Abstract
Galanin (GAL) and GAL receptors (GALR) are overexpressed in degenerating brain regions associated with cognitive decline in Alzheimer’s disease (AD). The functional consequences of GAL plasticity in AD are unclear. GAL inhibits cholinergic transmission in the hippocampus and impairs spatial memory in rodent models, suggesting that GAL overexpression exacerbates cognitive impairment in AD. By contrast, gene expression profiling of individual cholinergic basal forebrain (CBF) neurons aspirated from AD tissue revealed that GAL hyper-innervation positively regulates mRNAs that promote CBF neuronal function and survival. GAL also exerts neuroprotective effects in rodent models of neurotoxicity. These data support the growing concept that GAL overexpression preserves CBF neuron function, which may in turn delay the onset of symptoms of AD. Further elucidation of GAL activity in selectively vulnerable brain regions will help gauge the therapeutic potential of GALR ligands in the treatment of AD.
Keywords: Alzheimer’s disease, Galanin, Cholinergic basal forebrain, Hippocampus, Plasticity
Introduction
The neuropeptide galanin (GAL) and its cognate G protein-coupled receptors (GALR1–3) are widely distributed in the mammalian central nervous system (CNS) and modulate several ascending neurotransmitter systems including cholinergic, noradrenergic, serotonergic as well as neuroendocrine pathways [1, 2]. Notably, GAL activity regulates cognitive behaviors mediated by the basal fore-brain, amygdala, hippocampus, and entorhinal cortex [3–6]. GAL regulates cholinergic basal forebrain (CBF) neurons that provide the major cholinergic innervation to the cortex and hippocampus [7] and plays a key role in memory and attention functions [8]. CBF neurons undergo selective degeneration during later stages of Alzheimer’s disease (AD), which correlates with disease duration and degree of cognitive impairment [9]. Several groups have made a striking observation that hypertrophic GAL-containing fibers innervate surviving CBF neurons in end-stage AD [10–12]. GAL levels increase throughout the cortex in AD [13, 14] and GALR binding sites are amplified in the cortex, CBF, hippocampus, entorhinal cortex, and amygdala during the course of the disease [15–18]. However, the functional impact of GAL overexpression within the CBF in AD is not clear. For example, GAL inhibits acetylcholine (ACh) release in rodent hippocampal preparations, restricts long-term potentiation (LTP), and disrupts cognitive performance in animals [4, 6]; these observations support the notion that CBF GAL fiber hypertrophy exacerbates the cholinergic deficit seen in AD. This hypothesis has been challenged by recent findings which show that GAL protects the hippocampus from excitotoxic damage [19] and the CBF septal neurons from amyloid toxicity [20]. These observations raise the possibility that GAL upregulation may promote cholinergic neuronal survival in late-stage AD, and gene expression profiling of individual CBF neurons in AD tissue suggests that GAL hyperinnervation positively regulates mRNAs that promote cholinergic neuron function and survival [21–23]. This article reviews evidence that support the concept that GAL overexpression plays a role in the survival of select neuronal populations associated with cognitive decline in AD.
Galanin in Alzheimer’s Disease
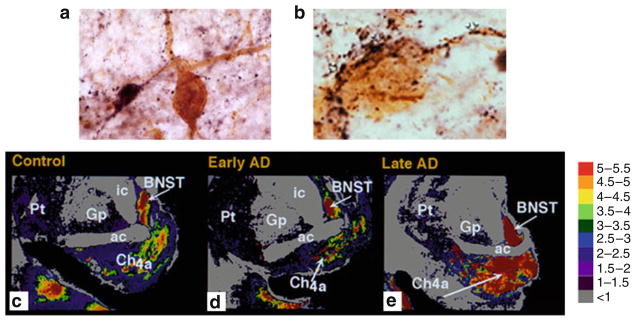
Galaninergic systems exhibit hypertrophy in brain regions that mediate cognition and are prone to neuropathological damage in AD. For instance, immunohistochemical studies in postmortem basal forebrain tissue from aged cognitively intact subjects, reveal a fine network of GAL-immunoreactive (-ir) fibers coursing through the CBF which often appear in direct apposition to CBF perikarya or dendrites (Fig. 1a). In contrast, end-stage AD tissue displays a dense plexus of enlarged GAL-ir fibers that hyperinnervate the surviving CBF neocortical and hippocampal projection neurons located within the nucleus basalis (NB, Fig. 1b) and septal diagonal band complex, respectively [10–12]. GAL radioimmunoassay (RIA) studies in autopsied CBF tissue revealed a 2-fold increase in GAL peptide levels within the NB of late stage AD subjects when compared to control subjects [24]. However, quantitative in vitro autoradiographic imaging of [125I]hGAL binding sites within the NB of pathologically defined early (mild) and late (severe) AD cases showed a significant increase in the density of GALR labeling within the anterior NB subfield of late AD subjects compared to controls and early AD subjects (Fig. 1c–e) [16]. In addition, a semiquantitative immunohistochemical study of GAL-ir profiles in the NB of subjects clinically diagnosed with mild cognitive impairment, a putative preclinical AD stage, or mild AD, revealed no evidence for GAL hyperinnervation of this CBF region [25]. Taken together, these findings indicate that GAL fiber and receptor overexpression occur within the anterior portion of the NB, when CBF neuron degeneration is advanced, during late-stage AD.
Fig. 1.
GAL plasticity in the basal forebrain nucleus basalis in AD. (a) Photomicrograph shows a magnocellular cholinergic NB neuron immunostained with the CBF neuronal marker p75NTR (brown reaction product) and innervated by GAL-ir fibers (dark blue reaction product) in aged control brain. Note the GAL-ir parvicellular neuron contacting the CBF neuron. (b) Photomicrograph shows a striking hyperinnervation of GAL fibers impinging upon a cholinergic NB neuron in AD. (c–e) Pseudocolor density maps showing the regional distribution of [125I]hGAL binding sites in the aged control NB (c) as compared to early stage (d) and late stage (e) AD. Note the increase in labeling in the anterior subfield of the nucleus basalis in late AD. Gray to red on the color scale indicates increasing GAL binding levels
There is evidence for GAL plasticity in other brain regions associated with cognitive dysfunction in AD. The noradrenergic locus coeruleus (LC), which is similar to the CBF, contains long neocortical and hippocampal projection neurons that modulate memory and attention. These degenerate in AD [26] and also exhibit prominent GAL-ir fiber hyperinnervation in postmortem AD tissue [27]. Two additional studies using GAL RIA, demonstrated a significant increase in GAL peptide concentration in frontal, temporal, and parietal neocortical association areas in end-stage AD, but not in controls [13, 14] or patients diagnosed with schizophrenia [14]. In addition, in vitro autoradiographic studies revealed a significant increase in GALR occupancy in the deep layers of the frontal cortex in AD subjects [15]. GALR binding sites were also detected in all neocortical areas and in layer II of the entorhinal cortex, the uncus, and the hippocampal-amygdala transition area in the human brain [17, 18, 28]. Autoradiographic localization of GALR binding sites in the entorhinal cortex layer II is intriguing as these neurons provide the major glutamatergic excitatory input to the hippocampus (i.e., the perforant pathway) but degenerate very early in AD [29]. In vitro autoradiographic studies of [125I]hGAL binding revealed a ~3-fold increase in GALR binding sites in the entorhinal cortex layer II in early-AD patients compared to those with late-stage AD or age matched control subjects [17]. [125I]hGAL binding sites were also localized to the central nucleus and corticoamygdaloid transition area of the amygdala, which have reciprocal connections with the basal forebrain, hippocampus, and the cortex [30]. These regions play a pivotal role in higher order cognitive processing and display extensive AD-related pathology early in the disease process [31]. Similar to the findings observed in layer II of the entorhinal cortex, [125I]hGAL binding was upregulated in the amygdala in early stage/probable AD but not in late-stage AD [17]. Hence, increased GALR binding occurs in select cognitive regions of the AD brain that are affected during the prodromal stages of AD [29, 31]. On the other hand, enhanced GALR binding and GAL fiber hyperinnervation are found within the CBF projection system in the later stages of the disease, when the remaining neurons succumb to the disorder [10–12, 16].
Potential Triggers of GAL Plasticity in AD
The pathophysiological factors that induce GAL plasticity in the brain in AD have been a matter of great speculation. As discussed above, the observed spatiotemporal pattern of increased GAL binding suggests that GAL hypertrophy occurs in response to neuronal injury. Along these lines, in rats, GAL is dramatically upregulated following several experimental injury paradigms in the central and peripheral nervous systems, including olfactory bulbectomy [32], hypophysectomy [33], neurochemical dorsal raphe lesions [34], immunotoxic basal forebrain lesions [35], potassium chloride-mediated cortical spreading depression [36], global ischemia [37], and sciatic [38] or dorsal root sensory [39] nerve transaction. Collectively, these observations support the notion that GAL overexpression is triggered by neuronal damage, suggesting that GAL fiber hyperinnervation of cell groups such as the CBF and LC in AD represents an intrinsic cellular program aimed at neuronal survival.
