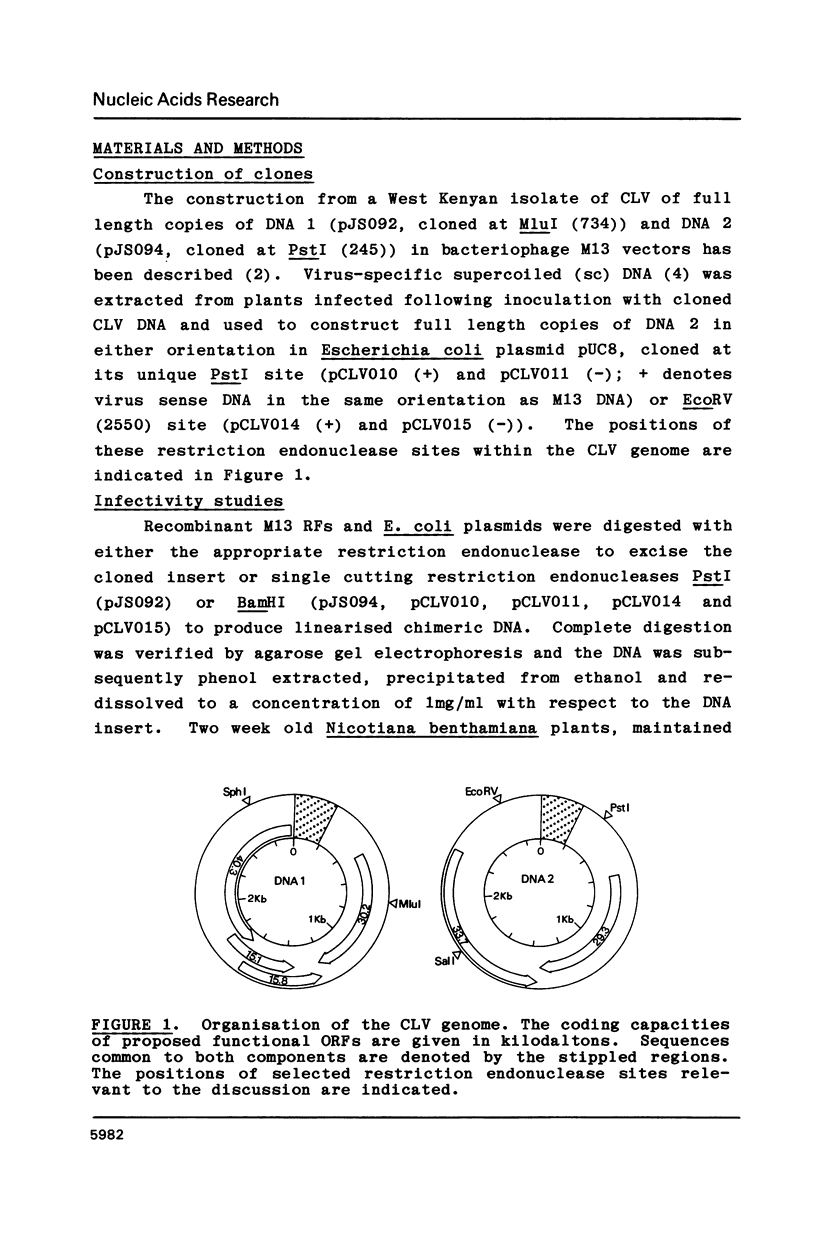
Abstract
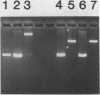
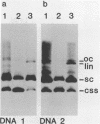
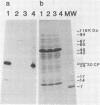
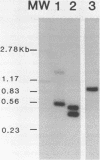
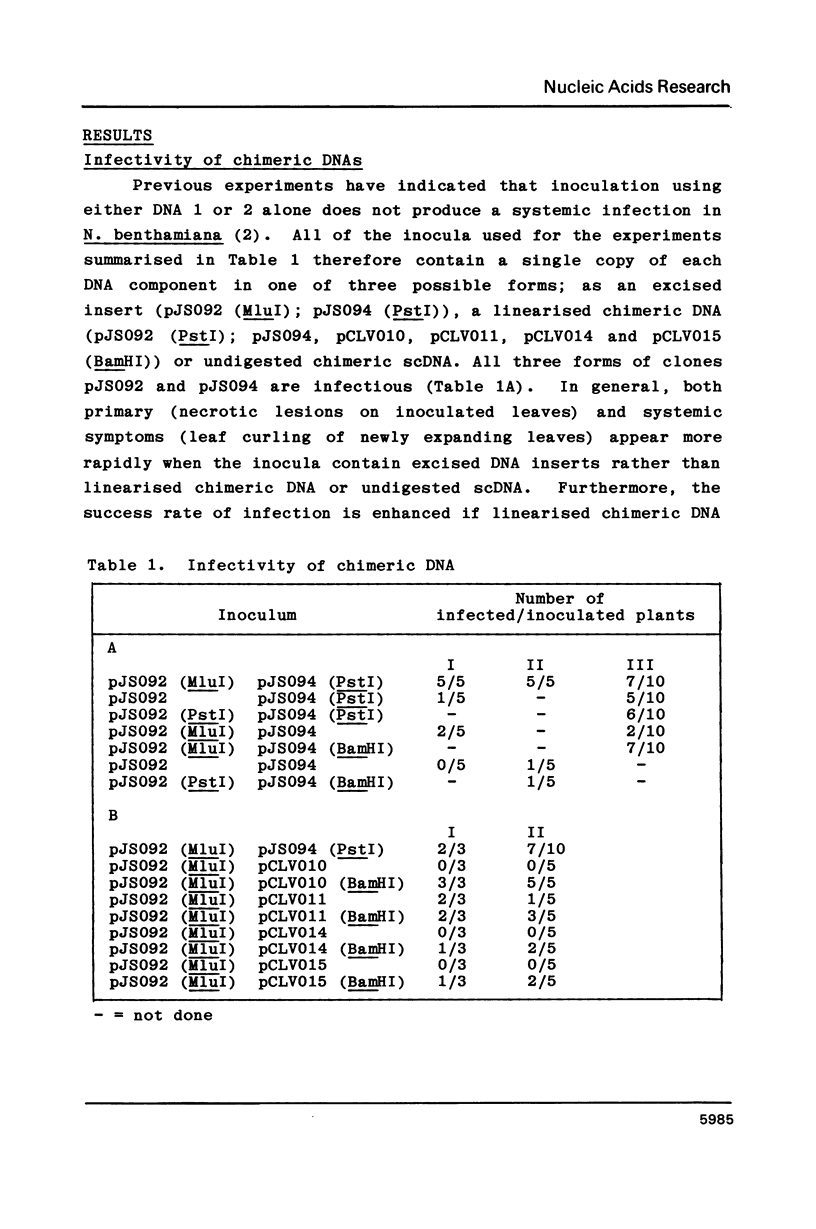
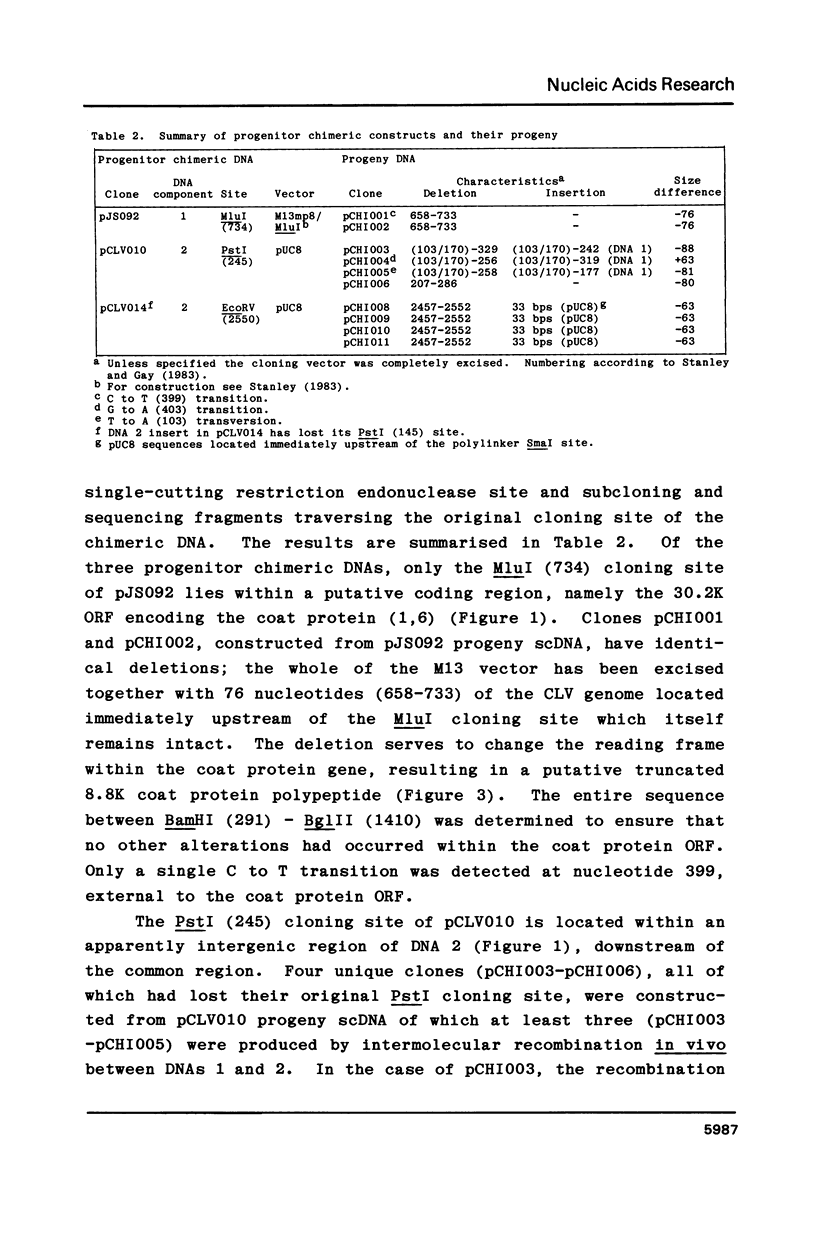
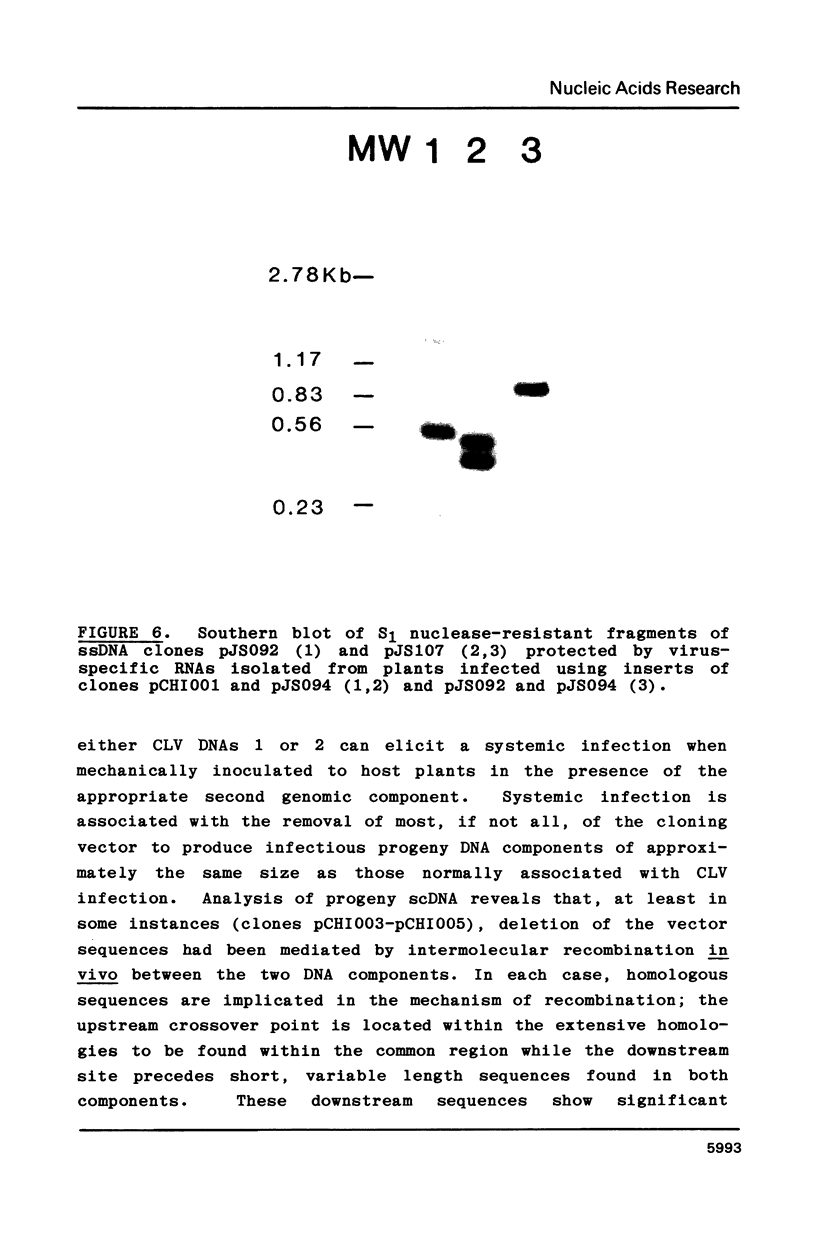
Intact recombinant DNAs containing single copies of either component of the cassava latent virus genome can elicit infection when mechanically inoculated to host plants in the presence of the appropriate second component. Characterisation of infectious mutant progeny viruses, by analysis of virus-specific supercoiled DNA intermediates, indicates that most if not all of the cloning vector has been deleted, achieved at least in some cases by intermolecular recombination in vivo between DNAs 1 and 2. Significant rearrangements within the intergenic region of DNA 2, predominantly external to the common region, can be tolerated without loss of infectivity suggesting a somewhat passive role in virus multiplication for the sequences in question. Although packaging constraints might impose