Abstract
In relating pollution to birth outcomes, maternal exposure has usually been described using monitoring data. Such characterization provides a misrepresentation of exposure as it (i) does not take into account the spatial misalignment between an individual’s residence and monitoring sites, and (ii) it ignores the fact that individuals spend most of their time indoors and typically in more than one location. In this paper, we break with previous studies by using a stochastic simulator to describe personal exposure (to particulate matter) and then relate simulated exposures at the individual level to the health outcome (birthweight) rather than aggregating to a selected spatial unit.
We propose a hierarchical model that, at the first stage, specifies a linear relationship between birthweight and personal exposure, adjusting for individual risk factors and introduces random spatial effects for the census tract of maternal residence. At the second stage, our hierarchical model specifies the distribution of each individual’s personal exposure using the empirical distribution yielded by the stochastic simulator as well as a model for the spatial random effects.
We have applied our framework to analyze birthweight data from 14 counties in North Carolina in years 2001 and 2002. We investigate whether there are certain aspects and time windows of exposure that are more detrimental to birthweight by building different exposure metrics which we incorporate, one by one, in our hierarchical model. To assess the difference in relating ambient exposure to birthweight versus personal exposure to birthweight, we compare estimates of the effect of air pollution obtained from hierarchical models that linearly relate ambient exposure and birthweight versus those obtained from our modeling framework.
Our analysis does not show a significant effect of PM2.5 on birthweight for reasons which we discuss. However, our modeling framework serves as a template for analyzing the relationship between personal exposure and longer term health endpoints.
Keywords: Conditionally autoregressive (CAR) model, exposure metrics, hierarchical model, stochastic simulator
1 Introduction
The contribution of this paper is to develop a prototype hierarchical model for assessing the effect of air pollution on a long-term health outcome such as birthweight. We use a stochastic human exposure simulator, captured through empirical distributions, driven by ambient exposure obtained through fusing monitoring data and atmospheric numerical model output. We model at the individual level rather than aggregating to counts at census units in order to incorporate individual level risk factors. However, spatial random effects are introduced at the census unit level. We offer novel exposure metrics beyond average concentration which can be created due to the high spatial and temporal resolution in our pollution data. We propagate uncertainty in exposure through the hierarchical model to obtain appropriate uncertainty in the effect of exposure as well as in prediction using the model.
Air pollution has an adverse effect on human health: increasing concentration of pollutants are associated with a significant increase in nonaccidental mortality (Goldberg et al. 2001), total mortality (Dockery et al. 1993; Schwartz 1994; Katsouyanni et al. 2001; Fuentes et al. 2006), cardiovascular disease (Hoek et al. 2001; Braga et al. 2001), respiratory deaths (Ostro et al. 2000; Braga et al. 2001), and morbity in general (Schwartz 1999; Pope et al. 1995; Samet et al. 2000; Zanobetti et al. 2000). Health risks are not homogeneously spread across the population: elderly (Saldiva et al. 1995; Katsouyanni et al. 2001), children (Dockery and Pope 1994; Heinrich et al. 1999; Schwartz et al. 1994), and infants (Bobak and Leon 1992; Woodruff et al. 1997) are more vulnerable to the harmful effects of air pollution than the general adult population.
More recently, it has been suggested that fetuses might also be susceptible to the noxious effects of air pollution and a growing body of research on air pollution and birth outcomes has been carried out to validate such hypothesis. Studies have been conducted in several parts of the United States as well as around the world. In the United States, researchers have examined the relationship between air pollution and birth outcomes in California (Basu et al. 2004; Ritz and Yu 1999; Ritz et al. 2000; Parker et al. 2005), Nevada (Chen et al. 2002), Georgia (Rogers and Dunlop 2000, 2005), Connecticut and Massachusets (Bell et al. 2007), Northeastern US (Maisonet et al. 2001), and the entire US (Woodruff et al. 1997). Internationally, studies have been conducted in Brazil (Gouveia et al. 2004), Canada (Liu et al. 2003; Dugandzic et al. 2006), Korea (Ha et al. 2001; Lee et al. 2003), Taiwan (Yang et al. 2003; Lin et al. 2004), Australia (Mannes et al. 2005; Hansen et al. 2006), China (Wang et al. 1997), and the Czech Republic (Bobak 2000). In each of these studies, the effects of exposure to several pollutants (ozone, particulate matter, total suspended particles, sulfure dioxide, nitrogen dioxide, and carbon monoxide) during different phases of the pregnancy (first trimester, last trimester, entire pregnancy) on birth outcomes (birth weight, low birth weight, preterm delivery, intrauterine growth restrictions and retardation, birth defects) have been examined with various results. Some authors have found an increase in the risk of low birth weight or premature birth as mothers are exposed to increasing concentrations of either pollutants during the entire pregnancy or earlier/later stages of the pregnancy. Others did not find a significant association between exposure and birth outcomes during any particular time window. Reviews on some of the literature on the effect of air pollution on pregnancy outcomes can be found in S̆rám et al. (2005); S̆rám (1999); Maisonet et al. (2004).
Despite differences in spatial locations, populations considered, temporal window of exposure and/or pollutants examined, all the studies on air pollution and birth outcomes share a similar characteristic. Maternal exposure to a pollutant is usually quantified by taking the average concentration, over the selected time window, of the pollutant under consideration as it is reported by outdoor monitors. While using monitoring data might seem a natural choice, there are two main problems with such a characterization of exposure: (i) monitors are not located where subjects live and (ii) individuals do not spend all of their time outdoors. As a consequence, ambient exposure, that is, exposure to an air pollutant in an outdoor environment, should not be considered equivalent to personal exposure and care should be taken in extrapolating the relationship between ambient exposure and birth outcomes to personal exposure and birth outcomes.
During the course of a day, an individual is exposed to pollution generated from sources that are located both outdoors and indoors. Therefore an individual’s daily personal exposure is the the sum of the exposure to pollutants accumulated in these two macroenvironments. It is clear then that personal exposure and ambient exposure are not equal (Dockery and Spengler 1981; Lioy et al. 1990; Spengler et al. 1985; Kinney et al. 2006), and studies have also shown that they are only weakly correlated (Lioy et al. 1990; Özkaynak et al. 1996; Wallace 1996). In light of this, using ambient exposure as a proxy for personal exposure in health studies might lead to biases both in the estimate of the effect of air pollution (Armstrong et al. 1992; Zeger et al. 2000) and in the assessment of its uncertainty (Samet et al. 2000; Gryparis et al. 2008).
To account for the discrepancy between ambient and personal exposure, Dominici et al. (2000) propose a hierarchical measurement error model that links personal exposure to mortality in Baltimore in two stages. In the first stage, Dominici et al. (2000) use external data from five different cities to estimate the relationship between average ambient concentration and average personal exposure. They use this relationship to derive personal exposures for Baltimore, given the ambient concentration. Then, they establish a linear relationship between the derived personal exposures and mortality. Using a similar framework and considering again the city of Baltimore, McBride et al. (2007) presents a Bayesian hierarchical model whose aim is to characterize the relationship between personal exposure to fine particulate matter, that is PM2.5, and ambient concentration.
In both of these studies, personal exposure is derived from ambient exposure using a regression model. A different approach to obtain personal exposures is by using exposure simulators. Exposure simulators are stochastic models that predict the average personal exposure to a pollutant for demographic subgroups by randomly sampling individuals from each demographic group and randomly associating to each individual a time activity pattern that matches the subject in terms of personal characteristics, day of the week, temperature, season, etc. A description of the theoretical probabilistic framework underlying exposure simulators such as pNEM (Law et al. 1997), SHEDS-PM (Burke et al. 2001), APEX (Ozone2006 2000) and pCNEM (Zidek et al. 2003, 2007) can be found in Zidek et al. (2003, 2007).
Initially, exposure simulators were developed with the goal of determining the effect of ambient regulatory strategies on human exposure by observing how different ambient concentration scenarios changed the predicted personal exposure for each demographic group. Recently, they have been used as a tool to estimate personal exposures that are subsequently incorporated into statistical models relating air pollution to health outcomes. Holloman et al. (2004) and Calder et al. (2008) use a deterministic simplified version of the SHEDS-PM simulator to derive personal exposures to PM2.5 for eight counties in North Carolina for which they analyze mortality data. Shaddick et al. (2008) obtain sets of personal exposures to PM10 for eight districts in the Greater London area using the pCNEM simulator. A parametric distribution is fitted to these exposures which provides a second stage to relate exposure to mortality counts for senior citizens using a Bayesian hierarchical framework. A similar approach is employed by Reich et al. (2008), who estimate personal exposures using the SHEDS-PM simulator and relate them to mortality in Fresno (CA) using a Bayesian hierarchical model.
Additionally, in this work, both the health outcome and the personal exposure are not modeled at the individual level, but rather they are aggregated over an area. As noted at the outset, we present a methodological template for employing contaminant exposure simulators to explain a health outcome when both the personal exposure and the health outcome are considered at the individual level. Again, this enables us to introduce individual level risk factors. In particular, we consider PM2.5 as the pollutant, birthweight as the health outcome and use the SHEDS-PM model (Burke et al. 2001) as the exposure simulator. SHEDS-PM enables us to account for variation in the daily activity of pregnant women and hence to capture uncertainty in exposure.
To assess the difference in relating ambient exposure and birthweight versus realting personal exposure and birthweight, we compare the use of ambient exposure developed through fusion of monitoring station data and computer model output with exposure developed through the simulator. This allows us to address a long-standing question of whether more reliable measures of exposure provide a stronger signal with regard to effect on birthweight outcomes. Additionally, to characterize maternal exposure over the course of a pregnancy we have built several metrics that take us beyond average concentration and allows us to investigate various windows of vulnerability. We introduce these, one at time, into a regression model to explain birthweight. The simulator provides us only with empirical prior distributions for these metrics, not actual realizations for a given pregnancy. The uncertainty in these exposure metrics is then propagated to uncertainty in birthweight via a Bayesian hierarchical model that we fit through MCMC methods.
