Abstract
Drosophila Homeodomain-interacting protein kinase (Hipk) has been shown to regulate in vivo, the stability of Armadillo, the transcriptional effector of Wingless signaling. The Wingless pathway culminates in the stabilization of Armadillo that, in the absence of signaling, is sequentially phosphorylated, polyubiquitinated and degraded. Loss-of-function clones for hipk result in reduced stabilized Armadillo, whereas overexpression of hipk elevates Armadillo levels to promote Wingless-responsive target gene expression. Here, we show that overexpression of hipk can suppress the effects of negative regulators of Armadillo to prevent its degradation in the wing imaginal disc. Hipk acts to stabilize Armadillo by impeding the function of the E3 ubiquitin ligase Skp1-Cul1-F-box (SCF)Slimb, thereby inhibiting Armadillo ubiquitination and subsequent degradation. Vertebrate Hipk2 displays a similar ability to prevent β-catenin ubiquitination in a functionally conserved mechanism. We find that Hipk's ability to inhibit SCFSlimb-mediated ubiquitination is not restricted to Armadillo and extends to other substrates of SCFSlimb, including the Hedgehog signaling effector Ci. Thus, similar to casein kinase 1 and glycogen synthase kinase 3, Hipk dually regulates both Wingless and Hedgehog signaling by controlling the stability of their respective signaling effectors, but it is the first kinase to our knowledge identified that promotes the stability of both Armadillo and Ci.
Keywords: Wnt, beta-TrCP
A small number of evolutionarily conserved cell signaling pathways are used reiteratively, both spatially and temporally, to control the development of multicellular animals. Canonical Wnt/Wingless (Wg) signaling represents one such pathway that has multiple essential roles during both embryogenesis and adult homeostasis to control cell proliferation and cell fate specification (1, 2). As a result, aberrant Wnt/Wg signaling is involved in myriad human diseases, ranging from developmental disorders to cancers (3). In the absence of the Wnt/Wg ligand, cytosolic β-catenin/Armadillo (Arm), the transcriptional effector of the pathway, is constitutively degraded by the action of a protein destruction complex composed of the scaffolding protein Axin, the tumor suppressor protein adenomatous polyposis coli (APC) and two serine/threonine kinases casein kinase 1 (CK1) and glycogen synthase kinase 3 (GSK3)/Shaggy (Sgg). Binding of the Wnt/Wg ligand to its cognate Frizzled receptor and LRP/Arrow (Arr) coreceptor activates the pathway through the Dishevelled-mediated recruitment of Axin to LRP/Arr at the cell membrane, thereby disassembling the destruction complex (2). This sequence of events allows stabilized β-catenin/Arm to translocate into the nucleus and bind the transcription factor T-cell factor (TCF) to direct the expression of Wnt/Wg target genes (4).
The regulation of the stability of the cytosolic pool of β-catenin/Arm is the central feature of Wnt/Wg signaling (5). In the absence of pathway activity, the members of the destruction complex interact with one another to direct the entry, phosphorylation, and exit of β-catenin/Arm from the complex (6). The binding of β-catenin/Arm to Axin allows for its sequential phosphorylation by CK1 and GSK3/Sgg at serine 45/56 and threonine 41/52, serine 37/48, serine 33/44, respectively (7, 8). These phosphorylation events trigger the APC-mediated extraction of β-catenin/Arm from the complex and its subsequent transfer to the Skp1-Cul1-F-box (SCF)β-TrCP/Slimb E3 ubiquitin ligase (9, 10). Targeted degradation via the 26S proteasome can be achieved by the covalent addition of multiple ubiquitin molecules (polyubiquitination) to one or more lysine residues on a substrate protein (11). This process requires the sequential actions of an E1 ubiquitin activating enzyme, an E2 ubiquitin conjugating enzyme and an E3 ubiquitin ligase, the latter being responsible for mediating the transfer of the ubiquitin moiety onto the recognized substrate destined for degradation (11, 12). The E3 ligase that poly-ubiquitinates β-catenin/Arm is SCFβ-TrCP/Slimb, a complex composed of the linker protein Skp1, the scaffold protein Cullin1, and the F-box protein β-TrCP/Slimb, a component that provides substrate specificity (13, 14). β-catenin/Arm phosphorylated at serine 33/44 and serine 37/48 provides a consensus phosphodegron motif that is recognized by SCFβ-TrCP/Slimb, leading to its polyubiquitination and rapid proteolysis by the 26S proteasome (14). Thus, β-catenin/Arm stability can be regulated at the level of either phosphorylation and/or ubiquitination (8, 13, 15, 16). We here provide evidence that Drosophila Homeodomain-interacting protein kinase (Hipk) regulates the ubiquitination of Arm in vivo.
