Abstract
Face individuation is one of the most impressive achievements of our visual system, and yet uncovering the neural mechanisms subserving this feat appears to elude traditional approaches to functional brain data analysis. The present study investigates the neural code of facial identity perception with the aim of ascertaining its distributed nature and informational basis. To this end, we use a sequence of multivariate pattern analyses applied to functional magnetic resonance imaging (fMRI) data. First, we combine information-based brain mapping and dynamic discrimination analysis to locate spatiotemporal patterns that support face classification at the individual level. This analysis reveals a network of fusiform and anterior temporal areas that carry information about facial identity and provides evidence that the fusiform face area responds with distinct patterns of activation to different face identities. Second, we assess the information structure of the network using recursive feature elimination. We find that diagnostic information is distributed evenly among anterior regions of the mapped network and that a right anterior region of the fusiform gyrus plays a central role within the information network mediating face individuation. These findings serve to map out and characterize a cortical system responsible for individuation. More generally, in the context of functionally defined networks, they provide an account of distributed processing grounded in information-based architectures.
The neural basis of face perception is the focus of extensive research as it provides key insights both into the computational architecture of visual recognition (1, 2) and into the functional organization of the brain (3). A central theme of this research emphasizes the distribution of face processing across a network of spatially segregated areas (4–10). However, there remains considerable disagreement about how information is represented and processed within this network to support tasks such as individuation, expression analysis, or high-level semantic processing.
One influential view proposes an architecture that maps different tasks to distinct, unique cortical regions (6) and, as such, draws attention to the specificity of this mapping (11–20). As a case in point, face individuation (e.g., differentiating Steve Jobs from Bill Gates across changes in expression) is commonly mapped onto the fusiform face area (FFA) (6, 21). Although recent studies have questioned this role of the FFA (14, 15), overall they agree with this task-based architecture as they single out other areas supporting individuation.
However, various distributed accounts have also been considered. One such account ascribes facial identity processing to multiple, independent regions. Along these lines, the FFA's sensitivity to individuation has been variedly extended to areas of the inferior occipital gyrus (5), the superior temporal sulcus (12), and the temporal pole (22). An alternative scenario is that identity is encoded by a network of regions rather than by any of its separate components—such a system was recently described for subordinate-level face discrimination (23). Still another distributed account attributes individuation to an extensive ventral cortical area rather than to a network of smaller separate regions (24). Clearly, the degree of distribution of the information supporting face individuation remains to be determined.
Furthermore, insofar as face individuation is mediated by a network, it is important to determine how information is distributed across the system. Some interesting clues come from the fact that right fusiform areas are sensitive to both low-level properties of faces (16, 25) and high-level factors (26, 27), suggesting that these areas may mediate between image-based and conceptual representations. If true, such an organization should be reflected in the pattern of information sharing among different regions.
The current work investigates the nature and the extent of identity-specific neural patterns in the human ventral cortex. We examined functional MRI (fMRI) data acquired during face individuation and assessed the discriminability of activation patterns evoked by different facial identities across variation in expression. To uncover the neural correlate of identity recognition, we performed dynamic multivariate mapping by combining information-based mapping (28) and dynamic discrimination analysis (29). The results revealed a network of fusiform and anterior temporal regions that respond with distinct spatiotemporal patterns to different identities. To elucidate the distribution of information, we examined the distribution of diagnostic information across these regions using recursive feature elimination (RFE) (30) and related the information content of different regions to each other. We found that information is evenly distributed among anterior regions and that a right fusiform region plays a central role within this network.
Results
Participants performed an individuation task with faces (Fig. 1) and orthographic forms (OFs) (Fig. S1). Specifically, they recognized stimuli at the individual level across image changes introduced by expression (for faces) or font (for OFs). Response accuracy was at ceiling (>95%) as expected given the familiarization with the stimuli before scanning and the slow rate of stimulus presentation. Thus, behavior exhibits the expected invariance to image changes, and the current investigation focuses on the neural codes subserving this invariance.
Fig. 1.
Experimental face stimuli (4 identities × 4 expressions). Stimuli were matched with respect to low-level properties (e.g., mean luminance), external features (hair), and high-level characteristics (e.g., sex). Face images courtesy of the Face-Place Face Database Project (http://www.face-place.org/) Copyright 2008, Michael J. Tarr. Funding provided by NSF Award 0339122.
Dynamic Multivariate Mapping.
The analysis used a searchlight (SL) with a 5-voxel radius and a 3-TR (Time to Repeat) temporal envelope to constrain spatiotemporal patterns locally. These patterns were submitted to multivariate classification on the basis of facial identity (Methods and SI Text). The outcome of the analysis is a group information-based map (28) revealing the strength of discrimination (Fig. 2). Each voxel in this map represents an entire region of neighboring voxels defined by the SL mask.