For the dataset we work with, we find a weak story regardless of how we introduce exposure; there seems to be no evidence of a relationship between PM2.5 exposure and birthweight. We discuss why, for our analysis, this might be expected. Still, taking a broader view, our approach can be viewed as a prototype for future investigations of this sort.
The format of the paper is as follows. In Section 2 we describe the birth outcome dataset we used as well as exposure data. In Section 3 we describe the SHEDS-PM model and how we used it here. Section 4 develops the various metrics we use to characterize exposure during a pregnancy. Section 5 develops the hierarchical model we employ while Section 6 summarizes the results of our analyses. We conclude with a summary and discussion in Section 7.
2 Data
2.1 Birth outcome data
The Detailed Birth Record (DBR) database documents every live birth occurring in the state of North Carolina since 1990. For each birth, the DBR database reports the weight, originally measured in pounds (0-14) and ounces (0-16) and subsequently transformed in grams, the plurality, the number of children born dead and alive, the sex, the presence/absence of congenital anomalies and/or maternal complications, and the gestation length, clinically estimated and measured in weeks. The database also contains extensive information on maternal and paternal demographics, such as maternal and paternal race, maternal education, maternal age, maternal tobacco and alcohol use, marital status and maternal residence. For our analysis, we have restricted our attention to birth outcome data for years 2001 and 2002 since these are the years for which fused PM2.5 exposure data was available to us. Since our interest is to study the relationship between air quality and birth outcomes, in this study we only consider births occurring in the counties in the corridor along the two major interstate highways that cross the center of the state, Interstate 85 and Interstate 40, where we would expect the air quality to be the poorest in the state. Those are also the counties that include the major urban areas and population centers in North Carolina. The fourteen North Carolina counties and associated census tracts included in this study are shown in Figure 1. They are, from South to North, Gaston, Mecklenburg, Cabarrus, Rowan, Davidson, Randolph, Guilford, Alamance, Orange, Durham, Granville and Vance.
Figure 1.
Fourteen counties in North Carolina included in this analysis (top panel) and, in detail, the census tracts contained in the fourteen counties (bottom panel).
As our goal is to determine the effect on birthweight of maternal exposure to PM2.5 over the course of the pregnancy on birthweight, we have only analyzed birth outcomes relative to pregnancies for which the entire gestation is fully included in the period January 1, 2001 - December 31, 2002. We have also restricted the birth outcome data based on personal characteristics of the mother. Precisely, we have only considered birth outcomes for mothers who self-described themselves as either “Non-Hispanic White”, “Non-Hispanic Black” or “Hispanics”, with an age between 15 and 44 years, who had a gestation of at least 24 weeks and at most 42 weeks, and for which information on maternal tobacco and alcohol use was available. Finally, we excluded from our study births that were less than 400 grams, births with congenital anomalies, and non-singleton births.
In total, our dataset comprises 49,869 births. Of these, the majority, about 59.4%, were from non-hispanic white mothers, while 25.7% were from non-hispanic black mothers and 15.0% from hispanic mothers. Maternal education was reported as number of years of schools completed. We subsequently grouped this variable in five categories: “middle school”, “some high school”, ”high school”, ”some college” and ”college”. About 35% of the mothers in our dataset graduated from college, while 20.4% went through some years of it but did not complete it. Among the remaining mothers, 24.1% graduated from high school, 14.1% did not complete it and only 6.7% has a middle school diploma. Maternal age was originally recorded in integer years, however to facilitate the analysis and the merging of SHEDS-PM simulated individuals with mothers in our birth outcomes dataset, we grouped maternal age into four age classes: 15 to 17 years, 18 to 24, 25 to 34 and 35 to 44. About 54% of the births in our dataset were from mothers aged between 25 to 34 years, while only 3.4% were from mothers aged between 15 to 17 years of age. Additionally, in our analysis we included marital status (32.5% are married) and self-reported maternal tobacco use during pregnancy (only 9.5%).
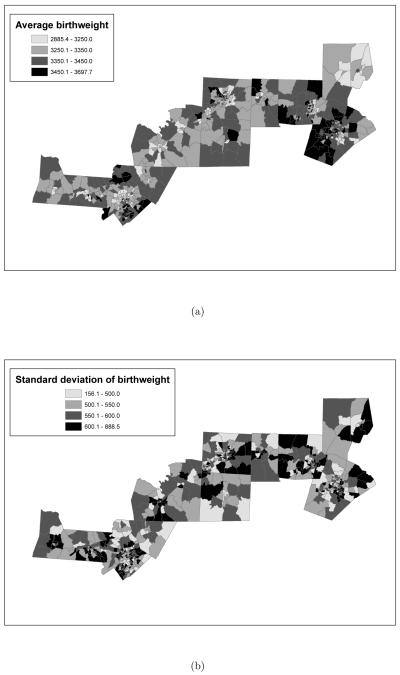
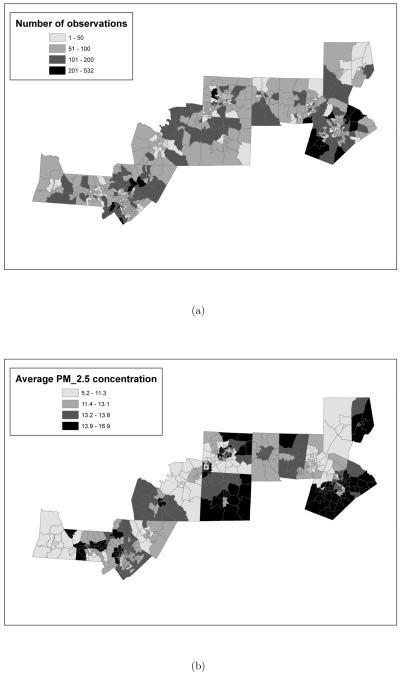
Finally, with the use of Geographical Information System (GIS) software, it has been possible to geocode every birth in the dataset to the census tract of residence of the mother. This has enabled us to analyze the birth outcome data spatially, as the model in Section 5 indicates. Spatial maps of the average birthweight with corresponding standard deviations for each census tract considered in this study are presented in Figure 2(a) and (b), while Figure 3(a) displays the number of observations in each census tract. As Figure 2(a) shows the average birthweight in the central counties of Alamance, Orange and Durham is among the largest. However, the same counties are also characterized by great variability in birthweight, as Figure 2(b) shows.
Figure 2.
(a) Average birthweight and (b) standard deviation of birthweigth (in grams) by census tract for births that occurred in years 2001 and 2002. In both panels the breakpoints of each class are the quartiles of the variable displayed.
Figure 3.
(a) Number of observations per census tract. (b) Average PM2.5 concentration (in μg/m3) by census tract in fourteen counties in North Carolina during years 2001-2002. In panel (b) the breakpoints are the quartiles of the distribution of average concentration.
2.2 Air pollution data
Particulate matter (PM) consists of small particles of solids and liquids that are suspended in the air. They are introduced in the atmosphere as a result of both biological and chemical processes and human activities (motor vehicles, power plants and wood burning, for example). In our study, we focus only on PM2.5 (also called fine particulate matter) and we examine the effect of exposure to fine particulate matter on birth outcomes. Our air pollution data refers to daily concentration of PM2.5. Two different types of data on PM2.5 concentration were available to us: monitoring data collected at sites sparsely located over North Carolina and gridded predictions of daily PM2.5 average concentration. The latter were produced by the Models-3/Community Mesoscale Air Quality (CMAQ; http://www.epa.gove/asmdnerl/CMAQ), a numerical model that estimates daily concentration of PM2.5 by integrating information coming from three different components: a meteorological component which accounts for the state and evolution in time of the atmosphere, an emission component which deals with emission injected in the atmosphere by both chemical plants and natural processes, and a component that accounts for the chemical and physical interactions occurring in the atmosphere. For our study we have used daily predictions of PM2.5 concentration obtained from the CMAQ numerical model run at the 12-km spatial resolution. The CMAQ output is available for each day and at higher spatial resolution than the monitoring network though it may have calibration issues. The monitoring station is not necessarily collected daily but, when available, it provides measurements that would be deemed more reliable than CMAQ output.
We use the fusion approach of McMillan et al. (2008) to exploit both sources of information (monitoring data and CMAQ output). Hence, we assume that there exists a true unobserved spatial process defined at the 12-km grid cell level that is related to both the observational data and the numerical model output via error measurement models. We use the median of the posterior predictive distribution of the unobserved spatial process given the observational data and the CMAQ model output as the daily “fused ambient concentration” of PM2.5. This fused data is defined at the grid cell level as the unobserved spatial process. Since the spatial resolution at which the SHEDS-PM exposure simulator operates is at the census tract level, we have further interpolated our daily fused predictive surfaces of PM2.5 concentration to the centroids of the census tracts considered in this study using ordinary kriging (Cressie 1993) with an exponential covariance function. Figure 3(b) displays the average PM2.5 concentration during years 2001-2002 as obtained from the fused surfaces for each census tract considered in this study. As the figure shows, there is considerable spatial variability in the level of air pollution, with the central counties Wake and Guilford having two of the highest average concentrations. The spatial map also displays some unexpected features, such as the moderate level of PM2.5 in Mecklenburg county, which contains the city of Charlotte, and the elevated concentration of PM2.5 in Vance county. The latter is likely explained by the presence of a power plant nearby.
3 SHEDS-PM
The Stochastic Human Exposure and Dose Simulator for PM (SHEDS-PM; Burke et al. 2001) is a stochastic model that estimates the distribution of average daily personal exposure for a population via a two-dimensional Monte Carlo sampling scheme that propagates the uncertainty in the input distributions to the predicted distribution of personal exposure. Several different inputs are needed by the SHEDS-PM model, including probability distributions of exposure factors and three types of databases. The latter include: 1) hourly or daily average of PM2.5 concentration for each census tract in the spatial domain of interest; 2) demographic information for each census tract; and 3) human activity diaries with data on the amount of time individuals spend in different locations (i.e. outdoors, indoors, in vehicle). Among the inputs to SHEDS-PM that are provided with a probability distribution are: parameters of equations for estimating indoor PM concentrations, such as residential air exchange rates, house volumes, penetration/deposition rates, and indoor source emission rates (e.g. cooking, cleaning, smoking). The distributions assigned to each of these inputs have been developed using data from previous studies and may characterize the variability in the data using multiple distributions that depend on season or housing type for example.
