Abstract
Defective homologous recombination (HR) DNA repair imposed by BRCA1 or BRCA2 deficiency sensitizes cells to poly (ADP-ribose) polymerase (PARP)-1 inhibition and is currently exploited in clinical treatment of HR-deficient tumors. Here we show that mild hyperthermia (41–42.5 °C) induces degradation of BRCA2 and inhibits HR. We demonstrate that hyperthermia can be used to sensitize innately HR-proficient tumor cells to PARP-1 inhibitors and that this effect can be enhanced by heat shock protein inhibition. Our results, obtained from cell lines and in vivo tumor models, enable the design of unique therapeutic strategies involving localized on-demand induction of HR deficiency, an approach that we term induced synthetic lethality.
Keywords: anti-cancer treatment, RAD51, double-strand break
Many anti-cancer therapies are based on cytotoxicity of DNA double strand breaks (DSBs) induced by ionizing radiation or, indirectly, by chemical agents. However, efficient DSB repair mechanisms protect cells from the genotoxic effects of DSBs, thereby reducing the effectiveness of the therapies. Two major pathways are involved in DSB repair in mammalian cells: homologous recombination (HR) and nonhomologous end joining (NHEJ). HR uses intact homologous DNA sequences, usually the sister chromatid in postreplicative chromatin, to faithfully restore DNA breaks (1), whereas NHEJ operates throughout the entire cell cycle and does not require a DNA template (2). Agents inhibiting DNA repair processes potentiate the cytotoxicity of DSBs in cancer therapy (3). Elevated temperature is one such agent that, via unclear mechanisms, interferes with multiple pathways of DNA repair (4–6) and is clinically applied (7).
Results
To investigate if HR, among other processes and DSB repair pathways, is influenced by elevated temperature, we used an isogenic set of mouse embryonic stem (ES) cells that are either HR proficient (wild-type) or HR deficient (Rad54−/−) due to the disruption of the Rad54 gene, which is important for HR activity (1). We compared radiosensitization of these cells by incubating them at 37 °C or 41 °C before irradiation. For this and subsequent experiments we chose temperatures below 43 °C, because they are relevant in clinical practice (8). Interestingly, we observed that wild-type but not Rad54−/− cells were radiosensitized by preincubation at 41 °C compared with cells incubated at 37 °C (Fig. 1A). Similarly, HeLa cells, in which the important HR factors XRCC3 or BRCA2 were down-regulated using siRNA, were refractory to further temperature-mediated radiosensitization (Fig. 1B and Fig. S1). These results suggest that elevated temperature inactivates HR. To directly measure the effect of temperature on HR, we quantitated HR-mediated gene targeting in ES cells (9) and found that the efficiency of gene targeting was significantly reduced by preincubation at 41 °C compared with 37 °C (Fig. 1C). Similarly, preincubation at 41 °C reduced the frequency of spontaneous and mitomycin C-induced sister chromatid exchanges in SW-1573 cells (Fig. S2A), which are to a large extent mediated by HR (10).
Fig. 1.
Mild increases in temperature radiosensitizes HR-proficient but not HR-deficient cells and inhibits HR repair. (A) Cloning efficiency of wild-type (Left) and Rad54−/− (Right) mouse ES cells incubated for 75 min at 37 °C (□) or 41 °C ( ) and subsequently exposed to the indicated dose of γ-rays. (B) Cloning efficiency of HeLa cells transfected with siRNA directed against luciferase (squares) or XRCC3 (circles), incubated for 75 min at 37 °C (open symbols) or 42.5 °C (filled symbols), and exposed to the indicated dose of γ-rays. Inset shows reduction of XRCC3 protein levels in HeLa cells transfected with siRNA directed against XRCC3. Cell lysates were analyzed by immunoblotting with antibodies against XRCC3. Equal sample loading was verified by probing for ORC2. (C) Efficiency of HR-mediated gene targeting in mouse ES cells. Cells were incubated for 7 h at 37 °C or 41 °C. At 2 h into this incubation period, cells were transfected with the hRad54GFP-puro knock-in targeting construct. Cells containing integrated construct were then selected in medium containing puromycin and analyzed by FACS. In this assay, GFP-positive cells arise when the targeting construct integrates in the mRad54 locus via HR. FACS profiles obtained in a single representative experiment show the percentage of GFP-positive cells upon incubation at the indicated conditions. The bar graph in Fig. 4A shows the relative average percentage of GFP-positive cells obtained from three independent experiments with the error bars representing SEM.
) and subsequently exposed to the indicated dose of γ-rays. (B) Cloning efficiency of HeLa cells transfected with siRNA directed against luciferase (squares) or XRCC3 (circles), incubated for 75 min at 37 °C (open symbols) or 42.5 °C (filled symbols), and exposed to the indicated dose of γ-rays. Inset shows reduction of XRCC3 protein levels in HeLa cells transfected with siRNA directed against XRCC3. Cell lysates were analyzed by immunoblotting with antibodies against XRCC3. Equal sample loading was verified by probing for ORC2. (C) Efficiency of HR-mediated gene targeting in mouse ES cells. Cells were incubated for 7 h at 37 °C or 41 °C. At 2 h into this incubation period, cells were transfected with the hRad54GFP-puro knock-in targeting construct. Cells containing integrated construct were then selected in medium containing puromycin and analyzed by FACS. In this assay, GFP-positive cells arise when the targeting construct integrates in the mRad54 locus via HR. FACS profiles obtained in a single representative experiment show the percentage of GFP-positive cells upon incubation at the indicated conditions. The bar graph in Fig. 4A shows the relative average percentage of GFP-positive cells obtained from three independent experiments with the error bars representing SEM.