Fig. 2.
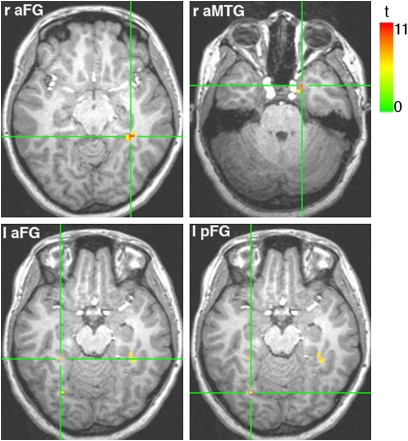
Group information-based map of face individuation. The map is computed using a searchlight (SL) approach and estimates the discriminability of facial identities across expression (q < 0.05). Each voxel in the map represents the center of an SL-defined region supporting identity discrimination. The four slices show the sensitivity peaks of the four clusters revealed by this analysis.
Our mapping revealed four areas sensitive to individuation: two located bilaterally in the anterior fusiform gyrus (aFG), one in the right anterior medial temporal gyrus (aMTG), and one in the left posterior fusiform gyrus (pFG). The largest of these areas corresponded to the right (r)aFG whereas the smallest corresponded to its left homolog (Table 1).
Table 1.
Areas sensitive to face individuation
| Coordinates (peak) |
|||||
| Region | x | y | z | SL centers (voxels) | Peak t value |
| raFG | 33 | −39 | −9 | 16 | 11.31 |
| raMTG | 19 | 6 | −26 | 8 | 9.90 |
| lpFG | −26 | −69 | −14 | 2 | 7.11 |
| laFG | −29 | −39 | −14 | 1 | 7.24 |
To test the robustness of our mapping, the same cortical volume (Fig. S2A) was examined with SL masks of different sizes. These alternative explorations produced qualitatively similar results (Fig. S3 A–C). In contrast, a univariate version of the mapping (SI Text) failed to uncover any significant regions, even at a liberal threshold (q < 0.10), attesting to the strength of multivariate; mapping.
To further evaluate these results, we projected the four areas from the group map back into the native space of each subject and expanded each voxel to the entire SL region centered on it. The resulting SL clusters (Fig. S3D) mark entire regions able to support above-chance classification. Examination of subject-specific maps revealed that the bilateral aFG clusters were consistently located anterior to the FFA [rFFA peak coordinates, 39, −46, and −16; left (l)FFA, −36, −47, and −18]. However, we also found they consistently overlapped with the FFA (mean ± SD: 24 ± 14% of raFG volume and 35 ± 20% of laFG).
Finally, multivariate mapping was applied to other types of discrimination: expression classification (across identities) and category-level classification (faces versus OFs). Whereas the former analysis did not produce any significant results, the latter found reliable effects extensively throughout the cortical volume analyzed (Fig. S2B). In contrast, a univariate version of the latter analysis revealed considerably less sensitivity than its multivariate counterpart (Fig. S2C).
The results above suggest that identity coding relies on a distributed cortical system. Clarifying the specificity of this system to face individuation is addressed by our region-of-interest (ROI) analyses.
ROI Analyses.
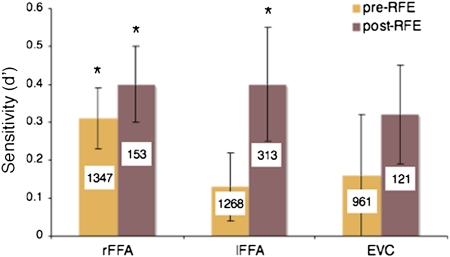
First, we examined whether the FFA supports reliable face individuation as tested with pattern classification. Bilateral FFAs were identified in each subject using a standard face localizer, and discrimination was computed across all features in a region—given the use of spatiotemporal patterns, our features are voxel X time-point pairings rather than voxels alone. The analysis revealed above-chance performance for rFFA (Fig. 3).
Fig. 3.
Sensitivity estimates in three ROIs. Facial identity discrimination was computed using both the entire set of features in an ROI and a subset of diagnostic features identified by multivariate feature selection (i.e., RFE), the two types of classification are labeled as pre- and post-RFE. The average number of features involved in classification is superimposed on each bar. The results indicate that the bilateral FFA, in contrast to an early visual area, contains sufficient information to discriminate identities above chance (P < 0.05).
To reduce overfitting, we repeated the analysis above using subsets of diagnostic features identified by multivariate feature selection, specifically RFE. The method works by systematically removing features, one at a time, on the basis of their impact on classification (SI Text). Following this procedure, we found above-chance performance bilaterally in the FFA (Fig. 3). In contrast, early visual cortex (EVC) did not exhibit significant sensitivity either before or after feature selection.