To estimate the distribution of average daily personal exposure for a population, SHEDS-PM simulates for each census tract, a representative number of individuals that match the census tract in demographic characteristics such as gender, age, employment status and housing type. Each simulated individual is randomly assigned a set of activity diaries for the time period of PM concentration data, based on the individual’s personal characteristics, day of the week and season. SHEDS-PM calculates the PM concentration for each location in the individual’s activity diary (microenvironment) using the time series of ambient PM concentrations in equations that estimate both the contribution due to outdoor sources, that is, ambient concentration, and the contribution due to indoor sources, or non-ambient concentration, which may depend on the individual’s activity within the microenvironment.
More specifically, let i denote a census tract, s an individual simulated by SHEDS-PM and Ci(d) the average daily PM2.5 concentration in census tract i on day d. Then, the ambient exposure or the exposure due to outdoor concentration for individual s living in census tract i on day d is:
| (1) |
An individual’s exposure in each of seven indoor microenvironments is derived using microenvironment specific equations. In particular, for the residence microenvironment (denoted by r), the average daily concentration of PM2.5 for simulated individual s living in census tract i is obtained using the single-compartment steady-state mass balance equation (Özkaynak et al. 1996):
| (2) |
where Ps, ks, achs and Vis denote, respectively, the penetration factor, the deposition rate, the air exchange rate and the volume of the residence of simulated individual s living in census tract i, while Esmk,s, Ncig,s, Ecook,s, tcook,s, and Eother,s indicate, respectively, the emission rate for smoking, the numbers of cigarette individual s smoked during the SHEDS-PM model time step T, the emission rate for cooking, the time simulated individual s spent cooking during model time step T, and the emission rate for other sources for simulated individual s.
On the other hand, the average daily concentration of PM2.5 in any other indoor microenvironment m different from residence (e.g., office, school, store, restaurant, bar and in vehicle) is given by:
| (3) |
where and represent, respectively, the contributions to average concentration in microenvironment m due to indoor and outdoor sources. The regression coeffcients in (3) are provided with probability distributions derived from previous exposure studies, and are randomly sampled for each simulated individual.
The average daily personal exposure for a simulated individual s is computed by summing the individual’s exposure to ambient concentration and the individual’s exposure in each of the seven indoor microenvironments. At their turn, each of these terms is obtained by multiplying, respectively, the average daily concentration of particulate matter either outdoor or in any of the seven indoor microenvironments by the amount of time the individual spends outdoor or in any of the indoor microenvironments.
More specifically, revising (1), if m = 1, … , 7 denotes an indoor microenvironment and indicates the average daily concentration of particulate matter in indoor microenvironment m for individual s, with , average daily concentration of PM2.5 in the residential microenvironment, then the average daily personal exposure for individual s is given by:
| (4) |
with fraction of time individual s spends outdoors and fraction of time individual spends in indoor microenvironment m, m = 1, … , 7.
Finally, the distribution of average daily personal exposure on day d for a population is obtained as the empirical distribution of average daily personal exposure for each simulated individual.
In addition to the average daily personal exposure for each simulated individual, SHEDS-PM also returns the decomposition of the exposure in terms of outdoor and indoor sources.
4 Characterizing exposure
During the course of a pregnancy, a woman undergoes physiological, biochemical and anatomical changes that might affect her overall response and susceptibility to external stimuli. To investigate whether there are periods during the course of a pregnancy in which a mother and her fetus are more vulnerable to the harmful effects of PM2.5, and to determine which aspects of the exposure to PM2.5 are more critical, we characterize maternal exposure to PM2.5 using different metrics examined in different time windows.
We can develop such metrics because of the high spatio-temporal resolution of our air pollution data. Normally, in studies of air pollution and birth outcomes, researchers have used only monitoring data to represent exposures. Due to the infrequent and irregular sampling of PM2.5 monitors and their sparse locations, most researchers have used as exposure metric the ambient average concentration reported by monitors located within a county (Chen et al. 2002; Basu et al. 2004; Bell et al. 2007), an area (Lee et al. 2003; Liu et al. 2003; Wang et al. 1997; Woodruff et al. 1997), or a city (Ha et al. 2001; Mannes et al. 2005; Gouveia et al. 2004; Hansen et al. 2006; Maisonet et al. 2001). Others have used as exposure metric the average obtained from the closest monitor(s) to the subject’s residence, but limiting their analysis only to birth outcomes for mothers who reside within a certain distance from monitoring sites (Basu et al. 2004; Dugandzic et al. 2006; Lin et al. 2004; Mannes et al. 2005; Parker et al. 2005; Ritz et al. 2000; Ritz and Yu 1999; Yang et al. 2003). In our case, the high spatio-temporal resolution of the air pollution data, permits us to use all the birth outcome data and allows us to investigate several research questions. More specifically, we can investigate whether it is the prolonged maternal exposure to PM2.5 or the exposure to extreme values of PM2.5 or the combined effect of both that is more harmful to the fetus.
To describe the three different metrics that we have developed, we follow the notation introduced in Section 3, denoting with i a census tract, j an individual, and Ci(d) the average daily PM2.5 concentration in census tract i on day d. We indicate with Tij the period of long-term exposure for individual j living in census tract i, while we use the symbol Wij to indicate any sub-window of exposure, i.e., . In the context of our study, the period of long-term exposure Tij coincides with subject j’s entire pregnancy; in other environmental exposure studies, the definition of the period of long-term exposure will be different and might not be subject-specific. As in (1), the daily ambient exposure for individual j on day d is given by . This implies that the ambient long-term exposure for individual j during Tij is given by the time series:
On the other hand, we denote with the daily personal exposure for individual j living in census tract i on day d. For each census tract i and individual j, we estimate using SHEDS-PM. More precisely, if we denote with the set of individuals sj simulated by SHEDS-PM in census tract i, that match subject j in terms of age, then we estimate using the set of personal exposure derived by SHEDS-PM using (4). It follows that the personal long-term exposure for individual j during Tij is estimated using the set of time series
In our study, for each i and j we took the set to be of cardinality n = 30, while we set the average daily ambient concentration Ci(d) of PM2.5 in census tract i on day d to be equal to the fused daily concentration of PM2.5.
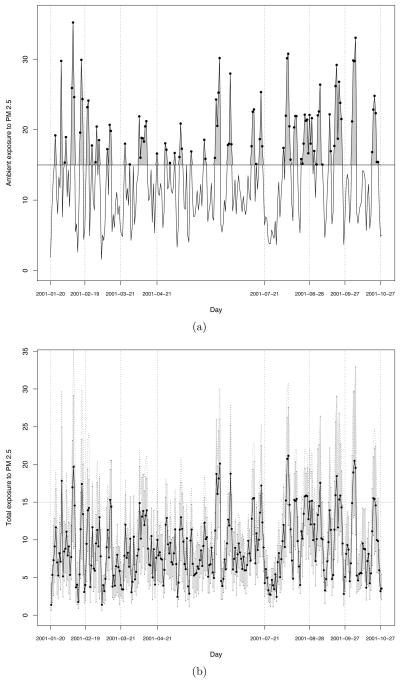
Figure 4(a) shows the time series for an illustrative mother in our database living in Mecklenburg county whose pregnancy Tij spans the period from January 20 to October 27, 2001. We call the time series displayed in Figure 4(a) the ambient exposure curve for subject j. On the other hand, Figure 4(b) displays, for each day d in Tij the range of the set for the same mother considered in Figure 4(a). The personal exposure curve for individual j is then obtained by linking for each day d the median of the set .
Figure 4.
(a) Ambient exposure curve or daily exposure to “fused” ambient PM2.5 concentration during the entire course of the pregnancy for a woman of living in a census tract in Mecklenburg. (b) Range of the 30 daily personal exposure values associated to the woman in consideration obtained from the SHEDS-PM simulator (gray vertical parallel lines) and median of the 30 daily personal exposure values (black solid line and black dots).
The vertical lines in Figure 4(a) and Figure 4(b) indicate the seven different time windows Wij that we have considered in our analysis for each i, j: respectively, the first 30 days, the first 60 days, the first trimester, the second trimester, the last 60 days, the last 30 days, and the entire pregnancy, Tij. Each of the metrics that we present below have been examined through these seven time windows.
First metric: average exposure
Following previous studies on air pollution and birthweight, we take as a first metric the average exposure to PM2.5 over any time window Wij. Thus, under the ambient exposure scenario, the first metric, the average ambient exposure, is given by
| (5) |
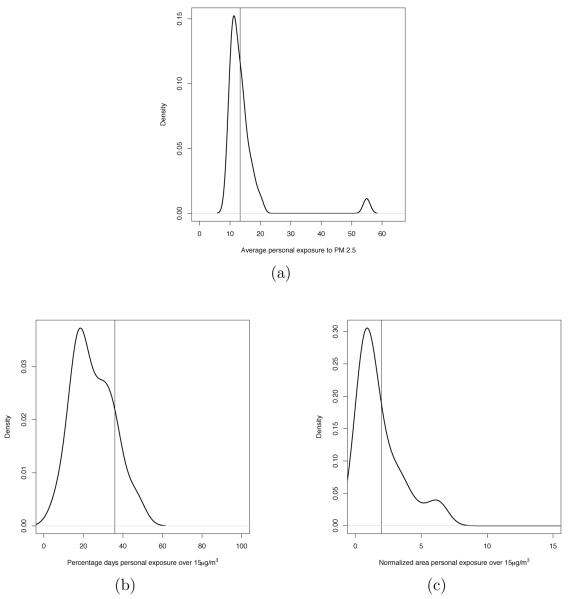
where |Wij| denotes the length of the time window Wij. For the illustrative mother in Mecklenburg, the value of this metric over the entire pregnancy is 13.36μg/m3. Under the personal exposure scenario the average personal exposure, , is estimated using the set , where, for each simulated individual , is computed using the analogous version of (5) with being replaced by . Thus, under the personal exposure scenario, the first metric is estimated with a distribution. Figure 5(a) displays a kernel density estimate of this distribution when Wij = Tij. The value of the average ambient exposure during the entire pregnancy Tij is indicated in Figure 5(a) with a vertical line.
