Abstract
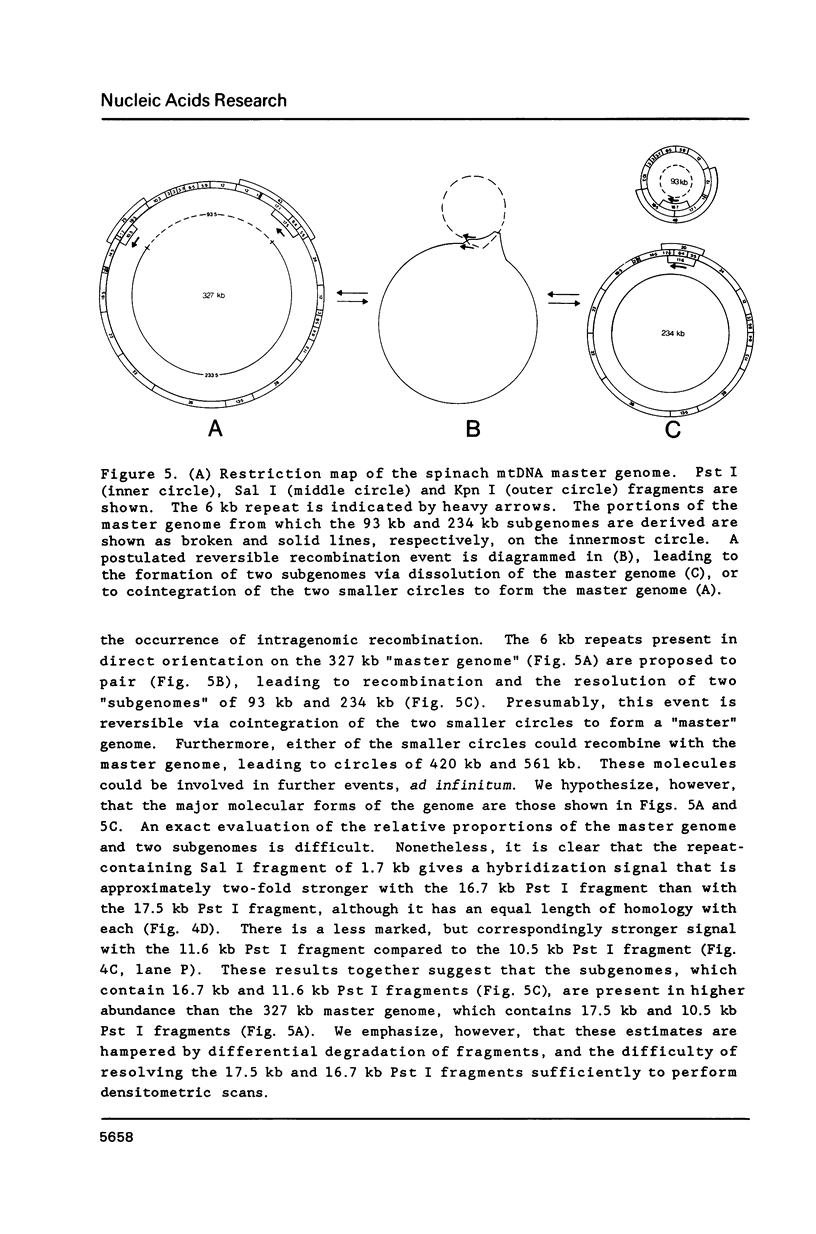
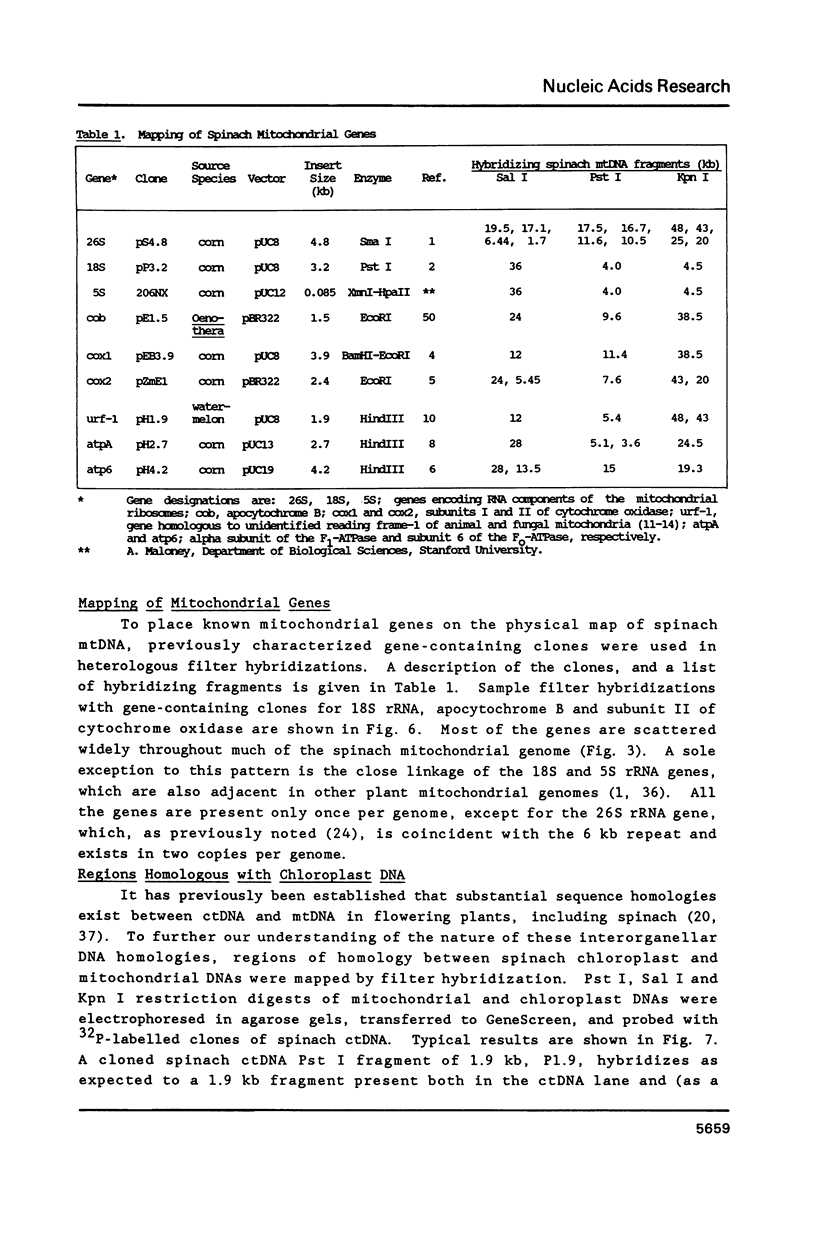
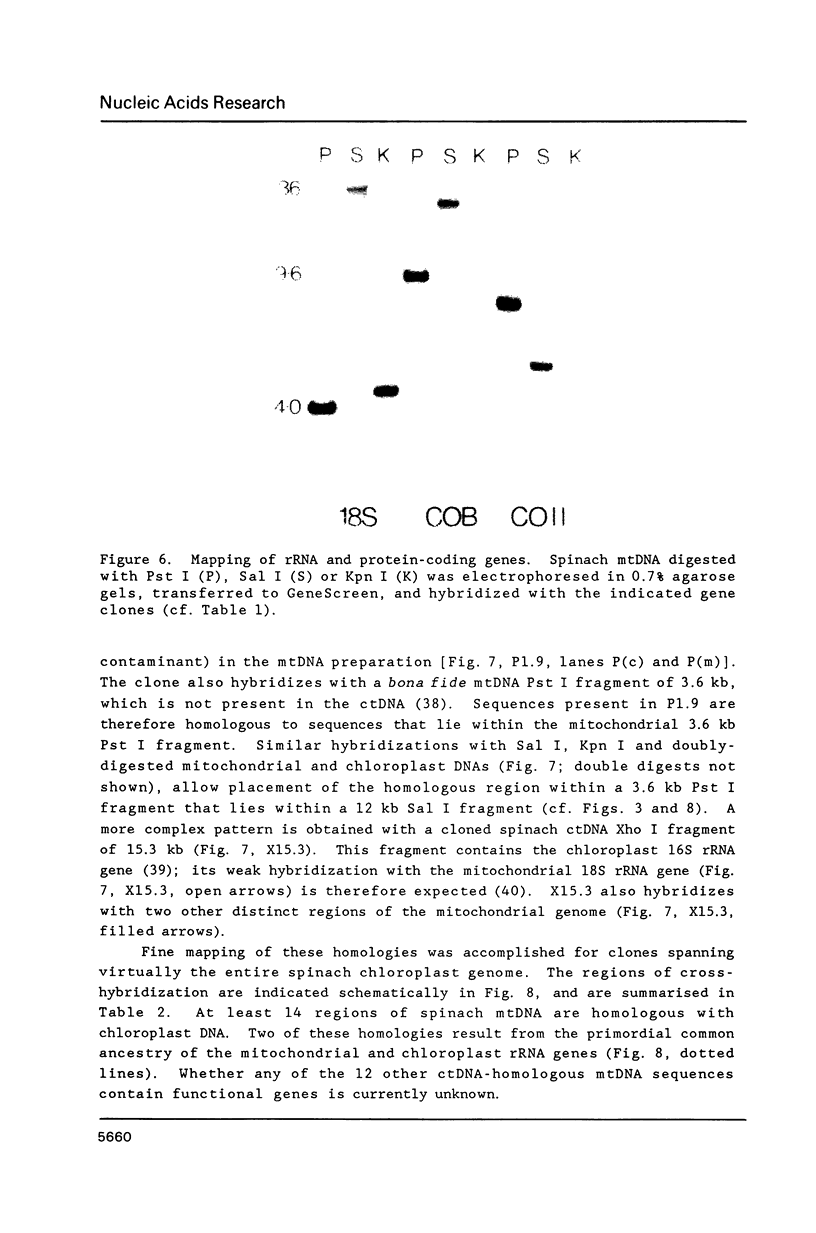
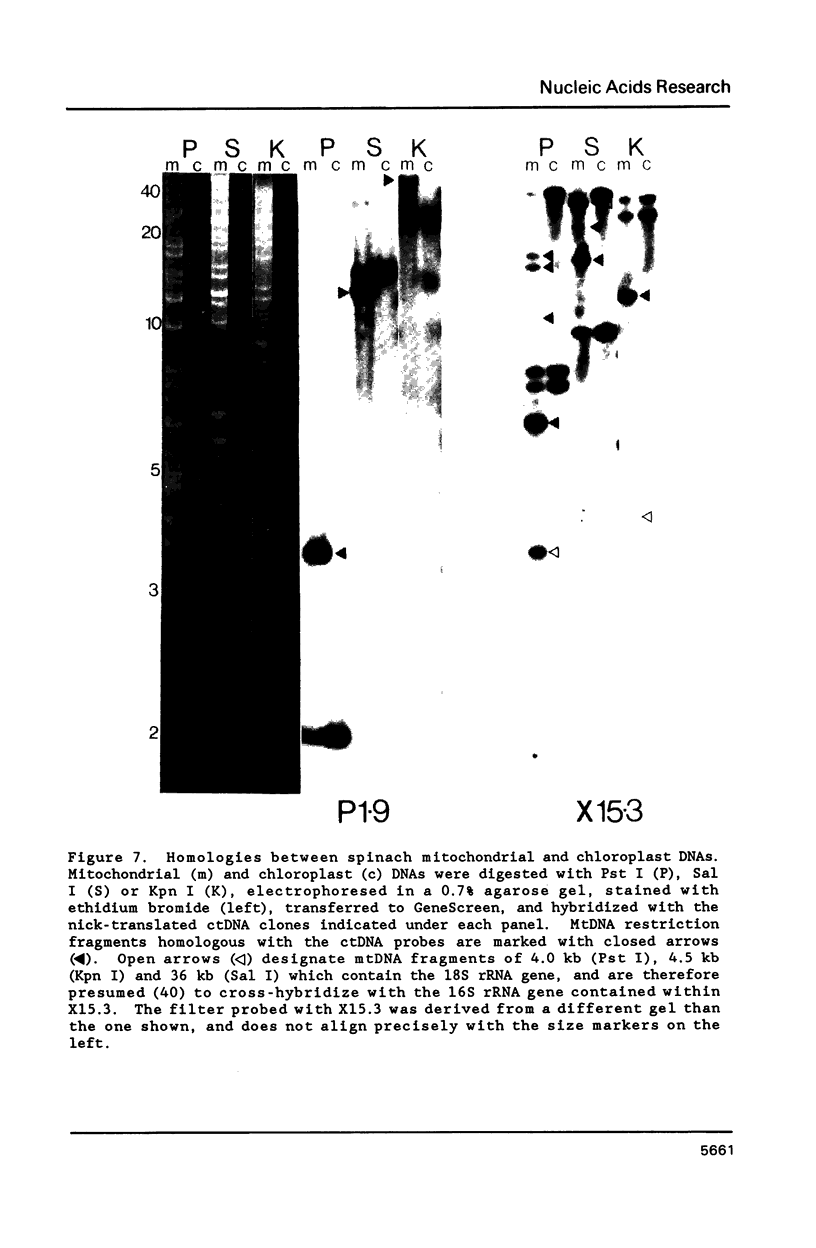
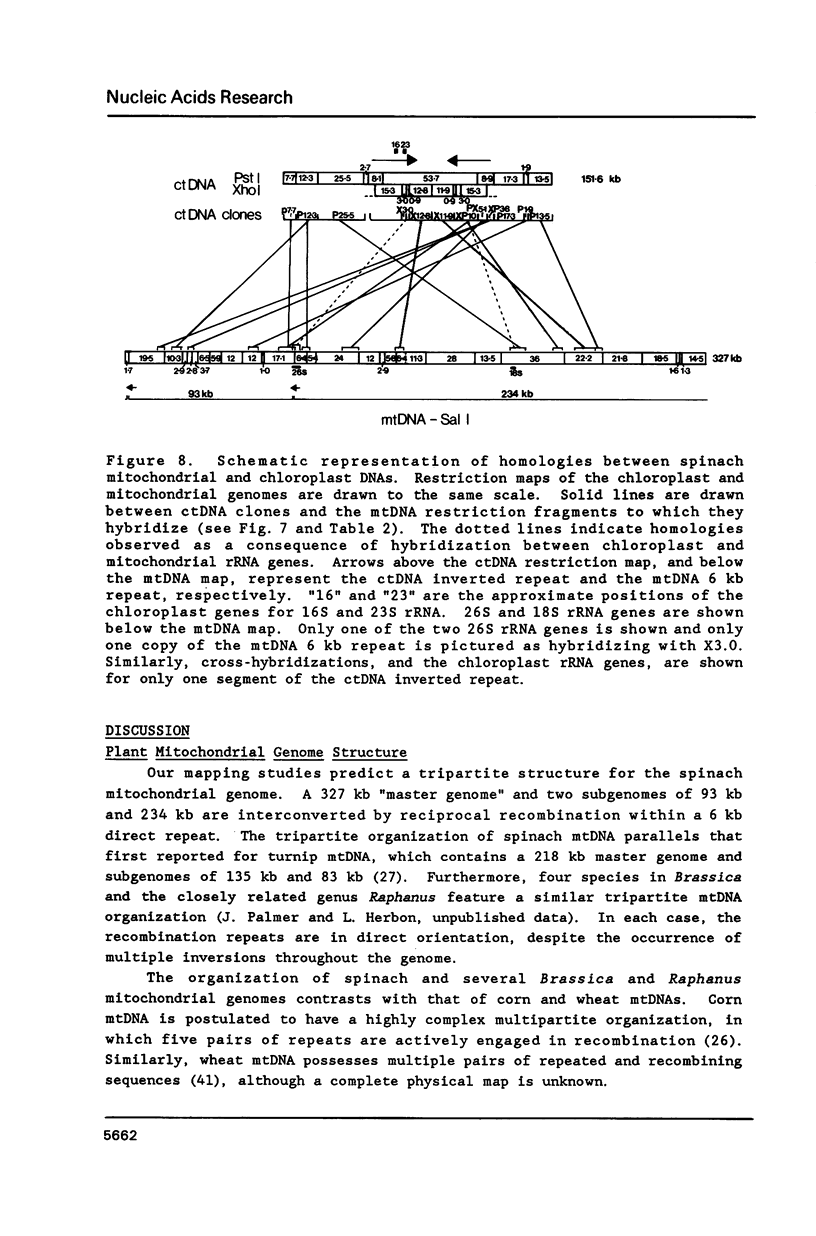
A complete physical map of the spinach mitochondrial genome has been established. The entire sequence content of 327 kilobase pairs (kb) is postulated to occur as a single circular molecule. Two directly repeated elements of approximately 6 kb, located on this "master chromosome", are proposed to participate in an intragenomic recombination event that reversibly generates two "subgenomic" circles of 93 kb and 234 kb. The positions of protein and ribosomal RNA-encoding genes, determined by heterologous filter hybridizations, are scattered throughout the genome, with duplicate 26S rRNA genes located partially or entirely within the 6 kb repeat elements. Filter hybridizations between spinach mitochondrial DNA and cloned segments of spinach chloroplast DNA reveal at least twelve dispersed regions of inter-organellar sequence homology.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Grivell L. A. The mitochondrial genome of yeast. Cell. 1978 Nov;15(3):705–723. doi: 10.1016/0092-8674(78)90257-x. [DOI] [PubMed] [Google Scholar]