Hipks constitute an evolutionarily conserved family of serine/threonine kinases (17). Members of this family have been shown to have roles in several developmental processes including cell proliferation, differentiation, and apoptosis (17). We have previously characterized Hipk as a positive regulator of Wg signaling (18). In the Drosophila wing imaginal disc, reduction of hipk function resulted in diminished Arm protein levels, whereas overexpression of hipk stabilized and increased Arm levels, resulting in the hyperactivation of Wg-responsive target genes. We demonstrated similar molecular interactions in cell culture between vertebrate Hipk2 and β-catenin, suggestive of a conserved role for Hipks in promoting Wnt/Wg signaling (18). We here provide evidence that Hipks stabilize β-catenin/Arm by binding and inhibiting the function of SCFβ-TrCP/Slimb, in a process that is kinase-dependent. Moreover, we find that Hipks can also block SCFβ-TrCP/Slimb-mediated ubiquitination to stabilize Gli/Ci, the signaling effector of the Hedgehog pathway.
Results
hipk Genetically Acts at the Level of the Destruction Complex and/or Ubiquitination Machinery.
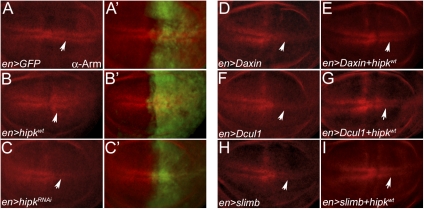
In our previous study, we revealed that Hipk functions downstream of the Wg ligand-Frizzled receptor interaction to stabilize cytosolic Arm in the Drosophila wing imaginal disc (18), thereby possibly acting to inhibit the destruction complex and/or ubiquitination machinery that control Arm phosphorylation and ubiquitination, respectively. The arm gene is transcribed ubiquitously, but a cytosolic pool of Arm protein is stabilized only in two stripes of cells spanning the dorsal/ventral (D/V) boundary of the wing disc that receive the highest levels of Wg signaling during the third instar stage (Fig. 1 A and A′) (19). Overexpression of a UAS-hipk transgene in the posterior compartment of the wing disc using en-Gal4 promoted Arm stability and broadened its domain (Fig. 1 B and B′) whereas knockdown of hipk using a UAS-hipkRNAi construct reduced Arm stability and constricted its domain in the posterior half of the wing disc (Fig. 1 C and C′). To assess the ability of Hipk to block the activity of the destruction complex and/or ubiquitination machinery, we expressed Drosophila axin (Daxin), cullin1 (Dcul1), and slimb, three negative regulators of Arm stability, and modulated hipk in these contexts to observe the effect on Arm protein levels. Overexpression of Daxin using en-Gal4 resulted in the loss of stabilized Arm at the D/V boundary in the posterior half of the wing disc (Fig. 1D). Coexpressing hipk suppressed this effect of Daxin and rescued the levels of stabilized Arm (Fig. 1E). Similarly, loss of stabilized Arm at the D/V boundary through the overexpression of Dcul1 (Fig. 1F) and slimb (Fig. 1H) could be rescued by coexpression of hipk (Fig. 1 G and I). These results suggest that Hipk functions to stabilize Arm through inhibition of the destruction complex and/or ubiquitination machinery. As a result, we would expect modulation of hipk to have minimal effect on Arm if it was already completely stabilized. This result can be observed through the generation of homozygous mutant MARCM clones for Daxin (Fig. S1A), Dcul1 (Fig. S1D), and slimb (Fig. S1G), which resulted in stabilized Arm anywhere in the wing disc. Overexpression (Fig. S1 B, E, and H) or knockdown (Fig. S1 C, F, and I) of hipk in these contexts had no effect on Arm stability. Thus, Hipk function is required for the inhibition of Arm phosphorylation and/or its ubiquitination. However, as Arm phosphorylation is a prerequisite for its ubiquitination (14), these genetic assays do not distinguish between these possibilities.
Fig. 1.
Hipk stabilizes Arm by inhibiting the function of members of the destruction complex and/or ubiquitination machinery. In wild-type w1118 wing discs, Arm is stabilized in two stripes of cells adjacent to the D/V boundary (A and A’). Overexpression of hipk using en-Gal4 (expression domain seen by GFP stain in A’–C’) enhances the levels of stabilized of Arm in the posterior compartment of the wing disc (B and B’), whereas the depletion of hipk through RNAi decreases the amount of stabilized Arm and constricts its domain at the D/V boundary in that compartment (C and C’). Using en-Gal4 to overexpress Daxin (D), the scaffold for the destruction complex, results in loss of stabilized Arm at the D/V boundary in the posterior compartment. This effect is rescued by the coexpression of hipk (E). Similarly, stabilized Arm is lost at the D/V boundary in the posterior compartment as a result of overexpression of members of the ubiquitination machinery, Dcul1 (F) and slimb (H). As in the case of Daxin, this effect is suppressed and the levels of stabilized Arm are rescued by the coexpression of hipk (G and I). Arrows are shown to indicate the position of the two stripes of stabilized Arm, which are affected in the experimental genotypes.
Hipk Does Not Inhibit Arm Phosphorylation by Members of the Destruction Complex.