Figure 5.
(a) Distributions of the average personal exposure to PM2.5 during the entire pregnancy for the mother considered in Figure 4(a). (b) Distribution of the percentage of days the personal exposure to PM2.5 was above 15 μg/m3 during the entire pregnancy for the mother considered in Figure 4(a). (c) Distributions of the normalized area under the personal exposure curve above 15 μg/m3 during the entire pregnancy for the mother considered in Figure 4(a). In panels (a)-(c) the vertical line display the value of the corresponding three metrics obtained using the ambient exposure.
Second metric: percentage of days over a threshold
The second metric finds its justification in the hypothesis that perhaps exposure to high values of PM2.5 is more deleterious for birthweight than a prolonged exposure to near average or lower concentrations. Therefore, for individual j living in census tract i, we define percentage of days daily ambient exposure was above a threshold level λ during time window Wij, the quantity:
| (6) |
where is the indicator function of the set . Note that in (6), we standardize the metric to account for differences in the length of pregnancies.
Given the frequency distribution of daily PM2.5 concentration in the census tracts in North Carolina considered in this study, we took as threshold the λ National Ambient Air Quality Standards (NAAQS) set by EPA for particulate matter for the yearly average, that is, 15μg/m3. Using this threshold, the value of the second metric for the illustrative mother in Mecklenburg is equal to 35.92% when Wij = Tij. In an analogous way, we define the second metric under the personal exposure scenario: the percentage of days daily personal exposure was above a threshold λ, . As for the first exposure metric, we estimate using the set where has been computed, for each by using an appropriately modified version of (6). A kernel density estimate of the distribution defined by the set for the illustrative mother living Mecklenburg is presented in Figure 5(b), with the in vertical line displaying the value of the second metric under the ambient exposure approach. Here, Wij = Tij.
Third metric: area above a threshold
The second metric, while accounting for extremes, does not take into consideration their magnitude. Additionally, it does not differentiate between situations where high concentration of particulate matter is an isolated event and situations where the elevated exposure is prolonged over time. To take into account both of these aspects, magnitude and length of duration, we have defined as third metric the area under the subject’s exposure curve above a threshold λ during a selected time window Wij. Also in this case, to account for difference in gestation lengths, we have standardized the metric by dividing it by the length of the time window. Therefore, in the case of ambient exposure, for any window of exposure Wij, we define the normalized area under the ambient exposure curve above the quantity
| (7) |
Analogously, we define the normalized area under the personal exposure curve above λ, , which we estimate via the distribution defined by the set . For the illustrative mother in consideration, taking λ = 15μg/m3, the normalized area under the ambient exposure curve above λ when , while the kernel density estimate of the distribution defined by the set is displayed in Figure 5(c).
Thus, under the ambient exposure scenario, to each mother is associated a set of 3 × 7 = 21 values, given by the three metrics evaluated over the seven time windows. On the other hand, under the personal exposure approach, to each mother is associated a set of 21 distributions.
Figure 5 presents kernel density estimates of three of these distributions for the illustrative mother that we took as example. In each panel, the corresponding value of the metric obtained using ambient exposure is indicated by a vertical line. As these plots seem to indicate, for this particular individual, the value of the exposure metrics obtained using ambient exposure are either about the same magnitude or larger than the median of the distributions of the corresponding exposure metrics obtained using the personal exposure curves. This is true for all the subjects in our study as pairwise scatterplots of the value of the three exposure metrics obtained using ambient exposures and the median of the distributions of the corresponding exposure metrics obtained using the personal exposure curves when the time window of exposure Wij is the entire pregnancy Tij indicate (figure not shown).
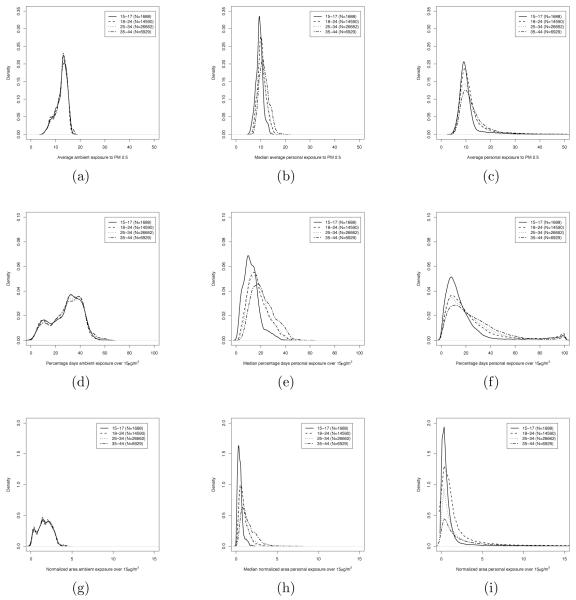
To further illustrate the relationship between the values of the exposure metrics obtained using the ambient exposure and the distributions of those metrics obtained under the personal exposure scenario, Figure 6 shows kernel density estimates of the distributions of the three metrics for all the women in our database grouped by age when the time selected time window is the entire pregnancy. More specifically, Figure 6(a) presents the distribution of the average ambient exposure, while panel (c) shows the distribution of the average personal exposure. To construct the distribution presented in panel (b) we have considered, for each woman, the median of the distribution of the average personal exposure. We have proceeded similarly for the other two metrics.
Figure 6.
Kernel density estimate of the distribution of the three metrics considered over the entire pregnancy. (a) Average ambient exposure, (b) median average personal exposure, and (c) average personal exposure; (d) percentage of days ambient exposure was above 15 μg/m3, (e) median percentage of days personal exposure was above 15 μg/m3, and (f) percentage of days personal exposure was above 15 μg/m3; (g) normalized area under ambient exposure curve above 15 μg/m3, (h) median normalized area under personal exposure curve above 15 μg/m3, and (i) normalized area under personal exposure curve above 15 μg/m3. In each panel, the kernel density estimates are grouped by age groups.
In accord with what was observed in Figure 5, the average ambient exposure metric and the median of the distribution of average personal exposure are fairly comparable in terms of magnitude and spread. On the other hand, the distribution of average personal exposure is more spread out than that of average ambient exposure as panel (c) in Figure 6 indicate allowing for the possibility of high personal exposures, which are not contemplated in the ambient exposure scenario. Also, as a natural consequence, the range of possible values for the second and third metrics are larger under the personal exposure approach than in the ambient exposure scenario, as panels (e) and (h) of Figure 6 show. Additionally, while ambient exposure doesn’t present any difference in the profile of the distributions for the different age groups, the distributions of the three metrics are different for the four age groups when exposure is characterized in terms of personal exposure. In particular, from Figure 6 it appears that, in general, personal exposures of younger mothers are lower than that of older mothers, even though there is positive probability that a younger mother might be subject to high concentrations of PM2.5 during the course of her pregnancy.
5 The Model
We start by presenting the model employed in the ambient exposure scenario and we subsequently show how the model is modified under the personal exposure approach. In both cases, the spatial unit at which we work is the census tract, a total of 616 in our study region.
5.1 Using ambient exposure
Let i be the index denoting a census tract in our area of study, with i = 1, … , M = 616, and let Yij denote the birthweight of a baby born to mother j living in census tract i. Then, for each census tract i, j ranges from 0 to Ni, Ni being the number of subjects in our birth outcome dataset living in census tract i. Let Xij be a vector with socio-economic information for subject j living in census tract i. Xij contains information on: gestation length, measured in weeks, maternal race, categorized into three classes (Non-Hispanic White, Non-Hispanic Black, and Hispanics), maternal age, grouped into four classes (15 to 17, 18 to 24, 25 to 34, and 35 to 44 years of age), maternal education, categorized into five classes (middle school, some high school, high school, some college, and college), maternal smoking status, grouped into two classes (smoker and non-smoker), marital status (married and not-married), birth order, grouped into two categories (first born and not-first born), sex of the baby. Let Oi denote the percentage of owner-occupied units in census tract i. In performing our analysis we take as baseline the following classes: Non-Hispanic White for maternal race, 25 to 34 for maternal age class, high school for maternal eduction, non-smoking for smoking status, married for marital status, non-first born for birth order, and female for sex of the baby. differently from some studies on the relationship between maternal exposure to a pollutant and birthweight (Woodruff et al. 1997; Ritz and Yu 1999; Ritz et al. 2000; Parker et al. 2005; Basu et al. 2004; Hansen et al. 2006; Yang et al. 2003; Chen et al. 2002), our model includes also gestation length. As Dugandzic et al. (2006); Bell et al. (2007); Wang et al. (1997); Maisonet et al. (2001); Liu et al. (2003); Lin et al. (2004); Lee et al. (2003); Gouveia et al. (2004); Ha et al. (2001); Mannes et al. (2005); Bobak (2000), we adjust for gestation length because the length of the gestation period is, evidently, a good predictor of birthweight. To conclude the list of explanatory variables in our model, let zij denote one of the exposure metrics defined in Section 4 for mother j living in census tract i evaluated over one of the seven time windows Wij. Thus, zij either , , or .