- Braun C. J., Levings C. S. Nucleotide Sequence of the F(1)-ATPase alpha Subunit Gene from Maize Mitochondria. Plant Physiol. 1985 Oct;79(2):571–577. doi: 10.1104/pp.79.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. A., Davies R. W., Ray J. A., Waring R. B., Scazzocchio C. The mitochondnal genome of Aspergillus nidulans contains reading frames homologous to the human URFs 1 and 4. EMBO J. 1983;2(3):427–435. doi: 10.1002/j.1460-2075.1983.tb01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger G., Werner S. The mitochondrial URF1 gene in Neurospora crassa has an intron that contains a novel type of URF. J Mol Biol. 1985 Nov 20;186(2):231–242. doi: 10.1016/0022-2836(85)90100-7. [DOI] [PubMed] [Google Scholar]
- Chao S., Sederoff R. R., Levings C. S. Partial Sequence Analysis of the 5S to 18S rRNA Gene Region of the Maize Mitochondrial Genome. Plant Physiol. 1983 Jan;71(1):190–193. doi: 10.1104/pp.71.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J., MacNeil I. A., Domenico J., Matsuura E. T. Excision-amplification of mitochondrial DNA during senescence in Podospora anserina. DNA sequence analysis of three unique "plasmids". J Mol Biol. 1985 Oct 20;185(4):659–680. doi: 10.1016/0022-2836(85)90052-x. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Mendu N., Ginsburg H., Kridl J. C. Sequence analysis of the maize mitochondrial 26 S rRNA gene and flanking regions. Plasmid. 1984 Mar;11(2):141–150. doi: 10.1016/0147-619x(84)90019-2. [DOI] [PubMed] [Google Scholar]
