Abstract
Herpes simplex virus type 1 (HSV-1) is a ubiquitously occurring pathogen that infects humans early in childhood. The virus persists as a latent infection in dorsal root ganglia, especially of the trigeminal nerve, and frequently becomes reactivated in humans under conditions of stress. Monocytic cells constitute an important component of the innate and adaptive immune responses. We show here for the first time that HSV-1 stimulates human FasL promoter and induces de novo expression of FasL on the surface of human monocytic cells, including monocytes and macrophages. This virus-induced FasL expression causes death of monocytic cells growing in suspension, but not in monolayers (e.g., macrophages). The addition of a broad-spectrum caspase inhibitor, as well as anti-FasL antibodies, reduced cell death but increased viral replication in the virus-infected cell cultures. We also show here for the first time that the virus-induced de novo expression of FasL on the cell surface acts as an immune evasion mechanism by causing the death of interacting human CD4+ T cells, CD8+ T cells, and natural killer (NK) cells. Our study provides novel insights on FasL expression and cell death in HSV-infected human monocytic cells and their impact on interacting immune cells.
Introduction
Herpes simplex virus type 1 (HSV-1; hereafter referred to as HSV) is a ubiquitously occurring human herpes virus that infects humans early in life (reviewed in 1–3). It is a member of the α-Herpesviridae subfamily. Primary infections with the virus usually occur in early childhood and are mild or symptomless. However, infected humans can never eliminate the virus and become lifelong carriers. The virus travels from the oral and facial skin nerve endings to dorsal root ganglia, especially of the trigeminal nerve, where it becomes latent. The latent infections frequently become reactivated under conditions of stress, immunosuppression, physical trauma, or exposure to UV radiation (4). These reactivations are often manifested as painful blisters or “cold sores” at the mucocutaneous junctions of the lips. The condition is called herpes labialis. The virus may also infect the cornea and cause keratitis. These conditions cause considerable discomfort and represent a serious health problem. Primary and reactivated latent infections may rarely cause encephalitis, especially in neonates and immunocompetent persons with unknown defects of the immune system (3). HSV infection is the most common cause of sporadic infectious encephalitis in apparently healthy individuals. Effective anti-HSV drugs have been developed; however, the emergence of drug-resistant viruses has also been documented, particularly in immunocompromised individuals (reviewed in 5). Unfortunately, effective vaccines against the virus are not yet available.
Monocytes and macrophages represent important cellular elements of the immune system. In response to a viral infection, they release a variety of proinflammatory cytokines and chemokines, and recruit inflammatory cells to the site of infection. Activated macrophages phagocytose pathogens and immune complexes, and present viral antigens to other immune cells. Unlike epithelial cells, in which HSV prevents apoptosis and causes cell death with predominant features of necrosis, HSV infects monocytic cells with different degrees of permissiveness, and appears to induce their cell death via apoptosis (6–8). However, little is known about the mechanism of this virus-induced apoptosis, or its consequences for antiviral immunity as well as for viral replication. We addressed these questions and show here that HSV infection causes apoptosis in human monocytic cells by inducing expression of FasL on their surface. Our data provide experimental evidence showing for the first time that the virus induces FasL at the transcriptional level by stimulating FasL promoter. Interference with this apoptotic pathway prevents cell death, but enhances viral replication. Furthermore, HSV-infected human monocytic cells were able to kill Fas-positive human CD4+ T cells, CD8+ T cells, and natural killer (NK) cells in in vitro co-culture assays. These observations provide valuable insights about the relevance of apoptosis to viral replication and immune evasion in this viral infection.
Materials and Methods
Cell culture
THP-1, U937, and Vero cells were obtained from ATCC. All cell lines were cultured in the culture medium RPMI-1640 containing 10% fetal calf serum (FCS), 2 mM L-glutamate, 100 U/mL of penicillin, and 100 μg/mL streptomycin (Gibco, Burlington, Ontario, Cananda), at 37°C in a 5% CO2 humidified atmosphere. Human peripheral blood mononuclear cells (PBMCs) were obtained by centrifugation of blood over Ficoll-Hypaque (Pharmacia, Montreal, Quebec, Canada) and washed with the culture medium without FCS and antibiotics. Human monocytes were isolated from PBMCs by negative selection using a commercially available kit (StemSep; Stem Cell Technology, Vancouver, British Columbia, Canada). Purity of the isolated cells was verified by fluorescence-activated cell sorting (FACS) analysis using FITC-conjugated anti-CD14 antibodies (BD Biosciences, Mississauga, Ontario, Canada), and was always ≥95%. In some experiments, monocytes were also isolated by adherence to a plastic surface. After removing the non-adherent cells, the adherent cells were induced to differentiate into macrophages by culturing them in culture medium containing 10% FCS, 5% human AB serum, and 2 ng/mL of recombinant human (rh) granulocyte-macrophage colony-stimulating factor (GM-CSF; BioSource, Camarillo, CA) for 5 d. For co-culturing with HSV-infected macrophages, CD4+ T cells, CD8+ T cells, and NK cells were purified from human PBMCs using kits (Stem Cell Technology).
Antibodies and reagents
The following antibodies were used in this study: mouse anti-human FasL from BD Biosciences, PE-conjugated mouse anti-FasL from eBioscience (San Diego, CA), mouse anti-Fas agonist from Upstate (Temecula, CA), and mouse anti-human glyceraldehyde phosphate dehydrogenase (GAPDH) from Ambion (Austin, TX). Recombinant human TNF-α was purchased from eBioscience. Acyclovir [9-(2-hydroxyethoxymethyl) guanine] was obtained from Calbiochem (San Diego, CA). Cell-permeable broad-spectrum caspase inhibitor N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (Z-VAD-fmk) was obtained from Calbiochem, and 4′-6-diamidino-2-phenylindole (DAPI) was obtained from Sigma-Aldrich (St. Louis, MO).
Virus preparation
Cell-free HSV-1 (McIntyre strain) was prepared from cell lysates and culture supernatants of HSV-1-infected Vero cells as previously described (9–11). The virus-containing culture medium was passed through 0.45-μm filters (Corning, Lowell, MA), and then centrifuged at 14000 × g for 90 min at 4°C. The viral pellets were resuspended in RPMI-1640 medium, titrated, and stored in aliquots at –80°C until use. The mock viral preparations were made like the viral preparations, but were from the culture supernatants obtained from uninfected Vero cells growing in culture. To prepare non-infectious HSV-1, the virus preparation was irradiated by a 60-min exposure to a UV source that delivered UV at 400 μJ per second. The loss of infectivity of the irradiated virus preparation was also tested by its plaque-forming ability on Vero cell monolayers. The viral stocks were aliquoted and kept at –80°C.
In some experiments, we used a recombinant fluorescent HSV-1 (KGFP-gB), which produces enhanced green fluorescent protein (EGFP), tagged with the ectodomain of its gB, as previously described (12). The fluorescent virus replicates less efficiently and produces smaller plaques than its wild-type parent strain KOS. The virus was also produced on Vero monolayers as previously described (9,12).