Then, our model relates ambient exposure to PM2.5 and birthweight in the following way:
| (8) |
for i = 1, … , M and j = 1, … , Ni with β0 representing an intercept term, β a vector of coeffcients for the socio-economic factors, α coeffcient of the effect of Oi, the percentage of owner-occupied units in census tract i, φi a spatial random effect for census tract i and πij error term that follows a normal distribution with mean zero and variance σ2. We include φi in (8) to model spatial variability between census tracts. Even though the ambient exposure term might account for some of the spatial variability in the response variable, we introduce spatial random effects in the model because we anticipate that there is additional residual spatial variability in the data that is not accounted for by the socio-economic factors or by the ambient exposure term. Indeed, these effects are at the census tract scale while the other covariate information is at the individual level. We note that we also fitted all of the models in the sequel without random effects, essentially using ordinary least squares (OLS). Summarizing, in the interest of space, these models do not predict as well (Table 2 below); introducing a spatially varying intercept for the census tracts provides improved model flexibility over a constant intercept. Figure 7 below reveals the substantial spatial variability in these intercepts and their consequential magnitude in grams of birthweight. Expressed in different terms, location matters though we do not know the spatial variables that account for this.
Table 2.
RMSPE (Root Mean Square Predictive Error), MAPE (Mean Absolute Predictive Error), average length of the 95% predictive intervals (PI) and empirical coverage of the 95% predictive intervals obtained using the model that characterizes exposure using the third metric, that is, respectively, the “normalized area ambient exposure was above 15 μg/m3” and the “normalized area personal exposure was above 15 μg/m3. The time window of exposure considered is the course of the entire pregnancy. Each statistic is reported in grams
| RMSPE | MAPE | Average length of 95% PI |
Empirical coverage of 95% |
|
|---|---|---|---|---|
| Ambient exposure (without spatial effects) |
428.52 | 334.71 | 1533.45 | 91.76% |
| Ambient exposure (with spatial effects) |
424.98 | 331.57 | 1632.32 | 94.11 % |
| Personal exposure (with spatial effects) |
393.19 | 310.67 | 1666.09 | 97.74 % |
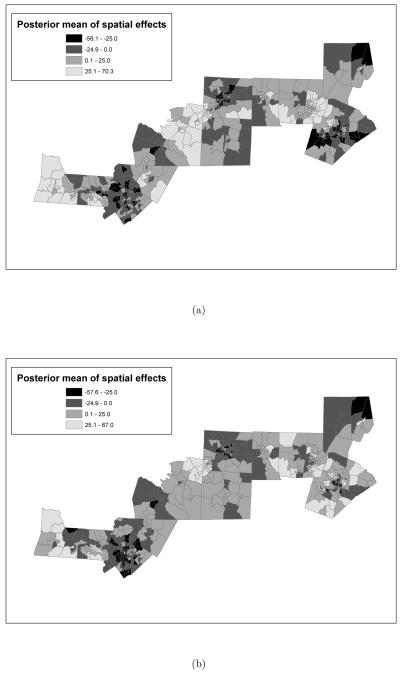
Figure 7.
Posterior means of the random spatial effects obtained from the model where the exposure metric is: (a) “normalized area ambient exposure curve was above 15μg/m3 versus (b) “normalized area personal exposure curve was above 15μg/m3. In both models, the time window of exposure is the entire pregnancy. Additionally, both posterior means are reported in grams.
Exposure metrics over different time windows enter (8) in different ways. Precisely, if the time window of exposure Wij is the entire pregnancy Tij, then zij can be, respectively, the average ambient exposure during the entire pregnancy, , the percentage of days during the entire pregnancy ambient exposure was above 15μg/m3, , or the normalized area under the ambient exposure curve above 15μg/m3, . However, if the time window is any of the subsets , then the exposure metrics appear in the model equation as a pair. More specifically, given an exposure metric we will introduce simultaneously in the model the value of the exposure metric evaluated over the first 30 and last 30 days of the pregnancy, for example, or over the first and last 60 days of the pregnancy, or over the first and second trimeste r. Hence, equation (8) presents the model for birthweight and ambient exposure when the time window of exposure Wij = Tij. For other time windows , equation (8) should be modified as:
| (9) |
where zij1 and zij2 indicate the two exposure metrics for mother j living in census tract i evaluated over the two ”paired” time windows. In total, we consider 12 models, four for each exposure metric.
We complete the specification of model (8) (respectively, (9)) by assigning prior distributions to the parameters in the model. Precisely:
| (10) |
while the spatial random effects for the census tract are modeled using a CAR model (Besag 1974; Banerjee et al. 2004). The prior specification for model (9) involves replacing the univariate normal distribution for γ with a bivariate normal distribution of γ1 and γ2. Hyperparameters of the priors were chosen so to have proper but diffuse priors. Estimates of the parameters in the model are obtained via MCMC using a Gibbs sampling algorithm (Gelfand and Smith 1990), with “centering on the fly” after each model-fitting iteration (Besag et al. 1995; Gelfand et al. 1996) to convert the improper CAR specification to a proper one.
5.2 Using personal exposure
Using personal exposure, we have a set of 21 distributions for the combinations exposure metric-time window of exposure. Therefore, in the personal exposure framework, models (8) and (9) need to be appropriately modified to accommodate for these distributions. We achieve this by modifying the second stage specification of models (9) and (10). More specifically, while equations (8) and (9) still hold, we add to (10) this additional distribution:
| (11) |
where Uniform30 denotes the discrete uniform distribution on a set of 30 values with support which changes for i = 1, … , M and j = 1, … , Ni, and is given respectively by , , or for any .
As under the exposure scenario, exposure metrics enter the model as a pair if the time windows considered are the first and last 30 days of the pregnancy, the first and last 60 days of the pregnancy, and the first and second trimester. In these cases, we add two additional distributions and to the specification of the second stage (10). Again, the resulting twelve models were fit via MCMC using a Gibbs sampling algorithm.
6 Results
We now present results of our analysis. Across all models, the estimates of the coeffcients of the socio-economic factors are rather consistent and consistent with previous findings. Additionally, with the exception of percentage of owner-occupied units in the census tract of maternal residence, marital status, and maternal age between 35-44, all of the coeffcients are significant for all the models. The significance of the coeffcient for the percentage of owner-occupied units in the census tract of maternal residence and marital status varies from model to model.
As previously commented, gestation length is probably the best predictor of birthweight and all models seem to indicate that for each additional week of gestation, birthweight increases of about 180 – 185 grams. All of our models indicate that Non-Hispanic Black women deliver babies that are on average about 165 – 180 grams lighter than babies of Non-Hispanic White mothers, while babies of Hispanic women are only 60–65 grams lighter than babies of Non-Hispanic White mothers. Lower levels of maternal education are negatively associated with birthweight: mothers with middle school education have babies that are on average 40 – 50 grams lighter than those of mothers with a high school education, while mothers that did not complete high school deliver babies that are on average 35–40 grams lighter than those of mothers who completed it. The situation is reversed for mothers with higher levels of education: birthweight increases by about 24 – 30 grams if the mother has done some college and by 25 – 35 grams if the mother has a college degree. Being too young at delivery also appears to be detrimental to birthweight. In particular, compared to the birthweight of a baby from a mother who is 25-34 years old at delivery, birthweight decreases by about 35 – 50 grams if the mother is between 15-17 years of age at delivery, and by 30 – 45 grams if maternal age is between 18 to 24 years of age. No significant difference in birthweight is found between mothers who deliver at 25-34 years of age and mothers who deliver at 35-44 years of age. Smoking and not being married are also risk factors. In particular, smoking during pregnancy is associated with a decrease of 180 – 190 grams, while not being married, in some models, is associated with a decrease of 30 – 40 grams in birthweight. Finally, not being the first baby and being a male are both associated with a significant increase in birthweight of about 120 – 135 grams.
Estimates of the coeffcients of maternal exposure to particulate matter are reported in Table 1. For each combination of exposure metric and time window of exposure, the table presents, in grams, posterior means and the 95% credible interval obtained by running the twelve models under the ambient exposure scenario and under the personal exposure framework. The difference in magnitude between the coeffcients of the exposure metrics obtained when Wij = Tij and when Wij ⊂ Tij is justified by an increased correlation between the exposure metrics for Wij ⊂ Tij and the intercept. The magnitude of the estimate of the intercept term drops by as many as 300g when we pass from a model where to a model with zij1 and zij2 terms evaluated for paired time windows Wij ⊂ Tij. Similarly, the estimate of the intercept drops by approximately 50g when or compared to models where Wij ⊂ Tij. A similar trend is observed R Punder the personal exposure framework.
Table 1.
Posterior means and 95% credible intervals for the coefficients of the different exposure metrics obtained using the two different approaches: the first one characterizes exposure using ambient exposure and the second one characterizes exposure using personal exposure. Covariates included in the model: gestation, maternal age, maternal race, maternal education, marital status, maternal smoking status, sex, birth order, percentage of owner-occupied units in the census tract and spatial random effect for census tract of maternal residence. Each coefficient is reported in grams
| Exposure metric | Time window | Ambient exposure | Personal exposure |
|---|---|---|---|
| Average exposure | Entire pregnancy | 20.27 (−179.23; 209.42) | 11.04 (−193.74; 160.65) |
| First 30 days Last 30 days |
−0.52 (−1.93; 0.86) −0.51 (−1.92; 0.88) |
1.09 (−0.13; 2.35) 1.10 (−0.12; 2.36) |
|
| First 60 days Last 60 days |
−0.64 (−2.21; 0.91) −0.63 (−2.24; 0.92) |
1.23 (−0.22; 2.59) 1.23 (−0.21; 2.58) |
|
| First trimester Second trimester |
−0.27 (−1.75; 1.17) −0.27 (−1.75; 1.19) |
1.05 (−0.19; 2.32) 1.05 (−0.21; 2.33) |
|
| Percentage days daily exposure over 15μg/m3 |
Entire pregnancy | 0.28 (−0.25; 0.84) | 0.27 (−0.28; 0.82) |
| First 30 days Last 30 days |
−0.16 (−0.38; 0.06) −0.15 (−0.38; 0.06) |
0.01 (−0.38; 0.45) 0.01 (−0.38; 0.45) |
|
| First 60 days Last 60 days |
−0.09 (−0.40; 0.23) −0.09 (−0.39; 0.25) |
−0.03 (−0.47; 0.37) −0.03 (−0.46; 0.39) |
|
| First trimester Second trimester |
−0.05 (−0.32; 0.30) −0.04 (−0.30; 0.31) |
0.01 (−0.40; 0.44) 0.01 (−0.40; 0.44) |
|
| Normalized area of daily exposure over 15 μg/m3 |
Entire pregnancy | 32.05 (−28.04; 96.18) | 19.57 (−89.17; 135.18) |
| First 30 days Last 30 days |
0.20 (−3.28; 3.64) 0.21 (−3.07; 3.62) |
2.48 (−4.72; 4.02) 2.48 (−4.74; 4.04) |
|
| First 60 days Last 60 days |
−0.35 (−4.78; 4.00) −0.34 (−4.53; 3.99) |
2.57 (−4.75; 4.15) 2.58 (−4.79; 4.14) |
|
| First trimester Second trimester |
−0.31 (−4.28; 3.66) −0.29 (−4.03; 3.61) |
2.19 (−5.28; 4.06) 2.19 (−5.26; 4.10) |
As Table 1 shows, none of the coeffcients of exposure is significant. There are several possible interpretations for such result. It is possible that the relationship between maternal exposure and birthweight is more complicated than just a simple linear relationship as it is implied in our model. It is also possible that the spatial random effects for the census tract of maternal residence are accounting for part of the effect of maternal exposure. It is also important to note the difference in the sign of the coeffcient for maternal exposure between models that use ambient exposure to characterize an individual’s exposure to PM2.5 and models that use personal exposure. With the exception of the model that introduces maternal exposure via the second metric (percentage of days maternal exposure, whether ambient or personal, was above 15μg/m3), every time the model using ambient exposure reports a negative (yet not significant) coeffcient, the corresponding model using personal exposure reports a positive, yet not significant coeffcient. We believe that this is explained mostly by the different estimates of the spatial random effects obtained under the two models.