- Dawson A. J., Jones V. P., Leaver C. J. The apocytochrome b gene in maize mitochondria does not contain introns and is preceded by a potential ribosome binding site. EMBO J. 1984 Sep;3(9):2107–2113. doi: 10.1002/j.1460-2075.1984.tb02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey R. E., Levings C. S., Timothy D. H. Nucleotide sequence of ATPase subunit 6 gene of maize mitochondria. Plant Physiol. 1985 Nov;79(3):914–919. doi: 10.1104/pp.79.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey R. E., Schuster A. M., Levings C. S., Timothy D. H. Nucleotide sequence of F(0)-ATPase proteolipid (subunit 9) gene of maize mitochondria. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1015–1019. doi: 10.1073/pnas.82.4.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. Promiscuous DNA--chloroplast genes inside plant mitochondria. Nature. 1982 Oct 21;299(5885):678–679. doi: 10.1038/299678a0. [DOI] [PubMed] [Google Scholar]
- Falconet D., Lejeune B., Quetier F., Gray M. W. Evidence for homologous recombination between repeated sequences containing 18S and 5S ribosomal RNA genes in wheat mitochondrial DNA. EMBO J. 1984 Feb;3(2):297–302. doi: 10.1002/j.1460-2075.1984.tb01799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenberg F. W., Mai U., Puppe C., Afifi M. S., Risse P., Grimm V., Harmann B., Herrmann G., Mondorf A. W. Urinary kidney-derived antigens determined by tests built on monoclonal antibodies: new markers for kidney damage. Uremia Invest. 1985;9(2):103–110. doi: 10.3109/08860228509088197. [DOI] [PubMed] [Google Scholar]