Titration of herpes simplex virus type 1
The viral preparations were titrated by plaque-forming assay as described elsewhere (9,13). Briefly, 2 million Vero cells were incubated in 100-mm-diameter culture dishes and infected with 100 μL of logarithmically diluted virus preparations for 2 h at 37°C with intermittent shaking. After removing the viral inoculum, 5 mL of 1% methylcellulose was added and the cells were incubated at 37°C in humidified 5% CO2 atmosphere. After 3 d, methylcellulose was removed by gentle suction, and the cell monolayers were washed with phosphate-buffered saline (PBS; pH 7.2), and fixed with formalin (diluted 1:5 with PBS). The fixed cells were stained with 0.1% crystal violet, and the plaques were counted under an inverted microscope. The plaques were counted only for the dilutions giving 20–100 plaques per culture dish. The viral stock used contained 108 plaque-forming units (PFU)/mL.
The supernatants from HSV-infected cells were titrated with the TCID50 method. Briefly, 2 × 104 cells were dispensed in 150 μL of the culture medium in duplicate in the wells of a flat-bottomed 96-well plate. Then the diluted (10–1 to 10–4) supernatants were added in 50 μL volume to each well. The microcultures were incubated for 3 d at 37°C in 5% CO2. The microcultures were examined under the microscope, and TCID50 was calculated by the Spearman-Kärber formula (14).
Herpes simplex virus type 1 infection
For HSV infection, cell pellets were incubated with the virus preparation with different multiplicities of infection (MOI) at 37°C for 90 min in 15-mL tubes, washed thrice, and resuspended in the culture medium. The cells were incubated at 37°C in a humidified 5% CO2 atmosphere for different time periods.
Western blotting
The expression of different proteins within cells was analyzed by Western blotting, as described in our earlier publications (15). Briefly, 5 × 106 cells were incubated in the culture medium with and without treatment as detailed in the individual experiments. The cells were then washed with PBS and lysed in a lysis buffer containing Tris-HCl (pH 6.8; 50 mM), sodium dodecyl sulfate (SDS; 2%), leupeptin (1 mg/mL), phenylmethylsulfonyl fluoride (1 mM), and pepstatin (1 mg/mL). The cell lysates were clarified by centrifugation, and protein concentrations were determined in the lysates by using a commercial kit (Pierce, Nepean, Ontario, Canada). Then 40 μg of the lysate proteins were mixed with 2 × SDS-polyacrylamide gel electrophoresis (PAGE) sample loading buffer containing 1 mM dithiothreitol, boiled, run on 12% SDS-PAGE gels, and electroblotted onto polyvinylidene difluoride membranes (Immobilon P; Millipore, Billerica, MA) by a semi-dry electroblotting system (Dryblot; Bio-Rad Laboratories, Inc., Hercules, CA). After blockage of the membranes in 1% casein in PBS for 2 h at room temperature, they were incubated on a shaker with protein-specific antibodies (i.e., anti-FasL or anti-GAPDH) at 4°C overnight. The protein bands were revealed by autoradiography by using biotinylated goat anti-mouse antibodies and a commercial chemiluminescent kit (Vectastain ABC-AmP; Vector Laboratories, Burlington, Ontario, Canada). Individual bands on the x-ray films were quantified into arbitrary units by densitometry.
Flow cytometry
For immunostaining, 106 cells were pelleted, washed with PBS, and resuspended in residual liquid. PE-conjugated anti-human FasL antibodies were added to the cell pellets after blocking Fc receptors with mouse IgG. The cells were incubated on ice for 45 min with intermittent shaking. After incubation, the stained cells were washed thrice with PBS 0.05% BSA and 0.002% sodium azide, and resuspended in PBS containing 2% paraformaldehyde. The cells were analyzed by flow cytometry using a FACSCalibur (BD Biosciences).
Detection of apoptosis
Apoptotic cells were detected by their ability to stain with FITC-conjugated annexin V and propidium iodide (PI) using a commercial kit (Cell Death Kit; BD Biosciences). Briefly, 105 cells were washed in PBS and resuspended in 100 μL of 1 × annexin V binding buffer. The cells were then stained with 10 μL of FITC-conjugated annexin V and 10 μL of PI for 15 min at room temperature in the dark. The cells were then diluted with 400 μL of 1 × binding buffer as recommended by the manufacturer, and analyzed by flow cytometry using a FACSCalibur (BD Biosciences). In all cases, the stained cells were analyzed within 15 min after the staining. The cell death in human CD4+ T cells, CD8+ T cells, and NK cells co-cultured with HSV-infected macrophages was detected by staining with annexin V/PI and flow cytometry as previously described (16).
Transfection and FasL reporter gene assay
The ability of HSV-1 to activate human FasL gene was investigated by the virus-induced transcriptional activation of a human FasL promoter-reporter gene construct. The construct was made using the plasmid pGL-3 basic (Promega Corp., Madison, WI), in which a 511-bp region upstream the FasL gene start codon was fused with the firefly luciferase gene as previously described (17). The cells were transiently transfected with 5 μg of the construct DNA using DMRIE-C transfection reagent (Invitrogen, Burlington, Ontario, Canada). After transfection, the cells were divided into two halves: one half was infected with HSV-1 and the second half was used for mock infection. The cells were harvested 18 h post-infection and subjected to the luminescence analyses by using a commercial kit (Luciferase Reporter Assay Kit; Pierce).
Confocal microscopy
Vero cells were cultured on cover-slips that were placed in 6-well culture plates and were pre-treated with poly-L-lysine (Invitrogen) for 10 min. After infection with KGFP-gB, the cells were fixed with 4% (wt/vol) paraformaldehyde in PBS for 20 min at room temperature, permeabilized with 0.05% Triton-X100, and stained with DAPI. The cells were washed three times with PBS. THP-1, U937, and primary cells were separately infected with KGFP-gB virus, cultured in the medium in suspension, and stained with DAPI as for the Vero cells. The DAPI-stained cells were mounted on glass slides with Vectashield (Vector Laboratories) and examined with a confocal microscope (LSM 510 META; Zeiss, Oakville, Ontario, Canada).
Statistical analysis
Standard Student's t-test and one-way analysis of variance (ANOVA) were performed using Prism software (GraphPad, San Diego, CA). The differences yielding p values ≤0.05 were deemed statistically significant.
