi: 10.1016/0031-9384(93)90102-l. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Ociepa D, Bihari F. Comparison between cholinergically and naturally induced ultrasonic vocalization in the rat. J Psychiatry Neurosci. 1991;16:221–226. [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J. Anticipation of rewarding electrical brain stimulation evokes ultrasonic vocalization in rats. Behav Neurosci. 2000;114:320–327. [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J, Ikemoto S. Nucleus accumbens amphetamine microinjections unconditionally elicit 50-kHz ultrasonic vocalizations in rats. Behav Neurosci. 2001a;115:940–944. doi: 10.1037//0735-7044.115.4.940. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J, Shippenberg TS. Evaluation of rat ultrasonic vocalizations as predictors of the conditioned aversive effects of drugs. Psychopharmacology (Berl) 2001b;155:35–42. doi: 10.1007/s002130100685. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Kroes RA, Moskal JR, Pfaus JG, Brudzynski SM, Panksepp J. Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: Behavioral concomitants, relationship to reward, and self-administration of playback. J Comp Psychol. 2008;122:357–367. doi: 10.1037/a0012889. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Wood PL, Kroes RA, Moskal JR, Panksepp J. Neurobiology of 50-kHz ultrasonic vocalizations in rats: electrode mapping, lesion, and pharmacology studies. Behav Brain Res. 2007;182:274–283. doi: 10.1016/j.bbr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Ciucci MR, Ahrens AM, Ma ST, Kane JR, Windham EB, Woodlee MT, Schallert T. Reduction of dopamine synaptic activity: degradation of 50-kHz ultrasonic vocalization in rats. Behav Neurosci. 2009;123:328–336. doi: 10.1037/a0014593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Francis RL. 22-kHz calls by isolated rats. Nature. 1977;265:236–238. doi: 10.1038/265236a0. [DOI] [PubMed] [Google Scholar]
- Hodgins DC, el-Guebaly N, Armstrong S. Prospective and retrospective reports of mood states before relapse to substance use. J Consult Clin Psychol. 1995;63:400–407. doi: 10.1037//0022-006x.63.3.400. [DOI] [PubMed] [Google Scholar]
- Kassel JD, editor. Substance abuse and emotion. Washington, D.C.: American Psychological Association; 2010. [Google Scholar]
- Kiyatkin EA, Stein EA. Conditioned changes in nucleus accumbens dopamine signal established by intravenous cocaine in rats. Neurosci Lett. 1996;211:73–76. doi: 10.1016/0304-3940(96)12731-2. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. J Comp Psychol. 1998;112:65–73. doi: 10.1037/0735-7036.112.1.65. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. High-frequency ultrasonic vocalizations index conditioned pharmacological reward in rats. Physiol Behav. 1999;66:639–643. doi: 10.1016/s0031-9384(98)00337-0. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull. 2002;128:961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- Koob GF. Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry. 2009;42 Suppl 1:S32–S41. doi: 10.1055/s-0029-1216356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert NM, McLeod M, Schenk S. Subjective responses to initial experience with cocaine: an exploration of the incentive-sensitization theory of drug abuse. Addiction. 2006;101:713–725. doi: 10.1111/j.1360-0443.2006.01408.x. [DOI] [PubMed] [Google Scholar]
- Ma ST, Maier EY, Ahrens AM, Schallert T, Duvauchelle CL. Repeated intravenous cocaine experience: development and escalation of pre-drug anticipatory 50-kHz ultrasonic vocalizations in rats. Behav Brain Res. 2010;212:109–114. doi: 10.1016/j.bbr.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier EY, Ahrens AM, Ma ST, Schallert T, Duvauchelle CL. Cocaine deprivation effect: cue abstinence over weekends boosts anticipatory 50-kHz ultrasonic vocalizations in rats. Behav Brain Res. 2010a;214:75–79. doi: 10.1016/j.bbr.2010.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier EY, Ma ST, Ahrens A, Schallert TJ, Duvauchelle CL. Assessment of ultrasonic vocalizations during drug self-administration in rats. J Vis Exp. 2010b doi: 10.3791/2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Tornatzky W, Vivian JA, editors. Ethology and neuropharmacology: Rodent ultrasounds. Basel: Birkhauser Verlag.; 2002. [Google Scholar]
- Mu P, Fuchs T, Saal DB, Sorg BA, Dong Y, Panksepp J. Repeated cocaine exposure induces sensitization of ultrasonic vocalization in rats. Neurosci Lett. 2009;453:31–35. doi: 10.1016/j.neulet.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler NH, Covington HE, 3rd, Miczek KA. Repeated self-administered cocaine "binges" in rats: effects on cocaine intake and withdrawal. Psychopharmacology (Berl) 2001;154:292–300. doi: 10.1007/s002130000646. [DOI] [PubMed] [Google Scholar]
- Mutschler NH, Miczek KA. Withdrawal from a self-administered or non-contingent cocaine binge: differences in ultrasonic distress vocalizations in rats. Psychopharmacology (Berl) 1998a;136:402–408. doi: 10.1007/s002130050584. [DOI] [PubMed] [Google Scholar]
