Abstract
Background and Purpose
Treatment of ischemic stroke by activation of endogenous plasminogen using tissue plasminogen activator (tPA) is limited by bleeding side effects. In mice, treatment of experimental ischemic stroke with activated protein C (APC) improves outcomes; however, APC also has bleeding side effects. In contrast, activation of endogenous protein C using thrombin mutant W215A/E217A (WE) is antithrombotic without hemostasis impairment in primates. Therefore, we investigated the outcome of WE-treated experimental ischemic stroke in mice.
Methods
The middle cerebral artery (MCA) was occluded with a filament for 60 minutes to induce ischemic stroke. Vehicle, recombinant WE, or tPA was administered during MCA occlusion (MCAO) or 2 hours post-MCAO. Neurological performance was scored daily. Intracranial bleeding and cerebral infarct size, defined by 2,3,5-triphenyltetrazolium chloride (TTC) exclusion, were determined on autopsy. Hemostasis was evaluated using tail bleeding tests.
Results
WE improved neurological performance scores, increased LDF-monitored post-MCAO reperfusion of the parietal cortex, and reduced TTC-defined cerebral infarct size versus vehicle controls. However, unlike tPA, WE did not increase tail bleeding or intracranial hemorrhage.
Conclusions
WE treatment is neuroprotective without hemostasis impairment in experimental acute ischemic stroke in mice; thus, may provide an alternative to tPA for stroke treatment.
Keywords: Ischemic Stroke, Thrombin, Thrombolysis, Antithrombotics
Introduction
Early thrombolysis with recombinant human tissue plasminogen activator (tPA) is currently the only FDA-approved causal treatment for acute ischemic stroke. Through enzymatic induction of endogenous plasmin-catalyzed fibrinolysis, tPA treatment promotes reperfusion and improves long term clinical outcomes.1 However, tPA increases the incidence of intracerebral hemorrhage and may have neurotoxic effects in experimental stroke.2-4
Experimental data suggest that the use of recombinant activated protein C (APC), which inhibits activated factors V and VIII, improves stroke outcomes.5,6 Beyond its anticoagulant activity, APC therapy is vasculoprotective and neuroprotective, and helps to maintain the integrity of the blood-brain barrier;7-10 however, systemic APC administration can also impair hemostasis.6,11 Potentially safer alternatives to recombinant APC administration include the use of innovative APC mutants with reduced anticoagulant activity,11 or the direct activation of endogenous protein C by thrombin in vivo.
The essential serine protease thrombin catalyzes site-specific procoagulant and anticoagulant events.12 Low doses of infused thrombin are antithrombotic through activation of endogenous protein C, however, thrombin also has concurrent prothrombotic effects.13 Alanine scanning studies identified several key residues involved in thrombin substrate specificity,14,15 and thrombin analogs with reduced procoagulant activity have been designed. The thrombin mutant W217A/E217A (WE) has significantly reduced catalytic activity toward fibrinogen and protease-activated receptors (PARs), but retains activity toward protein C in the presence of thrombomodulin.16 WE treatment is as antithrombotic as interventional doses of low-molecular weight heparin or APC but without significant hemostasis impairment in baboons.17,18
Based on the observed dissociation of antithrombotic and antihemostatic effects of WE in primates, we hypothesized that WE administration may provide a safe approach to early treatment of stroke. We therefore investigated the effect of WE administration in a murine middle cerebral artery occlusion (MCAO) model of acute ischemic stroke.