Second, we reversed our approach by using multivariate mapping to localize clusters and univariate analysis to assess face selectivity. Concretely, we examined the face selectivity of the SL clusters using the data from our functional localizers. No reliable face selectivity was detected for any cluster.
Third, SL clusters along with the FFA were tested for their ability to discriminate expressions across changes in identity. Above-chance discrimination was found in rFFA and raMTG (P < 0.05).
Finally, we tested our clusters for OF individuation across variation in font. The analysis found sensitivity in two regions: rFFA and lpFG.
These findings are important in several respects. They suggest conventionally defined face selectivity, although informative, may not be enough to localize areas involved in fine-level face representation. Also, they show that the identified network is not exclusively dedicated to individuation or even to face processing per se. One hypothesis, examined below, may explain this involvement in multiple types of perceptual discrimination simply by appeal to low-level image properties.
Impact of Low-Level Image Similarity on Individuation.
To determine the engagement of the network in low-level perceptual processing, image similarity was computed across images of different individuals using an L2 metric (Table S1). For each pair of face identities, the average distance was correlated with the corresponding discrimination score produced by every ROI (including the FFA). The only ROI susceptible to low-level image sensitivity was laFG (P < 0.05 uncorrected) (Fig. S4).
These results, along with the inability of the EVC to support individuation, suggest that low-level similarity is unlikely to be the main source of the individuation effects observed here.
Feature Ranking and Mapping.
Having located a network of regions sensitive to face identity, we set out to determine the spatial and temporal distribution of the features diagnostic of face individuation. Specifically, we performed RFE analysis jointly across all SL clusters and recorded the ranking of the features within each subject. To eliminate any spatial bias within the initial feature set, we started with an equally large number of features for each cluster: the 1,000 top-ranked ones based on single-cluster RFE computation.
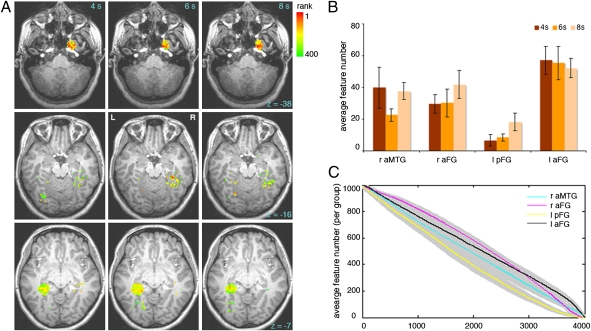
Fig. 4A shows the average ranking of the 400 most informative features across subjects and Fig. 4B summarizes their distribution across clusters and time points. We found a significant effect of cluster (two-way analysis of variance, P < 0.05) but no effect of time point and no interaction. Further comparisons revealed that lpFG contains fewer features than other clusters (P < 0.05), which did not differ among each other. The time course of feature elimination revealed that lpFG features were consistently eliminated at a higher rate than features from other clusters (Fig. 4C). A similar analysis across time points showed no substantial differences across time (Fig. S5).
Fig. 4.
Spatiotemporal distribution of information diagnostic for face individuation. (A) Group map of average feature ranking for the top 400 features—rows show different slices and columns different time points. Color codes the ranking of the features across space (the four regions identified by our SL analysis) and time (4–8 s poststimulus onset). The map shows a lower concentration of features in the lpFG relative to other regions but a comparable number of features across time. (B) Average feature distribution across subjects by cluster and time point (the bar graph quantifies the results illustrated in A). (C) Time course of feature elimination by ROI for 4,000 features (top 1,000 features for each ROI). This analysis confirms that lpFG features are eliminated at a higher rate, indicative of their reduced diagnosticity (shaded areas show ±1 SE across subjects).
Feature mapping provides a bird's eye view of information distribution across regions. In our case, it reveals a relatively even division of diagnostic information among anterior regions. Further pairwise comparisons of SL clusters were deployed to examine how areas share information with each other.
Information-Based Pairwise Cluster Analysis.
Whereas activation patterns in different areas are not directly comparable with each other (e.g., because they have different dimensionalities, there is no obvious mapping between them, etc.), the classification results they produce serve as convenient proxies (Methods). Here, we compared classification patterns for pairs of regions while controlling for the pattern of correct labels. Thus, the analysis focuses on common biases in misclassification.