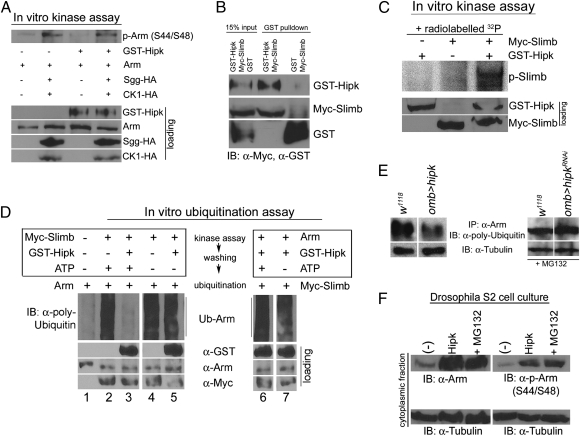
Our genetic analyses suggested that Hipk suppresses Arm phosphorylation and/or ubiquitination in the wing disc. We thus investigated whether Hipk could inhibit one or both of these processes by using biochemical assays. An in vitro kinase assay was conducted by using Arm and the destruction complex members, CK1 and Sgg. The ability of these kinases to phosphorylate Arm in the absence and presence of GST-Hipk was evaluated by using an anti-phospho-β-catenin (S33/S37) antibody. As the consensus phosphorylation sites (along with the region surrounding them) at the N terminus of β-catenin are perfectly conserved in Arm (8), the antibody that recognizes β-catenin phosphorylated at serine 33 and serine 37 also recognizes the corresponding phospho-epitope in Arm, serine 44 and serine 48 (20). We find that GST-Hipk did not inhibit the ability of CK1 and Sgg to phosphorylate Arm in vitro (Fig. 2A).
Fig. 2.
Hipk phosphorylates Slimb to inhibit Arm ubiquitination without any effect on Arm phosphorylation. (A) In an in vitro kinase assay, Arm is sequentially phosphorylated in the presence of CK1 and Sgg (lane 2), as detected by a phospho-specific antibody against Arm (S44/S48). The presence of GST-Hipk has no effect on the phosphorylation of Arm by these kinases (lane 4). Phospho-Arm (S44/S48) is not detected in the absence of CK1 and Sgg (lanes 1 and 3). (B) In a pull-down assay, GST-Hipk forms a complex with Myc-Slimb extracted from Drosophila adults. The GST moiety alone does not bind Myc-Slimb. (C) In an in vitro kinase assay using radiolabeled ATP, immunoprecipitated Myc-Slimb is phosphorylated in the presence of GST-Hipk (lane 3) but not in its absence (lane 2). (D) In an in vitro ubiquitination assay, purified Arm is ubiquitinated in the presence of Slimb and other components of the ubiquitination machinery (lane 2) but not in the absence of Slimb (lane 1). Preincubation of GST-Hipk with Slimb in a kinase assay inhibits its ability to ubiquitinate Arm (lane 3). Preincubation of GST-Hipk with Slimb in the absence of ATP does not reduce Arm ubiquitination in the subsequent assay (lane 5). Preincubation of GST-Hipk with Arm in a kinase assay does not inhibit its Slimb-mediated ubiquitination (lane 6). (E) Protein lysates from Drosophila wing discs were assayed for levels of ubiquitinated Arm. Using omb-Gal4 to overexpress hipk results in lower levels of ubiquitinated Arm relative to wild-type discs. In the presence of the proteasome inhibitor MG132, wing discs reduced in function for hipk have higher levels of ubiquitinated Arm compared with wild-type discs. (F) In Drosophila S2 cells, Hipk enhances both the levels of cytosolic Arm (lane 2) and phospho-Arm (S44/S48) (lane 5), an effect that resembles the treatment of S2 cells with MG132 (lanes 3 and 6).
Hipk Binds and Phosphorylates Slimb.
As Hipk did not influence Arm phosphorylation by the destruction complex in vitro, we next tested if it interacted with the ubiquitination machinery, thereby possibly affecting Arm ubiquitination. We observed that GST-Hipk could interact in a complex with Slimb protein extracted from Drosophila adults. This interaction is not a consequence of the GST-tag at the C terminus of Hipk, because the GST moiety alone did not bind Slimb (Fig. 2B). Moreover, we found that GST-Hipk could also phosphorylate Slimb in an in vitro kinase assay by using radiolabeled ATP (Fig. 2C).
Hipk Inhibits Arm Ubiquitination in a Kinase-Dependent Manner.