Neurodegenerative lesions observed in AD may also play a role in GAL fiber hypertrophy. For instance, neuropathological studies in humans have shown that AD-related neuritic plaques, which are composed chiefly of fibrillar deposits of β-amyloid (Aβ), are also GAL-positive [40]. A role for Aβ deposition in triggering GAL upregulation is supported by studies using transgenic mouse models of AD, which display prominent amyloidosis. Older (26-month old) mice that overexpress human amyloid precursor protein (APP) bearing the familial AD (FAD)-related V717F mutation, exhibit amyloid plaque deposition in the hippocampal stratum lacunosum-moleculare subfield and entorhinal cortex, concurrent with the appearance of dystrophic GAL-ir neurites in many of the plaques [41]. Occasional GAL-ir cell bodies were also observed in the hippocampus; these were not evident in wild-type mice [41]. In addition, APP23 mice bearing two FAD-related APP mutations (V717I and K670N/M671L), exhibited an increase of dystrophic GAL fibers and GAL-ir neurites apposing amyloid plaques in the supragranular layer of the hippocampus and ventral neocortex at 27 months compared to 21 months [42]. Interestingly, GAL immunoreactivity was reduced in the dorsolateral neocortex of 27-month-old APP23 mice [42], suggesting a dynamic age-related reorganization of cortical GAL-containing projections in the face of mounting amyloid deposition. Recently, we examined Aβ and GAL immunoreactivity in the brains of transgenic mice carrying the human APP K670N/M671L (APPswe) and presenilin 1 PS1ΔE9 FAD mutations, which display accelerated plaque deposition, compared to APP transgenic mice. In these mice, co-labeling of cortical and hippocampal amyloid plaques with GAL revealed peptide-containing dystrophic neurites at as early as 3 months of age (Fig. 2a–c). As neuronal loss was not evident at this age [43], these observations suggest that GAL expression is triggered by amyloidosis-related neurotoxicity rather than frank neurodegeneration in these transgenic animal models of AD. Whether amyloid plaque deposition, which is prominent in vulnerable cognitive brain regions in AD, also triggers GAL overexpression in these areas in the human condition remains an open question.
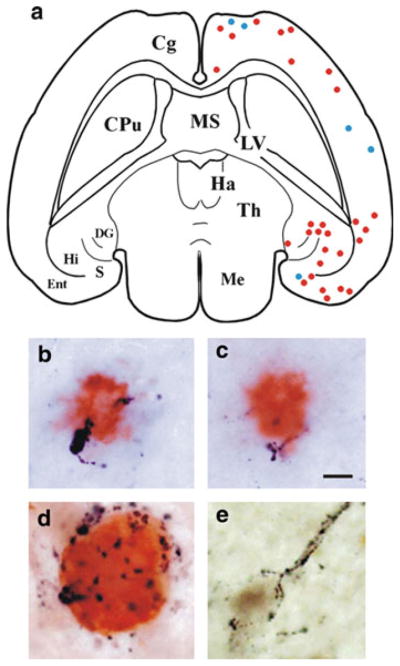
Fig. 2.
Association of GAL with AD-related lesions. (a) Schematic drawing of a horizontal brain section from a 3-month-old APPswe/PS1ΔE9 transgenic mouse showing the distribution of Aβ-ir plaques with adjacent GAL-ir dystrophic neurites (blue dots) or without dystrophic neurites (red dots). Abbreviations: Cg, cingulate cortex; CPu, caudate-putamen; DG, dentate gyrus; Ent, entorhinal cortex; Ha, habenula; Hi, hippocampus; LV, lateral ventricle; Me, mesencephalon; S, subiculum; Th, thalamus. (b, c) Photomicrographs show colocalization of GAL fibers (black) and Aβ (orange) in amyloid plaques located in the cortex (b) and hippocampus (c) of 3-month-old APPswe/PS1ΔE9 mice. Scale bar = 10 μm. (d) GAL hyperinnervation (black) of a cholinergic NB neuron dual stained for Tau C3 (orange), a tau epitope that appears early in the evolution of NFTs. (e) GAL hyperinnervation (black) upon a cholinergic NB neuron immunonegative for MN423, a late stage tau event in NFT formation. There was no evidence for GAL hyperinnervation of MN423-immnopositive CBF neurons
Neurofibrillary tangles (NFTs), filamentous deposits composed of aggregated tau microtubule-binding proteins, are also a cardinal neuropathological feature of AD. In the CBF, the evolution of NFT pathology within individual neurons follows a sequence of differentially expressed tau epitopes during the course of AD [44]. Using antibodies raised against different tau epitopes, we tested whether GAL plasticity is associated with the evolution of NFTs in the CBF neurons in AD [44]. It was observed that CBF neurons displaying the tau C3 epitope, a marker for early stage NFT formation, were often hyperinnervated by GAL-ir fibers (Fig. 2d), whereas CBF neurons displaying the tau epitope MN423, an end-stage NFT marker, were not associated with GAL (Fig. 2e). Gene expression studies in single cells in AD, have shown that the levels of mRNAs encoding selected subclasses of protein phosphatase subunits (PP1α and PP1γ) are stable in GAL hyperinnervated CBF neurons but downregulated in those that were not hyperinnervated by GAL[22]. Reduced activity of PP1 and PP2A subunits is implicated in tau hyperphosphorylation, which in turn precipitates NFT pathology and subsequent cytoskeletal destabilization in vulnerable neurons. Taken together, these observations suggest that GAL remodeling may delay NFT pathology in CBF neurons in AD.
Neuronal Origin of GAL Hyperinnervation in AD
The source of GAL hyperinnervation in AD remains unclear. For instance, it is unlikely that the few small GAL-ir neurons within the basal forebrain and preoptic area account for the rich galaninergic fiber plexus seen within this region of the human brain [45, 46]. One potential source of GAL-fiber innervation to the basal forebrain may be from the LC [47]. The coeruleo-forebrain pathway is well characterized in the mammalian CNS and GAL-ir cells within the LC also exhibit enhanced GAL immunoreactivity in AD [27]. Although the human LC does not contain numerous GAL-ir cells [46], as compared to rodents [48, 49], GAL-positive LC neurons are preserved in AD [47], suggesting LC neurons as a source of GAL forebrain hyperinnervation with a neuroprotective action for GAL as well. Another potential source of GAL fibers may be the central nucleus of the amygdala; and these fibers course through the basal forebrain en route to the substantia innominata, bed nucleus of the stria terminalis, and hypothalamus [12]. While the precise cells of origin of this GAL-ir forebrain bundle remain to be determined, they may also arise from the extended amygdaloid complex, which contains numerous GAL-ir cell bodies [12, 47] and displays hypertrophy of GAL-ir fibers in AD (Mufson unpublished observations).
Galanin Plasticity as a Detrimental Factor in AD
The functional consequences of GAL plasticity in AD remain an area of intense interest. The majority of evidence concerning the effects of GAL overexpression in AD is derived from rodent studies which show that GAL inhibits ACh release in the hippocampus [4, 50] and disrupts cognitive performance on emotional and spatial memory tasks [6, 51]. GAL inhibits the evoked release of ACh in the ventral hippocampus of the rat in a concentration-dependent manner and blocks the slow cholinergic excitatory postsynaptic potential (EPSP) induced by the release of endogenous ACh onto CA1 hippocampal pyramidal neurons [50]. Furthermore, microinjection of GAL into the medial septum/diagonal band complex or ventral hippocampus impairs cognitive performance of rats in several spatial learning and working memory tasks [6, 51]. GAL interference of cholinergic transmission during these tasks is particularly evident in the presence of muscarinic ACh receptor antagonists or cholinergic immunotoxin lesions [51, 52].
A role for GAL in glutamate-mediated LTP in the hippocampus may also contribute to the above mentioned effects of GAL on memory. Electrophysiological studies in rodent hippocampal slices show that GAL restricts LTP at both perforant path-dentate gyrus and Schaffer collateral-CA1 synapses [3, 5, 53]. GAL may affect glutamatergic transmission in the hippocampus by reducing evoked glutamate release [5, 54]. However, while GAL inhibits LTP at CA1 synapses, it has no effect on ionotropic AMPA or NMDA glutamate receptor-mediated EPSPs, suggesting that GAL acts through a postsynaptic GALR to inhibit LTP-related signaling cascades [3].