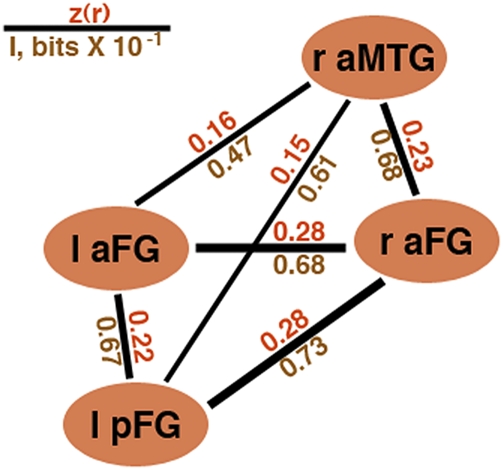
First, we computed the partial correlation between classification patterns corresponding to different clusters. Group results are displayed as a graph in Fig. 5. Similarity scores for all pairs of regions tested above chance (one-sample t test, P < 0.01). Similarity scores within the network were not homogeneous (one-way analysis of variance, P < 0.05) mainly because raFG evinced higher similarity estimates than the rest (P < 0.01). In addition, we computed similarity scores between the four clusters and the EVC. Average similarity scores within the network were significantly higher than scores with the EVC (paired-sample t test, P < 0.01). Second, to verify our findings using an information-theoretic measure, we computed the conditional mutual information between classification patterns produced by different clusters (Fig. 5). Examination of information estimates revealed a relational structure qualitatively similar to that obtained using correlation.
Fig. 5.
Pairwise ROI relations. The pattern of identity (mis)classifications is separately compared for each pair of regions using correlation-based scores (red) and mutual information (brown). Specifically, we relate classification results across regions while controlling for the pattern of true labels. These measures are used as a proxy for assessing similarity in the encoding of facial identity across regions. Of the four ROIs, the raFG produced the highest scores in its relationship with the other regions (connector width is proportional to z values).
We interpret the results above as evidence for the central role of the right FG in face individuation. More generally, they support the idea of redundant information encoding within the network.
Discussion
The present study investigates the encoding of facial identity in the human ventral cortex. Our investigation follows a multivariate approach that exploits multivoxel information at multiple stages from mapping to feature selection and network analysis. We favor this approach because multivariate methods are more successful at category discrimination than univariate tests (31) and possibly more sensitive to “subvoxel” information than adaptation techniques (32). In addition, we extend our investigation to take advantage of spatiotemporal information (29) and, thus, optimize the discovery of small fine-grained pattern differences underlying the perception of same-category exemplars.
Multiple Cortical Areas Support Face Individuation.
Multivariate mapping located four clusters in the bilateral FG and the right aMTG encoding facial identity information. These results indicate that individuation relies on a network of ventral regions that exhibit sensitivity to individuation independently of each other. This account should be distinguished both from local ones (6, 21) and from other versions of distributed processing (23, 24).
With regard to the specific clusters identified, previous work uncovered face-selective areas in the vicinity of the FFA, both posterior (9) and anterior (33) to it. Our clusters did not exhibit face selectivity when assessed with a univariate test. However, this lack of selectivity may reflect the variability of these areas (9, 33) and/or the limitations of univariate analysis (34). Alternatively, it is possible that face processing does not necessarily entail face selectivity (35). More relevantly here, face individuation, rather than face selectivity, was previously mapped to an area in the anterior vicinity of the FFA (14). Overall, our present results confirm the involvement of these areas in face processing and establish their role in individuation.
On a related note, the proximity of these fusiform clusters to the FFA may raise questions as to whether they are independent clusters or rather extensions of the FFA (7, 9). On the basis of differences in peak location and the lack of face selectivity, we treat them here as distinct from the FFA although further investigation is needed to fully understand their relationship with this area.
Unlike the FG areas discussed above, an anterior region of the right middle temporal cortex (36, 37) or temporal pole (22) was consistently associated with identity coding. Due to its sensitivity to higher-level factors, such as familiarity (22), and its involvement in conceptual processing (38, 39), the anterior temporal cortex is thought to encode biographical information (6). Whereas our ability to localize this region validates our mapping methodology, the fact that our stimuli were not explicitly associated with any biographical information suggests that the computations hosted by this area also involve a perceptual component. Consistent with this, another mapping attempt, based on perceptual discrimination (15), traced face individuation to a right anterior temporal area. Also, primate research revealed encoding of face–space dimensions in the macaque anterior temporal cortex (40) as well as different sensitivity to perceptual and semantic processing of facial identity (41). In light of these findings, we argue that this area is part of the network for perceptual face individuation although the representations it hosts are likely to also comprise a conceptual component.
In sum, our mapping results argue for a distributed account of face individuation that accommodates a multitude of experimental findings. Previous imaging research may have failed to identify this network due to limits in the sensitivity of the methods used in relation with the size of the effect. Inability to find sensitivity in more than one region can easily lead to a local interpretation. At the same time, combining information from multiple regions may counter the limitations of one method but overestimate the extent of distributed processing. Our use of dynamic multivariate mapping builds upon these previous findings and is a direct attempt to increase the sensitivity of these mapping methods at the cost of computational complexity.