To evaluate whether Hipk had any effect on the ubiquitination of Arm, an in vitro ubiquitination assay was performed with E1, an E2 enzyme specific to SCFβ-TrCP/Slimb (UbcH5), Slimb, and Arm. As Hipk might inhibit Slimb-mediated ubiquitination through phosphorylation, the immunoprecipitated E3 ligase and Hipk were first subjected to an in vitro kinase assay, before the ubiquitination assay with added Arm. In the presence of Slimb, Arm was abundantly ubiquitinated, as detected by an anti-poly-Ubiquitin antibody (Fig. 2D). Preincubation with GST-Hipk in a kinase assay reduced the ability of Slimb subsequently to ubiquitinate Arm. We found that this ability of GST-Hipk to impede Slimb function was kinase-dependent, because preincubation with GST-Hipk in a kinase assay in the absence of ATP did not block the subsequent Slimb-mediated ubiquitination of Arm (Fig. 2D). We have previously shown that Hipk can phosphorylate Arm in vitro (18). To confirm that Hipk's ability to reduce Arm ubiquitination was a consequence of its phosphorylation of Slimb and not Arm itself, the assay was repeated but with GST-Hipk and Arm in a kinase assay before the ubiquitination assay with added Slimb. As expected, preincubation of Hipk with Arm did not block its subsequent Slimb-mediated ubiquitination (Fig. 2D).
Hipk Overexpression Decreases Ubiquitinated Arm and Increases Phospho-Arm Levels.
Our results suggest that Hipk regulates Slimb function in a kinase-dependent manner to reduce Arm ubiquitination. Correspondingly, when we assayed levels of ubiquitinated Arm from Drosophila, there was less ubiquitinated Arm in wing discs overexpressing hipk and more ubiquitinated Arm in wing discs reduced for hipk function, relative to wild type (Fig. 2E). As reducing hipk function through RNAi results in less stabilized Arm due to enhanced degradation (18), in the latter assay the discs were treated with MG132 to block proteasome-mediated degradation. Further, because Hipk inhibits the ubiquitination of phosphorylation-primed Arm, transfection of Drosophila S2 cells with Hipk not only resulted in an increase in the levels of cytosolic, stabilized Arm, but also increased the levels of phospho-Arm (S44/S48). This effect was similar to that observed when S2 cells were treated with MG132, which blocks the proteasome-mediated degradation of ubiquitinated Arm (20) (Fig. 2F).
Conserved Mechanism of Regulation of β-catenin Degradation by Vertebrate Hipk2.
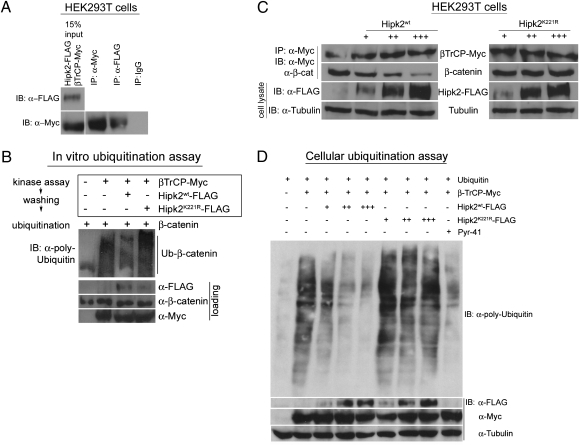
We showed that a vertebrate homolog of Drosophila Hipk, Hipk2, has a conserved function in positively regulating the stability of β-catenin and Wnt signaling in mammalian cell culture (18). Moreover, this ability to stabilize β-catenin is kinase-dependent, because only Hipk2WT but not Hipk2K221R, a kinase-inactive version, can stabilize β-catenin (18). To evaluate whether the mechanism by which Hipk2 stabilizes β-catenin is similar to its Drosophila counterpart, we carried out binding and ubiquitination assays. Hipk2 formed a complex with β-TrCP in HEK293T cells (Fig. 3A) and reduced the β-TrCP–mediated ubiquitination of β-catenin in vitro, in a process that depended on its kinase activity (Fig. 3B).
Fig. 3.
Vertebrate Hipk2 displays functional conservation with its Drosophila counterpart and inhibits β-catenin ubiquitination. (A) In a coimmunoprecipitation assay in HEK293T cells, Hipk2 forms a complex with β-TrCP, the substrate recognition domain of the E3 ligase complex. IgG was used as a negative control in the assay. (B) In an in vitro ubiquitination assay, β-catenin is ubiquitinated in the presence of β-TrCP (lane 2) but not in its absence (lane 1). Preincubation of β-TrCP in a kinase assay with Hipk2WT (lane 3) but not Hipk2K221R (lane 4) reduces the β-TrCP–mediated ubiquitination of β-catenin. (C) In HEK293T cells, an increasing dosage series of Hipk2WT proportionately reduces the amount of β-catenin bound to β-TrCP. This effect is kinase-dependent, because kinase-inactive Hipk2K221R has no effect on the amount of β-catenin bound to β-TrCP. (D) HEK293T cells transfected with β-TrCP results in the ubiquitination of multiple cellular substrates. The introduction of increasing amounts of Hipk2WT in the presence of β-TrCP leads to a gradual decline in levels of ubiquitinated cellular proteins, an effect similar to that produced by treating the cells with the E1 inhibitor PYR41. This ability of Hipk2 to inhibit the β-TrCP-mediated ubiquitination is kinase-dependent, because an increasing dosage series of Hipk2K221R has no effect on cellular ubiquitination levels.
