Abstract
Ovarian cancer remains a leading cause of death among women worldwide, and current treatment regimens for advanced disease are inadequate. Oligonucleotides with sequence homology to telomeres (called T-oligos) have been shown to mimic DNA damage responses in cells and induce cytotoxic effects in certain tumor cell lines. We studied the effects of 2 distinct 16 mer T-oligos in 4 human ovarian epithelial carcinoma cell lines. A T-oligo with perfect homology to the telomere overhang region demonstrated some cytotoxic activity in half of the cell lines. A G-rich T-oligo derivative showed more potency and broader cytotoxic activity in these lines than the parental T-oligo. Activation of apoptotic pathways in ovarian cancer cells by exposure to the T-oligo was demonstrated by multiple independent assays. T-oligo was shown to have additive, or more than additive, activity in combination with 2 different histone deacetylase drugs currently in clinical testing. T-oligos may therefore provide a new and tumor-targeted approach to ovarian cancers.
Introduction
Despite multidisciplinary approaches to the disease, ovarian epithelial cancer continues to have a poor prognosis. The majority of patients are found to have stage II or III disease, which is characterized by carcinomatosis throughout the peritoneal cavity (Guarneri et al., 2010). Conventional treatment combines surgery and systemic chemotherapy. The therapeutic goal of surgical management in ovarian cancer is optimal cytoreduction; however, in the most advanced cases, such cytoreductive surgery may not be technically possible. Further, although 70% of ovarian cancer patients initially respond to platinum- or taxane-based chemotherapy, the majority experience recurrence, and overall 5-year survival is only ∼20% for advanced-stage disease (Liu and Matulonis, 2010). Thus, current chemotherapeutic treatments for advanced ovarian cancer are relatively ineffective and are also relatively nonspecific, in that they damage normal tissues as well as malignant tissues, leading to the side effects and toxicities associated with radiation therapy and chemotherapy. More effective and more targeted therapies are urgently needed.
DNA oligos homologous to the telomere 3′ overhang region (T-oligos) induce a variety of protective DNA damage-like responses in normal cells (Eller et al., 1994, 1997; Goukassian et al., 2002), including transient cell cycle arrest in normal human cells of many lineages (Eller et al., 2002, 2003; Li et al., 2003; Puri et al., 2004). In contrast, T-oligos appear to exert selectively cytotoxic effects on malignant cells compared to their normal counterparts in vitro and in vivo. Treatment of melanoma or human breast cancer xenografts, or murine lymphomas, by systemic injection of various types of T-oligos resulted in reduced tumorigenicity and metastatic potential (Puri et al., 2004; Yaar et al., 2007; Longe et al., 2009).
In the current study, we exploited this novel approach of using oligonucleotides with sequence homology to human telomeres to mimic DNA damage and initiate a cytotoxic response, and demonstrate their activity in human ovarian cancer cells.
Materials and Methods
Reagents
Sodium butyrate and SAHA were purchased from Sigma. Other chemical reagents were purchased from Invitrogen or Sigma, or Pierce. The histone deacetylase (HDAC) inhibitors LBH589 (George et al., 2005) and MS275 (Saito et al., 1999) were synthesized. The annexin/propidium iodide (PI) apoptosis analysis kit was from BDR. The caspase-9 and caspase-3 activity measurement kit was from Promega.
Oligonucleotides
The sequence of 16 mer T-oligo was 5′-p-GGTTAGGTGTAGGTTT-3′; the 16 mer GT-oligo sequence was 5′-p-GGTTGGTTGGTTGGTT-3′ and 16 mer C-oligo sequence was 5′ p AAA CCT ACA CCT AAC C 3′. T-oligo, C-oligo and GT-oligo were obtained from Midland Certified Reagent. The oligonucleotides were purified from the trityl group by gel filtration chromatography. Purity was ∼98% for all oligonucleotides.
Cell culture
The human ovarian cancer cell lines PA1, CAOV-3, OVCAR-3, and SKOV-3 were obtained from ATCC. CAOV-3, OVCAR-3, and SKOV-3 cells were grown in RPMI 1640 media (Invitrogen) containing 5 mL penicillin/streptomycin and 10% heat-inactivated fetal bovine serum. PA1 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Cells at 70%–80% confluence were exposed to either vehicle (water or dimethyl sulfoxide) or various concentrations of reagents. Cells were incubated for 24, 48, or 72 hours before processing, as described in the figure legends.