The majority of DSB repair-related proteins accumulate at sites of DSBs, forming so-called ionizing radiation-induced foci (IRIF) (11). This accumulation is crucial for proper functioning of repair mechanisms, because disturbed formation of IRIF is associated with repair deficiencies and genome instability (1). To pinpoint the temperature-induced defect(s) in the HR pathway, we examined formation of IRIF by a range of DSB repair factors at α-particle induced DSBs (12) in U2OS cells incubated at 37 °C or 41 °C for 60 min before irradiation (Fig. 2 A and B and Fig. S2). Early in HR, the ends of DSBs are resected in a reaction involving the MRE11/RAD50/NBS1, CtIP, and BRCA1 complexes (13, 14) generating single-stranded DNA stretches, which are subsequently coated by RPA (1). MRE11 and replication protein A (RPA) efficiently accumulated at DSB sites regardless of whether the cells had been incubated at 41 °C (Fig. 2 A and B), suggesting that DSB end resection is unaffected by heat. In subsequent steps of HR, the RAD51 recombinase forms nucleoprotein filaments on single-stranded DNA with the help of BRCA2 (1, 15). Whereas RAD51 and BRCA2 IRIF could be detected 15 min post-irradiation in control cells, incubation at 41 °C completely abrogated accumulation of both proteins at DSB sites (Fig. 2 A and B). Similarly, elevated temperature prevented formation of RAD51 IRIF at α-particle- and γ-ray-induced DSBs in mouse ES cells (Fig. S2B). Moreover, incubation at 41 °C abrogated accumulation of RAD51-GFP in V79 cells (16) at laser-induced DNA damage (Fig. S2C). These results suggest that the formation of RAD51 nucleoprotein filaments, a pivotal step of HR (1), is sensitive to a mild temperature elevation in all cell lines tested. This could be due to defects in RAD51 itself or in BRCA2, which is essential for the loading of RAD51 on the proper DNA substrate (17, 18) and the accumulation of RAD51 in IRIF (19, 20).
Fig. 2.
Mild temperature increase interferes with the function and stability of HR proteins. (A) Visualization of accumulation of repair proteins at DSB sites in cells preincubated at 37 °C or 41 °C. U2OS cells were incubated at 37 °C or 41 °C for 60 min, irradiated with α-particles from a source positioned alongside the cells, resulting in linear arrays of DSBs, and then incubated for 15 min at 37 °C and fixed. Cells were stained for DNA (blue), γH2AX, or MDC1 (red), which were used as markers of the DSBs induced by α-particles, and either of the following proteins: MRE11, RPA34, BRCA2, or RAD51 (green). (Scale bar: 5 μm.) (B) Quantification of accumulation of repair proteins at DSB sites in cells preincubated at 37 °C or 41 °C. Cells treated and prepared as in A were scored as positive if they contained at least three IRIF of the indicated repair protein colocalizing with either γH2AX or MDC1 IRIF. Graphs represent average percentages of positive cells. Error bars represent the range of percentages obtained from two independent experiments. At least 50 cells containing damage induced by α-particles were scored per experiment. (C) Immunoblotting of cells subjected to mild heat and/or proteasome inhibitor. HeLa and BRO cells were incubated at 37 °C or 42.5 °C for 75 min in the presence or absence of 10 μM MG132. Next, cells were lysed and the lysates were analyzed by immunoblotting with antibodies against RAD51 (Upper, HeLa cells) or BRCA2 (Lower, BRO cells). (D) Immunoblotting of cells subjected to mild heat. BRO cells were incubated at 37 °C or 42.5 °C for 75 min. Next, cells were incubated for the indicated period at 37 °C and lysed. Lysates were analyzed by immunoblotting with antibodies against BRCA2 (Upper). Equal sample loading was verified by probing for ORC2 (Lower). (E) Visualization of accumulation of RAD51 at DSB sites in cells isolated from cervix carcinoma biopsies preincubated at 37 °C or 41 °C for 60 min, then irradiated with α-particles from a source positioned above the cells, incubated for 30 min at 37 °C or 41 °C, and fixed. Cells were stained for DNA (blue), γH2AX (red), and RAD51 (green). (Scale bar: 5 μm.)
To establish how these two proteins are affected by increased temperature, we analyzed cell extracts by immunoblotting. Incubating cells at 42.5 °C had no detectable effect on RAD51 (Fig. 2C) or PALB2 (Fig. S3), but did result in considerable reduction of BRCA2 levels (Fig. 2 C and D). This reduction could be a result of proteasome-mediated degradation, because we detected no decrease of BRCA2 levels when cells were exposed to 42.5 °C in the presence of proteasome inhibitor MG132 (21) (Fig. 2C). The extent of BRCA2 degradation was dependent on the temperature used as well as on the duration of the exposure (Fig. S3). The effects of temperature on BRCA2 levels were observed in multiple cell lines, including HeLa, U2OS, and human melanoma (BRO)-derived cells. Importantly, temperature-induced effects on HR proteins were not limited to cultured cells. They were also observed in cells from a fresh tumor biopsy of a cervix carcinoma, because preincubation at 41 °C eliminated accumulation of RAD51 on α-particle-induced DSBs (Fig. 2E). Taken together, these results suggest that a moderate (4–5.5 °C) elevation of temperature above the physiological level interferes with HR, likely by inducing degradation of BRCA2, in human cells, tissues, and tumors.