Hipk2 Reduces the Affinity of β-TrCP for β-Catenin.
In the presence of increasing amounts of Hipk2WT in HEK293T cells, there was less β-catenin bound to β-TrCP (Fig. 3C). This ability to reduce the amount of β-catenin bound to β-TrCP was kinase-dependent, because Hipk2K221R had no effect on the ability of β-TrCP to interact with β-catenin, and is possibly the mechanism through which Hipk2 blocks the ability of β-TrCP to ubiquitinate β-catenin. Neither Hipk2WT nor Hipk2K221R had any effect on the stability of β-TrCP in HEK293T cells (Fig. 3C).
Hipk2 Inhibits SCFβ-TrCP-Mediated Ubiquitination of Cellular Proteins.
In our analyses of the regulation of SCFβ-TrCP by Hipk2, we tested whether this effect was unique to β-catenin or whether Hipk2 played a broader role to control the ubiquitination and stability of other proteins regulated by the SCFβ-TrCP E3 ligase. When whole-cell lysate from HEK293T cells transfected with β-TrCP was probed with an anti-poly-Ubiquitin antibody, β-TrCP–mediated ubiquitination of cellular proteins was observed (Fig. 3D). We examined the effect of a dosage series of Hipk2 in the presence of β-TrCP and found that as the amount of Hipk2WT was increased, there was a reduction in overall levels of protein ubiquitination (Fig. 3D). This result suggests that Hipk2WT may regulate the β-TrCP–mediated ubiquitination of more than one substrate of the E3 ligase. This effect was similar to that observed when the cells were treated with the E1 inhibitor, PYR41. In contrast, transfection of cells with an increasing dosage of Hipk2K221R had no effect on the β-TrCP–mediated ubiquitination of cellular proteins (Fig. 3D).
Hipk2/Hipk Regulates the Stability of the Hedgehog Effector Gli/Ci.
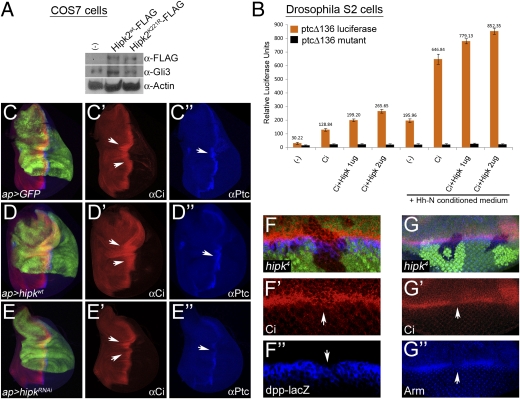
In addition to Wnt/Wg signaling, the SCFβ-TrCP/Slimb E3 ligase has been implicated in Hedgehog (Hh) signaling where it regulates the ubiquitination of the Hh signal transducer, Gli/Cubitus interruptus (Ci) (13). In the absence of Hh signaling, Gli/Ci is sequentially phosphorylated by PKA, GSK3/Sgg, and CK1, which targets it for polyubiquitination via SCFβ-TrCP/Slimb followed by incomplete proteolysis via the 26S proteasome. This incomplete proteolysis yields a truncated, repressor form of Gli/Ci, which moves into the nucleus to inhibit the expression of target genes of the pathway. Upon Hh pathway activation, the phosphorylation and, hence, subsequent ubiquitination/proteolysis of Gli/Ci is blocked, resulting in a full-length protein that translocates into the nucleus to activate gene transcription (21). As Hipk inhibits the function of Slimb in Wg signaling and Hipk2 reduces the β-TrCP–mediated ubiquitination of cellular proteins, we wanted to test whether Hipk2/Hipk could also block the ability of β-TrCP/Slimb to target Gli/Ci. As in the case of β-catenin (18), the introduction of Hipk2WT but not Hipk2K221R into mammalian cells increased the levels of endogenous stabilized, full-length Gli3 (Fig. 4A). We next examined Hipk's effect on Hh signaling in Drosophila S2 cells by using a reporter construct (ptcΔ136-Luc) that responds to Ci binding (22). Transfection of S2 cells with Hipk enhances the response of ptcΔ136-Luc in a dose-dependent manner, both in the absence and presence of Hh stimulation. To elucidate a response from ptcΔ136-Luc, it was necessary to introduce exogenous Ci because S2 cells have low endogenous levels of full-length Ci (22). A reporter construct with mutated Ci-binding sites (ptcΔ136-mut) did not show any response to Ci levels (22) (Fig. 4B). Lastly, we examined Hipk's effect on Ci and Hh signaling in various Drosophila tissues. In the third instar wing disc, ci is expressed throughout the anterior compartment (23) but full-length Ci is stabilized only along the anterior/posterior (A/P) boundary where it turns on expression of its target gene patched (ptc) (Fig. 4 C, C′, and C′′) (24). Overexpression of hipk using the ap-Gal4 driver increased the levels of stabilized, full-length Ci (and ptc expression), with a broadening of its domain along the A/P boundary in certain regions of the wing disc (Fig. 4 D, D′, and D′′). Conversely, reducing hipk function through RNAi [or clonal analysis (Fig. S2)] lowered the amount of stabilized Ci (and correspondingly ptc expression), resulting in a constriction of its domain along the A/P boundary (Fig. 4 E, E′, and E′′). In the eye imaginal disc, Hh signal transduction and full-length Ci are present in the morphogenetic furrow (MF), a physical indentation that moves across the disc to cause retinal specification (25). Loss-of-function clones for hipk that span the MF showed a reduction in full-length Ci and expression of its target gene dpp (Fig. 4 F, F′, and F′′). These analyses strongly suggest that Hipks have a conserved function from Drosophila to mammals in dually regulating the stability of Arm in the Wg pathway and Ci in the Hh pathway (Fig. 4 G, G′, and G′′).