- Mutschler NH, Miczek KA. Withdrawal from i.v. cocaine "binges" in rats: ultrasonic distress calls and startle. Psychopharmacology (Berl) 1998b;135:161–168. doi: 10.1007/s002130050497. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, O'Dell LE, Tran-Nguyen LT, Castaneda E, Fuchs RA. Dopamine overflow in the nucleus accumbens during extinction and reinstatement of cocaine self-administration behavior. Neuropsychopharmacology. 1996;15:506–514. doi: 10.1016/S0893-133X(96)00097-8. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Knutson B, Burgdorf J. The role of brain emotional systems in addictions: a neuro-evolutionary perspective and new 'self-report' animal model. Addiction. 2002;97:459–469. doi: 10.1046/j.1360-0443.2002.00025.x. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Moskal J. Dopamine and Seeking: Subcortical Reward Systems and Appetitive Urges. In: Elliot AJ, editor. Handbook of Approach and Avoidance Motivation. New York: Taylor & Francis Group, LLC; 2008. pp. 67–87. [Google Scholar]
- Panksepp J, Nocjar C, Burgdorf J, Panksepp JB, Huber R. The role of emotional systems in addiction: a neuroethological perspective. Nebr Symp Motiv. 2004;50:85–126. [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Sales GD. Ultrasound and aggressive behaviour in rats and other small mammals. Anim Behav. 1972a;20:88–100. doi: 10.1016/s0003-3472(72)80177-5. [DOI] [PubMed] [Google Scholar]
- Sales GD. Ultrasound and mating behaviour in rodents with some observations on other behavioural situations. Journal of Zoology. 1972b;168:149–164. [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiessen DD, Kittrell EM, Graham JM. Biomechanics of ultrasound emissions in the Mongolian gerbil, Meriones unguiculatus. Behav Neural Biol. 1980;29:415–429. doi: 10.1016/s0163-1047(80)92597-2. [DOI] [PubMed] [Google Scholar]
- Thomas DA, Takahashi LK, Barfield RJ. Analysis of ultrasonic vocalizations emitted by intruders during aggressive encounters among rats (Rattus norvegicus) J Comp Psychol. 1983;97:201–206. [PubMed] [Google Scholar]
- Thompson B, Leonard KC, Brudzynski SM. Amphetamine-induced 50 kHz calls from rat nucleus accumbens: a quantitative mapping study and acoustic analysis. Behav Brain Res. 2006;168:64–73. doi: 10.1016/j.bbr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. Imagery and smoking urges: the manipulation of affective content. Addict Behav. 1990;15:531–539. doi: 10.1016/0306-4603(90)90053-z. [DOI] [PubMed] [Google Scholar]
- Tonoue T, Ashida Y, Makino H, Hata H. Inhibition of shock-elicited ultrasonic vocalization by opioid peptides in the rat: a psychotropic effect. Psychoneuroendocrinology. 1986;11:177–184. doi: 10.1016/0306-4530(86)90052-1. [DOI] [PubMed] [Google Scholar]
- Van der Poel AM, Miczek KA. Long ultrasonic calls in male rats followning mating, defeat and aversive stimulation: Frequency modulation and bout structure. Behaviour. 1991;119:127–142. [Google Scholar]
- Vivian JA, Miczek KA. Effects of mu and delta opioid agonists and antagonists on affective vocal and reflexive pain responses during social stress in rats. Psychopharmacology (Berl) 1998;139:364–375. doi: 10.1007/s002130050727. [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci U S A. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SN, Undieh AS. Brain-derived neurotrophic factor signaling modulates cocaine induction of reward-associated ultrasonic vocalization in rats. J Pharmacol Exp Ther. 2010;332:463–468. doi: 10.1124/jpet.109.158535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohr M, Schwarting RK. Ultrasonic communication in rats: can playback of 50-kHz calls induce approach behavior? PLoS One. 2007;2:e1365. doi: 10.1371/journal.pone.0001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JM, Gourdon JC, Clarke PB. Identification of multiple call categories within the rich repertoire of adult rat 50-kHz ultrasonic vocalizations: effects of amphetamine and social context. Psychopharmacology (Berl) 2010;211:1–13. doi: 10.1007/s00213-010-1859-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Active and inactive nose-poke responding. Active nose-poke responding during (A) cocaine self-administration and (B) sucrose self-administration. Inactive nose-poke responding during (C) cocaine self-administration and (D) sucrose self-administration. Data are expressed as mean ± SEM. *Represents significant difference from Beginning Acquisition (ANOVA followed by LSD test,*P≤0.05). #Represents significant difference from prior extinction day (Paired t-test, # P≤0.05). $ Represents a trend towards significance (P=0.0759). Note: N=4 instead of 7 on the cocaine-reinstatement day due to equipment malfunction in which nose-pokes were not recorded.
Locomotor activity during cocaine and sucrose self-administration. Locomotor activity over 2 hr sessions in the (A) cocaine experiment and (B) sucrose experiment. Data are expressed as mean ± SEM of distance traveled (cm). *Represents significant difference from Beginning Acquisition (ANOVA followed by LSD test,*P≤0.05). # Represents significant difference from prior extinction day (Paired t-test, #P≤0.05). (C–F) Time course of locomotor activity during acquisition (C and D) and extinction and reinstatement (E and F) in the cocaine experiment (C and E) and the sucrose experiment (D and F). *Represents significant difference from Beginning Acquisition (ANOVA followed by LSD test,*P≤0.05). # Represents significant difference from prior extinction day (ANOVA followed by LSD test, #P≤0.05).