Results
Herpes simplex virus infection induces death of THP-1 cells, but not of U937 cells
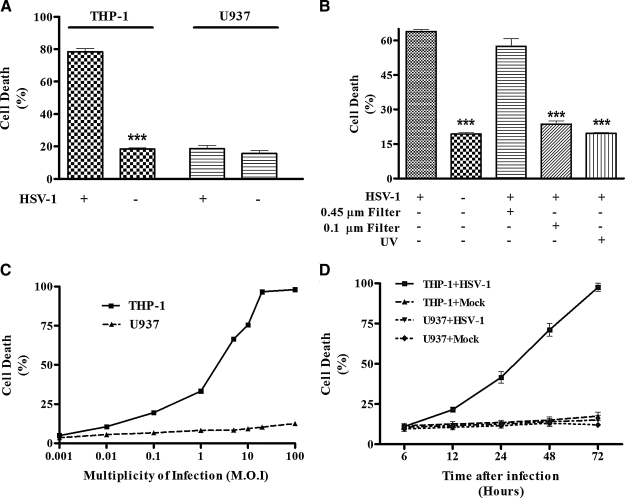
In order to learn about the interactions of HSV-1 with human monocytic cells, we infected THP-1 and U937 cells in vitro with the virus. As shown in Fig. 1A, HSV-1 infection resulted in the death of THP-1 cells, compared to the mock-infected cells (80% versus 18%). Interestingly, HSV infection of U937 cells did not affect their viability significantly. In order to confirm that this effect on cells was due to HSV infection and not due to the presence of any other soluble factor in the viral preparation, we infected THP-1 cells with UV-irradiated HSV-1 or a filtered viral preparation. As shown in Fig. 1B, the infection of THP-1 cells with the UV-irradiated virus abrogated the virus-induced cell death, resulting in a percentage of the dead cells equivalent to that seen in the mock-infected cells. Furthermore, we passed the viral preparation through a 0.1-μm filter, which should have retained the viral particles present in the preparation. As shown in Fig. 1B, the 0.1-μm filter-passed preparation failed to cause death in THP-1 cells compared to a 0.45-μm filter-passed preparation, in which the virus was not retained. These results show that HSV infection affects the two human monocytic cell lines differently; it infects and kills THP-1 but not U937 cells.
FIG. 1.
Infection with HSV induces THP-1 cell death in a dose- and time-dependent manner. (A) THP-1 and U937 cells were infected with HSV (MOI = 5) or mock-infected for 2 h at 37°C. The cells were then washed and put in culture for 24 h. After 24 h, total cells were counted and dead cells were discriminated using trypan blue. (B) Cells were infected as described above with HSV, with the HSV preparation previously passed through 0.45-μM and 0.1-μM filters, or with UV-treated viruses. (C) THP-1 and U937 cells were infected with different concentrations of HSV (MOI) for 24 h. For each condition, total cells were counted and dead cells were discriminated using trypan blue coloration. (D) THP-1 and U937 cells were mock- or HSV-infected with a low MOI (0.1), and the cells were incubated at 37°C for different time periods. For each time point total cells were counted, and the percentage of dead cells was calculated using trypan blue. All the experiments were repeated at least three times (***p < 0.001).
We next investigated if the cell death observed in THP-1 cells was dose- and time-dependent. We infected THP-1 cells with HSV-1 in vitro at different MOI (ranging from 0.001 to 100) for different lengths of time. Our data show that the percentages of dead cells correlate with the quantity of virus used for the infection (Fig. 1C). The numbers of dead cells were not significantly different in U937 cells between infected and mock-infected cells at all MOI. In order to know if the virus-induced cell death was time-dependent, we infected THP-1 cells with a low MOI (0.1) and counted dead cells at different time points (6 to 72 h). The cell death started 12 h post-infection and continued increasing until 72 h post-infection. It is noteworthy that by 24 h post-infection, during which time the virus completes its first round of infection, most of the cell death has already occurred. On the contrary, cell death in U937 cells remained stable during the entire culture period (Fig. 1D).
The HSV-induced cell death in THP-1 cells occurs via apoptosis
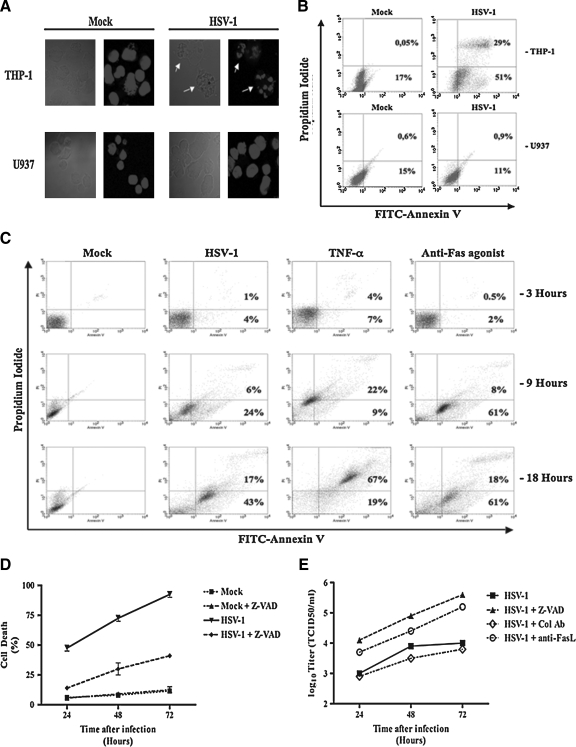
We performed experiments in order to determine whether the cell death in the THP-1 cells was due to apoptosis or necrosis. For this purpose, we stained the cells with DAPI and examined them under a fluorescence microscope. As shown in Fig. 2A, the nuclei of HSV-infected THP-1 cells showed chromatin condensation characteristic of apoptosis. We also stained cells with PI and FITC-conjugated annexin V to discriminate between cell death caused by apoptosis and that caused by necrosis. As shown in Fig. 2B, THP-1 cells infected with the virus, but not U937 cells, became positive for the two markers. It is noteworthy that the cells undergoing apoptosis and necrosis differ from each other temporally with respect to the sequence of expression of these two markers. The cells undergoing necrosis become positive for annexin V and PI simultaneously. However, the cells undergoing apoptosis show a time lag between staining for these two markers. First, they show staining for annexin V, and become PI-positive only several hours later (18). In order to determine whether HSV-infected THP-1 cells undergo sequential (a characteristic of apoptosis) or simultaneous (a characteristic of necrosis) staining with FITC-annexin V and PI, we harvested these cells at different time points (3, 9, and 18 h) after the infection, stained them with the two markers and examined them by flow cytometry. In this experiment, we treated THP-1 cells with anti-Fas-agonistic antibody and used them as positive controls for apoptosis. We also treated these cells separately with TNF-α and used them as positive controls for necrosis, as previously described (18). As shown in Fig. 2C, the virus-infected THP-1 cells followed a pattern of sequential staining with FITC-annexin V and PI, as was seen in the case of cells undergoing apoptosis due to treatment with anti-Fas antibodies. They shifted progressively from the lower left quadrant (PI-negative/FITC-annexin V-negative cells) to the lower right quadrant (PI-negative/FITC-annexin V-positive cells; t = 9 h), and with time to the upper right quadrant (PI-positive/FITC-annexin V-positive cells; t = 18 h). These data show that HSV-1 induced apoptosis of THP-1 cells.
FIG. 2.