Fig. 4.
Hipk2/Hipk inhibits SCFβ-TrCP/Slimb-mediated ubiquitination of Gli3/Ci to promote Hedgehog signaling. (A) Transfection of COS7 cells with Hipk2WT stabilizes endogenous, full-length Gli3 (lane 2), relative to the untransfected control (lane 1). Hipk2K221R does not have any effect on the levels of full-length Gli3 (lane 3). (B) A transcriptional assay performed in Drosophila S2 cells shows that Hipk significantly up-regulates the expression of a Ci-responsive reporter gene ptcΔ136-Luc in a dose-dependent manner, both in the absence (P < 0.0009) and presence (P < 0.01) of Hh pathway activity. The mutant reporter ptcΔ136-Luc shows no response to Ci levels and Hh pathway stimulation. (C–E) Expression of hipk using ap-Gal4 increases both the amount of stabilized Ci and ptc expression (D, D’, and D’’), relative to a wild-type wing disc (C, C’, and C’’). Conversely, knockdown of hipk reduces the levels of stabilized Ci and results in lower levels of ptc expression. (E, E’, and E’’). Arrows indicate the region of the disc in which stabilized Ci and Ptc are affected. Loss-of-function clones for hipk in the eye disc induced using the ey-flp strain show a reduction in the levels of stabilized, full-length Ci and the Hh target gene dpp (F, F’, and F’’). Loss-of-function clones for hipk in the eye disc show a reduction of both Arm and Ci, indicating that Hipk dually regulates both the Wg and Hh pathways (G, G’, and G’’). Arrows indicate locations of hipk loss of function clones.
Discussion
Our study elucidates the mechanistic details through which Hipk acts as a positive regulator of Arm stability in Wg signaling. We show that Hipk acts at the level of the ubiquitination machinery as opposed to the destruction complex, to control Arm degradation. Hipk does not affect the phosphorylation of Arm by CK1 and Sgg in the destruction complex. Rather, it binds Slimb, a component of the E3 ubiquitin ligase, to inhibit the polyubiquitination of phosphorylation-primed Arm. A functionally conserved molecular interaction is observed among the vertebrate homologs, Hipk2, β-catenin, and β-TrCP. Interestingly, we find that this ability of Hipk to inhibit Slimb-mediated ubiquitination is not substrate-specific. Hipk also stabilizes another Slimb substrate, Ci in the Hh pathway, thereby reflecting an ability to have a more universal inhibitory effect on Slimb. The Wnt/Wg and Hedgehog signaling pathways share many common features, including the negative regulation of the stability of their effector proteins through constitutive phosphorylation by the actions of the CK1 and GSK3/Sgg kinases (26, 27). Our study characterizes the role of a third kinase, Hipk, which functions in both pathways to directly promote rather than inhibit the stability of their respective transcriptional effectors.
As Hipk is involved in multiple signaling pathways, it is possible that its activity is selectively regulated by individual pathways. An emerging theme with respect to signaling redundancy is that kinases are present with their substrates in distinct complexes and subcellular locations and, as a consequence, are controlled by different upstream stimuli. Future studies should almost certainly entail how the activity of Hipk is regulated in response to the Wg and Hh signals. However, we cannot exclude the possibility that rather than being actively regulated by the Wg and Hh signals, Hipk plays a permissive role in these pathways.
A recent study performed in Xenopus suggests that Hipk2 promotes Wnt signaling by phosphorylating TCF3 to derepress the expression of target genes (28). Indeed, we have also observed an interaction between Hipk and TCF in the nucleus (18), suggestive of Hipks acting at multiple levels in the Wnt/Wg pathway, another feature reminiscent of the CK1 and GSK3/Sgg kinases (2).
Experimental Procedures
Details on fly strains and plasmid constructs can be found in SI Experimental Procedures.
Immunohistochemistry.
Wing and eye imaginal discs were dissected from Drosophila third instar larvae and antibody stainings were performed according to standard protocols by using anti-Arm N2 7A1 (1:200), anti-Ci 2A1 (1:40), anti-Ptc (1:50) (Developmental Studies Hybridoma Bank), and anti-β-galactosidase (1:1,500) (Promega). Discs were mounted in Vectashield (Vector Laboratories) and oriented with their anterior to the left.