Figures 7 and 8 show, respectively, the posterior means and standard deviations for the spatial random effects obtained from the model that employs the third metric, that is the normalized area under the ambient exposure curve, respectively, the personal exposure curve, above 15μg/m3 with Wij = Tij. We present results for this model because we believe that this metric has the advantage of combining both aspects of the first and second metrics, as it accounts for both magnitude and exceedances.
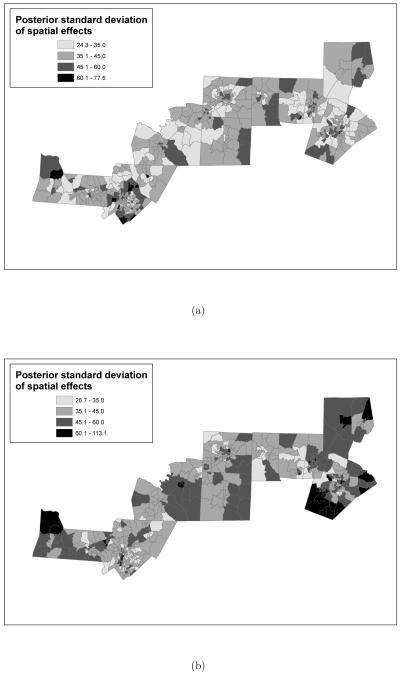
Figure 8.
Posterior standard deviations of the random spatial effects obtained from the models where the exposure metric is: (a) “normalized area ambient exposure curve was above 15μg/m3 versus (b) “normalized area personal exposure curve was above 15μg/m3. In both models the time window of exposure is the entire pregnancy. Additionally, both posterior standard deviations are reported in grams.
In Figure 7 darker shades are used to represent negative values, while the two lighter tones are used to represent positive values. As the figure shows, the two models imply a different spatial structure for the spatial random effects. In particular, the model employing ambient exposure seems to indicate more extreme values, both positive and negative, for the spatial random effects, while the spatial map for the model that employes personal exposure indicates more moderate values. Additionally, while both models associate large negative effects to census tracts in Mecklenburg, the two models disagree on the effects associated with census tracts in Wake county. In particular, while the model using personal exposure estimates the effect of some of the tracts in Wake county to be positive and between 0 to 25 grams, according to the model for ambient exposure, babies born from mothers residing in those census tracts are on average 25 to 56 grams lighter. Similar discrepancies can be observed in some of the western tracts in Randolph county: while the model for personal exposure associates a moderate positive effect to those tracts, the model for ambient exposure associates to them a moderate negative effect.
The spatial map of the posterior standard deviation of the random spatial effects indicates that there is much more uncertainty in the estimates of these effects under the personal exposure scenario than under the ambient exposure scenario. In Figure 8, lighter shades indicate smaller standard deviation, while darker shades indicate larger standard deviation. Such an increase in uncertainty under the personal exposure framework can be explained by the additional uncertainty in exposure provided by the distributions from the SHEDS-PM model.
We have also assessed the predictive ability of our models by predicting birthweight for each mother in the dataset. Table 2 reports, in grams, the root mean square predictive error, the mean absolute predictive error, the average length of the 95% predictive interval and the empirical coverage of the 95% predictive interval for the models that use the normalized area under the ambient exposure curve, respectively, the personal exposure curve above 15μg/m3 evaluated over the course of the entire pregnancy. To show how adding the spatial random effects improves the predictive performance of the model, in Table 2 we have also included results for a model that does not include spatial random effects. As the table shows, modeling the residual spatial variability does improve the predictive performance of the model. However, it is using personal exposure rather than ambient exposure that improves the results dramatically as the first model yields predictions that are on average closer to the observed values. Both models generate predictive intervals that have an empirical coverage very close to the nominal. In particular, the additional uncertainty in the personal exposure scenario is reflected in wider predictive intervals that yield an empirical coverage above the nominal. In both exposure scenarios, the predictive intervals are quite wide and this can be explained by the rather large residual standard standard deviation. After adjusting for socio-economic factors and maternal exposure, independently of the combination of exposure metric and time window of exposure used, the residual standard deviation of the models is estimated to be around 300 – 450 grams. Such large residual standard deviations are also obtained when no spatial random effects are included in the model.
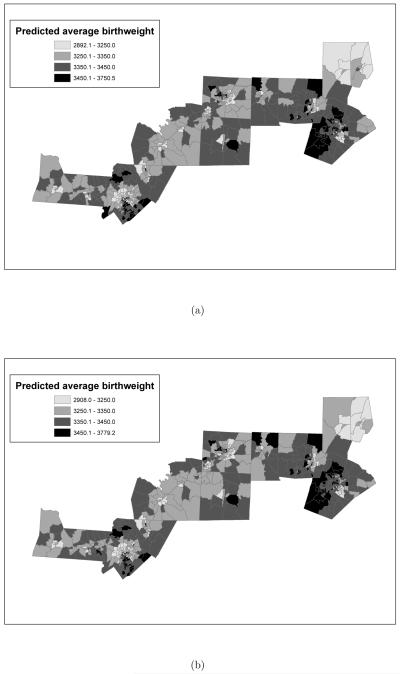
We conclude this section by presenting spatial maps of the predicted average birthweight and the standard deviation of the predicted average birthweight by census tract. Comparing Figure 2(a) and Figures 9(a) and (b), we can clearly see that the model that uses the normalized area under the personal exposure curve above 15μg/m3 reproduces the spatial pattern of average birthweight shown in Figure 2(a). On the other hand, the model that uses ambient exposure seems to underestimate average birthweight in some of the tracts, as the top right corner of the map indicates.
Figure 9.
Posterior predictive mean of average birth weight (in grams) by census tract as obtained from the model where the exposure metric is: (a) “normalized area ambient exposure curve was above 15μg/m3 versus (b) “normalized area personal exposure curve was above 15μg/m3. In both models the time window of exposure is the entire pregnancy. Additionally, both posterior predictive means are reported in grams.
7 Summary and Discussion
In this paper, we have presented a hierarchical modeling framework that illustrates how an exposure simulator can be used to relate a health outcome to personal exposure when both the health endpoint and personal exposure are modeled at the individual level. For our analysis, we have applied this modeling framework to birthweight, as health endpoint, and particulate matter, as pollutant. However the model is general and we believe that this type of approach could be useful in determining long-term effects of exposure on other health outcomes given a window of exposure, for example, a year before the onset of a medical complication.
Differently from the findings of Bell et al. (2007), Mannes et al. (2005), and Basu et al. (2004), our analysis did not reveal any significant effect of maternal exposure to PM2.5 on birthweight at any time window of exposure. In particular, both Bell et al. (2007) and Mannes et al. (2005) have reported a significant adverse effect of exposure to PM2.5 during the second and third trimester of the pregnancy.
A comparison between our results and previous results cannot easily be made as our model estimates maternal ambient exposure not in terms of county-wide average ambient concentration (Bell et al. 2007), city-wide average ambient concentration (Basu et al. 2004), or average concentration as reported by the closest monitors within a 5 mile radius from the mother’s residence (Mannes et al. 2005; Basu et al. 2004). Additionally, our study considers a spatial domain with lower concentration of fine particulate matter than the area considered by Bell et al. (2007), who study the relationship between maternal exposure to PM2.5 and birthweight in Connecticut and Massachusetts, and Mannes et al. (2005), who examines Sydney, Australia. Finally, our model includes spatial random effects for the census tract of maternal residence, that other studies have not adjusted for.
The spatial random effects play an important role in our model and we believe that they do affect the relationship between exposure and birthweight, as the estimates of exposure and birthweight change if the spatial random effects are not included in the model, even though they still remain non-significant. We believe that including these spatial random effects is appropriate as they account for the residual spatial variability in the response variable that is not accounted for by the socio-economic factors and by exposure itself. Additionally, even though not in line with the findings of Bell et al. (2007), Mannes et al. (2005), and Basu et al. (2004), our results agree with those of Parker et al. (2005) and Basu et al. (2004) who, characterizing exposure using the average ambient concentration of PM2.5 measured by the closest monitoring sites to a mother’s residence, didn’t find any significant adverse effect of particulate matter on birthweight.
Our analysis has also revealed that models using personal exposure yield better predictions than models that use ambient exposure, thus arguing that personal exposure offers a better representation of an individual’s exposure to particulate matter than ambient exposure.