Materials and Methods
Mouse model of acute ischemic stroke
Animal experiments were approved by the Institutional Animal Care and Use Committee. Three month-old male C57Bl/6 mice (Charles River Laboratories; Madison, WI) weighing 21 to 27 g were anesthetized with 5.0% isoflurane. Anesthesia was maintained with 1.0-1.5% isoflurane in 37% oxygen. Rectal temperature was maintained at 37.0±0.5°C. A laser Doppler flowmetry (LDF) probe (Moor Instruments Ltd; Oxford, UK) was secured over the right parietal bone to monitor focal changes of cortical perfusion. Ischemic stroke was induced by surgical deployment of a silicone-coated (Xantopren Comfort Light and hardener mix, Heraeus; Hanau, Germany), heat-blunted 6-0 nylon filament through the right external carotid artery to achieve middle cerebral artery occlusion (MCAO), as described.19 The beginning of focal cerebral ischemia was defined as a decrease in LDF signal to less than 20% of the pre-MCAO baseline. After 60 minutes, the filament was removed, and reperfusion was monitored with LDF. In selected experiments, extensive perfusion deficit of the affected hemisphere during MCAO was verified with optical microangiography (OMAG) as described.20
Treatment of stroke
Recombinant human WE was prepared by site directed mutagenesis, as described.16 Recombinant human tPA, Alteplase (Activase®), was purchased from Genentech (South San Francisco, CA). Treatments were randomly assigned and administered either during cerebral ischemia (before filament removal) or during reperfusion, two hours after removal of the filament from the MCA. For treatment during occlusion, single bolus vehicle (physiological saline with 2.5% dextrose), WE (25 μg/kg), or tPA (2.5 mg/kg) were administered in 185 μL volume through the isolated right femoral vein after 15 minutes of sustained ischemia as defined by LDF. For treatments administered 2 hours post-MCAO, animals received a 45 minute intravenous infusion of 185 μL of vehicle, WE (25 μg/kg) or tPA (10 mg/kg). The bolus and infusion doses of tPA and WE were selected based on previous studies in mice and primates.3,17,18
Neurological evaluation
Following MCAO and treatment administration, mice were allowed to recover, and neurological deficit was assessed the following day. Neurological performance scores were assessed daily using a modified five point Hara scale (0: no neurological signs, 1: flexion of the contralateral torso, 2: circling to the contralateral side but normal posture at rest, 3: leaning to the contralateral side at rest, 4: no spontaneous motor activity, 5: death).21 Scores were ranked from 0 to 5 at intervals of 0.5; animals demonstrating neurological signs between two categories were given an intermediate score.
Brain analysis
After neurological scoring, animals were euthanized, and brains were harvested and sectioned into 2 mm coronal sections. Sections were inspected for the presence of macroscopic intracranial hemorrhage, then stained with 1.2% 2,3,5-triphenyltetrazolium chloride (TTC) for 20 minutes (37°C), fixed with 10% formalin, and evaluated for infarct size by morphometric analysis (MCID software, InterFocus Imaging Ltd; Linton, Cambridge, UK) as described.22 The TTC exclusion area of the ipsilateral (ischemic) hemisphere was measured, and the total TTC exclusion volume was expressed as a percentage of the contralateral (non-ischemic) hemisphere to represent the TTC-defined infarct volume. Edema was indirectly calculated from morphometric data as the percent increase in size of the ipsilateral hemisphere over the contralateral hemisphere.
Evaluation of hemostasis
Naive mice (C57Bl/6, 19 to 34 g) were used for hemostasis assessment using the tail transection test as described.23 Anesthetized mice were administered 185 μL of vehicle (saline), WE (25 μg/kg), or tPA (2.5 mg/kg) intravenously. Tail transection (at 1.5 mm tail diameter) was performed 15 minutes after the end of vehicle, WE, or tPA administration, and the bleeding time (visual observation) and total volume of blood loss were recorded.
For activated partial thromboplastin time (APTT) and thrombin time tests, mice were euthanized following administration of vehicle, WE, or tPA, and blood was drawn by cardiac puncture into 3.2% sodium citrate (9:1, vol:vol). Plasma was prepared by centrifugation of blood for 3 minutes at 15,700g. Clotting and clot lysis times were measured with a KC4 Coagulation Analyzer (Trinity Biotech; Bray, Co Wicklow, Ireland). APTT measurements were performed at 10±1 and 60±5 minutes after the blood draw using the APTT-ES kit from Helena Laboratories (Beaumont, TX). Thrombin time measurements were performed at 10.5±1 minutes after the blood draw, with bovine thrombin (Sigma-Aldrich; St. Louis, MO) added to plasma at a final concentration of 5 U/mL.