Biochemical Assays.
The Slimb protein used in all assays was immunoprecipitated from lysate extracted from Drosophila Tub > Myc-Slimb adult flies. In the binding assay, GST-Hipk produced in Escherichia coli BL21 cells according to standard procedures was incubated with Slimb lysate, and the proteins were immunoprecipitated by using GST beads (Amersham) followed by SDS/PAGE/Western blotting. Kinase assays were performed by using kinase assay buffer/ATP (Cell Signaling Technology) with GST-Hipk and other proteins immunoprecipitated from Drosophila S2 cells. Kinase assay reactions were incubated at 30 °C for 30 min followed by SDS/PAGE/Western blotting and detection with a phospho-specific β-catenin (S33/S37) antibody (1:750) (Cell Signaling Technology) or autoradiography. The kinase assays that were performed immediately before the ubiquitination assays followed the same protocol with the relevant immunoprecipitated proteins. For the ubiquitination assays, E1, E2 (UbcH5), Mg-ATP solution, ubiquitin, and ubiquitination buffer (Enzo Life Sciences) were mixed with immunoprecipitated Arm/β-catenin, Slimb/β-TrCP, and GST-Hipk/Hipk2WT or Hipk2K221R. The reactions were incubated at 37 °C for 1 h followed by SDS/PAGE/Western blotting and detection with an anti-poly-Ubiquitin antibody (1:1,000) (Enzo Life Sciences).
Cell Culture.
Drosophila S2 cells were maintained at 25 °C in Schneider's medium supplemented with 10% heat-inactivated FCS (Invitrogen). Cells were transfected with Effectene reagent (Qiagen) according to the manufacturer's instructions. Twelve hours after transfection, genes with metallothionein promoters were induced by the addition of CuSO4 (final concentration of 0.5 mM). HEK293T and COS7 mammalian cells were cultured at 37 °C in DMEM supplemented with 10% FBS (Invitrogen). Transfection was performed with Polyfect reagent (Qiagen) according to the manufacturer's instructions. Where required, the final amount of DNA used for transfection was kept constant by the addition of empty vector DNA. All cells were harvested 48 h after transfection by using lysis buffer supplemented with protease inhibitors (Cell Signaling Technology). For the stability assay, MG132 (Calbiochem) was used to treat cells at a concentration of 25 μM for 6 h. For the cellular ubiquitination assay, PYR41 (Calbiochem) was used to treat cells for 1 h at a concentration of 50 μM. For the fractionation assay, the Subcellular Protein Fractionation Kit (Thermo Fisher) was used according to the manufacturer's instructions.
Transcriptional Assay.
Luciferase assays were performed with the Dual Luciferase Reporter assay system (Promega) according to manufacturer's instructions by using the reporter plasmids ptcΔ136-Luc and ptcΔ136-mut that contain a wild-type and mutated ptc promoter, respectively. A control reporter plasmid pDA-RL that expresses Renilla luciferase was used for normalizing transfection efficiencies. The values shown are the average of one representative experiment in which each transfection was performed in triplicate. Hh-N conditioned medium was produced by transfecting S2 cells with a plasmid that encodes the active Hh molecule and then recovering the medium 3 d after transfection.
Drosophila Lysate Extraction.
Extraction of lysate from adult flies was performed by homogenizing fly tissue in lysis buffer, followed by sonication and recovery of protein lysate. To detect levels of ubiquitinated Arm, 150 wing discs of each genotype were harvested in lysis buffer. To inhibit Arm degradation, wing discs were treated with MG132 at a concentration of 25 μM for 6 h in Schneider's medium.
Supplementary Material
Acknowledgments
We thank numerous people who provided fly strains and plasmid constructs: R. Nusse, C. T. Chien, F. Rouyer, I. Edery, J. Jiang, B. A. Edgar, C. Y. Choi, S. Ishii, X. He, G. D'Orazi, C. Kanei-Ishii, S. Yanagawa, P. Beachy, and R. Fukunaga, and we thank J. Gardner, M. Rahnama, V. Fernandes, C. J. Gottardi, and N. Harden for help with dissections, microscopy, and discussions on the manuscript. This work was supported by an operating grant from Canadian Institutes of Health Research.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017548108/-/DCSupplemental.