HSV-1 infection induces THP-1 cell apoptosis. (A) THP-1 and U937 cells were infected (MOI = 5) and put in culture. After 24 h, the cells were permeabilized and DNA was stained with DAPI. The fluorescence was observed by confocal microscopy (arrows indicate chromatin condensation). (B) THP-1 and U937 cells were mock- and HSV-infected (MOI = 5) for 2 h at 37°C and put in culture at 37°C. After 18 h, the cells were harvested and stained with annexin-V FITC and propidium iodide. (C) THP-1 cells were treated with 100 ng/mL TNF-α (to induce necrosis), and 1 μg/mL of a monoclonal anti-Fas agonist antibody (to induce apoptosis), or infected with HSV-1 (MOI = 5) for 3, 9, and 18 h. At each time point, the cells were harvested and stained with annexin V-FITC and propidium iodide. (D) THP-1 cells were infected with HSV (MOI = 0.1) or mock-infected for 2 h at 37°C. The cells were then washed and put in culture for different time periods, with or without 10 μg/mL of a broad-spectrum caspase inhibitor (Z-VAD). For each time point, the total cells were counted and the dead cells were discriminated using trypan blue. (E) For each time point, cell culture supernatants from HSV infection with or without 10 μg/mL of Z-VAD and anti-FasL neutralizing antibodies were also harvested and titered for their contents in newly-produced HSV particles using a standard TCID50 titration method as described in the materials and methods section. The figure shows data from one representative experiment out of three (Col Ab means control antibody).
Inhibition of HSV-induced apoptosis and its effects on viral replication
Caspases are the main effector molecules that execute apoptosis in human cells. Therefore, we investigated whether a broad-spectrum cell-permeable caspase inhibitor (Z-VAD-fmk) could block HSV-induced cell death in THP-1 cells. As shown in Fig. 2D, addition of the inhibitor reduced virus-induced cell death significantly (p < 0.01) at all tested time points.
Since HSV-induced apoptosis could be inhibited by caspase inhibitors, we sought to determine the effect of this inhibition on viral replication. As shown in Fig. 2E, addition of a broad-spectrum caspase inhibitor enhanced HSV production in THP-1 cells at all time points examined. Furthermore, similarly to the effect of the caspase inhibitor, addition of anti-FasL-antagonistic antibodies also enhanced HSV production in THP-1 cells at all tested time points (Fig. 2E). These results show that preventing apoptosis in HSV-infected THP-1 cells enhances viral replication.
HSV replicates in THP-1 cells with lower efficiency than in Vero cells
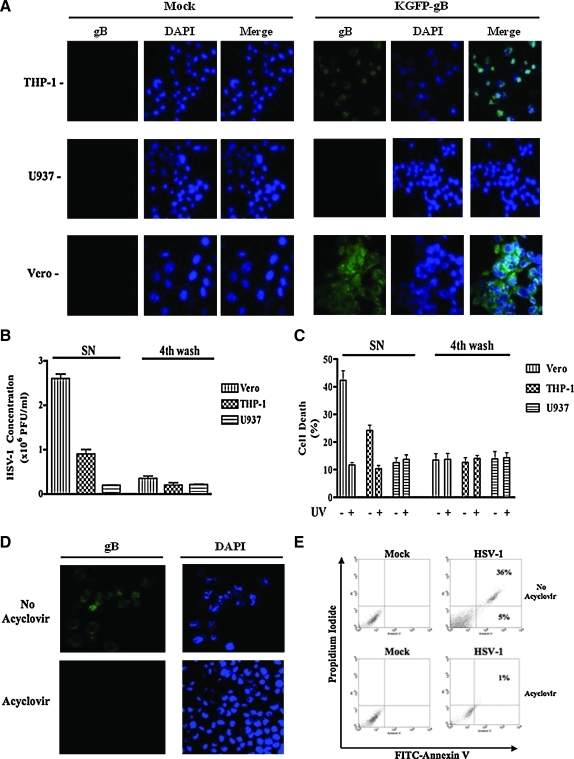
In order to determine if HSV infection was productive and resulted in the release of infectious virions, we infected THP-1, U937, and Vero cells with a recombinant HSV-1, KGFP-gB, as described in the materials and methods section, and analyzed them for the expression of the virus-encoded gB-GFP by confocal microscopy. Vero cells were used as a positive control for the viral replication due to their high permissivity for productive HSV-1 infection. When the infected cells were examined 3 h post-infection, no fluorescence signal was observed in any of the cell types, suggesting that any residual virus from the inoculum could not have given false-positive results (data not shown). When examined 18 h post-infection, green fluorescence was observed in Vero and THP-1 cells, but not in U937 cells (Fig. 3A). The numbers of GFP-positive cells were significantly lower (p < 0.01) in the THP-1 cultures than in the Vero cultures (5.0 ± 4.6% versus 86.0 ± 10.6% positive cells, respectively). Furthermore, the intensity of the signal emitted by the gB-GFP was much lower in the virus-infected THP-1 cells than in the infected Vero cells (Fig. 3A). No fluorescence was observed in U937 cells. These data suggest that HSV-1 replicates less efficiently in THP-1 cells than in Vero cells.
FIG. 3.
HSV replication occurring in THP-1 cells is needed to induce their apoptosis. (A) THP-1, U937, and Vero cells were infected with a fluorescent HSV-1 (KGFP-gB) (MOI = 5) for 2 h at 37°C. The cells were then extensively washed and put in culture for 18 h. After this time, the cells were harvested and permeabilized, and DNA was stained with DAPI. The fluorescence was observed by confocal microscopy. The representative figures from three sets of experiments are shown. (B) THP-1, U937, and Vero cells were infected with HSV-1 (MOI = 5). After 24 h, supernatants (SN) of these cell cultures were harvested and titrated using the standard plaque-forming unit method as described in the materials and methods section. The fourth wash after infection was kept at –20°C and was used as control for the inoculum background. (C) THP-1, U937, and Vero cells were infected with HSV-1 (MOI = 5). After 24 h, supernatants of these cell cultures were harvested, filtered through a 0.45-μM filter, and treated or not with UV light before infection with fresh THP-1 cells for 24 hours. After the infection, all cells were harvested and dead cells were counted using trypan blue. The fourth wash was kept at –20°C and was also used as control for the inoculum background. The results from three different experiments are shown. (D) THP-1 cells were infected as described above with a fluorescent HSV-1 (KGFP-gB) (MOI = 5). The cells were then extensively washed and put in culture for 18 h with or without 100 μM of acyclovir. After this time, the cells were permeabilized and DNA was stained with DAPI. The fluorescence was observed by confocal microscopy. In the same experiment, cells were also harvested, stained with annexin V-FITC and propidium iodide, and examined by flow cytometry (E). Similar results were obtained in three independent experiments. Color images available online at www.liebertonline.com/vim.
We also verified these results by measuring viral titers in the cell culture supernatants obtained from HSV-infected Vero, THP-1, and U937 cells. For this purpose, the cells were infected with the virus for 24 h, and the culture supernatants were titrated by plaque-forming assay as described in the materials and methods section. The cells were washed four times after the infection, and the fourth wash was also titrated to determine if residual HSV particles remained in the cell cultures. As shown in Fig. 3B, THP-1 cells produced three times fewer viral particles than Vero cells (0.9 ± 0.1 × 106 PFU/mL versus 2.6 ± 0.1 × 106 PFU/mL, respectively). These data show that THP-1 cells produce infectious virions, but were less permissive to viral replication than the Vero cells. We also collected cell-free culture supernatants from the three sets of infected cells and determined their ability to cause cell death in THP-1 cells. As shown in Fig. 3C, the culture supernatant-caused cell death correlated with their viral titers.