- Farrelly F., Butow R. A. Rearranged mitochondrial genes in the yeast nuclear genome. Nature. 1983 Jan 27;301(5898):296–301. doi: 10.1038/301296a0. [DOI] [PubMed] [Google Scholar]
- Fox T. D., Leaver C. J. The Zea mays mitochondrial gene coding cytochrome oxidase subunit II has an intervening sequence and does not contain TGA codons. Cell. 1981 Nov;26(3 Pt 1):315–323. doi: 10.1016/0092-8674(81)90200-2. [DOI] [PubMed] [Google Scholar]
- Gellissen G., Bradfield J. Y., White B. N., Wyatt G. R. Mitochondrial DNA sequences in the nuclear genome of a locust. Nature. 1983 Feb 17;301(5901):631–634. doi: 10.1038/301631a0. [DOI] [PubMed] [Google Scholar]
- Herrmann R. G., Whitfeld P. R., Bottomley W. Construction of a SalI/PstI restriction map of spinach chloroplast DNA using low-gelling-temperature-agarose electrophoresis. Gene. 1980 Jan;8(2):179–191. doi: 10.1016/0378-1119(80)90036-0. [DOI] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Isaac P. G., Jones V. P., Leaver C. J. The maize cytochrome c oxidase subunit I gene: sequence, expression and rearrangement in cytoplasmic male sterile plants. EMBO J. 1985 Jul;4(7):1617–1623. doi: 10.1002/j.1460-2075.1985.tb03828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs H. T., Posakony J. W., Grula J. W., Roberts J. W., Xin J. H., Britten R. J., Davidson E. H. Mitochondrial DNA sequences in the nuclear genome of Strongylocentrotus purpuratus. J Mol Biol. 1983 Apr 25;165(4):609–632. doi: 10.1016/s0022-2836(83)80270-8. [DOI] [PubMed] [Google Scholar]