Statistical Analysis
Data are presented as mean±SEM. Statistical significance between means was determined by one-way ANOVA with the Tukey post hoc test (for 3 conditions) or the Student's t-test (for 2 conditions). Statistical significance for hemorrhage rates was determined by the Kruskal-Wallis test and for survival curves by the Log-rank test. Significance for all statistical tests required P<0.05.
Results
WE is neuroprotective in a mouse model of MCAO-induced ischemic stroke
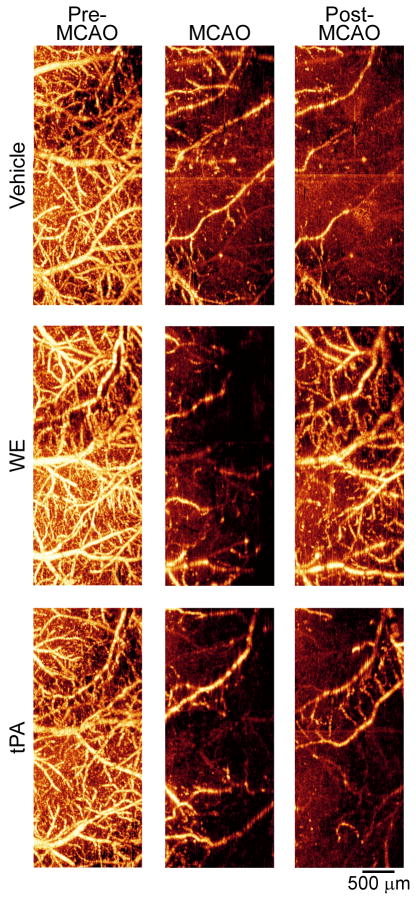
In order to visualize the extensive ischemia of the parietal region induced by MCAO, we performed OMAG imaging of animals that received a bolus of vehicle, WE (25 μg/kg), or tPA (2.5 mg/kg). Images obtained pre-, during and post-MCAO were consistent with LDF-probe data (Table 1), confirming that blood perfusion of the affected hemisphere was significantly decreased during MCAO (Figure 1).
Table 1. Parietal laser Doppler flowmetry (LDF) during and post-MCAO.
| Treatment | n | During MCAO, % baseline LDF | Post-MCAO, % baseline LDF |
|---|---|---|---|
| Vehicle | 5 | 8.7±1.6 | 35.4±13.0 |
| WE | 5 | 9.3±1.1 | 73.5±2.7* |
| tPA | 9 | 10.1±1.5 | 69.9±1.9* |
LDF was continuously recorded during cerebral ischemia and reperfusion. Start of MCAO was defined as stable signal for 5 minutes following decrease of pre-MCAO LDF to 20% or less of baseline. Reperfusion LDF values after 15 minutes of reperfusion following removal of the filament are shown. Values are mean±SEM.
P<0.05 versus vehicle.
Figure 1. Optical microangiography (OMAG) of the cerebral cortex verifies extensive cortical hypoperfusion during and after MCAO.
Transcranial non-invasive OMAG was used to image the brains of mice through intact skull before MCAO, during MCAO, and post-MCAO. Bright areas indicate perfused vessels (moving blood) and dark regions indicate absence or reduction of perfusion.