For each subject in our dataset, we have characterized personal exposure using the SHEDS-PM simulator, by associating to each mother a distribution based on a set of 30 personal exposures for 30 individuals simulated within SHEDS-PM, that match the selected mother in terms of age and census tract of residence. Since among its output, SHEDS-PM also reports the contribution of ambient exposure on each simulated individual’s personal exposure, it is possible to adapt the framework in this paper to assess the effect on birthweight of the contribution of a mother’s exposure to particulate matter due to outdoor sources on birth weight.
In our model, we have characterized each individual’s ambient exposure during a particular time window of exposure using single values and not distributions. As we did under the personal exposure scenario, it is possible to associate to each individual a distribution for each combination of ambient exposure-time window of exposure. This can easily be achieved by using the method of McMillan et al. (2008), that we have employed to combine monitoring data with numerical model’s gridded prediction of daily PM2.5 concentration. Instead of using the median of the predictive distribution yielded by the model of McMillan et al. (2008), we could use the entire distribution analogously to what is done in our model under the personal exposure scenario.
There are several ways in which our model can be extended: we have adjusted for gestational age using only a linear term. It would be possible to allow for a non-linear dependence of birth weight on gestational age, as Wang et al. (1997) have done. We have summarized the time series of both ambient and personal exposure using three metrics that highlight different aspects of an individual’s exposure; other metrics could be developed and tested. Finally, we have predicted the ambient concentration of particulate matter at the census tract level by employing the fusion method of McMillan et al. (2008). While this certainly breaks with the previous practice of characterizing ambient exposure using monitoring data only, there are alternative ways to integrate the information contained in the monitoring data and in the numerical model daily prediction of PM2.5.
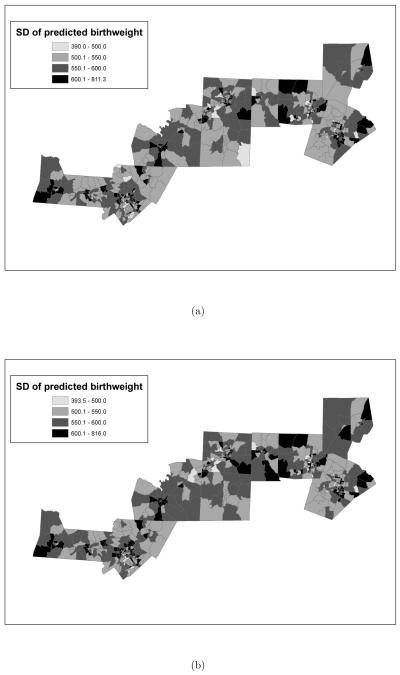
Figure 10.
Posterior predictive standard deviation of average birth weight (in grams) by census tract as obtained from the model where the exposure metric is: (a) “normalized area ambient exposure curve was above 15μg/m3 versus (b) “normalized area personal exposure curve was above 15μg/m3. Time window of exposure is the course of the entire pregnancy. Additionally, both posterior predictive standard deviations are reported in grams.
Footnotes
Disclaimer:
The U.S. Environmental Protection Agency through its Office of Research and Development partially collaborated in the research described here. Although it has been reviewed by the Agency and approved for publication, it does not necessarily reflect the Agency’s policies or views.
Contributor Information
Veronica J. Berrocal, Department of Statistical Science, Duke University, Durham, NC, USA (vjb2@stat.duke.edu).
Alan E. Gelfand, Department of Statistical Science, Duke University, Durham, NC, USA (alan@stat.duke.edu).
David M. Holland, U.S. Environmental Protection Agency, National Exposure Research Laboratory, Research Triangle Park, NC, USA (Holland.David@epa.gov).
Janet Burke, U.S. Environmental Protection Agency, National Exposure Research Laboratory, Research Triangle Park, NC, USA (Burke.Janet@epa.gov).
Marie Lynn Miranda, Nicholas School of the Environment (mmiranda@duke.edu).
References
- Armstrong BK, White E, Saracci R. Principles of exposure measurement in epidemiology. Oxford University Press; 1992. [Google Scholar]
- Banerjee S, Carlin BP, Gelfand AE. Hierarchical Modeling and Analysis for Spatial Data. Chapman & Hall/CRC; Boca Raton, Fla: 2004. [Google Scholar]
- Basu R, Woodruff TJ, Parker JD, Saulnier L, Schoendorf KC. Comparing exposure metrics in the relationship between PM2.5 and birth weight in California. Journal of Exposure Analysis and Environmental Epidemiology. 2004;14:391–396. doi: 10.1038/sj.jea.7500336. [DOI] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Belanger K. Ambient air pollution and low birth weight in Connecticut and Massachussetts. Environmental Health Perspectives. 2007;115:1118–1124. doi: 10.1289/ehp.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besag J. Spatial interaction and the statistical analysis of lattice systems. Journal of the Royal Statistical Society Series B. 1974;36:192–236. [Google Scholar]
- Besag J, Green PJ, Higdon D, Mengersen K. Bayesian computation and stochastic systems. Statistical Science. 1995;10:3–66. [Google Scholar]
- Bobak M. Outdoor air pollution, low birth weight, and prematurity. Environmental Health Perspectives. 2000;108:173–176. doi: 10.1289/ehp.00108173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobak M, Leon DA. Air pollution and infant mortality in the Czech Republic, 1986-1988. Lancet. 1992;340:1010–1014. doi: 10.1016/0140-6736(92)93017-h. [DOI] [PubMed] [Google Scholar]
- Braga ALF, Zanobetti A, Schwartz J. The lag structure between particulate air pollution and respiratory and cardiovascular deaths in ten U.S. cities. Journal of Occupational Environmental Medicine. 2001;43:927–933. doi: 10.1097/00043764-200111000-00001. [DOI] [PubMed] [Google Scholar]
- Burke JM, Zufall MJ, Özkaynak H. A population exposure model for particulate matter: case study results for PM2.5 in philadelphia, PA. Journal of Exposure and Analysis and Environmental Epidemiology. 2001;11:470–489. doi: 10.1038/sj.jea.7500188. [DOI] [PubMed] [Google Scholar]
- Calder CA, Holloman CH, Bortnick SM, Strauss WJ, Morara M. Relating ambient particulate matter concentration levels to mortality using an exposure simulator. Journal of the American Statistical Association. 2008;103:137–148. [Google Scholar]
- Chen L, Yang W, Jennison BJ, Goodrich A, Omaye ST. Air pollution and birth weight in Northern Nevada, 1991–1999. Inhalation Toxicology. 2002;14:141–157. doi: 10.1080/089583701753403962. [DOI] [PubMed] [Google Scholar]
- Cressie NAC. Statistics for Spatial Data. Wiley; New York: 1993. [Google Scholar]
- Dockery DW, Pope CAI. Acute respiratory effects of particulate air pollution. Annual Review of Public Health. 1994;15:107–132. doi: 10.1146/annurev.pu.15.050194.000543. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Pope CAI, Xu X, Spengler JD, Ware JH, Fay ME, Jr., B GF, Speizer FE. An association between air pollution and mortality in six US cities. New England Journal of Medicine. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Spengler J. Personal exposure to respirable particulates and sulfates. Journal of air pollution control association. 1981;31:153–159. doi: 10.1080/00022470.1981.10465205. [DOI] [PubMed] [Google Scholar]
- Dominici F, Zeger SL, Samet JM. A measurement error model for time-series studies of air pollution and mortality. Biostatistics. 2000;1:157–175. doi: 10.1093/biostatistics/1.2.157. [DOI] [PubMed] [Google Scholar]
- Dugandzic R, Dodds L, Stieb D, Smith-Doiron M. The association between low level exposures to ambient air pollution and term low birth weight: a retrospective cohort study. Environmental Health. 2006;5 doi: 10.1186/1476-069X-5-3. doi:10.1186/1476–069X–5–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes M, Song H-R, Ghosh SK, Holland DM, Davis JM. Spatial association between speciated fine particles and mortality. Biometrics. 2006;62:855–863. doi: 10.1111/j.1541-0420.2006.00526.x. [DOI] [PubMed] [Google Scholar]
- Gelfand AE, Sahu SK, Carlin BP. Efficient parametrization for generalized linear mixed models (with discussion) In: Bernardo JM, Berger JO, D AP, Smith AFM, editors. Bayesian Statistics 5. Vol. 1996. Oxford University Press; 1996. pp. 165–180. [Google Scholar]
- Gelfand AE, Smith AFM. Sampling-based approaches to calculating marginal densities. Journal of the American Statistical Association. 1990;85:398–409. [Google Scholar]
- Goldberg MS, Burnett RT, Bailar JCI, Brook J, Bonvalot Y, et al. The association between daily mortality and ambient air particle pollution in Montreal. Environmental Research. 2001;A86:12–25. doi: 10.1006/enrs.2001.4242. R. T. [DOI] [PubMed] [Google Scholar]
- Gouveia N, Bremner SA, Novaes HMD. Association between ambient air pollution and birth weight in Sao Paulo, Brazil. Journal of Epidemiology & Community Health. 2004;58:11–17. doi: 10.1136/jech.58.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryparis A, Paciorek CJ, Zeka A, Schwartz J, Coull BA. Measurement error caused by spatial misalignment in environmental epidemiology. BiostatisticsSubmitted. 2008 doi: 10.1093/biostatistics/kxn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha E-H, Hong Y-C, Lee B-E, Woo B-H, Schwarz J, Christiani DC. Is air pollution a risk factor for low birth weight in Seoul? Epidemiology. 2001;12:643–648. doi: 10.1097/00001648-200111000-00011. [DOI] [PubMed] [Google Scholar]
- Hansen C, Neller A, Williams G, Simpson R. Maternal exposure to low levels of ambient air pollution and preterm birth in Brisbane, Australia. Epidemiology. 2006:935–941. doi: 10.1111/j.1471-0528.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- Heinrich J, Hoelscher B, Wjst M, Ritz B, Cyrys J, Wichmann H. Respiratory diseases and allergies in two polluted areas in East Germany. Environmental Health Perspectives. 1999;107:53–62. doi: 10.1289/ehp.9910753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek G, Brunekreef B, Fisher P, Wijnen JV. The association between air pollution and heart failure, arrythmia, embolism, thromobosis, and other cardiovascular causes of death in a time series study. Epidemiology. 2001;12:355–357. doi: 10.1097/00001648-200105000-00017. [DOI] [PubMed] [Google Scholar]