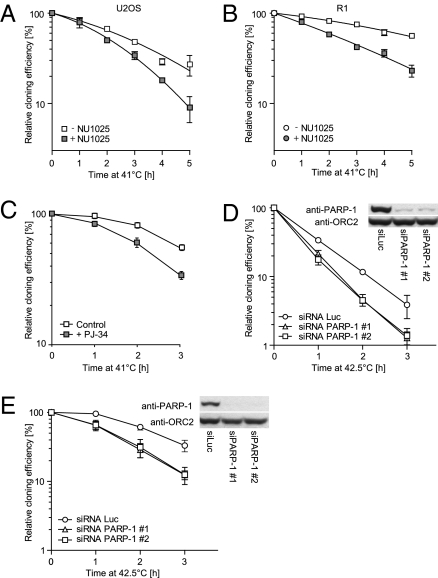
HR-deficient cell lines and tumors, particularly those defective in BRCA2, are extremely sensitive to replication-dependent DSBs induced indirectly by PARP-1 inhibitors (22, 23). Indeed, we observed a decrease in clonogenic survival of human U2OS (Fig. 3A) and rat R-1 (Fig. 3B) cells incubated at 41 °C in the presence of the PARP-1 inhibitor NU1025 (24) compared to cells subjected to 41 °C alone. A similar temperature-mediated sensitization was observed with another PARP-1 inhibitor, PJ-34 (Fig. 3C). To confirm that hyperthermia sensitizes cells to decreased PARP-1 activity, we analyzed HeLa and BRO cells in which PARP-1 was down-regulated by siRNA. In line with previous results, incubation at an elevated temperature resulted in diminished survival of these cells (Fig. 3 D and E).
Fig. 3.
A mild increase in temperature is toxic to cells lacking PARP-1 functionality. Cloning efficiency of U2OS (A) and R-1 (B) cells incubated at 41 °C for the indicated period in the absence (open symbols) or presence (filled symbols) of 100 μM NU1025. Graphs represent average cloning efficiencies corrected for the toxicity of NU1025. Error bars represent SEM from three independent experiments. (C) Cloning efficiency of BRO cells exposed to heat as described above, except for the use of 5 μM PJ-34 instead of 100 μM NU1025. Cloning efficiency of HeLa (D) and BRO (E) cells with reduced levels of PARP-1 protein subjected to mild heat. Cells were transfected with siRNA directed against luciferase (○) or PARP-1 (siRNA #1 △, siRNA #2 □) and incubated at 42.5 °C for the indicated period. Error bars represent SEM in a single experiment. The figure is representative for three independent experiments. Inset shows the efficiency of siRNA-induced reduction of PARP-1 protein levels. Cell lysates were analyzed by immunoblotting with antibodies against PARP-1. Equal sample loading was verified by probing for ORC2.
BRCA2 is a known client protein of HSP90 and the HSP90 inhibitor 17-DMAG moderately reduces BRCA2 levels (25, 26). Incubation of cells at elevated temperature in the presence of 17-DMAG resulted in a significant further reduction of BRCA2 level in BRO cells (Fig. 4A). More importantly, whereas treatment of cells with 17-DMAG at 37 °C did not reduce HR efficiency, incubation of cells with 17-DMAG at 41 °C resulted in a further reduction of HR compared with elevated temperature alone (Figs. 1C and 4B). Furthermore, 17-DMAG significantly prolonged the inhibitory effect of elevated temperature on the accumulation of RAD51 on DSBs in U2OS cells (Fig. S4). These results indicate that a combination of elevated temperature and 17-DMAG is a powerful tool to enhance heat-mediated degradation of BRCA2 and inhibition of HR. Notably, 17-DMAG dramatically enhanced the cytotoxic effects of PARP-1 inhibition in heated cells. The clonogenic survival of rat R-1 cells incubated at 41 °C was reduced ∼50-fold when they were treated in the presence of NU1025 and 17-DMAG (Fig. 4C). Additionally, HR deficiency induced by elevated temperature applied in the presence of 17-DMAG increased radiation sensitivity of HeLa cells by 7- to 10-fold compared with no treatment and by 3- to 4-fold compared with heat treatment alone (Fig. 4D).
Fig. 4.