The development of transgenic mice that overexpress GAL has facilitated the study of GAL overexpression in the brain and it provides a unique model for the investigation of this peptide in the area of cognition [55, 56]. For example, mice expressing GAL ectopically under the control of the dopamine β-hydroxylase promoter (GAL-tg mice), displayed increased GAL fiber density in the basal forebrain and a ~3-fold reduction in the number of ChAT-ir septohippocampal neurons in the horizontal limb of the diagonal band [56]. In situ hybridization experiments in these GAL-tg mice demonstrated a downregulation of the number of ChAT mRNA per cell within the horizontal limb without a difference in the number of ChAT mRNA-containing neurons [57]. Thus, GAL overexpression in the basal forebrain of GAL-tg mice may selectively reduce the expression of the cholinergic neuron phenotype. Spatial navigation testing with the Morris water task in GAL-tg mice showed a complete lack of selective search on the probe trial at 8, 16, and 24 months of age [56], indicating that the GAL-tg mice could not generate a cognitive map of the spatial environment to solve the probe test, which is the most challenging component of this task [56, 57]. As the Morris task requires an intact hippocampus [56], it seems likely that the mechanisms underlying the observed deficits in the GAL-tg mouse may include inhibitory neuromodulation by GAL in the hippocampus. Along these lines, GAL expression is increased by as much as ~4-fold in the hippocampus of GAL-tg mice compared to wild type (WT) mice [58]. In addition, GAL overexpression in these mice results in reduced ACh release in the ventral hippocampus in vivo [59], mimicking results from rats exposed to exogenous GAL administration (see above). Furthermore, hippocampal slices from GAL-tg mice show reduced glutamate release and have restricted LTP at perforant path-dentate gyrus synapses compared to WT mice [5].
A transgenic mouse that overexpresses GAL on a platelet-derived growth factor B promoter (GalOE mice) demonstrated a ~4-fold increase in hippocampal GAL. In addition, it also showed reduced frequency facilitation of field EPSPs – a form of short term synaptic plasticity – at mossy fiber-CA3 synapses in hippocampal slices [55]. Aged GalOE mice also display deficits in paired-pulse facilitation of field EPSP at perforant path-dentate gyrus synapses [60]. Similar to the GAL-tg mice, the GalOE mice also exhibited age-dependent impairments on the Morris water maze [61], possibly related to septal cholinergic function, as behavioral impairment was concomitant with decreased hippocampal ChAT activity [61]. Taken together, these data suggest that the GAL overexpression in these mouse models inhibits multiple neurotransmitter systems involved in cognitive function. As the organization of the galaninergic basal forebrain and LC systems differ between rodents and humans [46, 49, 62], whether the physiological actions seen in GAL transgenic mice or rat studies reflect the human condition, remains an open question.
Galanin Plasticity as a Neuroprotective Factor in AD
An alternative hypothesis to the suggestion that GAL is a neuroinhibitory factor in AD is that GAL overexpression is neuroprotective. With respect to the cholinergic NB in AD, it is intriguing to note that GAL hyperinnervation and GAL binding sites are greatest in the anterior NB subfield, where there is least amount of neural degeneration [16]. By contrast, there is no evidence of GAL fiber [11, 12] or GALR [15, 16] overexpression within the posterior NB subfields, where cholinergic neuron degeneration is greatest [63]. As described above, data from customized microarray experiments revealed that the expression of PP subunits is stabilized in single cholinergic NB neurons hyperinnervated by GAL, and this can potentially slow NFT formation. We also employed this technique to demonstrate that ChAT mRNA levels are selectively increased in GAL-hyperinnervated NB neurons in AD compared to non-innervated NB neurons in control or AD [21] (Fig. 3). These findings offer evidence to support the notion that GAL overexpression in NB neurons may result in the protection of cholinergic neuron function as the disease progresses.
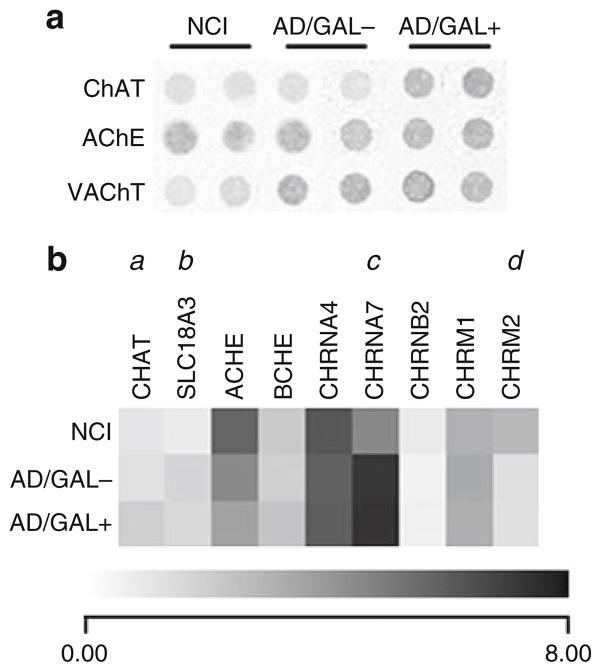
Fig. 3.
GAL hyperinnervation upregulates ChAT mRNA levels in single cholinergic NB neurons in AD. (a) Representative custom-designed microarray expression data showing relative hybridization signal intensities for ChAT, acetylcholinesterase (AChE), and the vesicular ACh transporter (VAChT) in non-GAL-innervated cholinergic NB neurons from control subjects with no cognitive impairment (NCI), non-GAL-innervated cholinergic NB neurons from AD subjects (AD/GAL−), and GAL-hyperinnervated cholinergic NB neurons from AD subjects (AD/GAL+). (b) Dendrogram illustrating relative mRNA expression levels (white to black = increasing levels) of ChAT (Unigene/NCBI notation CHAT), VAChT (SLC18A3), AChE (ACHE), butylcholinesterase (BCHE), nicotinic ACh receptor subunits α4 (CHRNA4), α7 (CHRNA7), β2 (CHRNB2), and muscarinic ACh receptor subtypes M1 (CHRM1) and M2 (CHRM2). a = AD/GAL+ > NCI, AD/GAL−, p < 0.01; b = AD/GAL+, AD/GAL− > NCI, p < 0.01; c = AD/GAL+, AD/GAL−> NCI, p < 0.001; d = NCI > AD/GAL−, AD/GAL+, p < 0.01
With respect to microarray analysis of other functional classes of genes related to CBF neuron function and survival in AD, GAL had no effect on the down-regulation of mRNAs encoding selected synaptic membrane proteins such as presynaptic synaptophysin (SYP) [64] and postsynaptic drebrin (DBN1) [65] (Fig. 4) [23]. Likewise, GAL had no effect on the AD-specific up-regulation of estrogen receptor β mRNA (ESR2, Fig. 4). Furthermore, an analysis of mRNAs encoding apoptotic regulators, including bcl2, bax, and bad (Fig. 4), as well as members of the caspase family of proapoptotic proteases (not shown), revealed no measurable difference in expression among control NB and AD-NB neurons, independent of GAL innervation.
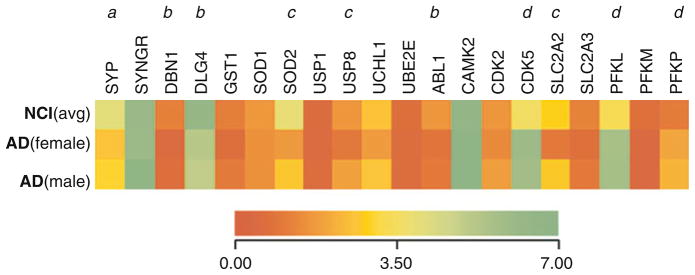
Fig. 4.
GAL hyperinnervated NB neurons display preserved antioxidant, deubiquitinating, and glucose transport transcripts in AD. Dendrogram illustrating relative mRNA expression levels (red to green = increasing levels) of synaptophysin (Unigene/NCBI notation SYP), synaptogyrin (SYNGR), drebrin 1 (DRB1), postsynaptic density PSD95 (DLG4), glutathione-S-transferase 1 (GST1), superoxide dismutase 1 (SOD1), superoxide dismutase 2 (SOD2), bcl2 (BCL2), bax (BAX), bad (BAD), ubiquitin-specific protease 1 (USP1), ubiquitin-specific protease 8 (USP8), ubiquitin carboxyl-terminal esterase L1 (UCHL1), ubiquitin-conjugating enzyme E2E 1 (UBE2E), abl1 tyrosine kinase (ABL1), Ca2+/calmodulin-dependent protein kinase II (CAMK2), cyclin-dependent kinase 2 (CDK2), cyclin-dependent kinase 5 (CDK5), p35 cdk5 regulatory subunit (CDK5R1), p75NTR neurotrophin receptor (NGFR), trkA (NTRK1), trkB (NTRK2), estrogen receptor α (ESR1), estrogen receptor β (ESR2), androgen receptor (AR), glucose transporters GLUT2 (SLC2A2) and GLUT3 (SLC2A3), and phosphofructokinase liver type (PFKL), muscle type (PFKM) and platelet type (PFKP) isozymes. a = NCI > AD/GAL−, AD/GAL+, p < 0.001; b = NCI > AD/GAL−, AD/GAL+, p < 0.01; c = NCI, AD/GAL+ > AD/GAL−, p < 0.01; d = AD/GAL−, AD/GAL+ > NCI, p < 0.01; e = NCI, AD/GAL− > AD/GAL+, p < 0.01
By contrast, an examination of mRNAs encoding antioxidant enzymes in NB neurons in AD revealed that manganese superoxide dismutase 2 (SOD2) expression levels were stable in GAL-hyperinnervated but downregulated in non-innervated neurons compared to control (Fig. 4). SOD2 protein products are targeted to mitochondria and their activity is critical for the detoxification of superoxide free radicals [66, 67]. Given the findings linking increased mitochondrial oxidative stress and reduced mitochondrial function in AD [68, 69], the preservation of SOD2 gene expression in GAL-hyperinnervated cells suggests that GAL exerts its neuroprotective effects upon CBF neurons through the modulation of select antioxidant genes [23].