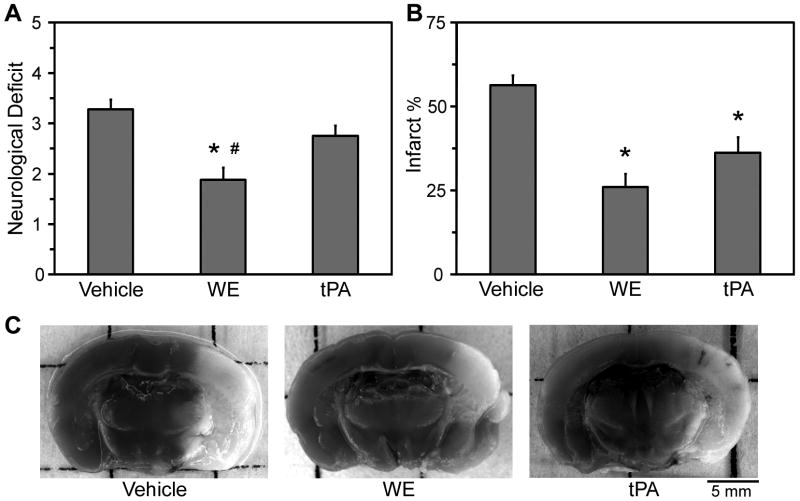
To evaluate the neurological outcomes of WE treatment of acute ischemic stroke, animals were administered vehicle, WE (25 μg/kg), or tPA (2.5 mg/kg) during cerebral ischemia. Performance scores on a modified Hara scale, assessed 24 hours after the MCAO procedure, were significantly better for WE-treated mice than for vehicle-treated or tPA-treated animals (Figure 2A).
Figure 2. WE treatment during MCAO improves neurological performance scores and reduces infarct size.
Mice were administered vehicle, WE (25 μg/kg), or tPA (2.5 mg/kg) during MCAO. A, Neurological deficits were scored 24 hours post-MCAO. Following scoring, autopsy was performed and brain sections were stained with TTC. B, Images of the sections were analyzed by morphometric analysis to determine the percentage of TTC-defined infarcted tissue. C, Representative images of brain sections show the presence of TTC-defined infarct (lighter areas) and viable tissue (darker areas). Values are mean±SEM, n=16-20. *P<0.05 versus vehicle treatment. #P<0.05 versus tPA.
WE reduces infarct size after cerebral ischemia
On morphometric analysis, the relative volume of TTC-defined infarction of the affected hemisphere was significantly smaller in both WE- and tPA-treated (26±4% and 36±5%, respectively) than in vehicle-treated (56±3%) mice, suggesting neuroprotection by both enzymes (Figure 2). No differences in the relative increase in size of the infarcted hemisphere (interpreted as edema) were observed between treatments (data not shown).
Effects of WE on post-MCAO cortical reperfusion
Infusion of tPA can support the breakdown of blood clots, increase reperfusion of ischemic regions, and lead to a reduction in infarct size in ischemic stroke.2 To determine whether the reduction in TTC-defined infarct size seen with WE treatment was also associated with an increase in LDF-defined reperfusion, we recorded cortical perfusion with LDF 15 minutes after removal of the intraluminal filament. Perfusion of the parietal cortex of both WE- and tPA-treated animals was restored to ∼70% of the baseline (pre-MCAO) LDF perfusion (Table 1). Post-MCAO reperfusion values were significantly lower in vehicle-treated mice averaging 35% of pre-MCAO baseline.
Post-MCAO WE treatment improves neurological outcome
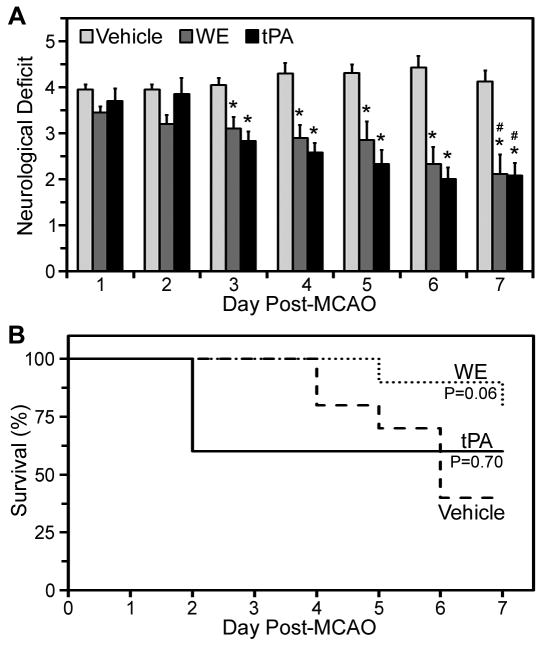
To evaluate the efficacy of WE administration post-MCAO, we infused vehicle, WE (25 μg/kg) or tPA (10 mg/kg) 2 hours after removal of the filament and monitored neurological deficits and survival for one week. On neurological assessment on days 3 to 7 after MCAO, performance scores in both WE- and tPA-treated mice were significantly better than in vehicle-treated mice (Figure 3A). Both WE- and tPA-treated mice showed significant improvement in neurologic scores over the 7 days of observation, while neurological scores in vehicle-treated mice were unchanged (Figure 3A). Survival curves of WE-treated animals trended toward an increase in survival beyond vehicle-treated animals, P=0.06 (Figure 3B). Therefore, administration of WE post-MCAO improves neurologic function over 7 days and may lead to an increased survival benefit.