References
- 1.Cadigan KM, Nusse R. Wnt signaling: A common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 2.Cadigan KM, Peifer M. Wnt signaling from development to disease: Insights from model systems. Cold Spring Harb Perspect Biol. 2009;1:a002881. doi: 10.1101/cshperspect.a002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 4.Daniels DL, Weis WI. Beta-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat Struct Mol Biol. 2005;12:364–371. doi: 10.1038/nsmb912. [DOI] [PubMed] [Google Scholar]
- 5.Verheyen EM, Gottardi CJ. Regulation of Wnt/beta-catenin signaling by protein kinases. Dev Dyn. 2010;239:34–44. doi: 10.1002/dvdy.22019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimelman D, Xu W. beta-catenin destruction complex: Insights and questions from a structural perspective. Oncogene. 2006;25:7482–7491. doi: 10.1038/sj.onc.1210055. [DOI] [PubMed] [Google Scholar]
- 7.Yost C, et al. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- 8.Liu C, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 9.Xing Y, Clements WK, Kimelman D, Xu W. Crystal structure of a beta-catenin/axin complex suggests a mechanism for the beta-catenin destruction complex. Genes Dev. 2003;17:2753–2764. doi: 10.1101/gad.1142603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su Y, et al. APC is essential for targeting phosphorylated beta-catenin to the SCFbeta-TrCP ubiquitin ligase. Mol Cell. 2008;32:652–661. doi: 10.1016/j.molcel.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Pickart CM. Ubiquitin enters the new millennium. Mol Cell. 2001;8:499–504. doi: 10.1016/s1097-2765(01)00347-1. [DOI] [PubMed] [Google Scholar]
- 12.Nagy V, Dikic I. Ubiquitin ligase complexes: From substrate selectivity to conjugational specificity. Biol Chem. 2010;391:163–169. doi: 10.1515/bc.2010.021. [DOI] [PubMed] [Google Scholar]
- 13.Jiang J, Struhl G. Regulation of the Hedgehog and Wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature. 1998;391:493–496. doi: 10.1038/35154. [DOI] [PubMed] [Google Scholar]
- 14.Hart M, et al. The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr Biol. 1999;9:207–210. doi: 10.1016/s0960-9822(99)80091-8. [DOI] [PubMed] [Google Scholar]
- 15.Korinek V, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 16.Yang J, et al. Adenomatous polyposis coli (APC) differentially regulates beta-catenin phosphorylation and ubiquitination in colon cancer cells. J Biol Chem. 2006;281:17751–17757. doi: 10.1074/jbc.M600831200. [DOI] [PubMed] [Google Scholar]
- 17.Rinaldo C, Siepi F, Prodosmo A, Soddu S. HIPKs: Jack of all trades in basic nuclear activities. Biochim Biophys Acta. 2008;1783:2124–2129. doi: 10.1016/j.bbamcr.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Lee W, Swarup S, Chen J, Ishitani T, Verheyen EM. Homeodomain-interacting protein kinases (Hipks) promote Wnt/Wg signaling through stabilization of beta-catenin/Arm and stimulation of target gene expression. Development. 2009;136:241–251. doi: 10.1242/dev.025460. [DOI] [PubMed] [Google Scholar]
- 19.Couso JP, Bishop SA, Martinez Arias A. The wingless signalling pathway and the patterning of the wing margin in Drosophila. Development. 1994;120:621–636. doi: 10.1242/dev.120.3.621. [DOI] [PubMed] [Google Scholar]
- 20.Matsubayashi H, et al. Biochemical characterization of the Drosophila wingless signaling pathway based on RNA interference. Mol Cell Biol. 2004;24:2012–2024. doi: 10.1128/MCB.24.5.2012-2024.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol. 2005;6:306–317. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- 22.Chen CH, et al. Nuclear trafficking of Cubitus interruptus in the transcriptional regulation of Hedgehog target gene expression. Cell. 1999;98:305–316. doi: 10.1016/s0092-8674(00)81960-1. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz C, Locke J, Nishida C, Kornberg TB. Analysis of cubitus interruptus regulation in Drosophila embryos and imaginal disks. Development. 1995;121:1625–1635. doi: 10.1242/dev.121.6.1625. [DOI] [PubMed] [Google Scholar]
- 24.Aza-Blanc P, Ramírez-Weber FA, Laget MP, Schwartz C, Kornberg TB. Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell. 1997;89:1043–1053. doi: 10.1016/s0092-8674(00)80292-5. [DOI] [PubMed] [Google Scholar]
- 25.Heberlein U, Wolff T, Rubin GM. The TGF beta homolog dpp and the segment polarity gene hedgehog are required for propagation of a morphogenetic wave in the Drosophila retina. Cell. 1993;75:913–926. doi: 10.1016/0092-8674(93)90535-x. [DOI] [PubMed] [Google Scholar]
- 26.Price MA, Kalderon D. Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by Glycogen Synthase Kinase 3 and Casein Kinase 1. Cell. 2002;108:823–835. doi: 10.1016/s0092-8674(02)00664-5. [DOI] [PubMed] [Google Scholar]
- 27.Jia J, et al. Shaggy/GSK3 antagonizes Hedgehog signalling by regulating Cubitus interruptus. Nature. 2002;416:548–552. doi: 10.1038/nature733. [DOI] [PubMed] [Google Scholar]
- 28.Hikasa H, et al. Regulation of TCF3 by Wnt-dependent phosphorylation during vertebrate axis specification. Dev Cell. 2010;19:521–532. doi: 10.1016/j.devcel.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.