An examination of mRNAs encoding enzymes involved in the ubiquitin-proteasome system showed a selective reduction in the expression of the deubiquitinating enzyme, ubiquitin-specific protease 8 (USP8), in GAL non-innervated NB neurons in AD; this was not evident in GAL-hyperinnervated neurons (Fig. 4). Functionally, posttranslational modification of proteins by ubiquitination regulates a wide range of cellular actions including protein stability and transport [70–72]. While the exact roles of the USP family of gene products in cell function are still under investigation, it has been shown that USP8 null mice exhibit embryonic lethality [73]. Furthermore, cultured fibroblasts and liver tissue from USP8 null mice display an accumulation of ubiquitinated proteins that colocalized with enlarged endosomes [73]. While it remains to be determined whether USP8 acts in a similar manner in cholinergic NB neurons, observations from peripheral tissue studies suggest that endosomal trafficking may be more efficient in GAL-hyperinnervated compared to non-innervated neurons in AD [23].
Finally, based on our finding that transcripts encoding two of the three phospho-fructokinase glycolytic isozymes [PFKL (isozyme B, liver type) and PFKP (isozyme C, platelet type)], are upregulated in AD cells compared to NCI (Fig. 4), we investigated the changes in other transcripts involved in glucose metabolism. In this case, mRNA levels of the glucose transporter GLUT2 (SLC2A2) were decreased in GAL non-innervated cells but not in GAL-hyperinnervated cells. GLUT2 protein is localized predominantly to neuronal dendrites in the mammalian brain [74] and in vivo cerebral blockade of GLUT2 induces aberrant insulin pathway signaling and spatial memory deficits in aged rats [75]. As such, reduced GLUT2 activity may contribute to the impairment in brain insulin signaling and glucose uptake observed in sporadic AD [76]. The preservation of GLUT2 mRNA in GAL-hyperinnervated NB neurons in AD may indicate relatively normal glucose metabolism in these cells relative to non-hyperinnervated NB neurons [23].
The results obtained from our postmortem tissue-based studies suggest a role for GAL in cholinergic cell survival. This concept is supported by findings from a knockout mouse model that carries a targeted loss-of-function mutation in the GAL gene (GAL-KO mice) [53, 77]. GAL-KO mice show a significant decrease in the number of ChAT-ir neurons in the CBF medial septum and vertical limb/diagonal band subfields. Moreover, these areas, as well as the NB, displayed a significant decrease in the number of neurons expressing TrkA, the high affinity receptor for the cholinergic cell survival substance, nerve growth factor (NGF) [53]. GAL-KO mice exhibit an age-related decrease in evoked hippocampal ACh release, inhibition of LTP in the CA1 region of the hippocampus, and an age-dependent decline on both the Morris water maze [53] and object-in-place [78] spatial memory tasks. Together, these findings indicate an excitatory role for GAL in hippocampal function in these mutant mice. Interestingly, electrophysiological investigations using primary CBF diagonal band neuron cultures from rats revealed that exogenous GAL reduced an array of inhibitory potassium currents in cholinergic neurons and increased the excitability of these cells under current-clamp conditions [79]. These findings complement in vivo studies which report that chronic infusion of 1–3 nM GAL into the rat medial septum/diagonal band resulted in increased ACh release in the ventral hippocampus and improved spatial memory performance on the water maze task [80]. This data, derived from awake and freely moving animals, stands in contrast to the inhibitory effects of GAL on ACh release described in rat hippocampal slices (see above) and suggests that the putative neuroprotective role for GAL may involve the survival and/or regulation of the tone of CBF neurons. In support of this hypothesis, GAL has been shown to protect CBF septal neuron cultures of rats from Aβ neurotoxicity by increasing prosurvival signaling (e.g., via phosphorylated Akt) and reducing apoptotic signaling (e.g., caspase 3 cleavage) [20].
The GALR(s) mediating these putative neuroprotective signals remain an area of active research. Single NB neuron gene expression studies from our laboratory failed to reveal any difference in the expression level of any of the GALR transcripts between control or AD CBF neurons (data not shown) [23, 25], warranting investigations into the GALR subtype(s) that mediate the putative prosurvival effects of GAL in AD (Figs. 3 and 4). However, various lines of research support a role for GALR2 in cholinergic neuroprotection. For example, the GALR2 agonist AR-M1896 mimicked the effect of GAL in protecting CBF septal neuron cultures of the rat from Aβ neurotoxicity [20]. This molecule also protected both mouse [19] and rat [81] primary neuronal hippocampal cultures from glutamate or staurosporine-induced [19] cell death. Recently, it has been shown that GAL failed to prevent glutamate-induced hippocampal cell death in cultures from GALR2 knockout (GALR2KO) mice [82]. In this study, GAL stimulated the neuroprotective Akt and Erk phosphorylation signaling cascades in hippocampal cultures from WT mice; this effect was significantly attenuated in GALR2KO cultures. Thus, GAL neuro-protection may involve GALR2-mediated stimulation of these pathways [82]. Supporting this concept is the observation that GAL, and the GALR2-prefering GAL-like peptide, induce neurite outgrowth in PC12 cells in an Erk-dependent manner [83].
The discordance in data regarding the putative function of GAL overexpression in AD is yet to be resolved, especially in light of similar detrimental phenotypes being observed in both the GAL-KO and GAL-tg/GAlOE mice. With respect to the CBF, results from the GAL-KO mouse suggest that GAL is important for the establishment of cholinergic basocortical and septohippocampal systems [53]. The hypertrophy of GAL fibers in AD may be an attempt by the brain to replicate developmental actions of this peptide in response to the degeneration of CBF cortical and hippocampal projection neurons. In support of this, GAL and its receptors are expressed in embryonic stem cells, suggesting that GAL may be a crucial factor in cell differentiation/survival during embryogenesis [84]. Data from human postmortem tissue studies, in vivo and in vitro models indicate a potential neuroprotective role for GAL plasticity in CBF neurons. On the other hand, GAL plasticity within the hippocampus may inhibit cholinergic transmission, as inferred from the phenotype of the GAL-tg/GAlOE mice and rodent hippocampal preparations. Therefore, GAL may induce a neuroprotective signal in the somatodendritic compartment of CBF neurons vulnerable to AD pathophysiology, but may play an inhibitory role in the axonal compartment of these neurons [85]. Given the diverse repertoire of context-dependent GALR signaling pathways (e.g., GALR1 inhibits adenylyl cyclase or activates Erk, GALR2 activates phospholipase C and activates or inhibits adenylyl cyclase [82, 86, 87]), specifically delineating the GALR(s) activated by GAL in the human CBF and hippocampus will be critical in clarifying the functional effects of GAL in AD. Knockout mice deficient for GALR subtypes may ultimately help to clarify the role(s) of GAL signaling in cognitive processes. However, findings from GALR1 knockout (GALR1KO) and GALR2KO mice have not yielded definitive results. For instance, studies in GALR1KO mice showed that GALR1 is required for anticonvulsant protection from spontaneous or experimentally induced seizures [88–90]. However, pharmacological [91] and molecular [92] manipulations of GALR2 implicate this receptor in anticonvulsant activities as well. Likewise, studies of GALR1KO and GALR2KO mouse strains implicate both receptors in mediating anxiolytic actions [93, 94]. With respect to learning and memory tasks, GALR1KO mice display an impairment in the fear conditioning emotional memory task [94]. In contrast, neither GALR knockout model exhibited deficits in spatial memory tasks [94, 95] suggesting that the effects of GAL on learning and memory involve a complex interplay between GALR1 and GALR2 receptors; perhaps at the level of the pre- and/or postsynaptic compartments of CBF and hippocampal neurons. It is also possible that the more sparsely distributed GALR3 receptor is involved in these cognitive processes.