Importantly, the regions uncovered by our analysis may represent only a subset of the full network of regions involved in identity processing. We allow for this possibility given our limited coverage (intended to boost imaging resolution in the ventral cortex) as well as the lower sensitivity associated with the imaging of the inferior temporal cortex. In particular, regions of the superior temporal sulcus and prefrontal cortex (4, 10) are plausible additions to the network uncovered here.
Individuation Effects Are Not Reducible to Low-Level Image Processing.
To distinguish identity representations from low-level image differences, we appealed to a common source of intraindividual image variations: emotional expressions. Also, identity was not predictable in our case by prominent external features, such as hair, or by image properties associated with higher-level characteristics (e.g., sex or age). Furthermore, we assessed the contribution of interindividual low-level similarity to discrimination performance. Of all regions examined, only laFG showed potential reliance on low-level properties. Finally, an examination of an early visual area did not produce any evidence for identity encoding. Taken together, these results render unlikely an explanation of individuation effects based primarily on low-level image properties.
The Face Individuation System Does Not Support General-Purpose Individuation.
To address the domain specificity of the system identified, we examined whether “abstract” OFs (i.e., independent of font) can be individuated within the regions mapped for faces. Although highly dissimilar from faces, OFs share an important attribute with them, by requiring fine-grained perceptual discrimination at the individual level. In addition, they appear to compete with face representations (42) and to rely on similar visual processing mechanisms (43). Thus, they may represent a more suitable contrast category for faces than other familiar categories, such as houses. Our attempt to classify OF identities revealed that two regions, the lpFG and the rFFA, exhibited sensitivity to this kind of OF information.
Furthermore, to examine the task specificity of the network, we evaluated the ability of its regions to support expression discrimination and found that the rFFA, along with the aMTG, was able to perform this type of discrimination.
Thus, it appears that the mapped network does not support general object individuation, although it may share resources with the processing of other tasks as well as of other visual categories.
FFA Responds to Different Face Identities with Different Patterns.
The FFA (44, 45) is one of the most intensely studied functional areas of the ventral stream. However, surprisingly, its role in face processing is far from clear. At one extreme, its involvement in face individuation (6, 18, 21) has been called into question (14–16); at the other, it has been extended beyond the face domain to visual expertise (17) and even to general object individuation (13).
Previous studies of the FFA using multivariate analysis have not been successful in discovering identity information (15, 24) or even subordinate-level information (23) about faces. The study of patient populations is also not definitive. FG lesions associated with acquired prosopagnosia (46) adversely impact face individuation, confirming the critical role of the FFA. However, individuals with congenital prosopagnosia appear to exhibit normal FFA activation profiles (47) in the presence of compromised fiber tracts connecting the FG to anterior areas (48). Thus, the question is more pertinent than ever: Does the FFA encode identity information?
Our results provide evidence that the FFA responds consistently across different images of the same individual, but distinctly to different individuals. In addition, we show that the right FFA can individuate OFs and decode emotional expressions, consistent with its role in expression recognition (12).
Thus, we confirm the FFA's sensitivity to face identity using pattern analysis. Moreover, we show that it extends beyond both a specific task, i.e., individuation, and a specific domain, i.e., faces. Further research is needed to determine how far its individuation capabilities extend and how they relate with each other.
Informative Features Are Evenly Distributed Across Anterior Regions.
How uniformly is information distributed across multiple regions? At one extreme, the system may favor robustness as a strategy and assign information evenly across regions. At the other, its structure may be shaped by the feed-forward flow of information and display a clear hierarchy of regions from the least to the most diagnostic. The latter alternative is consistent, for instance, with a posterior-to-anterior accumulation of information culminating in the recruitment of the aMTG as the endpoint of identity processing.
Our results fall in between these two alternatives. Anterior regions appear to be at an advantage compared with the left pFG. At the same time, there was no clear differentiation among anterior regions in terms of the amount of information represented, suggesting that information is evenly distributed across them.
The Right aFG May Be a Hub in the Facial Identity Network.
As different regions are not directly comparable as activation patterns, we used their classification results as a proxy for their comparison. Using this approach, we found that different regions do share information with each other, consistent with redundancy in identity encoding. Furthermore, we found that pairwise similarities are more prominent in relation with the right aFG than among other network regions, suggesting that the raFG plays a central role within the face individuation network. Thus, the raFG mirrors the role played by the right FFA among face-selective regions as revealed by functional connectivity (4). One explanation of this role is that a right middle/anterior FG area serves as an interface between low- and high-level information.
Two lines of evidence support this hypothesis. Recent results show, surprisingly, that the FFA exhibits sensitivity to low-level face properties (16, 25). Additionally, the right FG is subject to notable top–down effects (26, 27). Maintaining a robust interface between low-level image properties and high-level factors is likely a key requirement for fast, reliable face processing. Critical for our argument, this requirement would lead to the formation of an FG activation/information hub. Future combinations of information and activation-based connectivity analyses might be able to assess such hypotheses and provide full-fledged accounts of the flow of information in cortical networks.