An HSP90 inhibitor enhances temperature-induced degradation of BRCA2, reduction of HR efficiency, sensitivity of heat-treated cells to PARP-1 inhibitors and to ionizing radiation, and reduction in in vivo tumor outgrowth. (A) Immunoblotting of cells subjected to a mild temperature increase and/or HSP90 inhibitor. BRO cells were incubated at 37 °C or 42.5 °C for 75 min in the presence or absence of 100 nM 17-DMAG. Next, cells were lysed and lysates were analyzed by immunoblotting with antibodies against BRCA2. Equal sample loading was verified by probing for ORC2. (B) Effect of 17-DMAG on the efficiency of HR-mediated gene targeting. Mouse ES cells were incubated for 7 h at 37 °C or 41 °C in the presence of 100 nM 17-DMAG. Transfection with the targeting construct and analysis by FACS was done as described in the legend to Figure 1C. FACS profiles obtained in a single representative experiment (Left) show the percentage of GFP-positive cells upon incubation at the indicated conditions. The bar graph (Right) shows the relative average percentage of GFP-positive cells obtained from three independent experiments. Error bars represent SEM. (C) Cloning efficiency of R-1 cells incubated for 4 h at 41 °C in the presence or absence of 100 μM NU1025 and/or 100 nM 17-DMAG. Error bars represent the range of cloning efficiencies obtained in two independent experiments. (D) Cloning efficiency of HeLa cells incubated for 75 min at 42.5 or 37 °C and subsequently exposed to the indicated dose of γ-rays in the presence or absence of 100 nM 17-DMAG. Error bars represent SEM from three independent experiments. (E) Temperature-controlled sensitization of tumors to PARP-1 inhibition. BRO derived-tumor bearing animals were treated with either PJ-34 or vehicle and subsequently exposed to elevated temperature (1 h at 42 °C) on the days marked with arrows. The number of animals per group is indicated in brackets. Error bars represent SEM (P < 0.002). (F) R-1 rhabdomyosarcoma-bearing rats were subjected to the indicated treatment on the days marked with arrows. Heat treatment of tumor-bearing hind limb was applied for 1.5 h at 42 °C. Olaparib and 17-DMAG were used at 50 and 10 mg/kg, respectively. The number of tumors analyzed/number of animals per group are indicated in brackets. Error bars represent SEM (P < 0.0001). (G) Survival of rats during the experiment shown in E. Animals were killed when the tumors exceeded a volume of 3,500 mm3. The number of animals per group is indicated in brackets.
To test whether increased temperature sensitizes tumors to PARP-1 inhibition and reduces tumor outgrowth in vivo, we implanted small pieces of a BRO cell-derived tumor (1–5 mm3) s.c. in the hind limb of nude mice and started treatment of those tumors when their size reached 20–100 mm3. Animals were injected with 5 mg/kg PJ-34 and, while under anesthesia, the temperature in the tumor bearing legs was raised to 42 °C for 1 h. The localized temperature increase and systemic PJ-34 PARP inhibitor by themselves did not significantly influence tumor growth; however, their combination resulted in a significant decline in tumor outgrowth (Fig. 4E). To demonstrate the universality of our strategy, we used an unrelated rat tumor model (27) and a different PARP-1 inhibitor. We also tested in vivo antitumor activity of a trimodality treatment including heat, PARP-1 inhibition, and 17-DMAG. Fragments of R-1 cell-derived rhabdomyosarcoma tumors were implanted into hind limbs of rats. When the tumors attained a volume of 100–400 mm3, animals were divided randomly into groups and treated with 50 mg/kg of the PARP-1 inhibitor Olaparib (AZD2281) and/or 10 mg/kg of 17-DMAG. The tumor-bearing hind limbs were heated for 1.5 h at 42 °C. The tumors in the sham-treated group and in groups treated with either single agent showed typical exponential growth kinetics (Fig. 4F). The combination of heat and Olaparib, a PARP-1 inhibitor currently undergoing clinical trials (28), significantly (P < 0.001) inhibited outgrowth of the tumors. In addition, a relative reduction in tumor outgrowth was observed in animals treated with heat and 17-DMAG. Importantly, tumor outgrowth was completely halted when animals were subjected to trimodality therapy combining heat, 17-DMAG, and Olaparib (Fig. 4F). The treatments also delayed the time when animals had to be killed due to excessive tumor volume (>3,500 mm3). Compared with the separate single-agent treatments, the combination of heat with Olaparib or 17-DMAG considerably increased the number of surviving rats. Moreover, the trimodal therapy resulted in such an effective tumor growth control that all animals survived the course of the treatments (Fig. 4G).
Discussion
The results presented here indicate that elevated temperature at a clinically achievable range (41–42.5 °C) inhibits HR and also leads to degradation of BRCA2. Our results do not prove that degradation of BRCA2 is the sole or even most important effect of heat on DNA repair or on other cellular processes relevant for cell killing. In fact, multiple hyperthermia targets have been described (29–34). They do demonstrate, however, that hyperthermia can be a powerful, noninvasive tool to locally introduce BRCA2 degradation and HR deficiency. This induced synthetic lethality renders innately HR-proficient tumor cells highly sensitive to PARP-1 inhibitors. Our results might have direct clinical relevance, because recent trials demonstrated that both HSP90 and PARP-1 inhibitors are generally well tolerated (35, 36). Moreover, because our approach is not based on genetic deficiency, as is the case in hereditary BRCA2-deficient tumors, it is not likely that a previously described mechanism of resistance to the inhibitor will be relevant (37, 38). Our results could provide a rational basis for development of therapies for a wide range of tumors, exploiting local, on-demand induction of HR deficiency in combination with PARP-1 inhibitors, ionizing radiation, or other DSB-inducing chemotherapeutic agents.
Materials and Methods
Cell Culture.