- Kolodner R., Tewari K. K. Physicochemical characterization of mitochondrial DNA from pea leaves. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1830–1834. doi: 10.1073/pnas.69.7.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdale D. M., Hodge T. P., Fauron C. M. The physical map and organisation of the mitochondrial genome from the fertile cytoplasm of maize. Nucleic Acids Res. 1984 Dec 21;12(24):9249–9261. doi: 10.1093/nar/12.24.9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdale D. M., Thompson R. D., Hodge T. P. The integrated forms of the S1 and S2 DNA elements of maize male sterile mitochondrial DNA are flanked by a large repeated sequence. Nucleic Acids Res. 1981 Aug 11;9(15):3657–3669. doi: 10.1093/nar/9.15.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. D. Comparative organization of chloroplast genomes. Annu Rev Genet. 1985;19:325–354. doi: 10.1146/annurev.ge.19.120185.001545. [DOI] [PubMed] [Google Scholar]
- Palmer J. D. Physical and gene mapping of chloroplast DNA from Atriplex triangularis and Cucumis sativa. Nucleic Acids Res. 1982 Mar 11;10(5):1593–1605. doi: 10.1093/nar/10.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. D., Thompson W. F. Clone banks of the mung bean, pea and spinach chloroplast genomes. Gene. 1981 Oct;15(1):21–26. doi: 10.1016/0378-1119(81)90100-1. [DOI] [PubMed] [Google Scholar]