Galanin Receptors as Therapeutic Targets for AD
Currently approved drug treatments for AD include cholinesterase inhibitors which act by increasing the bioavailability of synaptic ACh, and memantine, a noncompetitive glutamatergic NMDA receptor antagonist that suppresses excitotoxicity. These drugs produce small but consistent improvements in memory and global cognitive function and positively influence activities of daily living. If GAL inhibits ACh release, then GALR subtype-specific antagonists may enhance cholinergic transmission by reducing the inhibitory influence of GAL on the firing rate of CBF neurons. Likewise, if GAL promotes the survival or cholinergic tone of CBF neurons, then a GALR agonist might prove efficacious. Gene expression profiling studies have revealed that human CBF neurons express mRNAs encoding all three GALRs [23, 25]; hence, the predominant GAL-mediated signal elicited in innervated and hyperinnervated cholinergic neurons is unclear. Moreover, unlike human CBF neurons, very few rodent CBF neurons express GALR1 [62]; this is a potential confounder in extrapolating results from animal models to humans. The ambiguous results from GALRKO mice with respect to rodent memory tasks analogous to human working memory function suggest that, in vivo, GALR subtype-specific pharmacological manipulations can potentially clarify the role of each GALR in the face of basal and augmented GAL signaling. Until recently, the only tools available for pharmacological differentiation of GALR subtypes have been synthetic GAL analogs with one or more amino acid substitution or chimeric GAL peptide ligands that show variable affinity for human and rat GALRs. These behave incongruently as antagonists at native receptors but work as partial or weak agonists at cloned receptors [96, 97]. However, two peptidergic compounds, AR-M1896 and AR-M961, which are selective agonists for GALR2 and GALR1/GALR2, respectively [98], have been used in rat models to identify GALR subtype specific activities in nociception (mediated by GALR2) and analgesia (GALR1) in the spinal cord [98], hyperpolarization of LC neurons (GALR1; [99]), and neuritogenesis in cholinergic sensory neuron explants (GALR2; [100]). Recently, a non-peptidergic GALR1 agonist, galmic, which mimics GAL in suppressing LTP and seizures in the rodent hippocampus [101] was discovered. The effects of these ligands on cognitive function have not been firmly established. The ongoing search for selective GALR ligands in drug discovery programs will hopefully provide new research tools needed to understand GALR subtype-specific pharmacology with respect to cognitive processes mediated by neuronal populations vulnerable to AD pathogenesis. Since AD appears to have multiple etiologies, a rational treatment strategy might include high-affinity GALR ligands used in combination with anticholinesterases and perhaps other compounds, such as memantine and modulators of Aβ aggregation or clearance. In this regard, intraventricular infusion of NGF increased GAL mRNA expression in the hippocampus of rats [102] suggesting that the use of NGF in the treatment of AD [103] may indirectly increase GAL in the brain, thus providing a dual therapeutic benefit for treatment of CBF dysfunction in AD. We suggest that the poly-pharmacological use of such compounds may ameliorate cholinergic hypofunction observed in AD, and perhaps benefit other aspects of this heterogeneous disorder.
Conclusions
The presentation of AD is likely precipitated by neuronal degeneration in selectively vulnerable regions of the limbic system and the brainstem involved in higher order cognitive processes. The observations discussed in this chapter reveal that GAL has important effects on cell survival, thus raising the intriguing possibility that pharmacological stimulation of GAL activity might be neuroprotective and slowdown the cognitive decline in AD. This concept is an alternative to the more traditional hypothesis that GAL inhibits neuronal function in relation to cognition. As there are major differences between species in the expression of GAL within the CBF and LC in the human and rodent brain [46, 48, 49, 62], the physiological actions of GAL may also have diverged during the evolution of the human brain in which GAL is required to fine tune the functional tone of select neuronal populations such as those involved in learning and memory [104]. Therefore, the consequences of GAL plasticity in AD must be explored further to guide the development of high-affinity GALR subtype-specific agonists or antagonists. Elucidation of GALR distribution through the development of subtype-specific antibodies and endogenous GALR activity via continued development of subtype-specific ligands will be critical in determining the therapeutic efficacy of GAL mimetics aimed at ameliorating symptoms of AD.
Acknowledgments
The authors would like to thank our colleagues we’ve worked with over the years in exploring the nature of GAL plasticity in AD: M. Basile, W.C. Benzing, L.I. Binder, R.P. Bowser, J.N. Crawley, S. De Lacalle, D.C. Deecher, D.L. Feinstein, I. Hartonian, J.H. Kordower, D.C. Mash, R.A. Steiner, and D. Wynick. Supported by NIH grants AG14449, AG09466, AG10161 (Dr. Mufson), AG03500 (Dr. Counts), the Illinois Department of Public Health (Dr. Counts), and the Rush University Medical Center Research Council (Drs. Perez and Counts).
Contributor Information
Scott E. Counts, Department of Neurological Sciences, Rush University Medical Center, 1735 West Harrison Street, Suite 300, Chicago, IL 60612, USA
Sylvia E. Perez, Department of Neurological Sciences, Rush University Medical Center, 1735 West Harrison Street, Suite 300, Chicago, IL 60612, USA
Stephen D. Ginsberg, Department of Psychiatry, Department of Physiology and Neuroscience, Center for Dementia Research, Nathan Kline Institute, New York University School of Medicine, Orangeburg, NY, USA
Elliott J. Mufson, Email: emufson@rush.edu, Department of Neurological Sciences, Rush University Medical Center, 1735 West Harrison Street, Suite 300, Chicago, IL 60612, USA
References
- 1.Bartfai T, Hokfelt T, Langel U. Galanin–a neuroendocrine peptide. Crit Rev Neurobiol. 1993;7:229–274. [PubMed] [Google Scholar]
- 2.Hokfelt T, Xu ZQ, Shi TJ, Holmberg K, Zhang X. Galanin in ascending systems. Focus on coexistence with 5-hydroxytryptamine and noradrenaline. Ann N Y Acad Sci. 1998;863:252–263. doi: 10.1111/j.1749-6632.1998.tb10700.x. [DOI] [PubMed] [Google Scholar]
- 3.Coumis U, Davies CH. The effects of galanin on long-term synaptic plasticity in the CA1 area of rodent hippocampus. Neuroscience. 2002;112:173–182. doi: 10.1016/s0306-4522(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 4.Fisone G, Wu CF, Consolo S, Nordstrom O, Brynne N, Bartfai T, Melander T, Hokfelt T. Galanin inhibits acetylcholine release in the ventral hippocampus of the rat: histochemical, autoradiographic, in vivo, and in vitro studies. Proc Natl Acad Sci USA. 1987;84:7339–7343. doi: 10.1073/pnas.84.20.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazarati AM, Hohmann JG, Bacon A, Liu H, Sankar R, Steiner RA, Wynick D, Wasterlain CG. Modulation of hippocampal excitability and seizures by galanin. J Neurosci. 2000;20:6276–6281. doi: 10.1523/JNEUROSCI.20-16-06276.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wrenn CC, Crawley JN. Pharmacological evidence supporting a role for galanin in cognition and affect. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:283–299. doi: 10.1016/s0278-5846(00)00156-1. [DOI] [PubMed] [Google Scholar]
- 7.Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- 8.Bartus RT. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol. 2000;163:495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- 9.Wilcock GK, Esiri MM, Bowen DM, Smith CC. Alzheimer’s disease. Correlation of cortical choline acetyltransferase activity with the severity of dementia and histological abnormalities. J Neurol Sci. 1982;57:407–417. doi: 10.1016/0022-510x(82)90045-4. [DOI] [PubMed] [Google Scholar]
