Abstract
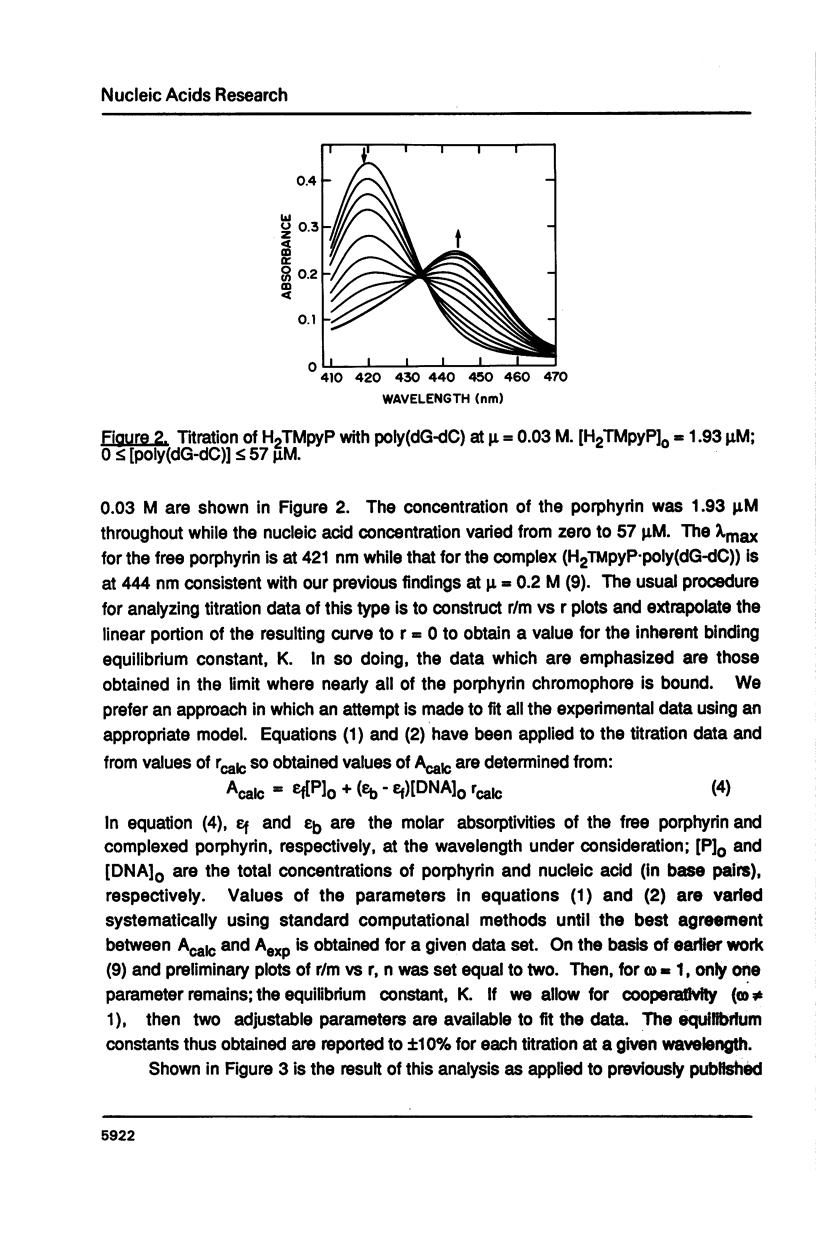
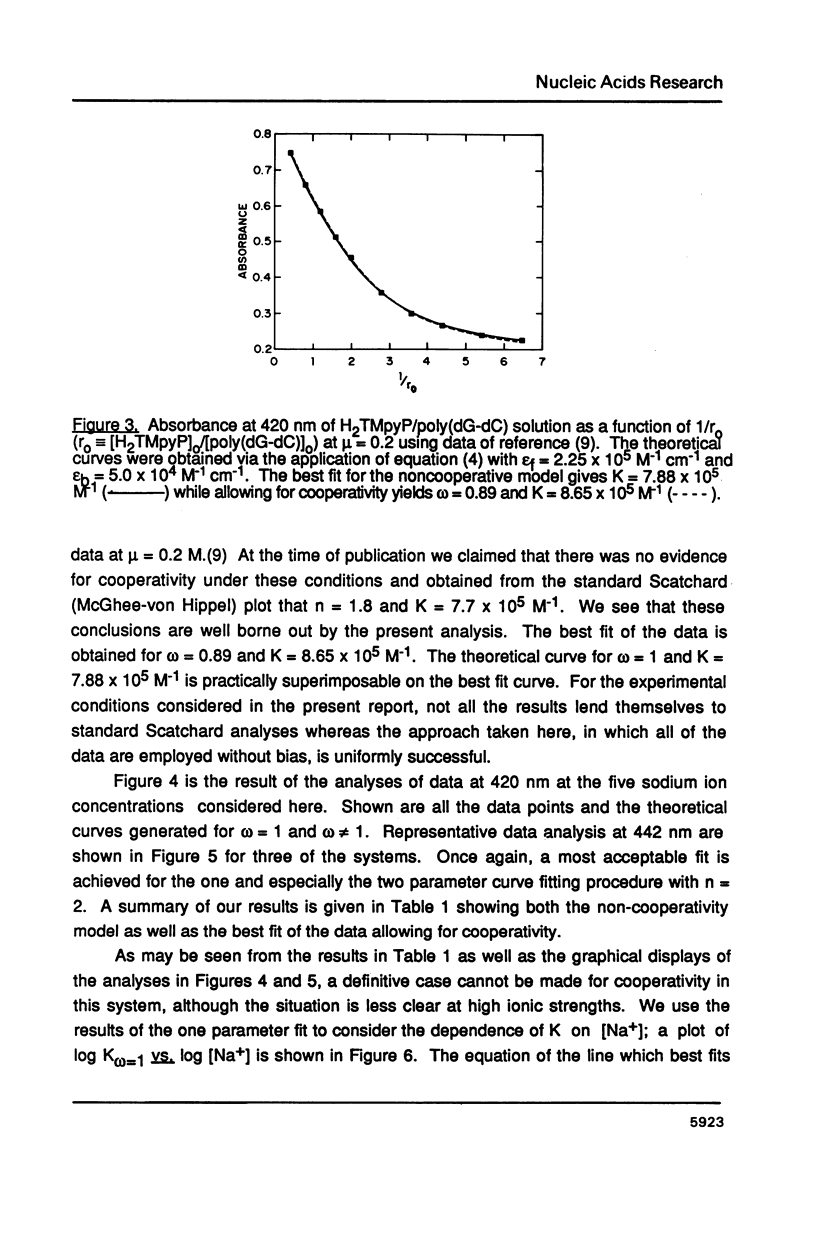
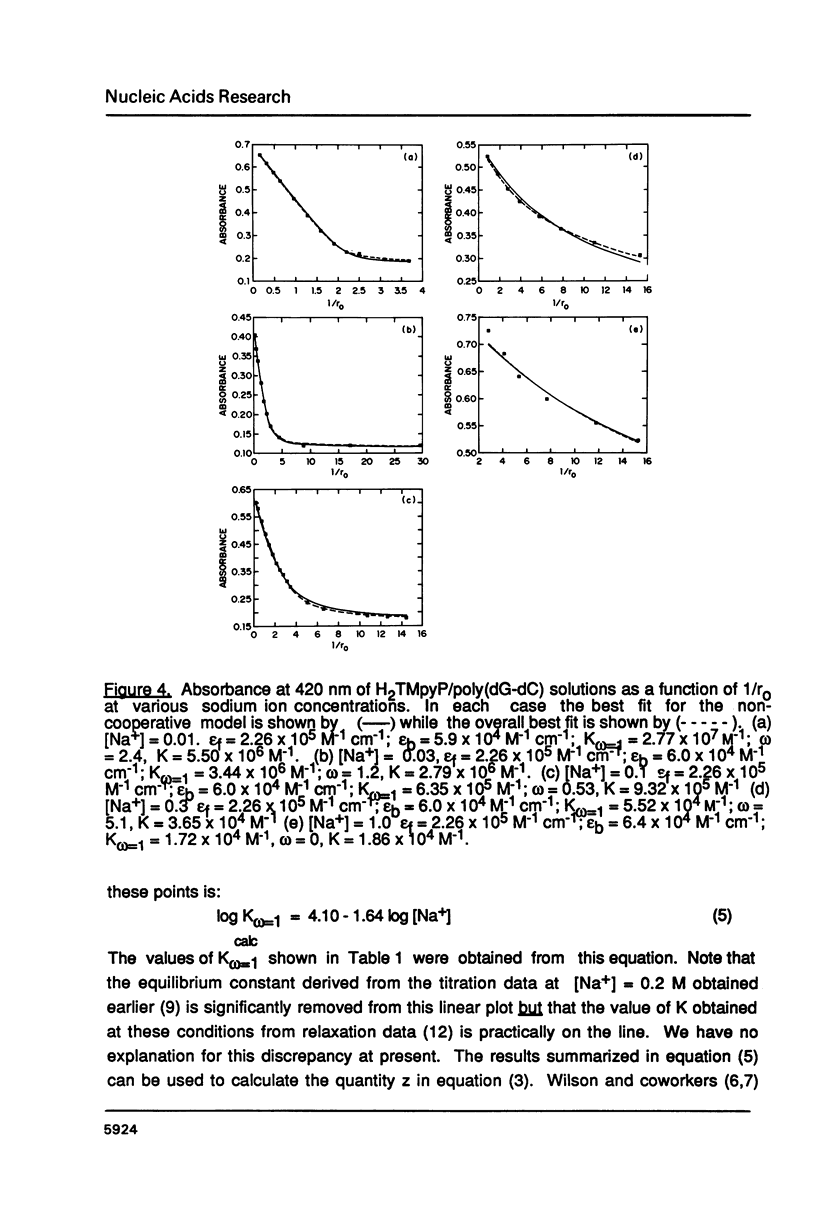
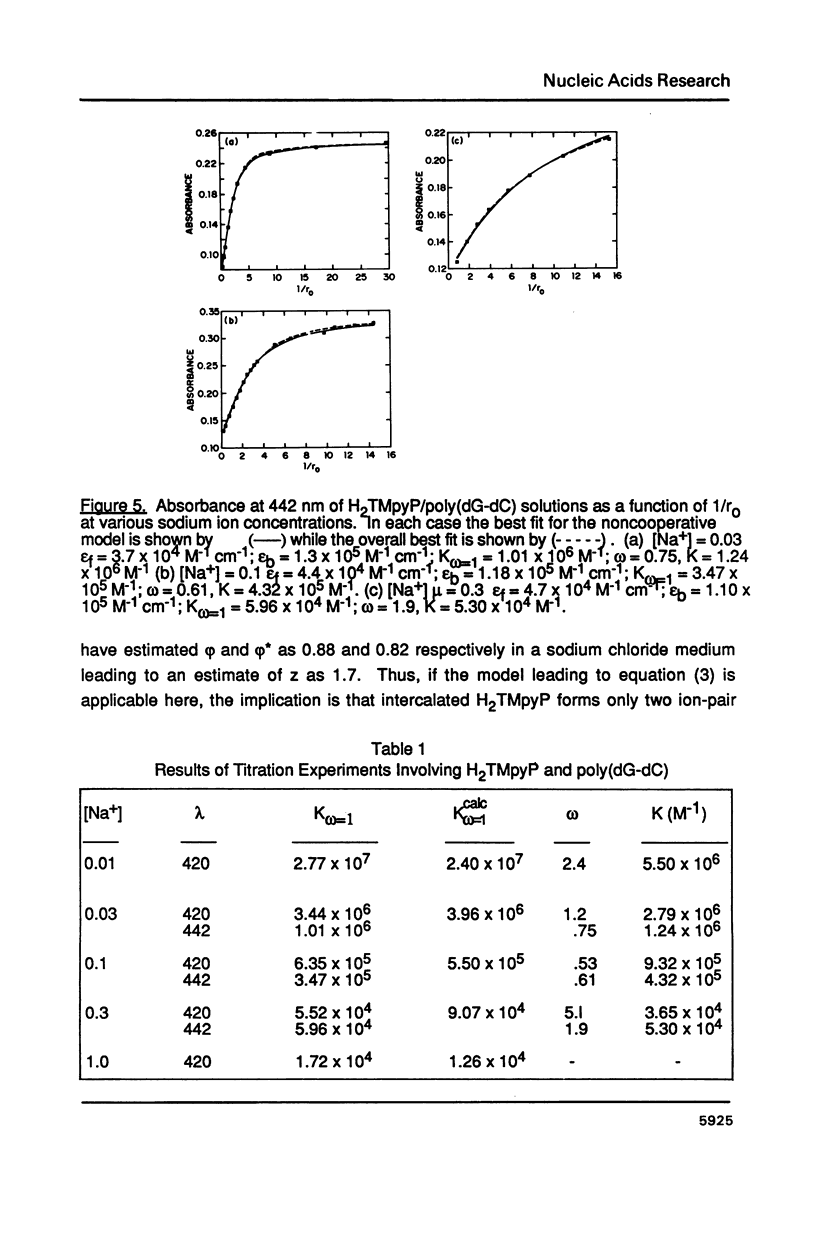
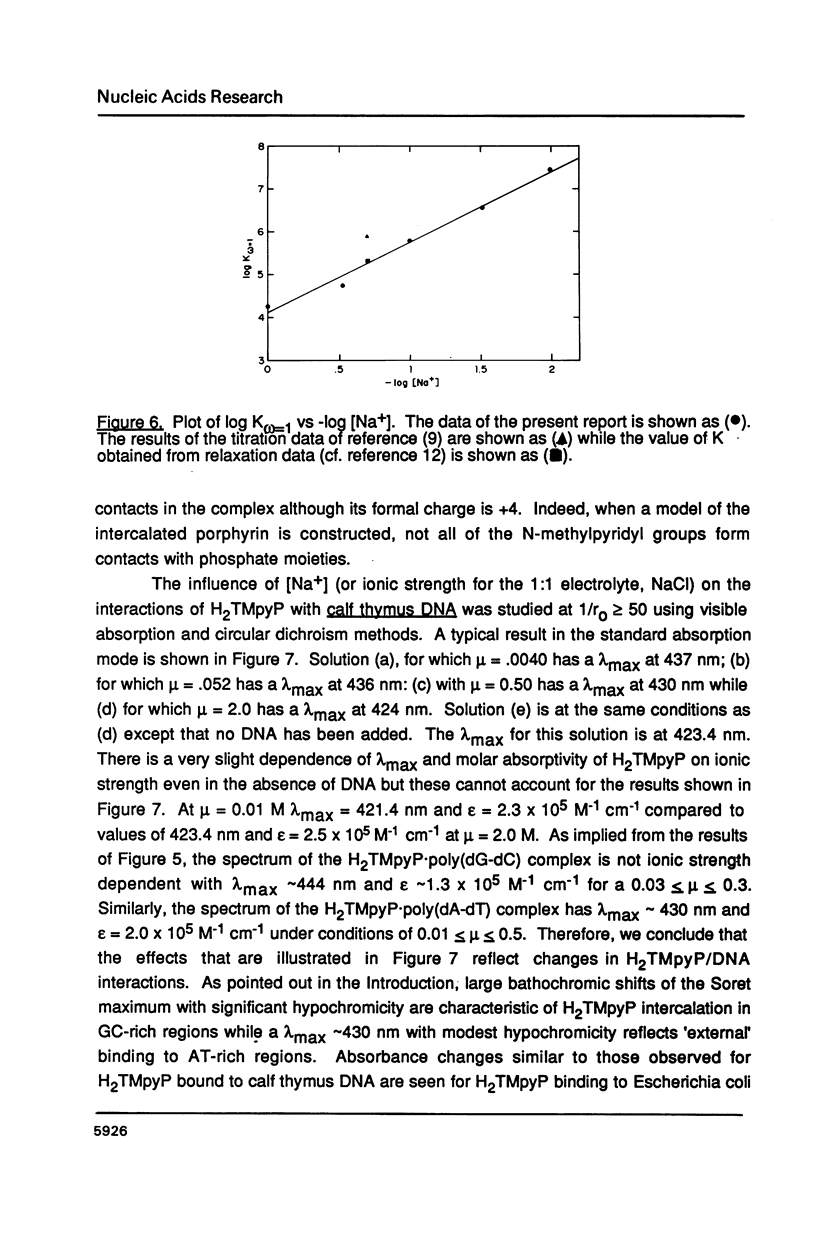
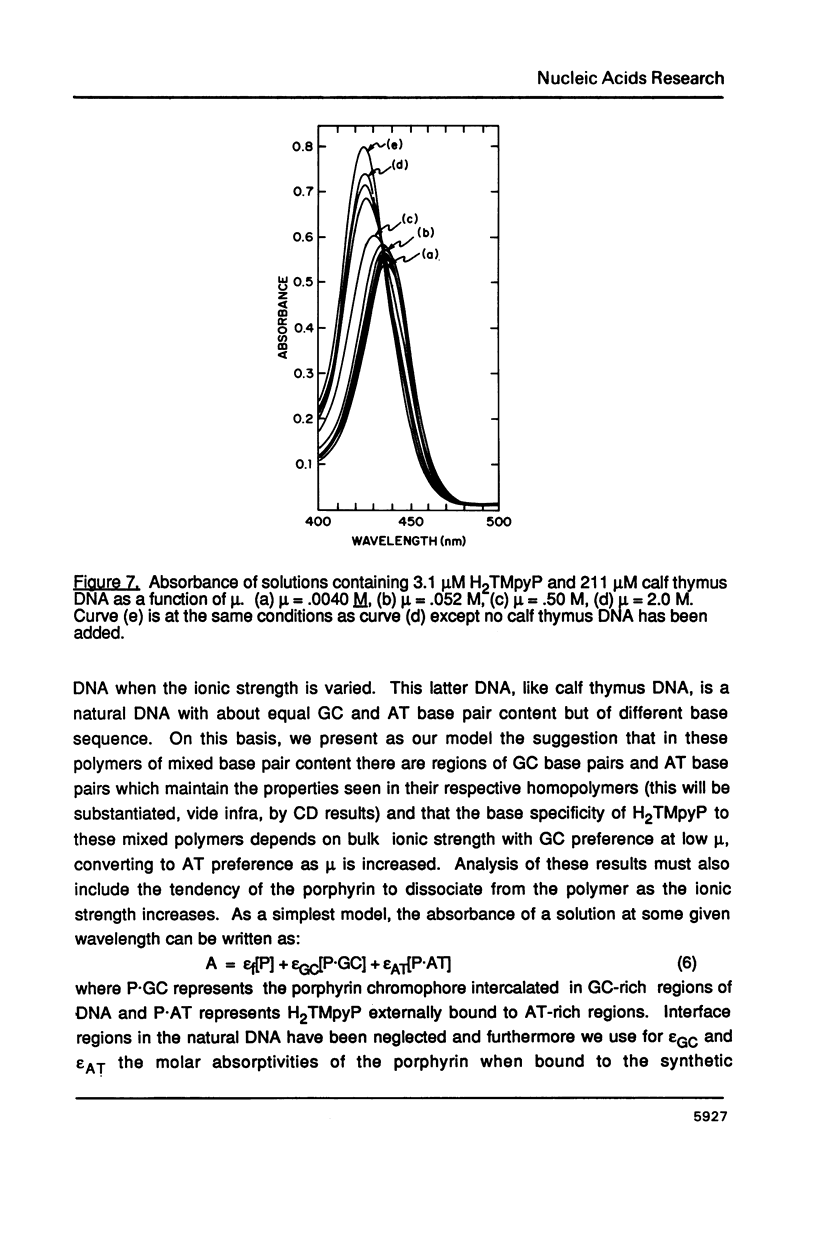
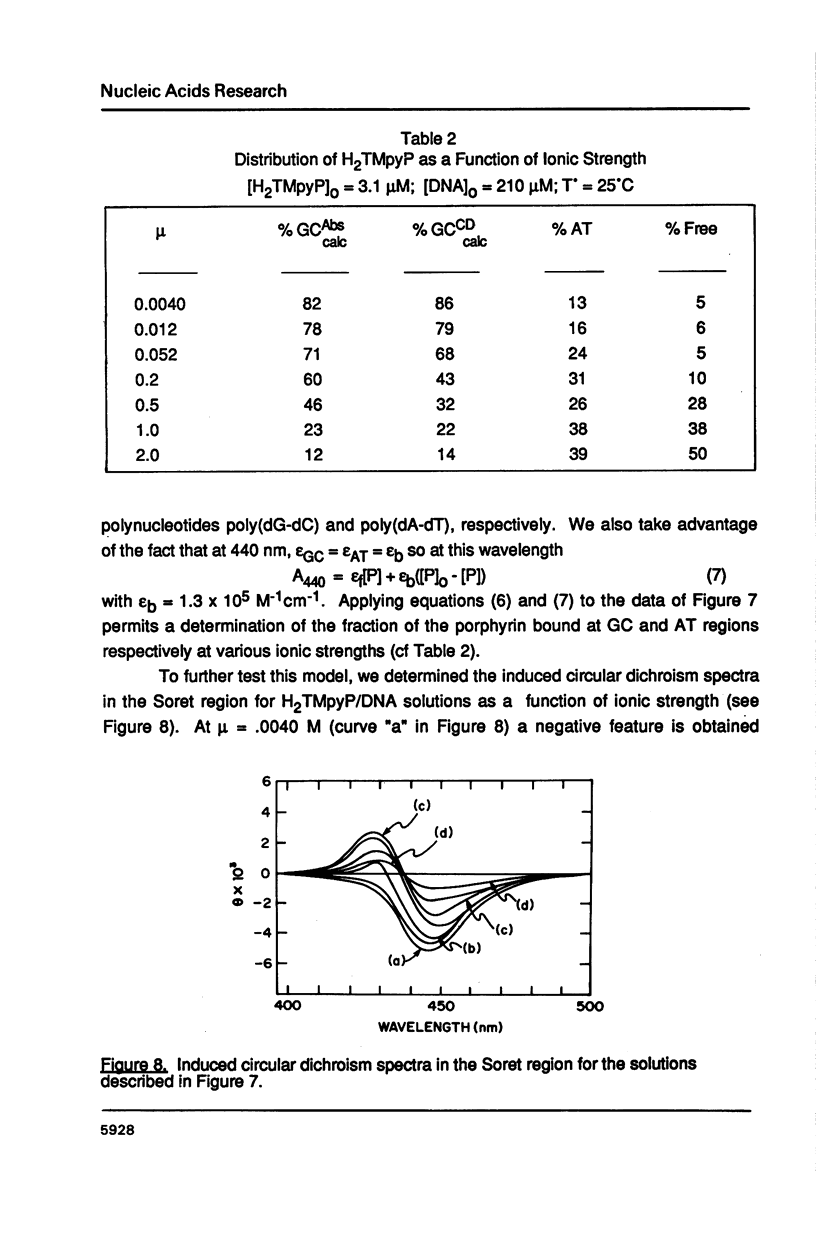
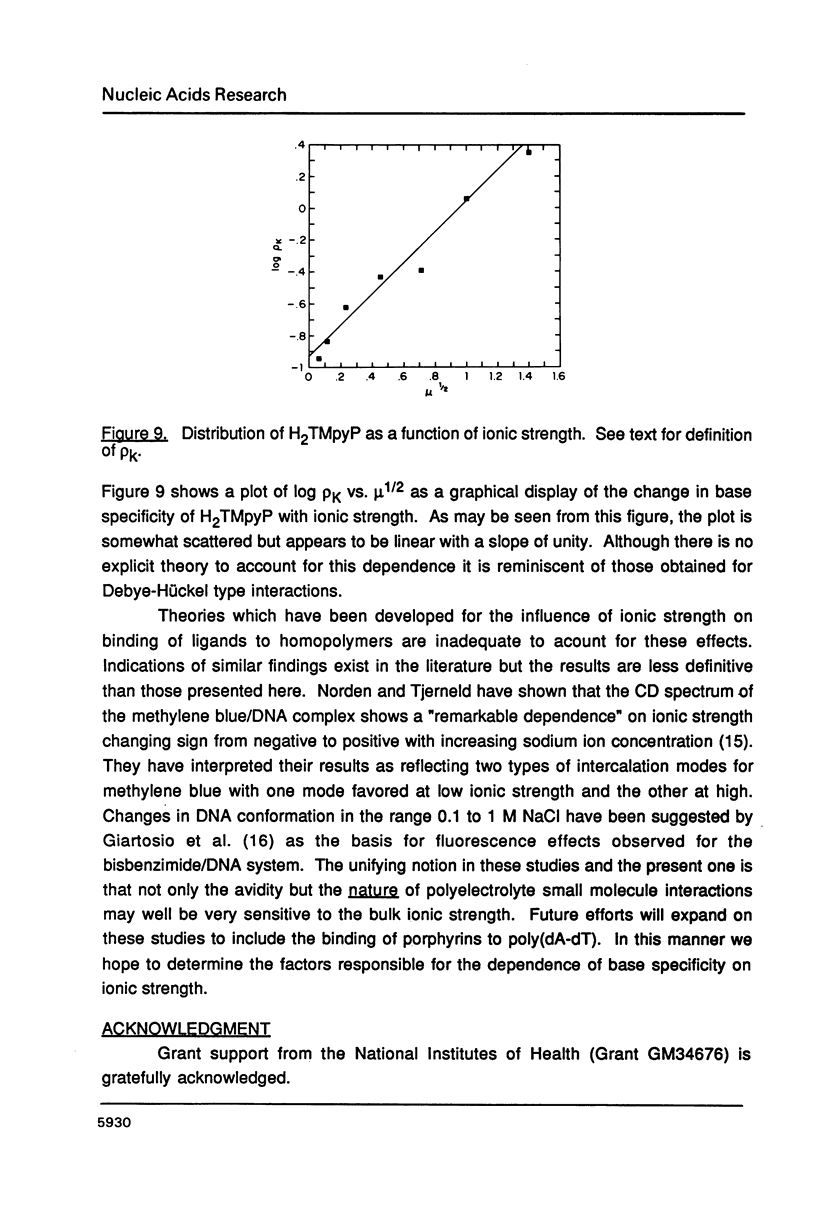
The ionic strength dependences of the binding of tetrakis (4-N-methylpyridyl)porphine (H2TMpyP) to poly(dG-dC) and calf thymus DNA have been determined. For the former