Figure 3. WE treatment post-MCAO improves 7-day neurological scores.
Mice were infused with vehicle, WE (25 μg/kg), or tPA (10 mg/kg) 2 hours post-MCAO. A, Neurological deficits were scored daily for one week beginning at 24 hours post-MCAO (Day 1). Values are mean±SEM, n=10. *P<0.05 versus vehicle. #P<0.05 versus corresponding treatment at Day 1. B, Animal survival was tracked for one week following MCAO and treatment.
WE treatment does not impair hemostasis
Hemostasis impairment tests were used to assess treatment safety. Upon autopsy, brain sections were examined for the presence of macroscopic intracranial hemorrhage. No instance of visible hemorrhage was observed in vehicle-treated animals (0/12). Macroscopic hemorrhage was seen in 14% (2/14) of mice treated with WE, with no statistical difference from vehicle treatment. Macroscopic intracranial hemorrhage was observed in 44% (7/16) of mice that received tPA (P=0.006 versus vehicle).
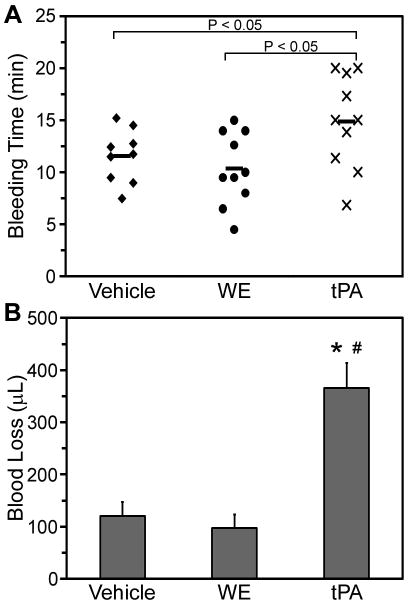
The effect of WE (25 μg/kg) on murine hemostasis was evaluated 15 minutes after administration. Tail bleeding times in WE-treated mice were comparable to vehicle-treated mice (Figure 4A). In contrast, tPA (2.5 mg/kg) significantly prolonged tail bleeding time in comparison to both vehicle and WE treatment. Further, the blood volume loss from tPA-treated mice was more than three-fold greater than the blood loss from vehicle- or WE-treated mice (Figure 4B).
Figure 4. WE treatment does not increase bleeding time and blood loss.
Anesthetized mice were administered a bolus of vehicle, WE (25 μg/kg), or tPA (2.5 mg/kg). Tails were transected where the diameter reached 1.5 mm and placed in a tube of water. The time until cessation of bleeding (A) and total blood loss volume (B) were recorded. Mean bleeding times are indicated by the bold horizontal lines in A. Values are mean±SEM. *P<0.05 versus vehicle. #P<0.05 versus WE.
No significant prolongation of APTT was observed for WE- or tPA-treated animals (Table 2), although a transient increase of APTT was observed following the administration of 10-fold higher doses of WE (data not shown), consistent with anticoagulant concentrations of endogenous APC in the circulation as seen in primates following WE administration.17 To examine the effects of WE and tPA on fibrinolysis, the plasma clot lysis time was recorded following APTT measurements. In plasma from tPA-treated mice, APTT clots lysed in 82.4±3.1 seconds, while no lysis was observed over a 240 second period of observation with vehicle or WE treatment. No differences in plasma thrombin times were observed in vehicle-, WE-, or tPA-treated animals (Table 2). These data suggest that administration of WE did not significantly interfere with the hemostasis of mice.