- 10.Bowser R, Kordower JH, Mufson EJ. A confocal microscopic analysis of galaninergic hyperinnervation of cholinergic basal forebrain neurons in Alzheimer’s disease. Brain Pathol. 1997;7:723–730. doi: 10.1111/j.1750-3639.1997.tb01058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan-Palay V. Galanin hyperinnervates surviving neurons of the human basal nucleus of Meynert in dementias of Alzheimer’s and Parkinson’s disease: a hypothesis for the role of galanin in accentuating cholinergic dysfunction in dementia. J Comp Neurol. 1988;273:543–557. doi: 10.1002/cne.902730409. [DOI] [PubMed] [Google Scholar]
- 12.Mufson EJ, Cochran E, Benzing W, Kordower JH. Galaninergic innervation of the cholinergic vertical limb of the diagonal band (Ch2) and bed nucleus of the stria terminalis in aging, Alzheimer’s disease and Down’s syndrome. Dementia. 1993;4:237–250. doi: 10.1159/000107329. [DOI] [PubMed] [Google Scholar]
- 13.Bierer LM, Haroutunian V, Gabriel S, Knott PJ, Carlin LS, Purohit DP, Perl DP, Schmeidler J, Kanof P, Davis KL. Neurochemical correlates of dementia severity in Alzheimer’s disease: relative importance of the cholinergic deficits. J Neurochem. 1995;64:749–760. doi: 10.1046/j.1471-4159.1995.64020749.x. [DOI] [PubMed] [Google Scholar]
- 14.Gabriel SM, Bierer LM, Davidson M, Purohit DP, Perl DP, Harotunian V. Galanin-like immunoreactivity is increased in the postmortem cerebral cortex from patients with Alzheimer’s disease. J Neurochem. 1994;62:1516–1523. doi: 10.1046/j.1471-4159.1994.62041516.x. [DOI] [PubMed] [Google Scholar]
- 15.McMillan PJ, Peskind E, Raskind MA, Leverenz JB. Increased galanin receptor occupancy in Alzheimer’s disease. Neurobiol Aging. 2004;25:1309–1314. doi: 10.1016/j.neurobiolaging.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Mufson EJ, Deecher DC, Basile M, Izenwasse S, Mash DC. Galanin receptor plasticity within the nucleus basalis in early and late Alzheimer’s disease: an in vitro autoradiographic analysis. Neuropharmacology. 2000;39:1404–1412. doi: 10.1016/s0028-3908(00)00011-3. [DOI] [PubMed] [Google Scholar]
- 17.Perez S, Basile M, Mash DC, Mufson EJ. Galanin receptor over-expression within the amygdala in early Alzheimer’s disease: an in vitro autoradiographic analysis. J Chem Neuroanat. 2002;24:109–116. doi: 10.1016/s0891-0618(02)00034-0. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Puertas R, Nilsson S, Pascual J, Pazos A, Hokfelt T. 125I-galanin binding sites in Alzheimer’s disease: increases in hippocampal subfields and a decrease in the caudate nucleus. J Neurochem. 1997;68:1106–1113. doi: 10.1046/j.1471-4159.1997.68031106.x. [DOI] [PubMed] [Google Scholar]
- 19.Elliott-Hunt CR, Marsh B, Bacon A, Pope R, Vanderplank P, Wynick D. Galanin acts as a neuroprotective factor to the hippocampus. Proc Natl Acad Sci USA. 2004;101:5105–5110. doi: 10.1073/pnas.0304823101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding X, MacTavish D, Kar S, Jhamandas JH. Galanin attenuates beta-amyloid (Abeta) toxicity in rat cholinergic basal forebrain neurons. Neurobiol Dis. 2006;21:413–420. doi: 10.1016/j.nbd.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Counts SE, He B, Che S, Ginsberg SD, Mufson EJ. Galanin hyperinnervation upregulates choline acetyltransferase expression in cholinergic basal forebrain neurons in Alzheimer’s disease. Neurodegener Dis. 2008;5:228–231. doi: 10.1159/000113710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Counts SE, Perez SE, Ginsberg SD, De Lacalle S, Mufson EJ. Galanin in Alzheimer disease. Mol Interv. 2003;3:137–156. doi: 10.1124/mi.3.3.137. [DOI] [PubMed] [Google Scholar]
- 23.Counts SE, He B, Che S, Ginsberg SD, Mufson EJ. Galanin fiber hyperinnervation preserves neuroprotective gene expression in cholinergic basal forebrain neurons in Alzheimer’s disease. J Alzheimers Dis. 2009;18(4):885–896. doi: 10.3233/JAD-2009-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beal MF, MacGarvey U, Swartz KJ. Galanin immunoreactivity is increased in the nucleus basalis of Meynert in Alzheimer’s disease. Ann Neurol. 1990;28:157–161. doi: 10.1002/ana.410280207. [DOI] [PubMed] [Google Scholar]
- 25.Counts SE, Chen EY, Che S, Ikonomovic MD, Wuu J, Ginsberg SD, Dekosky ST, Mufson EJ. Galanin fiber hypertrophy within the cholinergic nucleus basalis during the progression of Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;21:205–214. doi: 10.1159/000090906. [DOI] [PubMed] [Google Scholar]
- 26.Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol. 2003;60:337–341. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]
- 27.Chan-Palay V. Alterations in the locus coeruleus in dementias of Alzheimer’s and Parkinson’s disease. Prog Brain Res. 1991;88:625–630. doi: 10.1016/s0079-6123(08)63839-x. [DOI] [PubMed] [Google Scholar]
- 28.Deecher DC, Mash DC, Staley JK, Mufson EJ. Characterization and localization of galanin receptors in human entorhinal cortex. Regul Pept. 1998;73:149–159. doi: 10.1016/s0167-0115(97)01067-7. [DOI] [PubMed] [Google Scholar]
- 29.Gomez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amaral DG. Higher functions of the brain. In: Mountcastle VB, Plum F, Geiger SR, editors. Handbook of physiology. Section 1: The nervous system. American Physiological Society; Bethesda, MD: 1987. [Google Scholar]
- 31.Vereecken TH, Vogels OJ, Nieuwenhuys R. Neuron loss and shrinkage in the amygdala in Alzheimer’s disease. Neurobiol Aging. 1994;15:45–54. doi: 10.1016/0197-4580(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 32.Holmes PV, Crawley JN. Olfactory bulbectomy increases preprogalanin mRNA levels in the rat locus coeruleus. Brain Res Mol Brain Res. 1996;36:184–188. doi: 10.1016/0169-328x(95)00295-4. [DOI] [PubMed] [Google Scholar]
- 33.Villar MJ, Meister B, Hokfelt T. Reorganization of neural peptidergic systems in the median eminence after hypophysectomy. J Neurosci. 1994;14:5996–6012. doi: 10.1523/JNEUROSCI.14-10-05996.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gabriel SM, Knott PJ, Haroutunian V. Alterations in cerebral cortical galanin concentrations following neurotransmitter-specific subcortical lesions in the rat. J Neurosci. 1995;15:5526–5534. doi: 10.1523/JNEUROSCI.15-08-05526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartonian I, Mufson EJ, de Lacalle S. Long-term plastic changes in galanin innervation in the rat basal forebrain. Neuroscience. 2002;115:787–795. doi: 10.1016/s0306-4522(02)00453-0. [DOI] [PubMed] [Google Scholar]
- 36.Shen PJ, Larm JA, Gundlach AL. Expression and plasticity of galanin systems in cortical neurons, oligodendrocyte progenitors and proliferative zones in normal brain and after spreading depression. Eur J Neurosci. 2003;18:1362–1376. doi: 10.1046/j.1460-9568.2003.02860.x. [DOI] [PubMed] [Google Scholar]
- 37.Bond BC, Virley DJ, Cairns NJ, Hunter AJ, Moore GB, Moss SJ, Mudge AW, Walsh FS, Jazin E, Preece P. The quantification of gene expression in an animal model of brain ischaemia using TaqMan real-time RT-PCR. Brain Res Mol Brain Res. 2002;106:101–116. doi: 10.1016/s0169-328x(02)00417-5. [DOI] [PubMed] [Google Scholar]
- 38.Villar MJ, Cortes R, Theodorsson E, Wiesenfeld-Hallin Z, Schalling M, Fahrenkrug J, Emson PC, Hokfelt T. Neuropeptide expression in rat dorsal root ganglion cells and spinal cord after peripheral nerve injury with special reference to galanin. Neuroscience. 1989;33:587–604. doi: 10.1016/0306-4522(89)90411-9. [DOI] [PubMed] [Google Scholar]
- 39.Bacon A, Holmes FE, Small CJ, Ghatei M, Mahoney S, Bloom S, Wynick D. Transgenic over-expression of galanin in injured primary sensory neurons. Neuroreport. 2002;13:2129–2132. doi: 10.1097/00001756-200211150-00028. [DOI] [PubMed] [Google Scholar]
- 40.Kowall NW, Beal MF. Galanin-like immunoreactivity is present in human substantia innominata and in senile plaques in Alzheimer’s disease. Neurosci Lett. 1989;98:118–123. doi: 10.1016/0304-3940(89)90384-4. [DOI] [PubMed] [Google Scholar]
- 41.Diez M, Koistinaho J, Kahn K, Games D, Hokfelt T. Neuropeptides in hippocampus and cortex in transgenic mice overexpressing V717F beta-amyloid precursor protein–initial observations. Neuroscience. 2000;100:259–286. doi: 10.1016/s0306-4522(00)00261-x. [DOI] [PubMed] [Google Scholar]