Summary.
A broad body of research suggests that face perception relies on an extensive network of cortical areas. Our results show that a single face-processing task, individuation, is supported by a network of cortical regions that share resources with the processing of other visual categories (OFs) as well as other face-related (expression discrimination) tasks. Detailed investigation of this network revealed an information structure dominated by anterior cortical regions, and the right FG in particular, confirming its central role in face processing. Finally, we suggest that a full understanding of the operation of this system requires a combination of conventional connectivity analyses and information-based explorations of network structure.
Methods
An extended version of this section is available in SI Text.
Design.
Eight subjects were scanned across multiple sessions using a slow event-related design (10-s trials). Subjects were presented with a single face or OF stimulus for 400 ms and were asked to identify the stimulus at the individual level using a pair of response gloves. We imaged 27 oblique slices covering the ventral cortex at 3T (2.5-mm isotropic voxels, 2-s TR).
Dynamic Information-Based Brain Mapping.
The SL was walked voxel-by-voxel across a subject-specific cortical mask. The mask covered the ventral cortex (Fig. S2) and was temporally centered on 6-s poststimulus onset. At each location within the mask, spatiotemporal patterns (29) were extracted for each stimulus presentation. To boost their signal, these patterns were averaged within runs on the basis of stimulus identity. Pattern classification was performed using linear support vector machines (SVM) with a trainable c term followed by leave-one-run-out cross-validation. Classification was separately applied to each pair of identities (six pairs based on four identities). Discrimination performance for each pair was encoded using d′ and an average information map (28) was computed across all pairs. For the purpose of group analysis, these maps were normalized into Talairach space and examined for above-chance sensitivity (d′ > 0), using voxelwise t tests across subjects [false discovery rate (FDR) corrected]. Expression discrimination followed a similar procedure.
Analyses were carried out in Matlab with the Parallel Processing Toolbox running on a ROCKS+ multiserver environment.
ROI Localization.
Three types of ROIs were localized as follows: (i) We identified locations of the group information map displaying above-chance discriminability and projected their coordinates in the native space of each subject. ROIs were constructed by placing spherical masks at each of these locations—a set of overlapping masks gave rise to a single ROI. (ii) Bilateral FFAs were identified for each subject by standard face–object contrasts. ROIs were constructed by applying a spherical mask centered on the FG face-selective peak. (iii) Anatomical masks were manually drawn around the calcarine sulcus of each subject and a mask was placed at the center of these areas. The results serve as a rough approximation of EVC. All masks used for ROI localization had a 5-voxel radius.
RFE-Based Analysis.
SVM-based RFE (30) was used for feature selection and ranking—the order of feature elimination provides an estimate of feature diagnosticity for a given type of discrimination. To obtain unbiased estimates of performance, we executed two types of cross-validation. We performed cross-validation, first, at each RFE iteration step to measure performance and, second, across iteration steps to find the best number of features. RFE analysis was applied to all types of ROIs described above.
Pairwise ROI Analysis.
We computed the similarity of classification patterns produced by each pair of ROIs, namely the patterns of classification labels obtained with test instances during cross-validation. However, patterns are likely correlated across regions by virtue of the ability of SVM models to approximate true labels. Therefore, we measured pattern similarity with partial correlation while controlling for the pattern of true labels. Correlations were computed for each pair of facial identities, transformed using Fisher's z, and averaged within subjects. Critically, to eliminate common biases based on spatial proximity between regions (because of spatially correlated noise) all activation patterns were z-scored before classification. To obtain estimates of the information shared between ROIs we also computed the conditional mutual information between ROI-specific classification patterns given the pattern of true labels.
Supplementary Material
Acknowledgments
This work was funded by National Science Foundation Grant SBE-0542013 to the Temporal Dynamics of Learning Center and by National Science Foundation Grant BCS0923763. M.B. was partially supported by a Weston Visiting Professorship at the Weizmann Institute of Science.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The fMRI dataset has been deposited with the XNAT Central database under the project name “STSL.”
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102433108/-/DCSupplemental.