system the results are typical of other intercalators, i.e., a plot of log K vs log [Na+] is linear albeit with a slope which suggests that the "effective charge" of the porphyrin is closer to two than the formal charge of +4. For calf thymus DNA, the binding profile is not completely compatible with the predictions of condensation theory. Whereas the avidity of binding does decrease with increasing [Na+] as predicted, of greater interest is the relocation of the porphyrin from GC-rich regions to AT-rich regions as the ionic strength increases.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fiel R. J., Howard J. C., Mark E. H., Datta Gupta N. Interaction of DNA with a porphyrin ligand: evidence for intercalation. Nucleic Acids Res. 1979 Jul 11;6(9):3093–3118. doi: 10.1093/nar/6.9.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. A., Manning G. S. Polyelectrolyte effects on site-binding equilibria with application to the intercalation of drugs into DNA. Biopolymers. 1984 Dec;23(12):2671–2714. doi: 10.1002/bip.360231202. [DOI] [PubMed] [Google Scholar]
- Giartosio A., Ferraro A., Lavaggi M. V., Allegra P., Turano C. The interaction of bisbenzimide with DNA. Physiol Chem Phys Med NMR. 1984;16(6):481–490. [PubMed] [Google Scholar]
- Manning G. S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys. 1978 May;11(2):179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J Mol Biol. 1974 Jun 25;86(2):469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- Müller W., Crothers