- 42.Diez M, Danner S, Frey P, Sommer B, Staufenbiel M, Wiederhold KH, Hokfelt T. Neuropeptide alterations in the hippocampal formation and cortex of transgenic mice overexpressing beta-amyloid precursor protein (APP) with the Swedish double mutation (APP23) Neurobiol Dis. 2003;14:579–594. doi: 10.1016/j.nbd.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Perez SE, Dar S, Ikonomovic MD, DeKosky ST, Mufson EJ. Cholinergic forebrain degeneration in the APPswe/PS1DeltaE9 transgenic mouse. Neurobiol Dis. 2007;28:3–15. doi: 10.1016/j.nbd.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mufson EJ, Counts SE, Perez SE, Binder L. Galanin plasticity in the cholinergic basal forebrain in Alzheimer’s disease and transgenic mice. Neuropeptides. 2005;39:232–236. doi: 10.1016/j.npep.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Chan-Palay V. Neurons with galanin innervate cholinergic cells in the human basal forebrain and galanin and acetylcholine coexist. Brain Res Bull. 1988;21:465–472. doi: 10.1016/0361-9230(88)90160-8. [DOI] [PubMed] [Google Scholar]
- 46.Kordower JH, Mufson EJ. Galanin-like immunoreactivity within the primate basal forebrain: differential staining patterns between humans and monkeys. J Comp Neurol. 1990;294:281–292. doi: 10.1002/cne.902940211. [DOI] [PubMed] [Google Scholar]
- 47.Miller MA, Kolb PE, Leverenz JB, Peskind ER, Raskind MA. Preservation of noradrenergic neurons in the locus ceruleus that coexpress galanin mRNA in Alzheimer’s disease. J Neurochem. 1999;73:2028–2036. [PubMed] [Google Scholar]
- 48.Perez SE, Wynick D, Steiner RA, Mufson EJ. Distribution of galaninergic immunoreactivity in the brain of the mouse. J Comp Neurol. 2001;434:158–185. doi: 10.1002/cne.1171. [DOI] [PubMed] [Google Scholar]
- 49.Skofitsch G, Jacobowitz DM. Immunohistochemical mapping of galanin-like neurons in the rat central nervous system. Peptides. 1985;6:509–546. doi: 10.1016/0196-9781(85)90118-4. [DOI] [PubMed] [Google Scholar]
- 50.Dutar P, Lamour Y, Nicoll RA. Galanin blocks the slow cholinergic EPSP in CA1 pyramidal neurons from ventral hippocampus. Eur J Pharmacol. 1989;164:355–360. doi: 10.1016/0014-2999(89)90477-9. [DOI] [PubMed] [Google Scholar]
- 51.McDonald MP, Gleason TC, Robinson JK, Crawley JN. Galanin inhibits performance on rodent memory tasks. Ann N Y Acad Sci. 1998;863:305–322. doi: 10.1111/j.1749-6632.1998.tb10704.x. [DOI] [PubMed] [Google Scholar]
- 52.Robinson JK, Crawley JN. Intraseptal galanin potentiates scopolamine impairment of delayed nonmatching to sample. J Neurosci. 1993;13:5119–5125. doi: 10.1523/JNEUROSCI.13-12-05119.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Meara G, Coumis U, Ma SY, Kehr J, Mahoney S, Bacon A, Allen SJ, Holmes F, Kahl U, Wang FH, et al. Galanin regulates the postnatal survival of a subset of basal forebrain cholinergic neurons. Proc Natl Acad Sci USA. 2000;97:11569–11574. doi: 10.1073/pnas.210254597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zini S, Roisin MP, Langel U, Bartfai T, Ben-Ari Y. Galanin reduces release of endogenous excitatory amino acids in the rat hippocampus. Eur J Pharmacol. 1993;245:1–7. doi: 10.1016/0922-4106(93)90162-3. [DOI] [PubMed] [Google Scholar]
- 55.Kokaia M, Holmberg K, Nanobashvili A, Xu ZQ, Kokaia Z, Lendahl U, Hilke S, Theodorsson E, Kahl U, Bartfai T, et al. Suppressed kindling epileptogenesis in mice with ectopic overexpression of galanin. Proc Natl Acad Sci USA. 2001;98:14006–14011. doi: 10.1073/pnas.231496298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steiner RA, Hohmann JG, Holmes A, Wrenn CC, Cadd G, Jureus A, Clifton DK, Luo M, Gutshall M, Ma SY, et al. Galanin transgenic mice display cognitive and neurochemical deficits characteristic of Alzheimer’s disease. Proc Natl Acad Sci USA. 2001;98:4184–4189. doi: 10.1073/pnas.061445598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crawley JN, Mufson EJ, Hohmann JG, Teklemichael D, Steiner RA, Holmberg K, Xu ZQ, Blakeman KH, Xu XJ, Wiesenfeld-Hallin Z, et al. Galanin overexpressing transgenic mice. Neuropeptides. 2002;36:145–156. doi: 10.1054/npep.2002.0891. [DOI] [PubMed] [Google Scholar]
- 58.Wrenn CC, Marriott LK, Kinney JW, Holmes A, Wenk GL, Crawley JN. Galanin peptide levels in hippocampus and cortex of galanin-overexpressing transgenic mice evaluated for cognitive performance. Neuropeptides. 2002;36:413–426. doi: 10.1016/s0143-4179(02)00111-7. [DOI] [PubMed] [Google Scholar]
- 59.Laplante F, Crawley JN, Quirion R. Selective reduction in ventral hippocampal acetylcholine release in awake galanin-treated rats and galanin-overexpressing transgenic mice. Regul Pept. 2004;122:91–98. doi: 10.1016/j.regpep.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 60.Zheng K, Kuteeva E, Xia S, Bartfai T, Hokfelt T, Xu ZQ. Age-related impairments of synaptic plasticity in the lateral perforant path input to the dentate gyrus of galanin overexpressing mice. Neuropeptides. 2005;39:259–267. doi: 10.1016/j.npep.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 61.Pirondi S, D’Intino G, Gusciglio M, Massella A, Giardino L, Kuteeva E, Ogren SO, Hokfelt T, Calza L. Changes in brain cholinergic markers and spatial learning in old galanin-overexpressing mice. Brain Res. 2007;1138:10–20. doi: 10.1016/j.brainres.2006.12.069. [DOI] [PubMed] [Google Scholar]
- 62.Miller MA, Kolb PE, Planas B, Raskind MA. Few cholinergic neurons in the rat basal forebrain coexpress galanin messenger RNA. J Comp Neurol. 1998;391:248–258. [PubMed] [Google Scholar]
- 63.Mufson EJ, Bothwell M, Kordower JH. Loss of nerve growth factor receptor-containing neurons in Alzheimer’s disease: a quantitative analysis across subregions of the basal forebrain. Exp Neurol. 1989;105:221–232. doi: 10.1016/0014-4886(89)90124-6. [DOI] [PubMed] [Google Scholar]
- 64.Mufson EJ, Counts SE, Ginsberg SD. Gene expression profiles of cholinergic nucleus basalis neurons in Alzheimer’s disease. Neurochem Res. 2002;27:1035–1048. doi: 10.1023/a:1020952704398. [DOI] [PubMed] [Google Scholar]
- 65.Ginsberg SD, Che S, Wuu J, Counts SE, Mufson EJ. Down regulation of trk but not p75NTR gene expression in single cholinergic basal forebrain neurons mark the progression of Alzheimer’s disease. J Neurochem. 2006;97:475–487. doi: 10.1111/j.1471-4159.2006.03764.x. [DOI] [PubMed] [Google Scholar]
- 66.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 67.Liang LP, Patel M. Mitochondrial oxidative stress and increased seizure susceptibility in Sod2(−/+) mice. Free Radic Biol Med. 2004;36:542–554. doi: 10.1016/j.freeradbiomed.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 68.Perry G, Castellani RJ, Hirai K, Smith MA. Reactive oxygen species mediate cellular damage in Alzheimer disease. J Alzheimers Dis. 1998;1:45–55. doi: 10.3233/jad-1998-1103. [DOI] [PubMed] [Google Scholar]
- 69.Wang J, Xiong S, Xie C, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer’s disease. J Neurochem. 2005;93:953–962. doi: 10.1111/j.1471-4159.2005.03053.x. [DOI] [PubMed] [Google Scholar]
- 70.Choi J, Levey AI, Weintraub ST, Rees HD, Gearing M, Chin LS, Li L. Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson’s and Alzheimer’s diseases. J Biol Chem. 2004;279:13256–13264. doi: 10.1074/jbc.M314124200. [DOI] [PubMed] [Google Scholar]
- 71.Keller JN, Hanni KB, Markesbery WR. Impaired proteasome function in Alzheimer’s disease. J Neurochem. 2000;75:436–439. doi: 10.1046/j.1471-4159.2000.0750436.x. [DOI] [PubMed] [Google Scholar]
- 72.Pasinetti GM. Use of cDNA microarray in the search for molecular markers involved in the onset of Alzheimer’s disease dementia. J Neurosci Res. 2001;65:471–476. doi: 10.1002/jnr.1176. [DOI] [PubMed] [Google Scholar]
- 73.Niendorf S, Oksche A, Kisser A, Lohler J, Prinz M, Schorle H, Feller S, Lewitzky M, Horak I, Knobeloch KP. Essential role of ubiquitin-specific protease 8 for receptor tyrosine kinase stability and endocytic trafficking in vivo. Mol Cell Biol. 2007;27:5029–5039. doi: 10.1128/MCB.01566-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arluison M, Quignon M, Thorens B, Leloup C, Penicaud L. Immunocytochemical localization of the glucose transporter 2 (GLUT2) in the adult rat brain. II. Electron microscopic study. J Chem Neuroanat. 2004;28:137–146. doi: 10.1016/j.jchemneu.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 75.Salkovic-Petrisic M, Tribl F, Schmidt M, Hoyer S, Riederer P. Alzheimer-like changes in protein kinase B and glycogen synthase kinase-3 in rat frontal cortex and hippocampus after damage to the insulin signalling pathway. J Neurochem. 2006;96:1005–1015. doi: 10.1111/j.1471-4159.2005.03637.x. [DOI] [PubMed] [Google Scholar]