ES cells were cultured on gelatin-coated dishes in a 1:1 mixture of DMEM and buffalo rat liver-conditioned medium, supplemented with 10% FBS (HyClone), 0.1 mM nonessential amino acids (Biowhittaker), 50 mM β-mercaptoethanol (Sigma), and 500 U mL−1 leukemia inhibitory factor. Other cells were cultured in the following media supplemented with 10% (vol/vol) FCS and streptomycin/penicillin: 1:1 mixture of DMEM and Ham's F-10 (HeLa), DMEM [BRO-derived human melanoma (39, 40) (BRO), osteosarcoma (U2OS), cervix carcinoma cells], l-15 [human squamous lung carcinoma (SW-1573)], Eagle's MEM [rat rhabdomyosarcoma (R-1)], Mc Coy's 5A with 25 mM HEPES [human colon cancer (RKO)], and Ham's F-10 (MRC5, EUFA1341). Cells were maintained at 37 °C in an atmosphere containing 5% (HeLa, BRO, MRC5, EUFA1341), 10% (U2OS, cervix carcinoma cells), 2% (R-1, RKO), or 0% (SW-1573) CO2. For the experiments, cell lines were exposed to increased temperature in the range of 41–42.5 °C. In this range, only the magnitude rather than the nature of all described cellular responses depended on temperature and duration of treatment. Patients with squamous cell carcinoma of the cervix expressed written informed consent to provide fresh biopsies during an investigation under general anesthesia and for its use of the tumor specimens in this study. The biopsies were minced using a scalpel and were then incubated in Liberase Blendzyme enzyme mixture (Roche) in DMEM for 60 min at 37 °C and plated on glass coverslips in fresh culture medium. Incubations at elevated temperatures were performed in a heated waterbath or in an incubator set to the required temperature in an atmosphere containing an appropriate CO2 concentration.
Antibodies.
The following antibodies were used for immunoblotting: mouse and rabbit anti-BRCA2 (Ab-1/OP-95 and PC146/CA1033, Calbiochem), rabbit anti-PALB2 (A301-246A, Bethyl Laboratories), rabbit anti-RAD51 (41), rabbit anti-XRCC3 (ab6494, Abcam), rabbit anti-ORC2 (#559266) and mouse anti-GRB2 (#610112) (BD Pharmingen), mouse anti-PARP-1 (C2-10, Alexis Biochemicals), and relevant horseradish peroxidase-conjugated secondary antibodies (Jackson Immunoresearch). The following antibodies were used for immunofluorescence: mouse-anti BRCA2 (Ab-1, Calbiochem), mouse-anti RPA34 (Ab-2, Oncogene), mouse anti-γH2AX (05-636, Millipore), rabbit anti-MDC1 (A300-051A, Bethyl Laboratories), rabbit anti-RAD51 (41), rabbit anti-MRE11 (42), goat anti-mouse Cy3 (115-165-166), and goat anti-rabbit-FITC (111-095-144) (Jackson Immunoresearch).
Chemical Agents.
The following chemical agents were used at the indicated final concentrations: 17-DMAG, NU1025, PJ-34, mitomycin C, BrdU, colcemid (all from Sigma-Aldrich), MG-132 (Calbiochem) and Olaparib (AZD2281, from Astra Zeneca/KuDOS).
Cell Lysis and Immunoblotting.
Cells were lysed in SDS sample buffer (2% SDS, 10% glycerol, 60 mM Tris-HCl, pH 6.8). Protein concentration was determined by the Lowry protein assay and extracts were supplemented with 0.5% β-mercaptoethanol and 0.02% bromophenol blue. After fractionation by SDS/PAGE, proteins were transferred to a nitrocellulose membrane and probed with the relevant antibodies. For immunoblotting of BRCA2, a 3–8% Tris-acetate gel system was used. BRCA2 was transferred onto a PVDF membrane.
siRNA Treatment.
Transfection of siRNA duplexes was carried out using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Cells transfected with 200 pmol siRNA per 60-mm culture dish were used for cloning efficiency assays or immunoblotting 48 h after transfection. Sense sequences of siRNA were GAAAGUGUGUUCAACUAAUUUdTdT (#1) and GAAGUCA-UCGAUAUCUUUAdTdT (#2) against PARP-1 (43), GGACCUGAAUCCCAGAAUUUU against XRCC3 (44), mix of UAAAUGAAUUUGACAGGAUUU and UUUUUAGCACAGCAAGUGGUU against BRCA2, and CGUACGCGGAAUACUUCGAdTdT against luciferase.
Clonogenic Assays.
In experiments involving NU1025 or irradiation, cells were trypsinized, counted, and plated at appropriate concentrations into 60- or 35-mm dishes. After a 4- to 6-h attachment period, if required, cells were irradiated and incubated at various temperatures for various periods of time in the presence or absence of the indicated inhibitors. Cells were then incubated for 7–14 d, fixed, and stained and colonies exceeding 50 cells were counted. NU1025 and PJ-34 were added to the cells 24 h before seeding and remained present during the entire duration of the experiment. In experiments involving a combination of NU1025 and 17-DMAG, cells were first treated as indicated and subsequently trypsinized, counted, and plated at various concentrations. 17-DMAG was added to the cells 1 h before and removed 30–60 min after the incubation at elevated temperature.
Gene Targeting Efficiency and Sister-Chromatid Exchange Assays.