Cell survival assays
Viable cell enumeration was performed using a trypan blue exclusion assay in 6-well plates. Cells were trypsinized and 20 μL cell suspensions were mixed with 20 μL trypan blue and viable cells (dye-excluding cells) enumerated. The percent viable cell counts were plotted against time of incubation. An increase in viable cell counts over time provides a measure of growth.
Washout studies
The conditioned medium was prepared by harvesting the medium from growing cultures of the relevant cell lines, and filtering. Cell cultures were initially exposed to oligo or vehicle in fresh medium. After specific intervals of exposure to the oligos (12, 24, 48, or 72 hours), the oligo-containing medium was removed off and replaced with the conditioned, depleted medium. Control cultures contained vehicle rather than oligo, and at 12 hours the medium was replaced by the conditioned medium. Cultures were carried for a total of 96 hours whereupon live cells were enumerated. The “96 hr” values are derived from cultures exposed to the GT-oligo throughout.
Flow cytometric and apoptosis assays
For cell cycle analyses, cells were treated with different inhibitors for the indicated times and stained with PI as described (Vaziri et al., 1995) and then analyzed for DNA content by flow cytometry. Briefly, cells were washed with phosphate-buffered saline, fixed in the medium containing 35% ethanol for 5 min at room temperature, and stained for 30 min in the dark with 25 μg PI/mL and 50 μg RNAse/mL in phosphate-buffered saline, before flow cytometric analysis.
Apoptosis assays were performed by annexin/PI assay, as described (Sarkar et al., 2002). In short, treated and untreated cells were harvested and suspended in 100 μL 1× binding buffer. Two microliters annexin reagent (conjugated to fluorescein isothiocyanate) and 2 μL PI was added to each sample, mixed well, and kept in the dark for 30 min at room temperature. Nine hundred microliters of 1× binding buffer was added, and the dual fluorescence of fluorescein isothiocyanate and PI was measured by flow cytometry.
Caspase-3/7 and caspase-9 assays
Cells were treated with GT-oligo for 72 hours and lysed, and protein was quantitated. One microgram protein in each sample was incubated with the synthetic substrate in the assay buffer in a volume of 200 μL for 1 hour. The substrate containing assay buffer served as a blank control. The light was measured in a luminometer. The results were presented in arbitrary units and were the average of 3 independent assays.
Results
Effects of oligos on the growth of human ovarian cancer cell lines
The 16-mer “T-oligo” 5′-p-GGTTAGGTGTAGGTTT-3′ has previously demonstrated cytostatic and cytotoxic activity against melanoma, breast cancer, and lymphoma cells and cell lines. At concentrations of 40 μM, the T-oligo inhibited the growth of the human ovarian cancer cell line PA1 (Fig. 1A). In contrast, exposure of the PA1 cell line to the 16-mer control oligo did not alter the proliferation of these cells relative to vehicle control. The T-oligo, however, demonstrated no significant inhibitory activity on the growth of the CAOV-3, OVCAR-3, and SKOV-3 ovarian cancer cell lines.
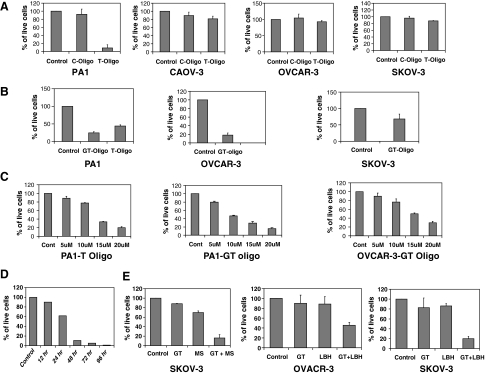
FIG. 1.