Viral replication is needed for HSV-induced apoptosis of THP-1 cells
In order to know if the viral replication was needed to induce apoptosis in THP-1 cells, we infected cells with and without adding acyclovir for 24 h. Acyclovir, a guanine analogue, is one of the most commonly used drugs against HSV infections (19). It inhibits replication of the virus by inhibiting the activity of viral DNA polymerase. As shown in Fig. 3D, no fluorescence was seen in THP-1 and Vero cells infected with the KGFP-gB virus when they were treated with 300 μg/mL of acyclovir, compared to untreated cells. These data confirm the efficacy of treatment with acyclovir in abrogating viral replication in THP-1 and Vero cells. We treated HSV-infected THP-1 cells with this drug, stained them with FICT-annexin V and PI, and examined by flow cytometry. The drug inhibited virus-induced cell death (1% versus 41% dead cells; Fig. 3E). These data clearly show that viral replication is needed to induce apoptosis in THP-1 cells.
The virus-induced apoptosis in THP-1 cells is mediated by Fas/FasL interactions
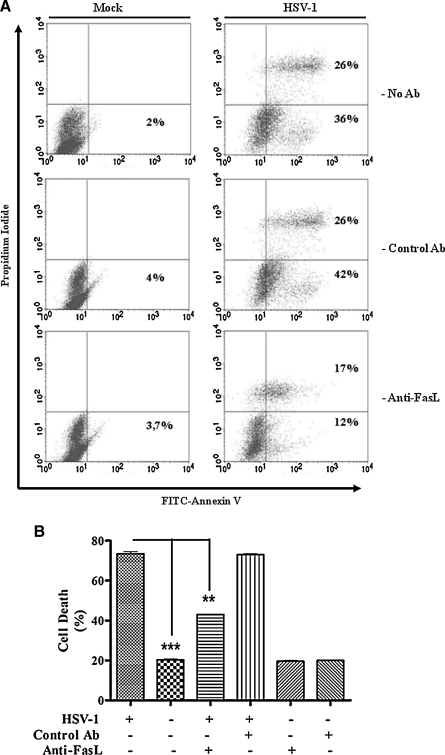
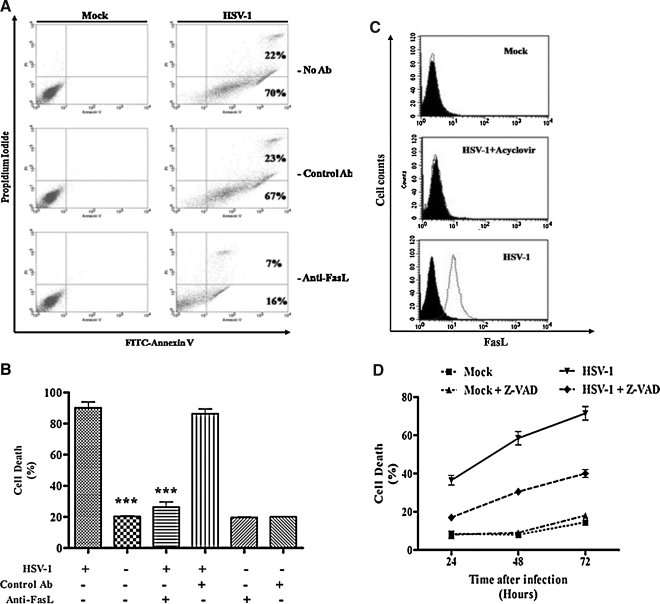
In order to understand the molecular mechanisms involved in the HSV-induced cell death in THP-1 cells, we attempted to block apoptosis by using anti-FasL antibodies that inhibit Fas/FasL interactions. The addition of anti-FasL antibody significantly reduced apoptosis in HSV-1-infected cells compared to isotype-matched control antibodies (Fig. 4A). Only 29% of the infected cells incubated with anti-FasL antibody were positively stained with FITC-annexin-V, compared to 68% of cells treated with the isotype control antibody. The experiment was repeated and dead cells were counted using the trypan blue exclusion assay. As shown in Fig. 4B, the viability of THP-1 cells infected with HSV-1 and treated with 1 μg/mL anti-FasL antibody was increased by almost 30% compared to the isotype-treated cells (43 ± 0.17% versus 73 ± 1%, respectively; p < 0.01). We conclude from these experiments that the Fas/FasL pathway represents one of the mechanisms involved in viral-induced THP-1 apoptosis.
FIG. 4.
The Fas/FasL apoptotic pathway is involved in HSV-1-induced THP-1 apoptosis. (A) THP-1 cells were mock- or HSV-1-infected (MOI = 5) as described above for 18 h at 37°C, with and without 1 μg/mL of a neutralizing monoclonal anti-FasL (Anti-FasL) antibody. Mouse IgG (1 μg/mL; Control Ab) was also used as a control. After 18 h, the cells were stained with annexin V-FITC and propidium iodide and analyzed by flow cytometry. The experiment was repeated three times and the figure shows results from a representative experiment. (B) This graph shows percentages of trypan blue–retaining dead THP-1 cells after 18 h of infection with the virus from three different experiments. (**p < 0.01; ***p < 0.001).
HSV-1 infection induces de novo expression of FasL expression on THP-1 cells
Based on these observations, we investigated whether HSV-1 infection induced FasL expression in THP-1 cells. For this purpose, we infected THP-1 and U937 cells for 18 h, and analyzed FasL expression by flow cytometry. As shown in Fig. 5A, the infection induced FasL expression on the surface of THP-1 cells, but not on U937 cells. Interestingly, no FasL expression was observed after treating HSV-infected THP-1 cells with acyclovir (Fig. 5B), suggesting that viral replication is required to induce the expression of FasL. These results also confirm our above-mentioned observations that the viral replication was needed to induce THP-1 cell apoptosis. We then investigated whether the viral infection was inducing expression of the FasL gene. For this purpose, we determined the effect of the viral infection on the transcription of a reporter gene placed under the control of the FasL promoter as described in the materials and methods section. As shown in Fig. 5C, the FasL promoter activity is significantly increased in HSV-infected cells compared to mock-treated cells (46,057 ± 686 versus 6870 ± 70 relative luciferase activity in arbitrary units, respectively; p < 0.01). Similarly to our flow cytometry data, no FasL promoter activity was observed after treating HSV-infected THP-1 cells with acyclovir. We also compared the levels of FasL expression in HSV- and mock-infected THP-1 cells by Western blots 18 h after infection. As shown in Fig. 5D, HSV-1 infection caused increased expression of FasL in the virus-infected cells. The results also suggest that both THP-1 and U937 constitutively express FasL intracellularly. Since FasL is known to be shed into culture medium by proteolytic cleavage of the surface-expressed FasL (20), we measured soluble (sFasL) using a commercial ELISA kit. In repeated experiments, no significant increase in the concentrations of the sFasL could be detected in the supernatants harvested from THP-1 and U937 cells that were mock- or HSV-infected (data not shown). Taken together, our data suggest that viral infection leads to FasL expression on the surface of THP-1 cells, which is implicated in the apoptosis of these cells. In separate experiments, we compared the expression of Bcl-2 and Bcl-XL between HSV-infected and mock-infected THP-1 cells 18 h post-infection by Western blots, and found very little difference between them (data not shown).