Table 2. Plasma APTT and thrombin time measurements following administration of vehicle, WE or tPA.
| Treatment | APTT | Thrombin time |
|---|---|---|
| Vehicle | 25.2±0.9 | 13.6±0.5 |
| WE | 26.2±1.1 | 14.7±0.6 |
| tPA | 27.8±0.8 | 13.3±0.4 |
Anesthetized mice were administered a bolus of vehicle, WE (25 μg/kg), or tPA (2.5 mg/kg). Fifteen minutes after administration, blood was drawn into sodium citrate and plasma prepared by centrifugation. Plasma APTT and thrombin times were measured at 10.0±1.0 and 10.5±1.0 minutes, respectively, after the blood draw. Values are mean±SEM from 5-6 experiments.
Discussion
We evaluated the efficacy and safety of WE treatment of MCAO-induced ischemic stroke in mice. Our data show that WE administration during MCAO improved neurological outcomes and reduced TTC-defined infarct size 24 hours after induction of ischemic stroke, with an efficacy that was comparable to tPA. Moreover, WE treatment 2 hours after MCAO also improved neurological performance over one week of observation.
Our information about the pathomechanism of progressive cerebral infarctions and ischemic stroke induced by surgical placement of the filament into the MCA in our model remains limited. The reduced LDF post-MCAO reperfusion in vehicle-treated mice could have various explanations, ranging from distal plugs due to denudation of the endothelium during introduction of the filament, vasospasm, or progressive distal thrombosis due to ischemic endothelial injury. As measured by LDF, significant reperfusion benefit achieved by both a thrombolytic (tPA) and an antithrombotic (WE) agent is suggestive of the formation of distal thrombotic occlusions during the development of progressive cerebral infarction. However, efficacy of a treatment in this model may be unrelated to antithrombotic activity. While the enzyme WE is antithrombotic in animal models,17,18 its molecular mechanisms of action in vivo are still not fully understood. The two point mutations, W215A and E217A, stabilize the enzyme in the inactive E* form of thrombin.24 Enzymatic activity is restored in the presence of thrombomodulin, and, as protein C is a natural substrate for the complex, the mutant effectively generates APC, in vitro as well as in vivo.16,17,25 In addition to anticoagulant properties, APC is directly cytoprotective, exerting neuroprotective effects in experimental ischemic stroke.5,7-9 The neurological outcome benefit observed with WE treatment of stroke may have resulted from neuroprotective effects of the intravascular generation of endogenous APC.
While systemic administration of APC is neuroprotective in this model, circulating APC can disrupt hemostasis.6,11 As an alternative to using the native enzyme, APC variants with reduced anticoagulant activity have been engineered to exploit the antiapoptotic activity of the enzyme. In a mouse model of ischemic stroke, variant 3K3A-APC, with reduced anticoagulant activity (∼90% reduction from wildtype), confers a comparable degree of neuroprotection to wildtype APC, without bleeding risks.11,26 Therefore, in addition to in vivo activation of protein C by thombin mutants, APC variants may provide a promising alternative to wildtype APC for treatment of ischemic stroke utilizing the protein C pathway.
Antithrombotic agents have proven effective at reducing infarct size and improving neurological outcomes. However, the associated bleeding risks can outweigh the benefits. Balancing the antithrombotic and neuroprotective benefits while minimizing the antihemostatic risks remains the critical hurdle in acute ischemic stroke treatment. The ability of WE to act safely with efficacy that may be comparable to tPA, makes it a promising candidate for the treatment of ischemic stroke. Further studies are needed to optimize the methods, duration, dosing and timing of WE administration to improve the long term outcomes in various models of stroke that assess both antithrombotic and neuroprotective activities of the treatment.