(A) Effects of T-oligo on the growth of ovarian cancer cells: 4 different ovarian cancer cell lines were exposed to either T-oligo or control oligo (C-oligo) at 40 μM. After 96 hours, viable cells were enumerated. Results are expressed relative to vehicle-treated (control) cells, arbitrarily assigned a value of 100%. Bars indicate standard error of the mean. PA1 cells: P value for C-oligo = 0.07, P value for T-oligo = 0.002; CAOV-3 cells: P value for C-oligo = 0.098, P value for T-oligo = 0.35; OVCAR-3 cells: P value for C-oligo = 0.387, P value for T-oligo = 0.238; SKOV-3 cells: P value for C-oligo = 0.263, P value for T-oligo = 0.9. (B) Effects of T-oligo and GT-oligo on the growth of ovary cancer cells: PA1 ovarian cancer cells were exposed to either T-oligo (20 μM) or GT-oligo (10 μM). After 96 hours, viable cells were enumerated. The results are expressed as described in (A). P value for GT-oligo compared to control = 0.05; P value for T-oligo = 0.05. OVCAR-3 and SKOV-3 cells were treated with 20 μM GT-oligo for 96 hours, viable cells were enumerated, and results expressed as described in (A). OVCAR-3 cells: P value for GT-oligo = 0.001; SKOV-3 cells: P value for GT-oligo = 0.05. (C) PA1 ovarian cancer cells were exposed to either T-oligo (left panel) or GT-oligo (middle panel) at concentrations ranging from 5 to 20 μM. OVCAR-3 cancer cells were exposed to GT-oligo at concentrations ranging from 5 to 20 μM. After 96 hours, viable cells were enumerated. Results are expressed relative to vehicle-treated (control) cells, arbitrarily assigned a value of 100%. (D) GT-oligo exposure time required to initiate growth inhibition: PA1 cells were exposed to the GT-oligo (20 μM) for different intervals of time (12, 24, 48, or 72 hours). The oligo was then washed out of the culture, and the effects on cell number were assessed over time. In the 96-hour exposure condition, the oligo was not washed out. The results shown are the average of 2 independent experiments. (E) Effect of GT-oligo and histone deacetylase inhibitors on the growth of ovary cancer cells: different ovarian cancer cell lines were exposed to either GT-oligo (10 μM) or histone deacetylase inhibitors MS275 (200 nM) or LBH589 (7.5 nM) or the combination of GT-oligo and MS275 (“MS”) or LBH589 (“LBH”). After 96 hours, viable cells were enumerated. Results are expressed relative to vehicle-treated (control) cells, arbitrarily assigned a value of 100%. SKOV-3 cells: P value for GT-oligo + MS 275 = 0.002; OVCAR-3 cells: P value for GT-oligo + LBH589 = 0.004; SKOV-3 cells: P value for GT-oligo + LBH589 = 0.001.
A more GT-rich oligo, designated “GT-oligo” (5′-p-GGTTGGTTGGTTGGTT-3′), has recently also been shown to exert anti-tumor cell activity (Ohashi et al., 2007), and was studied in comparison with the T-oligo. At suboptimal concentrations of 10 and 20 μM, the GT-oligo was more effective than the T-oligo at inhibiting growth of the PA1 cell line (Fig. 1B). In addition, the GT-oligo also showed activity against OVCAR-3 cell line, whereas 4-fold higher concentrations of the T-oligo had no effect on proliferation of these cells (see above). The growth-inhibitory effects of the GT-oligo on the SKOV-3 cell line were more modest, but consistently observed. Because of the more potent and broader activity of the GT-oligo than the T-oligo, the GT oligo was employed in the remainder of the studies described. Exposure to either the T-oligo or the GT-oligo produced concentration-dependent growth inhibition of PA1 ovarian cancer cells (Fig. 1C). Concentration-dependent growth inhibition of OVCAR-3 cells was produced by exposure to the GT-oligo, whereas they were relatively resistant to the T-oligo (see Fig. 1A).