FIG. 5.
HSV-1 infection of THP-1 cells induces FasL expression. (A) THP-1 and U937 cells were infected with HSV-1 (MOI = 5). After 18 h, the cells were harvested and Fc-receptors were blocked with 1 μg of mouse IgG. The cells were then stained using an anti-FasL PE-conjugated monoclonal antibody and analyzed by flow cytometry. (B) THP-1 and U937 cells were infected as described above and treated with or without 100 μM of acyclovir. After 18 h, the cells were harvested and Fc-receptors were blocked with 1 μg of mouse IgG before staining with a PE-conjugated anti-FasL monoclonal antibody. The cells were then analyzed by flow cytometry. Filled and empty histograms in A and B indicate staining with control and anti-FasL antibodies, respectively. (C) THP-1 cells were transfected with a FasL promoter-reporter construct containing the human FasL promoter region (–511 before ATG), fused with the firefly luciferase gene. SV-40-luc (Positive control) and Basic-luc (Negative control) constructs were also transfected. Twelve hours after transfection, the cells were infected with HSV or the mock viral preparation with or without 100 μM of acyclovir. After 18 h, the cells were washed and lysed in lysis buffer, and the luminescence was measured as described in the materials and methods section. (D) Cell lysates from THP-1 and U937 cells that were mock- (lanes 1 and 3) or HSV-infected (lanes 2 and 4) for 18 h were analyzed by Western blotting using anti-FasL monoclonal antibodies. Individual bands were quantified by densitometry, and the ratios between the band densities of the FasL proteins and GAPDH are also indicated in the figure panel below the Western blots. All experiments were repeated at least three times.
HSV infects and induces apoptosis in purified human monocytes via the Fas/FasL pathway
We further wanted to know whether HSV-1 infects and causes apoptosis in purified human monocytes. For this purpose, we isolated monocytes from PBMCs as described in the materials and methods section, and infected them with KGFP-gB and examined them under a fluorescence microscope. The virus underwent replicative cycles as evidenced by the expression of the protein GFP-gB (Supplementary Fig. 1; see online supplementary material at http://www.liebertonline.com). These results conclusively show permissivity of human monocytes to HSV. As shown in Fig. 6A, the viral infection caused apoptosis in these cells, and the neutralizing anti-FasL antibody significantly reduced this apoptosis in HSV-1-infected cells compared to control antibody-treated cells. Indeed, only 23% (7% + 16%) of the infected cells incubated with anti-FasL antibody were positively stained with annexin-V, compared to 92% (22% + 70%) of the infected cells alone, and 90% (23% + 67%) of the cells treated with the isotype control antibody. The experiments were repeated and dead cells were counted using the trypan blue exclusion assay. As shown in Fig. 6B, the viability of monocytes infected with HSV and treated with 1 μg/mL of anti-FasL antibody was significantly increased (21 ± 0.55% versus 86 ± 3% for untreated and treated cells, respectively; p < 0.001). Furthermore, we also determined the expression of FasL on the surface of HSV-1-infected and mock-infected human monocytes. As shown in Fig. 6C, HSV-infected cells expressed FasL on their surface, and the caspase inhibitor significantly (p < 0.01) reduced cell death at 24, 48, and 72 h after infection (Fig. 6D). Taken together, these data show that HSV also induces apoptosis in human monocytes, and the Fas/FasL pathway represents the main mechanism of this apoptosis.
FIG. 6.
HSV infection of freshly isolated human monocytes induces their apoptosis via the Fas/FasL pathway. (A) Isolated human monocytes were mock- or HSV-infected (MOI = 5) with and without the addition of isotype-control and anti-FasL antibodies (1 μg/mL each). After 18 h, the cells were stained with FITC-conjugated annexin V and propidium iodide, and analyzed by flow cytometry. (B) Cell death was also measured by counting dead cells using the trypan blue exclusion assay. The results from three different experiments are shown. (C) Monocytes were infected with HSV and treated with or without 100 μM acyclovir. After 18 h, the cells were harvested and Fc-receptors were blocked with 1 μg of mouse IgG. The cells were then stained using an anti-FasL PE-conjugated monoclonal antibody and analyzed by flow cytometry. The figure shows results from a typical experiment that was repeated three times. (D) Monocytes were infected with HSV at a low MOI (0.1), or mock-infected for 2 h at 37°C. The cells were then washed and put in culture for different time periods with or without 10 μg/mL of a broad-spectrum caspase inhibitor (Z-VAD). The figure shows results from three different experiments. (***p < 0.001). Filled and empty histograms in C indicate staining with a control and anti-FasL antibodies, respectively.
In vitro infection with HSV induces FasL expression in infected cells, but not in bystander cells
We next wanted to know whether HSV-1 induces FasL expression in the virus-infected cells and/or in uninfected bystander cells. For this purpose, we infected THP-1 cells as well as freshly isolated monocytes from two healthy donors with KGFP-gB, and measured expression of FasL by flow cytometry on GFP-positive (infected) cells, and on GFP-negative (uninfected bystander) cells. In these experiments, productively-infected (GFP-positive) THP-1 cells and human monocytes, but not GFP-negative bystander cells, were found to express FasL (Fig. 7). The culture supernatants from these experiments did not differ in their soluble FasL content, when they were tested by a commercial ELISA kit (data not shown).
FIG. 7.
HSV induces FasL expression on the cell surface in infected, but not in uninfected bystander, cells. THP-1 cells and isolated human monocytes from two different healthy donors were infected with a fluorescent HSV-1 (KGFP-gB) at MOI of 1 for 2 h at 37°C. The cells were then extensively washed and cultured at 37°C in the incubator. After 18 h, the cells were incubated on ice with mouse IgG to block Fc receptors, and stained with a PE-conjugated anti-FasL monoclonal antibody, or with an isotype-matched control antibody. The stained cells were analyzed by flow cytometry. The left and right rectangles in the top panel show gated uninfected (GFP-negative) and infected (GFP-positive) cells. For each staining, 10,000 gated cells were analyzed for the expression of FasL. Dark solid-line histograms indicate staining with the PE-conjugated isotype-matched control antibody, and clear dashed-line histograms indicate staining with PE-conjugated anti-FasL antibody. Note the expression of FasL on the virus-infected, but not on uninfected bystander, cells.