The gene targeting assay has been described previously (9, 45). Cells were incubated at elevated temperature from 2 h before transfection until 5 h post transfection. 17-DMAG was added 4 h before the heat treatment and removed 5 h after transfection. The sister-chromatid exchange assay was performed as previously published (46).
Irradiation.
Unless stated otherwise, cells were irradiated directly after incubation at elevated temperature by γ-rays from a cesium source (137Cs, 0.70 Gy/min) or by α-particles as previously described (15). Cells from the cervix carcinoma biopsies were irradiated for 1 min with α-particles by placing coverslips with cells upside-down on a 1.8-μm-thick polyester membrane and irradiating from below, through the membrane.
Laser Microirradiation and Time-Lapse Microscopy.
Cells were cultured on glass coverslips for 24 h, then the medium was changed to CO2-independent medium, and cells were incubated at 41 °C or 37 °C for 1 h and subsequently placed under a Leica SP2 confocal microscope equipped with heated stage. Cells were then microirradiated with 365-nm line of Innova II argon laser (Coherent) in predefined areas of nuclei. Next, the cells were imaged for the required period. At least 10 cells were analyzed for each condition tested.
Image Acquisition and Processing.
Unless stated otherwise, 3-D images of immunofluorescently stained cells were acquired using a DMRA fluorescence microscope (Leica) with cooled CCD camera (Apogee), deconvolved using Huygens software (SVI), and processed using Photoshop CS3 (Adobe Systems). Wide-field images represent maximum intensity projections of the respective 3-D images, whereas images and movies acquired using confocal microscope represent one confocal slice. Accumulation of RAD51-GFP and EGFP-KU80 at the areas exposed to UVA light was analyzed by measuring the mean intensity of the GFP signal in the selected areas using ImageJ.
Xenograft Studies.
The BRO melanoma tumor model.
Three million BRO cells were s.c. injected in nude mice (nu/nu). BRO cells grew in ∼3 wk into bulk tumors of 300 mm3. Tumor pieces of 1–5 mm3 were excised and subsequently implanted in the thigh of a cohort of nude (nu/nu) mice. The implanted tumors were grown to 20–100 mm3 and subsequently mock treated or exposed to 1 h 42 °C under anesthesia (isoflurane) either in the absence of presence of 5 mg/kg PARP inhibitor PJ-34, which was injected 24 and 1 h before heat treatment. Treatments were repeated three times at the days indicated in Figure 4D. Tumor size was measured using a vernier caliper 3 times/wk for a period of 3 wk. Statistical analysis was carried out by applying LN transformation and the effect of the different treatments on tumor growth was subsequently evaluated by ANCOVA linear regression analysis (Bonferonni correction).
R-1 rhabdomyosarcoma tumor model.
Female WAG/Rij rats weighing 140–180 g were used. The transplantable, low-immunogenic R-1 rhabdomyosarcoma model was used as previously described (27). Briefly, ∼3 × 106 R-1 cells were s.c.injected in the flank of a rat. After 3 wk, the tumors reached the volume of ∼3,000 mm3, at which point they were surgically removed, cut into 1-mm3 pieces, and implanted s.c. in the hind limbs of a cohort of 64 animals. After 18 d, when the implanted tumors reached volumes in the range of 100–400 mm3, animals were randomly assigned to the indicated treatment groups and the treatments were initiated. Olaparib was dissolved in solution of 10% 2-hydroxy-propyl-β-cyclodextrin and 10% DMSO in PBS and administered p.o. at the dosage of 50 mg/kg 16 and 2 h before the heating. 17-DMAG was dissolved in PBS and administered i.p. at the dose of 10 mg/kg 2 h before heat treatment. The hind limbs bearing tumors were heated for 1.5 h by submersion in a waterbath at 42.7 °C, resulting in an intratumor temperature of 42 °C. The animals were cooled, which prevented an increase of the core body temperature. The animals were anesthetized with a mixture of 2.5% isoflurane in oxygen during all treatments. Tumor volume was measured using a Vernier caliper. Treatments were performed on days indicated in Figure 4E. Statistical analysis of tumor growth was performed as described before (47). Animals were killed when the tumor volume exceeded 3,500 mm3.
Supplementary Material
Acknowledgments
We thank E. Appeldoorn, P. van Heijningen, and M. H. J. Selman for experimental and technical support. We thank Johan de Winter for providing EUFA1341 cells. The research leading to these results has received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) under Grant Agreement HEALTH-F2-2010-259893. This work was supported by grants from the Netherlands Organization for Scientific Research (NWO), Netherlands Genomics Initiative/NWO, the Maurits and Anna de Kock Foundation, and the Dutch Cancer Society.
Footnotes
Conflict of interest statement: Mark J. O'Connor is an employee of AstraZeneca.
See Commentary on page 9731.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1101053108/-/DCSupplemental.