Effects of oligos on cell cycle progression and apoptosis
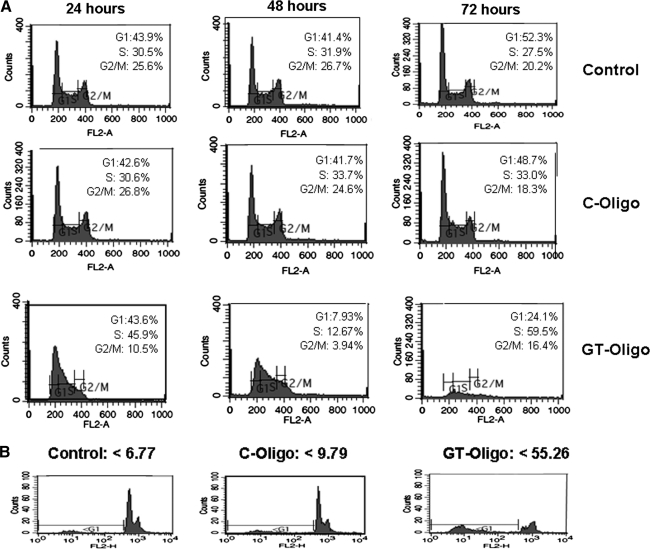
To determine the effects of oligonucleotide exposure on cell cycle progression, the DNA content of the tumor cells was analyzed by PI staining and flow cytometry. Cell lines were exposed to GT oligo, a control oligo, or vehicle for 24, 48, and 72 hours. Exposure to the GT-oligo produced a decrease in the fraction of cells with 4N DNA content (G2/M phase) within 24 hours (Fig. 2A). By 48 and 72 hours of exposure, the cells exposed to the GT-oligo demonstrated significant distortions of their DNA-content profile, indicative of cytotoxicity. In contrast, cells exposed to vehicle or control-oligo did not display significant differences in cell cycle profiles throughout the 72 hour period of analysis. Analysis of cells exposed to GT-oligo for 72 hours demonstrated a significant accumulation of cells with a <2 N DNA content, indicative of apoptosis, whereas this hypodiploid cell population was not observed in cells exposed to the control oligo (Fig. 2B).
FIG. 2.
(A) Effect of GT-oligo on cell cycle profile. PA1 cells were exposed to GT-oligo or C-oligo (20 μM). After 24, 48, or 72 hours, cells were stained with propidium iodide (PI) and cell cycle profiles were analyzed by flow cytometry. Vehicle-treated cells served as a control. Results are expressed as the percentage of cells in a particular phase of the cell cycle. (B) Fraction of cells with subdiploid DNA content. Flow cytometric profiles were also recorded using a log scale for FL2 to determine the fraction of cells with subdiploid DNA content.
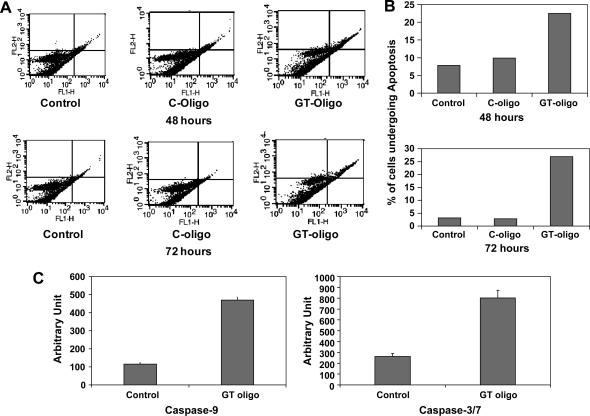
As an independent assay for induction of apoptosis, annexin/PI staining and analysis was employed. Evidence of a significant increase in an early apoptotic population (annexin-positive/PI-negative) and a later apoptotic population (annexin-positive/PI-positive) was evident at 48 hours in cells exposed to GT-oligo compared to controls, and this fraction was higher at the 72 hour time point (Fig. 3A, B).
FIG. 3.
(A) Induction of apoptosis by GT-oligo. PA1 cells were exposed to GT-oligo (20 μM) or C-oligo (20 μM). After 48 or 72 hours, cells were stained with fluorescein isothiocyanate-conjugated annexin antibody (FL1 channel) and PI (FL2 channel). Apoptosis profiles were analyzed by flow cytometry. Vehicle- and control oligo- (C-oligo)-exposed cells served as controls. (B) Graphical depiction of apoptotic fractions after exposure to GT-oligo. The data from (A) were expressed as percent of cells undergoing apoptosis (annexin-positive and annexin/PI-positive fractions). (C) Activation of caspase-9 and caspase-3/7 by GT-oligo. PA1 cells were exposed to GT-oligo (20 μM). After 72 hours, cell lysates were assayed for caspase activity using a synthetic substrate, and fluorescence was quantitated. Lysates from vehicle-treated cultures served as control. P value for caspase-9 assay, GT oligo treatment = 0.001; P value for caspase-3/7 assay, GT-oligo treatment = 0.005.
The length of exposure to oligo required for antitumor cell line activity was next assessed. PA1 cells were exposed to the GT-oligo for different intervals, followed by washout, and the effects on cell growth were assessed over time. Decreases in cell number between oligo- and vehicle-treated cultures became apparent between 24 and 48 hours of exposure to oligo, and remained lower for all longer periods of exposure (Fig. 1D).