- Parks T. D., Dougherty W. G., Levings C. S., Timothy D. H. Identification of two methionine transfer RNA genes in the maize mitochondrial genome. Plant Physiol. 1984 Dec;76(4):1079–1082. doi: 10.1104/pp.76.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Stern D. B., Dyer T. A., Lonsdale D. M. Organization of the mitochondrial ribosomal RNA genes of maize. Nucleic Acids Res. 1982 Jun 11;10(11):3333–3340. doi: 10.1093/nar/10.11.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. B., Lonsdale D. M. Mitochondrial and chloroplast genomes of maize have a 12-kilobase DNA sequence in common. Nature. 1982 Oct 21;299(5885):698–702. doi: 10.1038/299698a0. [DOI] [PubMed] [Google Scholar]
- Stern D. B., Palmer J. D. Extensive and widespread homologies between mitochondrial DNA and chloroplast DNA in plants. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1946–1950. doi: 10.1073/pnas.81.7.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. B., Palmer J. D. Recombination sequences in plant mitochondrial genomes: diversity and homologies to known mitochondrial genes. Nucleic Acids Res. 1984 Aug 10;12(15):6141–6157. doi: 10.1093/nar/12.15.6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Ward B. L., Anderson R. S., Bendich A. J. The mitochondrial genome is large and variable in a family of plants (cucurbitaceae). Cell. 1981 Sep;25(3):793–803. doi: 10.1016/0092-8674(81)90187-2. [DOI] [PubMed] [Google Scholar]