Acknowledgments
Sources of Funding: This work was supported in part by NIH grants EB009682 (R.K.W.), HL093140 (R.K.W., A.G.), HL049413, HL058141, HL073813 (E.D.C.), R01HL101972 (O.J.T.M., A.G.), HL095315 (A.G., E.I.T.), a Collins Medical Trust award (O.J.T.M., A.G.), American Heart Association awards 09PRE2230117 (M.A.B.), 0855733G (R.K.W.), 09GRNT2150003 (O.J.T.M.), 0850056Z (A.G.), and an unrestricted grant from Bayer Schering Pharma (A.G., E.I.T.). M.A.B. is a Whitaker and ARCS scholar.
Footnotes
Conflicts of Interest/Disclosures: A.G., E.I.T., and Oregon Health & Science University (OHSU) have a significant financial interest in ARONORA, LLC, a company that may have a commercial interest in the result of this research. This potential conflict of interest has been reviewed and managed by the OHSU Conflict of Interest in Research Committee.
References
- 1.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, Boysen G, Bluhmki E, Hoxter G, Mahagne MH, Hennerici M. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS) JAMA. 1995;274:1017–1025. [PubMed] [Google Scholar]
- 3.Kilic E, Hermann DM, Hossmann KA. Recombinant tissue plasminogen activator reduces infarct size after reversible thread occlusion of middle cerebral artery in mice. Neuroreport. 1999;10:107–111. doi: 10.1097/00001756-199901180-00021. [DOI] [PubMed] [Google Scholar]
- 4.Lo EH, Broderick JP, Moskowitz MA. tPA and proteolysis in the neurovascular unit. Stroke. 2004;35:354–356. doi: 10.1161/01.STR.0000115164.80010.8A. [DOI] [PubMed] [Google Scholar]
- 5.Shibata M, Kumar SR, Amar A, Fernandez JA, Hofman F, Griffin JH, Zlokovic BV. Anti-inflammatory, antithrombotic, and neuroprotective effects of activated protein C in a murine model of focal ischemic stroke. Circulation. 2001;103:1799–1805. doi: 10.1161/01.cir.103.13.1799. [DOI] [PubMed] [Google Scholar]
- 6.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ., Jr Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 7.Cheng T, Liu D, Griffin JH, Fernandez JA, Castellino F, Rosen ED, Fukudome K, Zlokovic BV. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat Med. 2003;9:338–342. doi: 10.1038/nm826. [DOI] [PubMed] [Google Scholar]
- 8.Guo H, Liu D, Gelbard H, Cheng T, Insalaco R, Fernandez JA, Griffin JH, Zlokovic BV. Activated protein C prevents neuronal apoptosis via protease activated receptors 1 and 3. Neuron. 2004;41:563–572. doi: 10.1016/s0896-6273(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 9.Liu D, Cheng T, Guo H, Fernandez JA, Griffin JH, Song X, Zlokovic BV. Tissue plasminogen activator neurovascular toxicity is controlled by activated protein C. Nat Med. 2004;10:1379–1383. doi: 10.1038/nm1122. [DOI] [PubMed] [Google Scholar]
- 10.Cheng T, Petraglia AL, Li Z, Thiyagarajan M, Zhong Z, Wu Z, Liu D, Maggirwar SB, Deane R, Fernandez JA, LaRue B, Griffin JH, Chopp M, Zlokovic BV. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med. 2006;12:1278–1285. doi: 10.1038/nm1498. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Thiyagarajan M, Chow N, Singh I, Guo H, Davis TP, Zlokovic BV. Differential neuroprotection and risk for bleeding from activated protein C with varying degrees of anticoagulant activity. Stroke. 2009;40:1864–1869. doi: 10.1161/STROKEAHA.108.536680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Cera E. Thrombin as procoagulant and anticoagulant. J Thromb Haemost. 2007;5 1:196–202. doi: 10.1111/j.1538-7836.2007.02485.x. [DOI] [PubMed] [Google Scholar]