Activation of caspase-3/7 is the terminal step in the process of apoptosis for both the intrinsic and extrinsic apoptotic effector pathways. The PA1 ovarian cancer cells were exposed to GT-oligo for 72 hours (a time point where apoptosis and significant growth inhibition was observed; eg, Figs. 1C and 3B), and cell lysates were assayed for caspase 3/7 activity. Exposure to GT-oligo induced caspase-3/7 activation significantly compared to the untreated cells (Fig. 3C). Caspase-9 activation is generally induced by the mitochondrial-mediated pathway (intrinsic mechanism) of apoptosis. Assay of PA1 ovarian cancer cells exposed to GT-oligo showed significant activation of caspase-9 at 72 hours (Fig. 3C).
Effects of oligos in combination with HDAC inhibitors
The ability to use oligo-based therapeutic strategies in combination with other antitumor agents would increase their potential application. HDAC inhibitors have shown some activity as single agents against ovarian carcinoma cells in culture (Takai and Narahara, 2010). Whether these T-oligos would have additive activity with HDAC inhibitors currently in clinical testing was therefore studied. Suboptimal concentrations of each agent were established to make it possible to observe any additive effects of the combinations. The GT-oligo at suboptimal concentrations provided at least additive activity in combination with the hydroxamic acid-class HDAC inhibitor LBH589 (Fig. 1E). The ovarian cancer cell line SKOV-3 had proven completely resistant to the T-oligo, and relatively resistant to the GT-oligo, compared to other human ovarian cancer cell lines. The combination of a suboptimal concentration of GT-oligo with suboptimal concentrations of the HDAC inhibitor LBH589, however, produced a more-than-additive inhibition of growth of the SKOV-3 cell line (Fig. 1E). Parallel studies using an HDAC inhibitor of a different chemical class (MS275, a benzamide) similarly showed additive or more-than-additive effects on growth inhibition of these resistant ovarian carcinoma cells (Fig. 1E). OVCAR-3 cell lines also showed significant growth inhibition when LHB589 and GT-oligo in combination were used in suboptimal concentrations.
Discussion
T-oligos may provide a novel and unique tumor-specific therapeutic approach (Puri et al., 2004; Aoki et al., 2007; Ohashi et al., 2007). During their exploration of DNA damage and differentiation responses in melanocytes, via introduction of ssDNA oligomers into cells, our colleagues discovered that 2–9 base DNA oligos homologous to the telomere 3′ overhang region induced a variety of protective DNA damage-like responses in normal cells and intact rodent skin (Eller et al., 1994, 1997; Goukassian et al., 2002). 11-Base T-oligos of specific sequences induced transient cell cycle arrest in human cells of most lineages (Eller et al., 2002, 2003; Li et al., 2003; Puri et al., 2004). In human tumor cell types, such as human T cell lymphomas and melanomas, however, 11-mer T-oligos induced apoptosis (Eller et al., 2002), mimicking responses of those cells to acute DNA damage or telomere loop disruption (Karlseder et al., 1999). These data strongly suggested that 11-mer T-oligos activate multiple, normally redundant, tumor-suppressive programs in both normal and malignant cells, in the apparent absence of DNA damage (Eller et al., 2003; Puri et al., 2004).
T-oligos appear to exert selectively greater effects on malignant cells than on their normal counterparts in vitro. A single 48-hour exposure of human melanoma cells to 11-mer T-oligos in vitro reduced their tumorigenicity and metastatic potential in mice and led to differentiation of remaining melanoma cells, strongly suggesting a permanent effect on cellular growth potential (Puri et al., 2004). In contrast, normal human melanocytes exposed to the same or higher concentration of T-oligo showed only transient cell cycle arrest (Puri et al., 2004). Treatment of melanoma or human breast cancer xenografts that had been previously established in SCID mice by systemic injection of T-oligo resulted in significant inhibition of growth (Puri et al., 2004; Yaar et al., 2007). We have more recently shown that a 16-mer T-oligo, which is more potent in vitro than the 11-mer previously used, when given systemically to mice with an aggressive spontaneous leukemia/lymphoma, demonstrated substantial antitumor activity, alone or in combination with chemotherapy (Longe et al., 2009). Importantly, T-oligos have been well-tolerated after systemic administration to immunocompromised mice at doses sufficient to block development of human tumor xenografts (Puri et al., 2004), or spontaneous leukemias (Longe et al., 2009).