limits on the amount of DNA within geminate particles, isolation of an infectious coat protein mutant defective in virion production suggests that packaging is not essential for systemic spread of the viral DNA.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Daubert S., Shepherd R. J., Gardner R. C. Insertional mutagenesis of the cauliflower mosaic virus genome. Gene. 1983 Nov;25(2-3):201–208. doi: 10.1016/0378-1119(83)90224-x. [DOI] [PubMed] [Google Scholar]
- Dixon L. K., Koenig I., Hohn T. Mutagenesis of cauliflower mosaic virus. Gene. 1983 Nov;25(2-3):189–199. doi: 10.1016/s0378-1119(83)80001-8. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hamilton W. D., Bisaro D. M., Coutts R. H., Buck K. W. Demonstration of the bipartite nature of the genome of a single-stranded DNA plant virus by infection with the cloned DNA components. Nucleic Acids Res. 1983 Nov 11;11(21):7387–7396. doi: 10.1093/nar/11.21.7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W. D., Stein V. E., Coutts R. H., Buck K. W. Complete nucleotide sequence of the infectious cloned DNA components of tomato golden mosaic virus: potential coding regions and regulatory sequences. EMBO J. 1984 Sep;3(9):2197–2205. doi: 10.1002/j.1460-2075.1984.tb02114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Otsuki Y., Takebe I. Fluorescent antibody staining of tobacco mosaic virus antigen in tobacco mesophyll protoplasts. Virology. 1969 Jul;38(3):497–499. doi: 10.1016/0042-6822(69)90167-6. [DOI] [PubMed] [Google Scholar]
- SIEGEL A., ZAITLIN M., SEHGAL O. P. The isolation of defective tobacco mosaic virus strains. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1845–1851. doi: 10.1073/pnas.48.10.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. A new computer method for the storage and manipulation of DNA gel reading data. Nucleic Acids Res. 1980 Aug 25;8(16):3673–3694. doi: 10.1093/nar/8.16.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J. The molecular biology of geminiviruses. Adv Virus Res. 1985;30:139–177. doi: 10.1016/s0065-3527(08)60450-9. [DOI] [PubMed] [Google Scholar]