References
- 1.San Filippo J, Sung P, Klein H. Mechanism of eukaryotic homologous recombination. Annu Rev Biochem. 2008;77:229–257. doi: 10.1146/annurev.biochem.77.061306.125255. [DOI] [PubMed] [Google Scholar]
- 2.Weterings E, Chen DJ. The endless tale of non-homologous end-joining. Cell Res. 2008;18:114–124. doi: 10.1038/cr.2008.3. [DOI] [PubMed] [Google Scholar]
- 3.O'Connor MJ, Martin NMB, Smith GCM. Targeted cancer therapies based on the inhibition of DNA strand break repair. Oncogene. 2007;26:7816–7824. doi: 10.1038/sj.onc.1210879. [DOI] [PubMed] [Google Scholar]
- 4.Iliakis G, Wu W, Wang M. DNA double strand break repair inhibition as a cause of heat radiosensitization: re-evaluation considering backup pathways of NHEJ. Int J Hyperthermia. 2008;24:17–29. doi: 10.1080/02656730701784782. [DOI] [PubMed] [Google Scholar]
- 5.Kampinga HH, Dikomey E. Hyperthermic radiosensitization: mode of action and clinical relevance. Int J Radiat Biol. 2001;77:399–408. doi: 10.1080/09553000010024687. [DOI] [PubMed] [Google Scholar]
- 6.Kampinga HH, Dynlacht JR, Dikomey E. Mechanism of radiosensitization by hyperthermia (> or = 43 degrees C) as derived from studies with DNA repair defective mutant cell lines. Int J Hyperthermia. 2004;20:131–139. doi: 10.1080/02656730310001627713. [DOI] [PubMed] [Google Scholar]
- 7.Issels RD, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol. 2010;11:561–570. doi: 10.1016/S1470-2045(10)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myerson RJ, Roti Roti JL, Moros EG, Straube WL, Xu M. Modelling heat-induced radiosensitization: clinical implications. Int J Hyperthermia. 2004;20:201–212. doi: 10.1080/02656730310001609353. [DOI] [PubMed] [Google Scholar]
- 9.Budzowska M, et al. Mutation of the mouse Rad17 gene leads to embryonic lethality and reveals a role in DNA damage-dependent recombination. EMBO J. 2004;23:3548–3558. doi: 10.1038/sj.emboj.7600353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonoda E, et al. Sister chromatid exchanges are mediated by homologous recombination in vertebrate cells. Mol Cell Biol. 1999;19:5166–5169. doi: 10.1128/mcb.19.7.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bekker-Jensen S, et al. Spatial organization of the mammalian genome surveillance machinery in response to DNA strand breaks. J Cell Biol. 2006;173:195–206. doi: 10.1083/jcb.200510130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stap J, et al. Induction of linear tracks of DNA double-strand breaks by alpha-particle irradiation of cells. Nat Methods. 2008;5:261–266. doi: 10.1038/nmeth.f.206. [DOI] [PubMed] [Google Scholar]
- 13.D'Amours D, Jackson SP. The Mre11 complex: at the crossroads of dna repair and checkpoint signalling. Nat Rev Mol Cell Biol. 2002;3:317–327. doi: 10.1038/nrm805. [DOI] [PubMed] [Google Scholar]
- 14.Sartori AA, et al. Human CtIP promotes DNA end resection. Nature. 2007;450:509–514. doi: 10.1038/nature06337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorslund T, West SC. BRCA2: a universal recombinase regulator. Oncogene. 2007;26:7720–7730. doi: 10.1038/sj.onc.1210870. [DOI] [PubMed] [Google Scholar]
- 16.Essers J, et al. Nuclear dynamics of RAD52 group homologous recombination proteins in response to DNA damage. EMBO J. 2002;21:2030–2037. doi: 10.1093/emboj/21.8.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, Yang H, Pavletich NP. Mechanism of homologous recombination from the RecA-ssDNA/dsDNA structures. Nature. 2008;453:489–4. doi: 10.1038/nature06971. [DOI] [PubMed] [Google Scholar]
- 18.San Filippo J, et al. Recombination mediator and Rad51 targeting activities of a human BRCA2 polypeptide. J Biol Chem. 2006;281:11649–11657. doi: 10.1074/jbc.M601249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jasin M. Homologous repair of DNA damage and tumorigenesis: the BRCA connection. Oncogene. 2002;21:8981–8993. doi: 10.1038/sj.onc.1206176. [DOI] [PubMed] [Google Scholar]
- 20.Yuan SS, et al. BRCA2 is required for ionizing radiation-induced assembly of Rad51 complex in vivo. Cancer Res. 1999;59:3547–3551. [PubMed] [Google Scholar]
- 21.Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/s0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- 22.Bryant HE, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 23.Farmer H, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 24.Bowman KJ, Newell DR, Calvert AH, Curtin NJ. Differential effects of the poly (ADP-ribose) polymerase (PARP) inhibitor NU1025 on topoisomerase I and II inhibitor cytotoxicity in L1210 cells in vitro. Br J Cancer. 2001;84:106–112. doi: 10.1054/bjoc.2000.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dote H, Burgan WE, Camphausen K, Tofilon PJ. Inhibition of Hsp90 compromises the DNA damage response to radiation. Cancer Res. 2006;66:9211–9220. doi: 10.1158/0008-5472.CAN-06-2181. [DOI] [PubMed] [Google Scholar]
- 26.Noguchi M, et al. Inhibition of homologous recombination repair in irradiated tumor cells pretreated with Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin. Biochem Biophys Res Commun. 2006;351:658–663. doi: 10.1016/j.bbrc.2006.10.094. [DOI] [PubMed] [Google Scholar]