- 13.Hanson SR, Griffin JH, Harker LA, Kelly AB, Esmon CT, Gruber A. Antithrombotic effects of thrombin-induced activation of endogenous protein C in primates. J Clin Invest. 1993;92:2003–2012. doi: 10.1172/JCI116795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall SW, Nagashima M, Zhao L, Morser J, Leung LL. Thrombin interacts with thrombomodulin, protein C, and thrombin-activatable fibrinolysis inhibitor via specific and distinct domains. J Biol Chem. 1999;274:25510–25516. doi: 10.1074/jbc.274.36.25510. [DOI] [PubMed] [Google Scholar]
- 15.Tsiang M, Jain AK, Dunn KE, Rojas ME, Leung LL, Gibbs CS. Functional mapping of the surface residues of human thrombin. J Biol Chem. 1995;270:16854–16863. doi: 10.1074/jbc.270.28.16854. [DOI] [PubMed] [Google Scholar]
- 16.Cantwell AM, Di Cera E. Rational design of a potent anticoagulant thrombin. J Biol Chem. 2000;275:39827–39830. doi: 10.1074/jbc.C000751200. [DOI] [PubMed] [Google Scholar]
- 17.Gruber A, Cantwell AM, Di Cera E, Hanson SR. The thrombin mutant W215A/E217A shows safe and potent anticoagulant and antithrombotic effects in vivo. J Biol Chem. 2002;277:27581–27584. doi: 10.1074/jbc.C200237200. [DOI] [PubMed] [Google Scholar]
- 18.Gruber A, Marzec UM, Bush L, Di Cera E, Fernandez JA, Berny MA, Tucker EI, McCarty OJ, Griffin JH, Hanson SR. Relative antithrombotic and antihemostatic effects of protein C activator versus low-molecular-weight heparin in primates. Blood. 2007;109:3733–3740. doi: 10.1182/blood-2006-07-035147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, Pieper A, Wang ZQ, Dawson TM, Snyder SH, Dawson VL. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- 20.Wang RK, Jacques SL, Ma Z, Hurst S, Hanson SR, Gruber A. Three dimensional optical angiography. Opt Express. 2007;15:4083–4097. doi: 10.1364/oe.15.004083. [DOI] [PubMed] [Google Scholar]
- 21.Tabrizi P, Wang L, Seeds N, McComb JG, Yamada S, Griffin JH, Carmeliet P, Weiss MH, Zlokovic BV. Tissue plasminogen activator (tPA) deficiency exacerbates cerebrovascular fibrin deposition and brain injury in a murine stroke model: studies in tPA-deficient mice and wild-type mice on a matched genetic background. Arterioscler Thromb Vasc Biol. 1999;19:2801–2806. doi: 10.1161/01.atv.19.11.2801. [DOI] [PubMed] [Google Scholar]
- 22.Zeynalov E, Nemoto M, Hurn PD, Koehler RC, Bhardwaj A. Neuroprotective effect of selective kappa opioid receptor agonist is gender specific and linked to reduced neuronal nitric oxide. J Cereb Blood Flow Metab. 2006;26:414–420. doi: 10.1038/sj.jcbfm.9600196. [DOI] [PubMed] [Google Scholar]
- 23.Thomas SG, Calaminus SD, Auger JM, Watson SP, Machesky LM. Studies on the actin-binding protein HS1 in platelets. BMC Cell Biol. 2007;8:46. doi: 10.1186/1471-2121-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gandhi PS, Page MJ, Chen Z, Bush-Pelc L, Di Cera E. Mechanism of the anticoagulant activity of thrombin mutant W215A/E217A. J Biol Chem. 2009;284:24098–24105. doi: 10.1074/jbc.M109.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pineda AO, Chen ZW, Caccia S, Cantwell AM, Savvides SN, Waksman G, Mathews FS, Di Cera E. The anticoagulant thrombin mutant W215A/E217A has a collapsed primary specificity pocket. J Biol Chem. 2004;279:39824–39828. doi: 10.1074/jbc.M407272200. [DOI] [PubMed] [Google Scholar]
- 26.Guo H, Singh I, Wang Y, Deane R, Barrett T, Fernandez JA, Chow N, Griffin JH, Zlokovic BV. Neuroprotective activities of activated protein C mutant with reduced anticoagulant activity. Eur J Neurosci. 2009;29:1119–1130. doi: 10.1111/j.1460-9568.2009.06664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]