- 76.Hoyer S. Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur J Pharmacol. 2004;490:115–125. doi: 10.1016/j.ejphar.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 77.Wynick D, Bacon A. Targeted disruption of galanin: new insights from knock-out studies. Neuropeptides. 2002;36:132–144. doi: 10.1054/npep.2002.0888. [DOI] [PubMed] [Google Scholar]
- 78.Massey PV, Warburton EC, Wynick D, Brown MW, Bashir ZI. Galanin regulates spatial memory but not visual recognition memory or synaptic plasticity in perirhinal cortex. Neuropharmacology. 2003;44:40–48. doi: 10.1016/s0028-3908(02)00297-6. [DOI] [PubMed] [Google Scholar]
- 79.Jhamandas JH, Harris KH, MacTavish D, Jassar BS. Novel excitatory actions of galanin on rat cholinergic basal forebrain neurons: implications for its role in Alzheimer’s disease. J Neurophysiol. 2002;87:696–704. doi: 10.1152/jn.00416.2001. [DOI] [PubMed] [Google Scholar]
- 80.Elvander E, Ogren SO. Medial septal galanin and acetylcholine: influence on hippocampal acetylcholine and spatial learning. Neuropeptides. 2005;39:245–248. doi: 10.1016/j.npep.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 81.Pirondi S, Fernandez M, Schmidt R, Hokfelt T, Giardino L, Calza L. The galanin-R2 agonist AR-M1896 reduces glutamate toxicity in primary neural hippocampal cells. J Neurochem. 2005;95:821–833. doi: 10.1111/j.1471-4159.2005.03437.x. [DOI] [PubMed] [Google Scholar]
- 82.Elliott-Hunt CR, Pope RJ, Vanderplank P, Wynick D. Activation of the galanin receptor 2 (GalR2) protects the hippocampus from neuronal damage. J Neurochem. 2007;100:780–789. doi: 10.1111/j.1471-4159.2006.04239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hawes JJ, Narasimhaiah R, Picciotto MR. Galanin and galanin-like peptide modulate neurite outgrowth via protein kinase C-mediated activation of extracellular signal-related kinase. Eur J Neurosci. 2006;23:2937–2946. doi: 10.1111/j.1460-9568.2006.04828.x. [DOI] [PubMed] [Google Scholar]
- 84.Tarasov KV, Tarasova YS, Crider DG, Anisimov SV, Wobus AM, Boheler KR. Galanin and galanin receptors in embryonic stem cells: accidental or essential? Neuropeptides. 2002;36:239–245. doi: 10.1016/s0143-4179(02)00050-1. [DOI] [PubMed] [Google Scholar]
- 85.Hokfelt T. Galanin and its receptors: Introduction to the Third International Symposium, San Diego, California, USA, 21–22 October 2004. Neuropeptides. 2005;39:125–142. doi: 10.1016/j.npep.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 86.Smith KE, Forray C, Walker MW, Jones KA, Tamm JA, Bard J, Branchek TA, Linemeyer DL, Gerald C. Expression cloning of a rat hypothalamic galanin receptor coupled to phosphoinositide turnover. J Biol Chem. 1997;272:24612–24616. doi: 10.1074/jbc.272.39.24612. [DOI] [PubMed] [Google Scholar]
- 87.Wang S, Hashemi T, Fried S, Clemmons AL, Hawes BE. Differential intracellular signaling of the GalR1 and GalR2 galanin receptor subtypes. Biochemistry. 1998;37:6711–6717. doi: 10.1021/bi9728405. [DOI] [PubMed] [Google Scholar]
- 88.Jacoby AS, Hort YJ, Constantinescu G, Shine J, Iismaa TP. Critical role for GALR1 galanin receptor in galanin regulation of neuroendocrine function and seizure activity. Brain Res Mol Brain Res. 2002;107:195–200. doi: 10.1016/s0169-328x(02)00451-5. [DOI] [PubMed] [Google Scholar]
- 89.Mazarati A, Lu X, Shinmei S, Badie-Mahdavi H, Bartfai T. Patterns of seizures, hippocampal injury and neurogenesis in three models of status epilepticus in galanin receptor type 1 (GalR1) knockout mice. Neuroscience. 2004;128:431–441. doi: 10.1016/j.neuroscience.2004.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McColl CD, Jacoby AS, Shine J, Iismaa TP, Bekkers JM. Galanin receptor-1 knockout mice exhibit spontaneous epilepsy, abnormal EEGs and altered inhibition in the hippocampus. Neuropharmacology. 2006;50:209–218. doi: 10.1016/j.neuropharm.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 91.Mazarati A, Lundstrom L, Sollenberg U, Shin D, Langel U, Sankar R. Regulation of kindling epileptogenesis by hippocampal galanin type 1 and type 2 receptors: the effects of subtype-selective agonists and the role of G-protein-mediated signaling. J Pharmacol Exp Ther. 2006;318:700–708. doi: 10.1124/jpet.106.104703. [DOI] [PubMed] [Google Scholar]
- 92.Mazarati A, Lu X, Kilk K, Langel U, Wasterlain C, Bartfai T. Galanin type 2 receptors regulate neuronal survival, susceptibility to seizures and seizure-induced neurogenesis in the dentate gyrus. Eur J Neurosci. 2004;19:3235–3244. doi: 10.1111/j.0953-816X.2004.03449.x. [DOI] [PubMed] [Google Scholar]
- 93.Holmes A, Kinney JW, Wrenn CC, Li Q, Yang RJ, Ma L, Vishwanath J, Saavedra MC, Innerfield CE, Jacoby AS, et al. Galanin GAL-R1 receptor null mutant mice display increased anxiety-like behavior specific to the elevated plus-maze. Neuropsychopharmacology. 2003;28:1031–1044. doi: 10.1038/sj.npp.1300164. [DOI] [PubMed] [Google Scholar]
- 94.Wrenn CC, Kinney JW, Marriott LK, Holmes A, Harris AP, Saavedra MC, Starosta G, Innerfield CE, Jacoby AS, Shine J, et al. Learning and memory performance in mice lacking the GAL-R1 subtype of galanin receptor. Eur J Neurosci. 2004;19:1384–1396. doi: 10.1111/j.1460-9568.2004.03214.x. [DOI] [PubMed] [Google Scholar]
- 95.Bailey KR, Pavlova MN, Rohde AD, Hohmann JG, Crawley JN. Galanin receptor subtype 2 (GalR2) null mutant mice display an anxiogenic-like phenotype specific to the elevated plus-maze. Pharmacol Biochem Behav. 2007;86:8–20. doi: 10.1016/j.pbb.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bartfai T, Langel U, Bedecs K, Andell S, Land T, Gregersen S, Ahren B, Girotti P, Consolo S, Corwin R, et al. Galanin-receptor ligand M40 peptide distinguishes between putative galanin-receptor subtypes. Proc Natl Acad Sci USA. 1993;90:11287–11291. doi: 10.1073/pnas.90.23.11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Branchek TA, Smith KE, Gerald C, Walker MW. Galanin receptor subtypes. Trends Pharmacol Sci. 2000;21:109–117. doi: 10.1016/s0165-6147(00)01446-2. [DOI] [PubMed] [Google Scholar]
- 98.Liu HX, Brumovsky P, Schmidt R, Brown W, Payza K, Hodzic L, Pou C, Godbout C, Hokfelt T. Receptor subtype-specific pronociceptive and analgesic actions of galanin in the spinal cord: selective actions via GalR1 and GalR2 receptors. Proc Natl Acad Sci USA. 2001;98:9960–9964. doi: 10.1073/pnas.161293598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ma X, Tong YG, Schmidt R, Brown W, Payza K, Hodzic L, Pou C, Godbout C, Hokfelt T, Xu ZQ. Effects of galanin receptor agonists on locus coeruleus neurons. Brain Res. 2001;919:169–174. doi: 10.1016/s0006-8993(01)03033-5. [DOI] [PubMed] [Google Scholar]
- 100.Mahoney SA, Hosking R, Farrant S, Holmes FE, Jacoby AS, Shine J, Iismaa TP, Scott MK, Schmidt R, Wynick D. The second galanin receptor GalR2 plays a key role in neurite outgrowth from adult sensory neurons. J Neurosci. 2003;23:416–421. doi: 10.1523/JNEUROSCI.23-02-00416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bartfai T, Lu X, Badie-Mahdavi H, Barr AM, Mazarati A, Hua XY, Yaksh T, Haberhauer G, Ceide SC, Trembleau L, et al. Galmic, a nonpeptide galanin receptor agonist, affects behaviors in seizure, pain, and forced-swim tests. Proc Natl Acad Sci USA. 2004;101:10470–10475. doi: 10.1073/pnas.0403802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Planas B, Kolb PE, Raskind MA, Miller MA. Nerve growth factor induces galanin gene expression in the rat basal forebrain: implications for the treatment of cholinergic dysfunction. J Comp Neurol. 1997;379:563–570. [PubMed] [Google Scholar]
- 103.Tuszynski MH, Thal L, Pay M, Salmon DP, HSU, Bakay R, Patel P, Blesch A, Vahlsing HL, Ho G, et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11:551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- 104.Counts SE, Perez SE, Kahl U, Bartfai T, Bowser RP, Deecher DC, Mash DC, Crawley JN, Mufson EJ. Galanin: neurobiologic mechanisms and therapeutic potential for Alzheimer’s disease. CNS Drug Rev. 2001;7:445–470. doi: 10.1111/j.1527-3458.2001.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]