References
- 1.DiCarlo JJ, Cox DD. Untangling invariant object recognition. Trends Cogn Sci. 2007;11:333–341. doi: 10.1016/j.tics.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Jiang X, et al. Evaluation of a shape-based model of human face discrimination using FMRI and behavioral techniques. Neuron. 2006;50:159–172. doi: 10.1016/j.neuron.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Kanwisher N. Functional specificity in the human brain: A window into the functional architecture of the mind. Proc Natl Acad Sci USA. 2010;107:11163–11170. doi: 10.1073/pnas.1005062107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fairhall SL, Ishai A. Effective connectivity within the distributed cortical network for face perception. Cereb Cortex. 2007;17:2400–2406. doi: 10.1093/cercor/bhl148. [DOI] [PubMed] [Google Scholar]
- 5.Gauthier I, et al. The fusiform “face area” is part of a network that processes faces at the individual level. J Cogn Neurosci. 2000;12:495–504. doi: 10.1162/089892900562165. [DOI] [PubMed] [Google Scholar]
- 6.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 7.Pinsk MA, et al. Neural representations of faces and body parts in macaque and human cortex: A comparative FMRI study. J Neurophysiol. 2009;101:2581–2600. doi: 10.1152/jn.91198.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossion B, et al. A network of occipito-temporal face-sensitive areas besides the right middle fusiform gyrus is necessary for normal face processing. Brain. 2003;126:2381–2395. doi: 10.1093/brain/awg241. [DOI] [PubMed] [Google Scholar]
- 9.Weiner KS, Grill-Spector K. Sparsely-distributed organization of face and limb activations in human ventral temporal cortex. Neuroimage. 2010;52:1559–1573. doi: 10.1016/j.neuroimage.2010.04.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsao DY, Schweers N, Moeller S, Freiwald WA. Patches of face-selective cortex in the macaque frontal lobe. Nat Neurosci. 2008;11:877–879. doi: 10.1038/nn.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calder AJ, Young AW. Understanding the recognition of facial identity and facial expression. Nat Rev Neurosci. 2005;6:641–651. doi: 10.1038/nrn1724. [DOI] [PubMed] [Google Scholar]
- 12.Fox CJ, Moon SY, Iaria G, Barton JJ. The correlates of subjective perception of identity and expression in the face network: An fMRI adaptation study. Neuroimage. 2009;44:569–580. doi: 10.1016/j.neuroimage.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haist F, Lee K, Stiles J. Individuating faces and common objects produces equal responses in putative face-processing areas in the ventral occipitotemporal cortex. Front Hum Neurosci. 2010;4:181. doi: 10.3389/fnhum.2010.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nestor A, Vettel JM, Tarr MJ. Task-specific codes for face recognition: How they shape the neural representation of features for detection and individuation. PLoS ONE. 2008;3:e3978. doi: 10.1371/journal.pone.0003978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kriegeskorte N, Formisano E, Sorger B, Goebel R. Individual faces elicit distinct response patterns in human anterior temporal cortex. Proc Natl Acad Sci USA. 2007;104:20600–20605. doi: 10.1073/pnas.0705654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu X, Yue X, Lescroart MD, Biederman I, Kim JG. Adaptation in the fusiform face area (FFA): Image or person? Vision Res. 2009;49:2800–2807. doi: 10.1016/j.visres.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Gauthier I, Skudlarski P, Gore JC, Anderson AW. Expertise for cars and birds recruits brain areas involved in face recognition. Nat Neurosci. 2000;3:191–197. doi: 10.1038/72140. [DOI] [PubMed] [Google Scholar]
- 18.Freiwald WA, Tsao DY, Livingstone MS. A face feature space in the macaque temporal lobe. Nat Neurosci. 2009;12:1187–1196. doi: 10.1038/nn.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrews TJ, Ewbank MP. Distinct representations for facial identity and changeable aspects of faces in the human temporal lobe. Neuroimage. 2004;23:905–913. doi: 10.1016/j.neuroimage.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 20.Pourtois G, Schwartz S, Seghier ML, Lazeyras F, Vuilleumier P. View-independent coding of face identity in frontal and temporal cortices is modulated by familiarity: An event-related fMRI study. Neuroimage. 2005;24:1214–1224. doi: 10.1016/j.neuroimage.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 21.Kanwisher N, Yovel G. The fusiform face area: A cortical region specialized for the perception of faces. Philos Trans R Soc Lond B Biol Sci. 2006;361:2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rotshtein P, Henson RN, Treves A, Driver J, Dolan RJ. Morphing Marilyn into Maggie dissociates physical and identity face representations in the brain. Nat Neurosci. 2005;8:107–113. doi: 10.1038/nn1370. [DOI] [PubMed] [Google Scholar]
- 23.Op de Beeck HP, Brants M, Baeck A, Wagemans J. Distributed subordinate specificity for bodies, faces, and buildings in human ventral visual cortex. Neuroimage. 2010;49:3414–3425. doi: 10.1016/j.neuroimage.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 24.Natu VS, et al. Dissociable neural patterns of facial identity across changes in viewpoint. J Cogn Neurosci. 2010;22:1570–1582. doi: 10.1162/jocn.2009.21312. [DOI] [PubMed] [Google Scholar]