- Holloman CH, Bortnick SM, Morara M, Strauss WJ, Calder CA. A Bayesian hierarchical approach for relating PM2.5 exposure to Cardiovascular mortality in North Carolina. Environmental Health Perspectives. 2004;112:1282–1288. doi: 10.1289/ehp.6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsouyanni K, Touloumi G, Samoli E, Gryparis A, Tertre AL, et al. Confounding and effect modification in the short-term effects of ambient particles on total mortality: results from 29 European cities within the APHEA2 project. Epidemiology. 2001;12:521–531. doi: 10.1097/00001648-200109000-00011. Y. M. [DOI] [PubMed] [Google Scholar]
- Kinney P, Spengler J, Brown K. Assessing population exposures in studies of human health effects of PM2.5. Air & Waste Management Association - 2006:16–33. [Google Scholar]
- Law PL, Lioy PJ, Zelenka MP, huber AH, McCurdy TR. Evaluation of a probabilistic exposure model applied to carbon monoxide (pNEM/CO) using Denver personal exposure monitoring data. Journal of the Air and Waste Management Association. 1997;47:491–500. doi: 10.1080/10473289.1997.10464416. [DOI] [PubMed] [Google Scholar]
- Lee BE, Ha EH, Park HS, Kim YJ, Hong YC, Kim H, Lee JT. Exposure to air pollution during different gestational phases contributes to risks of low birth weight. Human Reproduction. 2003;18:638–643. doi: 10.1093/humrep/deg102. [DOI] [PubMed] [Google Scholar]
- Lin C-M, Li C-Y, Yang G-Y, Mao I-F. Association between maternal exposure to elevated ambient sulfure dioxide during pregnancy and term low birth weight. Environmental Research. 2004;96:41–50. doi: 10.1016/j.envres.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Lioy PJ, Waldman BT, Butler J, Piertarinen C. The personal, indoor, and outdoor concentration of PM10 measured in an industrial community during the winter. Atmospheric Environment. 1990;24:57–66. [Google Scholar]
- Liu S, Krewski D, Shi Y, Chen Y, Burnett RT. Association between gaseous ambient air pollutants and adverse pregnancy outcomes in Vancouver, Canada. Environmental Health Perspectives. 2003;111:1773–1778. doi: 10.1289/ehp.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonet M, Bush T, Correa A, Jaakkola JJK. Relation between ambient air pollution and low birth weight in the Northeastern United States. Environmental Health Perspectives. 2001;109(S3):351–356. doi: 10.1289/ehp.01109s3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonet M, Correa A, Misra D, Jaakkola JJK. A review of the literature on the effects of ambient air pollution on air growth. Environmental Research. 2004;95:106–115. doi: 10.1016/j.envres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Mannes T, Jalaludin B, Morgan G, Lincoln D, Sheppeard V, Corbett S. Impact of ambient air pollution on birth weight in Sydney, Australia. Occupational & Environmental Medicine. 2005;62:524–530. doi: 10.1136/oem.2004.014282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride SJ, Williams RW, Creason J. Bayesian hierarchical modeling of personal exposure to particulate matter. Atmospheric Environment. 2007;41:6143–6155. [Google Scholar]
- McMillan N, Holland DM, Morara M, Feng J. Combining numerical model output and particulate data using Bayesian space-time modeling. 2008 Submitted. [Google Scholar]
- Ostro BD, Broadwin R, Lipsett MJ. Coarse and fine particles and daily mortality in the Coachella Valley: a follow-up study. Journal of Exposure Analysis and Environmental Epidemiology. 2000;10:412–419. doi: 10.1038/sj.jea.7500094. [DOI] [PubMed] [Google Scholar]
- Özkaynak H, Sue J, Spengler J, Wallace L, Pellizzari E, Jenkins P. Personal exposure to airborne particles and metals: Results from the particle team study in Riverside, California. Journal of Exposure Analysis and Environmental Epidemiology. 1996;6:57–78. [PubMed] [Google Scholar]
- Ozone2006 . Air quality criteria for ozone and related photochemical oxidants (Final) U.S. Environmental Protection Agency; Washington, DC: 2000. EPA/600/R-05/004aF-cF. [Google Scholar]
- Parker JD, Woodru TJ, Basu R, Schoendorf KC. Air pollution and birth weight among term infants in California. Pediatrics. 2005;115:121–128. doi: 10.1542/peds.2004-0889. [DOI] [PubMed] [Google Scholar]
- Pope CA, Dockery DW, Schwartz J. Review of epidemiological evidence of health-effects of particulate air-pollution. Inhalation Toxicology. 1995;7:1–18. [Google Scholar]
- Reich BJ, Fuentes M, Burke J. Analysis of the effects of ultrafine particulate matter while accounting for human exposure. EnvironmetricsSubmitted. 2008 doi: 10.1002/env.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, Yu F. Effect of ambient carbon monoxide on low birth weight among children born in Southern California between 1989 and 1993. Environmental Health Perspectives. 1999;107:17–25. doi: 10.1289/ehp.9910717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, Yu F, Chapa G, Fruin S. Effect of air pollution on preterm birth among children born in Southern California between 1989 and 1993. Epidemiology. 2000;5:502–511. doi: 10.1097/00001648-200009000-00004. [DOI] [PubMed] [Google Scholar]
- Rogers JF, Dunlop AL. Association of very low birth weight with exposures to environmental sulfur dioxide and total suspended particulates. American Journal of Epidemiology. 2000;151:602–612. doi: 10.1093/oxfordjournals.aje.a010248. [DOI] [PubMed] [Google Scholar]
- Rogers JF, Dunlop AL. Air pollution and very low birth weight infants: a target population. Pediatrics. 2005:156–165. doi: 10.1542/peds.2005-2432. [DOI] [PubMed] [Google Scholar]
- Saldiva PHN, Pope CAI, Schwartz J, Dockery DW, Lichtenfels AJF, et al. Air pollution and mortality in elderly people: a time series study in Sã0 Paulo, Brazil. Archives of Environmental Health. 1995;50:159–163. doi: 10.1080/00039896.1995.9940893. J. M. S. [DOI] [PubMed] [Google Scholar]
- Samet JM, Zeger SL, Dominici F, Curriero F, Coursac I, Dockery DW. The national morbidity, mortality and air pollution study. Part I: methods and methodologic issues. Part II: morbidity, mortality, and air pollution in the United States. Health Effects Institute; Cambridge, MA: 2000. Research Report No. 9. [PubMed] [Google Scholar]
- Schwartz J. Air pollution and daily mortality: a review and meta analysis. Environmental Research. 1994;64:36–52. doi: 10.1006/enrs.1994.1005. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Air pollution and hospital admissions for heart diseases in eight U.S. counties. Epidemiology. 1999;10:17–22. [PubMed] [Google Scholar]
- Schwartz J, Dockery DW, Neas LM, Wypij D, Ware JH, et al. Acute effects of summer air pollution on respiratory symptom reporting in children. American Journal of Respiratory Critical Care Medicine. 1994;150:1234–1242. doi: 10.1164/ajrccm.150.5.7952546. J. D. S. [DOI] [PubMed] [Google Scholar]
- Shaddick G, Lee D, Zidek JV, Salway R. Estimating exposure response functions using ambient pollution concentration. Annals of Applied Statisticssubmitted. 2008 [Google Scholar]
- Spengler JD, Treitman RD, Tosteson TD, Mage DT, Soczek ML. Personal exposure to respirable particulates and implications for air pollution epidemiology. Environmental Science Technology. 1985;19:700–707. doi: 10.1021/es00138a008. [DOI] [PubMed] [Google Scholar]
- S̆rám RJ. Impact of air pollution on reproductive health. Environmental Health Perspectives. 1999;107:542–543. doi: 10.1289/ehp.107-1566709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S̆rám RJ, Blinková B, Dejmek J, Bobak M. Ambient air pollution and pregnancy outcomes: a review of the literature. Environmental Health Perspectives. 2005;113:375–382. doi: 10.1289/ehp.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace L. Indoor particles: a review. Journal of the air and waste management association. 1996;46:98–126. doi: 10.1080/10473289.1996.10467451. [DOI] [PubMed] [Google Scholar]
- Wang X, Ding H, Ryan L, Xu X. Association between air pollution and low birth weight: a community based study. Environmental Health Perspectives. 1997;105:514–521. doi: 10.1289/ehp.97105514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Grillo J, Schoendorf KC. The relationship between selected causes of postnatal infant mortality and particulate air pollution in the United States. Environmental Health Perspectives. 1997;105:608–612. doi: 10.1289/ehp.97105608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CY, Tseng YT, Chang CC. Effects of air pollution on birth weight among children born between 1995 and 1997 in Kaohsiung, Taiwan. Journal of Toxicology and Environmental Health. 2003;66:807–816. doi: 10.1080/15287390306385. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J, Dockery DW. Airborne particles area risk factor for hospital admissions for heart and lung disease. Environmental Health Perspectives. 2000;108:1071–1077. doi: 10.1289/ehp.001081071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger S, Thomas D, Dominici F, Cohen A, Schwartz J, Dockery D, Samet J. Measurement error in time-series studies of air pollution: concepts and consequences. Environmental Health Perspectives. 2000;108:419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidek JV, Meloche J, Chatfield C, White R. A computational model for estimating personal exposure to air pollutants with application to London’s PM10 in 1997. 2003 Technical Report of the Statistical and Applied Mathematical Sciences Institute. [Google Scholar]
- Zidek JV, Shaddick G, Meloche J, Chatfield C, White R. A framework for predicting personal exposures to environmental hazards. Environmental and Ecological Statistics. 2007;14:411–431. [Google Scholar]