HSV infection induces apoptosis in monocyte-derived macrophages
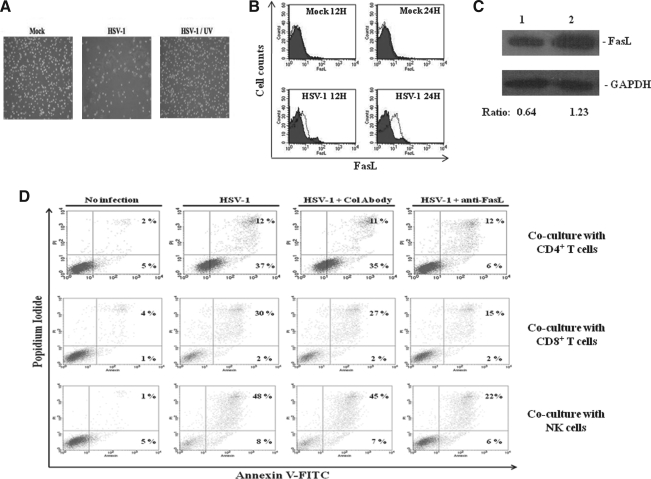
In vivo monocytes differentiate into macrophages, which play an important role in regulating inflammatory and immune responses in body tissues in response to pathogens. In order to investigate whether HSV infects and induces apoptosis in these cells, we generated macrophages from purified human monocytes as described in the materials and methods section. We infected the macrophages with HSV-1 or UV-irradiated HSV-1 for 24 h, and examined them with light microscopy to see if the virus was able to induce cytopathic effects in them. Interestingly, cytopathic effects (rounding, detachment, and cell death) were observed in the cells 24 h after infection with the virus, but not with the mock-infected or UV-inactivated viral preparations (Fig. 8A). In fact, many of the infected cells became detached and were floating in the culture medium, leaving visible empty surfaces in the cell monolayers, whereas the monolayers were intact in mock-inactivated HSV-1-infected cells. These data show that HSV is able to induce cell death in human macrophages. In contrast to monocytes, the addition of the antagonistic anti-FasL antibodies did not reduce cell death in HSV-infected monocyte-derived macrophages (data not shown), which suggests that the virus-induced cell death occurs due to viral replication, and was not due to Fas-FasL interactions. We further wanted to know whether monocyte-derived macrophages were permissive to HSV-1. For this purpose, we infected them with KGFP-gB and examined them under a fluorescence microscope. The virus underwent replicative cycles, as evidenced by the expression of GFP-gB and the shedding of virions into the culture medium, as was seen in the case of isolated human monocytes (Supplementary Fig. 2; see online supplementary material at http://www.liebertonline.com). We stained the macrophages 12 and 24 h post-infection, and determined the expression of FasL on their surface by flow cytometry. The infection induced expression of FasL on the surface of human macrophages (Fig. 8B). Western blots for FasL expression showed that mock-infected macrophages also expressed this molecule, and that HSV infection increased this expression (Fig. 8C). Finally, we determined whether HSV-infected and FasL-expressing human macrophages could induce death of Fas-positive human lymphocytes in co-cultures. For this purpose, we co-cultured purified human CD4+ T cells, CD8+ T cells, and NK cells in separate experiments with HSV or mock-infected macrophages. In separate wells, we also added anti-FasL or control antibodies to these co-cultures. After separating CD4+ T cells, CD8+ T cells and NK cells from these co-cultures, we determined their staining for anexin V and PI. As shown in Fig. 8D, HSV-infected macrophages induced apoptosis of Fas-positive human CD4+ T cells, CD8+ T cells, and NK cells. The death induced by the infected macrophages was inhibited by anti-FasL, but not by control, antibodies.
FIG. 8.
HSV-1 infection of human macrophages induces FasL expression and apoptosis of co-cultured Fas-positive cells. (A) Monocytes were isolated from PBMCs by their adhesion onto plastic dishes, and differentiated into macrophages by incubating them in RPMI 10% FCS, 5% human AB serum, and 2 ng/mL of GM-CSF. After 5 d, monocyte-derived macrophages were mock- or HSV-infected (MOI = 5) for 2 h at 37°C. Infection with UV-treated HSV-1 was performed as a control. After 2 h of infection, the cells were washed and put in culture for 24 h. The cytopathic effect was observed by light microscopy after 24 h of culture. (B) Macrophages were infected with HSV for 12 and 24 h as described above. The cells were then harvested and Fc-receptors were blocked with 1 μg of mouse IgG. The cells were then stained using a PE-conjugated anti-FasL monoclonal antibody and analyzed by flow cytometry. Filled and empty histograms indicate staining with the control and anti-FasL antibodies, respectively. (C) Cell lysates from macrophages mock-infected (lane 1) or HSV-infected (lane 2) for 24 h were analyzed by Western blotting using a monoclonal anti-FasL antibody. Individual bands were quantified by densitometry, and the ratios between the band densities of the FasL and GAPDH proteins are also indicated. (D) Macrophages were mock-infected or HSV-infected (MOI = 5) for 15 h as previously described, washed with PBS to remove unbound dead cells, and fixed with PBS and 2% paraformaldehyde for 30 min. Purified autologous human NK cells, CD4+ T cells, or CD8+ T cells, with or without 1 μg/mL anti-FasL or control antibodies (mouse IgG, Col Abody) were then added to the monolayers. The cells were allowed to settle and remain in contact with the monolayers for 4 h. Thereafter, floating cells were harvested by gentle washing of the monolayers with PBS, stained with annexin V-FITC and propidium iodide, and analyzed by flow cytometry. Percentages of apoptotic cells as determined by annexin V and propidium iodide positivity are shown. Each part of the figure shows results of a typical experiment, each of which was repeated at least three times.
Discussion
We have shown here that HSV-1 productively infects human monocytes and macrophages, as well as a monocytic human cell line THP-1. These conclusions were reached using both a wild-type HSV-1, as well as a recombinant virus in which the coding sequences for green fluorescent protein (GFP) were fused in frame with those of the viral gB (12). It is noteworthy that gB is transcribed as a late (γ1) gene. GFP expression could clearly be seen under a confocal microscope in human monocytes, macrophages, and THP-1 cells. Furthermore, these cell types also produced infectious virions, as shown by the ability of their culture supernatants to infect Vero cells, which are known to be very permissive for replication of HSV-1. Previous studies showed that monocytes were resistant to HSV-1 infection, and only become susceptible to it upon differentiation towards macrophages (21–23). Another study showed that human peritoneal macrophages become partially permissive to viral infection upon treatment with thioglycollate, and are fully permissive after prior infection with Corynebacterium parvum (24). We show here that freshly isolated human monocytes and monocyte-derived macrophages, as well as the monocytic cell line THP-1, are susceptible to infection with the virus, albeit with different degrees of permissiveness. The rate of virus replication is much lower in these cells than in Vero cells. Furthermore, we show here that another human monocytic cell line, U937, is not permissive to HSV-1 replication. These results are in agreement with those of Feng et al. (25), who showed that the virus did not replicate in U937 cells, but was able to induce activation of NF-κB in them. The cell line, however, also becomes permissive to viral replication upon differentiation with phorbol myristate acetate, but not with dimethyl sulfoxide or all-trans retinoic acid (23). The cell line expresses the herpesvirus entry mediator (HVEM), a specific receptor for gD of the virion. The viral glycoprotein gD binds HVEM and activates NF-κB (26). Collectively, these data suggest that freshly isolated human CD14+ monocytes, macrophages, and certain monocytic cell lines can be productively infected with HSV-1.