- 25.Yue X, Cassidy BS, Devaney KJ, Holt DJ, Tootell RB. Lower-level stimulus features strongly influence responses in the fusiform face area. Cereb Cortex. 2011;21:35–47. doi: 10.1093/cercor/bhq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bar M, et al. Top-down facilitation of visual recognition. Proc Natl Acad Sci USA. 2006;103:449–454. doi: 10.1073/pnas.0507062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox D, Meyers E, Sinha P. Contextually evoked object-specific responses in human visual cortex. Science. 2004;304:115–117. doi: 10.1126/science.1093110. [DOI] [PubMed] [Google Scholar]
- 28.Kriegeskorte N, Goebel R, Bandettini P. Information-based functional brain mapping. Proc Natl Acad Sci USA. 2006;103:3863–3868. doi: 10.1073/pnas.0600244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mourão-Miranda J, Friston KJ, Brammer M. Dynamic discrimination analysis: A spatial-temporal SVM. Neuroimage. 2007;36:88–99. doi: 10.1016/j.neuroimage.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 30.Hanson SJ, Halchenko YO. Brain reading using full brain support vector machines for object recognition: There is no “face” identification area. Neural Comput. 2008;20:486–503. doi: 10.1162/neco.2007.09-06-340. [DOI] [PubMed] [Google Scholar]
- 31.Haxby JV, et al. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- 32.Sapountzis P, Schluppeck D, Bowtell R, Peirce JW. A comparison of fMRI adaptation and multivariate pattern classification analysis in visual cortex. Neuroimage. 2010;49:1632–1640. doi: 10.1016/j.neuroimage.2009.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajimehr R, Young JC, Tootell RB. An anterior temporal face patch in human cortex, predicted by macaque maps. Proc Natl Acad Sci USA. 2009;106:1995–2000. doi: 10.1073/pnas.0807304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kriegeskorte N, Bandettini P. Analyzing for information, not activation, to exploit high-resolution fMRI. Neuroimage. 2007;38:649–662. doi: 10.1016/j.neuroimage.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hadj-Bouziane F, Bell AH, Knusten TA, Ungerleider LG, Tootell RBH. Perception of emotional expressions is independent of face selectivity in monkey inferior temporal cortex. Proc Natl Acad Sci USA. 2008;105:5591–5596. doi: 10.1073/pnas.0800489105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leveroni CL, et al. Neural systems underlying the recognition of familiar and newly learned faces. J Neurosci. 2000;20:878–886. doi: 10.1523/JNEUROSCI.20-02-00878.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugiura M, et al. Activation reduction in anterior temporal cortices during repeated recognition of faces of personal acquaintances. Neuroimage. 2001;13:877–890. doi: 10.1006/nimg.2001.0747. [DOI] [PubMed] [Google Scholar]
- 38.Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92:179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Simmons WK, Reddish M, Bellgowan PS, Martin A. The selectivity and functional connectivity of the anterior temporal lobes. Cereb Cortex. 2010;20:813–825. doi: 10.1093/cercor/bhp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leopold DA, Bondar IV, Giese MA. Norm-based face encoding by single neurons in the monkey inferotemporal cortex. Nature. 2006;442:572–575. doi: 10.1038/nature04951. [DOI] [PubMed] [Google Scholar]
- 41.Eifuku S, Nakata R, Sugimori M, Ono T, Tamura R. Neural correlates of associative face memory in the anterior inferior temporal cortex of monkeys. J Neurosci. 2010;30:15085–15096. doi: 10.1523/JNEUROSCI.0471-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dehaene S, et al. How learning to read changes the cortical networks for vision and language. Science. 2010;330:1359–1364. doi: 10.1126/science.1194140. [DOI] [PubMed] [Google Scholar]
- 43.Hasson U, Levy I, Behrmann M, Hendler T, Malach R. Eccentricity bias as an organizing principle for human high-order object areas. Neuron. 2002;34:479–490. doi: 10.1016/s0896-6273(02)00662-1. [DOI] [PubMed] [Google Scholar]
- 44.Puce A, Allison T, Asgari M, Gore JC, McCarthy G. Differential sensitivity of human visual cortex to faces, letterstrings, and textures: A functional magnetic resonance imaging study. J Neurosci. 1996;16:5205–5215. doi: 10.1523/JNEUROSCI.16-16-05205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barton JJ, Press DZ, Keenan JP, O'Connor M. Lesions of the fusiform face area impair perception of facial configuration in prosopagnosia. Neurology. 2002;58:71–78. doi: 10.1212/wnl.58.1.71. [DOI] [PubMed] [Google Scholar]
- 47.Avidan G, Behrmann M. Functional MRI reveals compromised neural integrity of the face processing network in congenital prosopagnosia. Curr Biol. 2009;19:1146–1150. doi: 10.1016/j.cub.2009.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas C, et al. Reduced structural connectivity in ventral visual cortex in congenital prosopagnosia. Nat Neurosci. 2009;12:29–31. doi: 10.1038/nn.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.