- 27.Barendsen GW, Broerse JJ. Experimental radiotherapy of a rat rhabdomyosarcoma with 15 MeV neutrons and 300 kV x-rays. I. Effects of single exposures. Eur J Cancer. 1969;5:373–391. doi: 10.1016/0014-2964(69)90051-6. [DOI] [PubMed] [Google Scholar]
- 28.Fong PC, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 29.Dynlacht JR, Bittner ME, Bethel JA, Beck BD. The non-homologous end-joining pathway is not involved in the radiosensitization of mammalian cells by heat shock. J Cell Physiol. 2003;196:557–564. doi: 10.1002/jcp.10334. [DOI] [PubMed] [Google Scholar]
- 30.Iliakis G, Seaner R. A DNA double-strand break repair-deficient mutant of CHO cells shows reduced radiosensitization after exposure to hyperthermic temperatures in the plateau phase of growth. Int J Hyperthermia. 1990;6:801–812. doi: 10.3109/02656739009140827. [DOI] [PubMed] [Google Scholar]
- 31.Kampinga HH, Kanon B, Konings AW, Stackhouse MA, Bedford JS. Thermal radiosensitization in heat- and radiation-sensitive mutants of CHO cells. Int J Radiat Biol. 1993;64:225–230. doi: 10.1080/09553009314551331. [DOI] [PubMed] [Google Scholar]
- 32.Raaphorst GP, Thakar M, Ng CE. Thermal radiosensitization in two pairs of CHO wild-type and radiation-sensitive mutant cell lines. Int J Hyperthermia. 1993;9:383–391. doi: 10.3109/02656739309005038. [DOI] [PubMed] [Google Scholar]
- 33.Raaphorst GP, Maude-Leblanc J, Li L. Evaluation of recombination repair pathways in thermal radiosensitization. Radiat Res. 2004;161:215–218. doi: 10.1667/rr3103. [DOI] [PubMed] [Google Scholar]
- 34.Yin HL, et al. Radiosensitization by hyperthermia in the chicken B-lymphocyte cell line DT40 and its derivatives lacking nonhomologous end joining and/or homologous recombination pathways of DNA double-strand break repair. Radiat Res. 2004;162:433–441. doi: 10.1667/rr3239. [DOI] [PubMed] [Google Scholar]
- 35.Modi S, et al. Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is safe and active in trastuzumab-refractory HER-2 overexpressing breast cancer: a phase I dose-escalation study. J Clin Oncol. 2007;25:5410–5417. doi: 10.1200/JCO.2007.11.7960. [DOI] [PubMed] [Google Scholar]
- 36.Plummer R, et al. Phase I study of the poly(ADP-ribose) polymerase inhibitor, AG014699, in combination with temozolomide in patients with advanced solid tumors. Clin Cancer Res. 2008;14:7917–7923. doi: 10.1158/1078-0432.CCR-08-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edwards SL, et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451:1111–1115. doi: 10.1038/nature06548. [DOI] [PubMed] [Google Scholar]
- 38.Sakai W, et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 2008;451:1116–1120. doi: 10.1038/nature06633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quax PH, et al. Metastatic behavior of human melanoma cell lines in nude mice correlates with urokinase-type plasminogen activator, its type-1 inhibitor, and urokinase-mediated matrix degradation. J Cell Biol. 1991;115:191–199. doi: 10.1083/jcb.115.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lockshin A, et al. Exceptional lethality for nude mice of cells derived from a primary human melanoma. Cancer Res. 1985;45:345–350. [PubMed] [Google Scholar]
- 41.Essers J, et al. Analysis of mouse Rad54 expression and its implications for homologous recombination. DNA Repair (Amst) 2002;1:779–793. doi: 10.1016/s1568-7864(02)00110-6. [DOI] [PubMed] [Google Scholar]
- 42.de Jager M, et al. DNA-binding and strand-annealing activities of human Mre11: implications for its roles in DNA double-strand break repair pathways. Nucleic Acids Res. 2001;29:1317–1325. doi: 10.1093/nar/29.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathieu J, Flexor M, Lanotte M, Besançon F. A PARP-1/JNK1 cascade participates in the synergistic apoptotic effect of TNFalpha and all-trans retinoic acid in APL cells. Oncogene. 2008;27:3361–3370. doi: 10.1038/sj.onc.1210997. [DOI] [PubMed] [Google Scholar]
- 44.Modesti M, et al. RAD51AP1 is a structure-specific DNA binding protein that stimulates joint molecule formation during RAD51-mediated homologous recombination. Mol Cell. 2007;28:468–481. doi: 10.1016/j.molcel.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 45.Essers J, et al. Disruption of mouse RAD54 reduces ionizing radiation resistance and homologous recombination. Cell. 1997;89:195–204. doi: 10.1016/s0092-8674(00)80199-3. [DOI] [PubMed] [Google Scholar]
- 46.Lin MS, Alfi OS. Detection of sister chromatid exchanges by 4′-6-diamidino-2-phenylindole fluorescence. Chromosoma. 1976;57:219–225. doi: 10.1007/BF00295208. [DOI] [PubMed] [Google Scholar]
- 47.Koziol JA, Maxwell DA, Fukushima M, Colmerauer ME, Pilch YH. A distribution-free test for tumor-growth curve analyses with application to an animal tumor immunotherapy experiment. Biometrics. 1981;37:383–390. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.