- Stanley J., Townsend R. Characterisation of DNA forms associated with cassava latent virus infection. Nucleic Acids Res. 1985 Apr 11;13(7):2189–2206. doi: 10.1093/nar/13.7.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sänger H. L. Functions of the two particles of tobacco rattle virus. J Virol. 1969 Mar;3(3):304–312. doi: 10.1128/jvi.3.3.304-312.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend R., Stanley J., Curson S. J., Short M. N. Major polyadenylated transcripts of cassava latent virus and location of the gene encoding coat protein. EMBO J. 1985 Jan;4(1):33–37. doi: 10.1002/j.1460-2075.1985.tb02313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend R., Watts J., Stanley J. Synthesis of viral DNA forms in Nicotiana plumbaginifolia protoplasts inoculated with cassava latent virus (CLV); evidence for the independent replication of one component of the CLV genome. Nucleic Acids Res. 1986 Feb 11;14(3):1253–1265. doi: 10.1093/nar/14.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhof E., Altschuh D., Moras D., Bloomer A. C., Mondragon A., Klug A., Van Regenmortel M. H. Correlation between segmental mobility and the location of antigenic determinants in proteins. Nature. 1984 Sep 13;311(5982):123–126. doi: 10.1038/311123a0. [DOI] [PubMed] [Google Scholar]