In this report, we have established the activity of a 16-mer T-oligo and a GT-rich 16-mer derivative against a number of human ovarian carcinoma cell lines, either as single agents or in combination with HDAC inhibitors. The GT-rich oligo is active at lower concentrations in the ovarian cancer cell lines studied than the parental 16-mer T oligo, and shows activity against individual ovarian carcinoma cell lines that are resistant to the T-oligo. In prior studies, the 16-mer T-oligo was shown to exhibit more antitumor activity than 11-mer oligos (Yaar et al., 2007; Longe et al., 2009).
The sensitivity of individual ovarian cancer cell lines to the oligos varied, however, ranging from the very sensitive PA1 line to the relatively resistant SKOV-3 line. The basis for these differences in sensitivities among tumor cell lines is not yet understood. T-oligos are thought to generate their tumor-specific cytotoxic activity through mimicking DNA damage, which initiates a transient cell cycle arrest in cells. Nonmalignant cells escape from this transient arrest without consequence, but an apoptotic process is initiated in tumor cells (see Rankin et al., 2008 for review). Activation of the ATM/p53 pathway in normal and malignant human cells by exposure to T-oligos has been demonstrated (Li et al., 2003; Longe et al., 2009). Others have therefore postulated a dependency upon p53 for the tumor-specific arrest and apoptotic response. Our previous work (Longe et al., 2009) and this current report, however, indicate that functional p53 is not required for T- or GT-oligo-induced cell cycle arrest or the subsequent initiation of apoptosis. For example, of the cell lines studied here, PA1 is reported to have wild-type p53, whereas the p53 proteins in CAOV-3 and OVCAR-3 cells are inactivated by mutations, and the SKOV-3 line lacks p53 expression. Thus, antitumor response to the GT-oligo in ovarian cancer cells is not dependent upon functional p53 signaling. The differential tumor cytotoxic potency of T-oligo and GT-oligo may be the result of the differing base compositions of each oligo. The higher G-content of GT-oligo increases the likelihood of forming G-quadruplex secondary structure, which may contribute to its enhanced activity, relative to T-oligo.
Cell cycle analysis of the ovarian carcinoma cell lines exposed to the GT-oligos or T-oligos were consistent with an initial transient S phase arrest, followed by apoptosis, and this same sequential pattern has been observed in human melanoma and murine and human lymphoma cells in our other studies (Eller et al., 2002, 2003; Li et al., 2004; Yaar et al., 2007; Longe et al., 2009). New results in human cancer cell lines demonstrate the dependence of the initial S-phase arrest on inhibition of the cyclin-dependent kinase cdk2 (A. Rankin and D.V. Faller, in preparation).
The 24–48-hour continuous exposure to T-oligo required for induction of cytotoxicity was initially unexpected, in light of expectations that oligos would degrade rapidly in media containing serum. Our data tracking a fluorescent-tagged oligo, however, demonstrate persistence of the oligo in media, and accumulation in exposed cells over 48 hours (S. Sarkar and D.V. Faller, unpublished).
Most of the drug-induced apoptotic events in cancer cells are mediated by the intrinsic or extrinsic signaling pathways, and some agents involve both pathways in different capacities (Frew et al., 2008). Both pathways culminate in the activation of caspase-3, and we observed significant activation of caspase-3/7 in response to GT-oligo. Our finding that caspase-9 is also activated by exposure to GT-oligos suggests a contribution of the mitochondrial-mediated intrinsic pathway in the induction of apoptosis. In new work, we have observed that exposure of ovarian carcinoma cells to GT-oligo induced the expression of TNF family receptors (unpublished). This finding poses an interesting scenario where GT-oligo-mediated cytotoxicity may be the result of activation of multiple apoptotic pathways.
The general current approaches for cancer therapy almost invariably employ combinations of cytotoxic agents, usually with different underlying modes of action. It is therefore of note that the oligos show at least additive activity with the class of HDAC inhibitory agents, including HDACi of different chemical classes. Further, exposure to HDACi rendered a relatively resistant ovarian cancer cell line (SKOV-3) sensitive to the oligo. HDACi agents have shown only modest clinical activity in cancer treatment, including in combination with conventional chemotherapeutic agents, so the potential to combine them with oligos may be of utility in the future. We had previously shown more-than-additive effects in combining T-oligos with specific chemotherapeutic agents like vinblastine (Longe et al., 2009). However, the ability of T-oligos to produce additive antitumor activity appears specific for the agent used in combination, as our unpublished studies did not show even additive activity when employing oligos in combination with paclitaxel in ovarian cancer cell lines.