D. M. Studies of the binding of actinomycin and related compounds to DNA. J Mol Biol. 1968 Jul 28;35(2):251–290. doi: 10.1016/s0022-2836(68)80024-5. [DOI] [PubMed] [Google Scholar]
- Müller W., Crothers D. M., Waring M. J. A non-intercalating proflavine derivative. Eur J Biochem. 1973 Nov 1;39(1):223–234. doi: 10.1111/j.1432-1033.1973.tb03120.x. [DOI] [PubMed] [Google Scholar]
- Nordén B., Tjerneld F. Structure of methylene blue-DNA complexes studied by linear and circular dichroism spectroscopy. Biopolymers. 1982 Sep;21(9):1713–1734. doi: 10.1002/bip.360210904. [DOI] [PubMed] [Google Scholar]
- Pasternack R. F., Gibbs E. J., Villafranca J. J. Interactions of porphyrins with nucleic acids. Biochemistry. 1983 May 10;22(10):2406–2414. doi: 10.1021/bi00279a016. [DOI] [PubMed] [Google Scholar]
- Pasternack R. F., Gibbs E. J., Villafranca J. J. Interactions of porphyrins with nucleic acids. Biochemistry. 1983 Nov 8;22(23):5409–5417. doi: 10.1021/bi00292a024. [DOI] [PubMed] [Google Scholar]
- Quadrifoglio F., Ciana A., Crescenzi V. Letter: On the interaction between actinomycin D and DNA. Biopolymers. 1976 Mar;15(3):595–597. doi: 10.1002/bip.1976.360150316. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Larson J. E., Grant R. C., Shortle B. E., Cantor C. R. Physicochemical studies on polydeoxyribonucleotides containing defined repeating nucleotide sequences. J Mol Biol. 1970 Dec 28;54(3):465–497. doi: 10.1016/0022-2836(70)90121-x. [DOI] [PubMed] [Google Scholar]
- Wilson W. D., Krishnamoorthy C. R., Wang Y. H., Smith J. C. Mechanism of intercalation: ion effects on the equilibrium and kinetic constants for the interaction of propidium and ethidium with DNA. Biopolymers. 1985 Oct;24(10):1941–1961. doi: 10.1002/bip.360241008. [DOI] [PubMed] [Google Scholar]
- Wilson W. D., Lopp I. G. Analysis of cooperativity and ion effects in the interaction of quinacrine with DNA. Biopolymers. 1979 Dec;18(12):3025–3041. doi: 10.1002/bip.1979.360181210. [DOI] [PubMed] [Google Scholar]