In summary, we have demonstrated the susceptibility of multiple human ovarian cancer cell lines to 2 distinct T-oligos, and shown the activity of these T-oligos in combination with a new class of targeted tumor agents, the HDAC inhibitors. We and others have previously demonstrated the safety and activity of T-oligos in animal models (Puri et al., 2004; Yaar, et al., 2007; Longe et al., 2009). These T-oligos may therefore hold potential as therapeutics, either as single agents or in combination regimens, for the treatment of ovarian cancer.
Acknowledgments
Supported by the Department of Defense (contract grant number: W81XWH-06-1-0408; D.V.F.) the National Cancer Institute (CA133654, D.V.F.) and the Karin Grunebaum Cancer Research Foundation (D.V.F.).
Author Contributions
S.S. and D.V.F. designed the studies and evaluated the results. S.S. performed experiments. S.S. and D.V.F. wrote the article. Both authors read and approved the final article.
Author Disclosure Statement
Drs. Sarkar and Faller declare that they have no competing interests.
References
- AOKI H. IWADO E. ELLER M.S. KONDO Y. FUJIWARA K. LI G.Z. HESS K.R. SIWAK D.R. SAWAYA R. MILLS G.B. GILCHREST B.A. KONDO S. Telomere 3′ overhang-specific DNA oligonucleotides induce autophagy in malignant glioma cells. FASEB J. 2007;21:2918–2930. doi: 10.1096/fj.06-6941com. [DOI] [PubMed] [Google Scholar]
- ELLER M.S. LI G.Z. FIROOZABADI R. PURI N. GILCHREST B.A. Induction of a p95/Nbs1-mediated S phase checkpoint by telomere 3′ overhang specific DNA. FASEB J. 2003;17:152–162. doi: 10.1096/fj.02-0197com. [DOI] [PubMed] [Google Scholar]
- ELLER M.S. MAEDA T. MAGNONI C. ATWAL D. GILCHREST B.A. Enhancement of DNA repair in human skin cells by thymidine dinucleotides: evidence for a p53-mediated mammalian SOS response. Proc. Natl. Acad. Sci. U. S. A. 1997;94:12627–12632. doi: 10.1073/pnas.94.23.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLER M.S. PURI N. HADSHIEW I.M. VENNA S.S. GILCHREST B.A. Induction of apoptosis by telomere 3′ overhang-specific DNA. Exp. Cell Res. 2002;276:185–193. doi: 10.1006/excr.2002.5531. [DOI] [PubMed] [Google Scholar]
- ELLER M.S. YAAR M. GILCHREST B.A. DNA damage and melanogenesis. Nature. 1994;372:413–414. doi: 10.1038/372413a0. [DOI] [PubMed] [Google Scholar]
- FREW A.J. LINDEMANN R.K. MARTIN B.P. CLARKE C.J.P. SHARKEY J. ANTHONY D.A. BANKS K.-M. HAYNES N.M. GANGATIRKAR P. STANLEY K. BOLDEN J.E. TAKEDA K. YAGITA H. SECRIST J.P. SMYTH M.J. JOHNSTONE R.W. Combination therapy of established cancer using histone deacetylase inhibitor and TRAIL receptor agonist. Proc. Natl. Acad. Sci. U. S. A. 2008;105:11317–11322. doi: 10.1073/pnas.0801868105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEORGE P. BALI P. ANNAVARAPU S. SCUTO A. FISKUS W. GUO F. SIGUA C. SONDARVA G. MOSCINSKI L. ATADJA P. BHALLA K. Combination of the histone deacetylase inhibitor LBH589 and the hsp90 inhibitor 17-AAG is highly active against human CML-BC cells and AML cells with activating mutation of FLT-3. Blood. 2005;105:1768–1776. doi: 10.1182/blood-2004-09-3413. [DOI] [PubMed] [Google Scholar]
- GOUKASSIAN D.A. BAGHERI S. EL KEEB L. ELLER M.S. GILCHREST B.A. DNA oligonucleotide treatment corrects the age-associated decline in DNA repair capacity. FASEB J. 2002;16:754–756. doi: 10.1096/fj.01-0829fje. [DOI] [PubMed] [Google Scholar]
- GUARNERI V. PIACENTINI F. BARBIERI E. CONTE P.F. Achievements and unmet needs in the management of advanced ovarian cancer. Gynecol. Oncol. 2010;117:152–158. doi: 10.1016/j.ygyno.2009.11.033. [DOI] [PubMed] [Google Scholar]
- KARLSEDER J. BROCCOLI D. DAI Y. HARDY S. DE LANGE T. p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science. 1999;283:1321–1325. doi: 10.1126/science.283.5406.1321. [DOI] [PubMed] [Google Scholar]
- LI G.Z. ELLER M.S. FIROOZABADI R. GILCHREST B.A. Evidence that exposure of the telomere 3′ overhang sequence induces senescence. Proc. Natl. Acad. Sci. U. S. A. 2003;100:527–531. doi: 10.1073/pnas.0235444100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI G.Z. ELLER M.S. HANNA K. GILCHREST B.A. Signaling pathway requirements for induction of senescence by telomere homolog oligonucleotides. Exp. Cell Res. 2004;301:189–200. doi: 10.1016/j.yexcr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- LIU J. MATULONIS U.A. New advances in ovarian cancer. Oncology. 2010;24:721–728. [PubMed] [Google Scholar]
- LONGE H.O. ROMESSER P.B. RANKIN A.M. FALLER D.V. ELLER M.S. GILCHREST B.A. DENIS G.V. Telomere homolog oligonucleotides induce apoptosis in malignant but not in normal lymphoid cells: mechanism and therapeutic potential. Int. J. Cancer. 2009;124:473–482. doi: 10.1002/ijc.23946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHASHI N. YAAR M. ELLER M.S. TRUZZI F. GILCHREST B.A. Features that determine telomere homolog oligonucleotide-induced therapeutic DNA damage-like responses in cancer cells. J. Cell. Physiol. 2007;210:582–595. doi: 10.1002/jcp.20848. [DOI] [PubMed] [Google Scholar]
- PURI N. ELLER M.S. BYERS H.R. DYKSTRA S. KUBERA J. GILCHREST B.A. Telomere-based DNA damage responses: a new approach to melanoma. FASEB J. 2004;18:1373–1381. doi: 10.1096/fj.04-1774com. [DOI] [PubMed] [Google Scholar]
- RANKIN A.M. FALLER D.V. SPANJAARD R.A. Telomerase inhibitors and “T-oligo” as cancer therapeutics: contrasting molecular mechanisms of cytotoxicity. Anticancer Drugs. 2008;19:329–338. doi: 10.1097/CAD.0b013e3282f5d4c2. [DOI] [PubMed] [Google Scholar]
- SAITO A. YAMASHITA T. MARIKO Y. NOSAKA Y. TSUCHIYA K. ANDO T. SUZUKI T. TSURUO T. NAKANISHI O. A synthetic inhibitor of histone deacetylase, MS-27-275, with marked in vivo antitumor activity against human tumors. Proc. Natl. Acad. Sci. U. S. A. 1999;96:4592–4597. doi: 10.1073/pnas.96.8.4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARKAR S. SVOBODA M. DE BEAUMONT R. FREEDMAN A.S. The role of Akt and RAFTK in beta1 integrin mediated survival of precursor B-acute lymphoblastic leukemia cells. Leuk. Lymphoma. 2002;43:1663–1671. doi: 10.1080/1042819021000003009. [DOI] [PubMed] [Google Scholar]
- TAKAI N. NARAHARA H. Histone deacetylase inhibitor therapy in epithelial ovarian cancer. J. Oncol. 2010;2010:458431. doi: 10.1155/2010/458431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAZIRI C. FALLER D.V. Repression of platelet-derived growth factor beta-receptor expression by mitogenic growth factors and transforming oncogenes in murine 3T3 fibroblasts. Mol. Cell. Biol. 1995;15:1244–1253. doi: 10.1128/mcb.15.3.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAAR M. ELLER M.S. PANOVA I. KUBERA J. WEE L.H. COWAN K.H. GILCHREST B.A. Telomeric DNA induces apoptosis and senescence of human breast carcinoma cells. Breast Cancer Res. 2007;9:R13. doi: 10.1186/bcr1646. [DOI] [PMC free article] [PubMed] [Google Scholar]