We show here for the first time that monocytic cells undergo apoptosis upon infection with HSV-1, and that this occurs due to the virus-induced expression of FasL on the surface of these cells. The addition of anti-FasL antibodies reduced virus-induced cell death in our study. Similar effects of the virus were reported earlier on human monocyte-derived macrophages (27,28). The infected macrophages were reported to induce death of interacting CD8+ T cells. The induction of FasL by these cells served as an immune evasion strategy. We extend these observations and show here for the first time that FasL-expressing HSV-infected macrophages also kill human CD4+ T cells and NK cells. In this regard another human virus, the human immunodeficiency virus type 1 (HIV-1), has also been reported to induce FasL expression on infected macrophages (28a).
Given the widespread expression of Fas on the surface of human cells, the HSV-induced expression of FasL on human monocytic cells may induce death of the virus-infected cells via apoptosis, and the infected cells may also kill Fas-positive immune cells (e.g., T cells, neutrophils, and NK cells), and thus evade the antiviral immune response. The first consequence may benefit the host by causing early death of the infected cells, and hence may result in reduced viral production. Indeed our results show that treating HSV-1-infected monocytic cells with a broad-spectrum caspase inhibitor reduces cell death, and increases viral titers in the culture medium. In this respect we have shown that the virus-induced apoptosis is a host beneficial response, as its inhibition with a broad-spectrum caspase inhibitor or with antagonistic anti-FasL antibodies decreases cell death, but increases viral replication. This conclusion is supported by an in-vivo study with HSV-2, a virus that is very closely related to HSV-1, showing that the virus causes more deaths and replicates to higher titers in mice lacking Fas or the FasL gene than in wild-type mice (29). The FasL-positive CD4+ T cells were shown to play a protective role in this study.
We also show here for the first time that HSV-1 enhances FasL expression in monocytic cells at the transcriptional level, as the infection stimulates FasL promoter and increases expression of the human FasL promoter-driven reporter gene. Interestingly, replication of the virus seems to be absolutely essential for the virus-mediated induction of FasL expression and cell death in human monocytic cells. Treating the infected cells with acyclovir, which inhibits viral DNA polymerase and viral DNA replication (19), inhibited FasL expression and prevented cell death. Furthermore, we show here that the virus induces de novo FasL expression on the surface of virus-infected, but not on uninfected bystander, cells.
It is noteworthy that in addition to monocytic cells, HSV-1 has been shown to induce FasL expression in other cell types. For example, the virus was shown to induce FasL expression and fratricide death in activated human CD8+ T lymphocytes (30). The virus induced FasL expression on various cell types in the eye when mice were infected via its anterior chamber. The infection resulted in enhanced apoptosis of various cell types in the eye and brain of the infected mice (31). A relatively recent study has shown that HSV-1 induces expression of FasL on neonatal, but not on adult, neutrophils (32). The study demonstrated hastened death of the virus-infected neonatal neutrophils that could be inhibited with antagonistic anti-Fas or anti-FasL antibodies. Interestingly, HSV-1 does not induce FasL expression in all cell types. For example, the infection induced cell death in immature dendritic cells not by inducing expression of FasL, but rather by causing enhanced proteasomal degradation of the long form of the cellular FILCE (or pro-caspase 8) inhibitory protein (c-FLIP-L) (33,34).
Several early and immediate early proteins of HSV-1 have been shown to induce apoptosis in different human cell types, although their exact mode of action remains unknown. The pro-apoptotic action of these early viral proteins is countered by subsequently-expressed gene products (e.g., α-2, US3, and gJ). The net result is that HSV-1-infected cells become resistant to several exogenous death-inducing stimuli (e.g., osmotic shock, thermal shock, Fas, TNF-α and C2 ceramide; 35–37). Furthermore, a micro RNA encoded by exon 1 of the LAT gene of HSV-1 protects neuroblastoma cells from apoptosis by downregulating TGF-β and SMAD3 expression (38). Interestingly, the resistance of HSV-1-infected cells to the death-inducing stimuli depends upon the cell type. Furthermore, the pro-apoptotic and anti-apoptotic effects of different viral proteins also varied with the cell type. It appears that these viral proteins exert their effects by interacting with different cellular factors. The differential expression of these factors in different human cells may be responsible for the differential effects of the viral proteins with respect to their effect on cell death (reviewed in 39).
We have shown here that HSV-1 replicates in human monocyte-derived macrophages (MDM), and induces de novo FasL expression on their surface. The virus-infected cells become rounded and detach from monolayers and die. The cell death in these cells could not be prevented by the addition of anti-FasL antibodies (unpublished data), despite the fact that these cells express FasL. The virus-infected cells retain their sensitivity to death by anti-Fas agonistic antibodies (unpublished data). The lack of Fas-FasL-mediated cell death in these cells may be due to the fact that they grow in monolayers and do not interact with each other via Fas-FasL. The inability of anti-FasL antibodies to reduce their death suggests that their ultimate death may be due to virus-induced changes occurring intrinsically, as happens in epithelial cells (35). We have demonstrated in this study that the virus-infected FasL-expressing macrophages are able to induce apoptosis in Fas-positive cells that interact with them. Thus the virus-induced expression of FasL may be more important for the infected cells in evading natural and virus-specific adaptive immunity of the host.
It is noteworthy that because of its strong capacity to kill Fas-positive cells, FasL is stored in secretory lysosomes in hematopoietic cells, and is translocated to the cell surface upon activation of the cell, or upon its interaction with a target cell (reviewed in 39). In line with this paradigm, our Western blot and flow cytometry results showed that THP-1 cells constitutively express FasL intracellularly, but not on the cell surface. The HSV-mediated de novo expression of FasL on the cell surface shows that, in addition to increasing FasL expression at the transcriptional level, the virus is also able to translocate intracellular FasL to the cell surface. Further studies are required to understand the mechanism behind the virus-induced translocation of FasL to the cell surface.
Overall, our study provides novel insights into HSV-induced apoptosis in human monocytic cells and its impact on viral replication and antiviral immunity.
Supplementary Material
Acknowledgments
We thank Dr. Jonathan Ashwell of the Immune Cell Biology Laboratory at the National Cancer Institute/National Institutes of Health, Bethesda, Maryland, for providing us with the plasmid –511FasLpGL-3. We also thank the Canadian Institutes for Health Research (CIHR) for their support, and the Fonds de recherche en santé du Québec (FRSQ) for a doctoral research award to A.I.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Corey L. Spear PG. Infections with herpes simplex viruses (2) N Engl J Med. 1986;314:749–757. doi: 10.1056/NEJM198603203141205. [DOI] [PubMed] [Google Scholar]
- 2.Corey L. Spear PG. Infections with herpes simplex viruses (1) N Engl J Med. 1986;314:686–691. doi: 10.1056/NEJM198603133141105. [DOI] [PubMed] [Google Scholar]
- 3.Whitley R. Field's Virology. 4th. Lippincott-Raven Publishers; Philadelphia: 2006. Herpes simplex viruses. [Google Scholar]
- 4.Posavad CM. Koelle DM. Corey L. Tipping the scales of herpes simplex virus reactivation: the important responses are local. Nat Med. 1998;4:381–382. doi: 10.1038/nm0498-381. [DOI] [PubMed] [Google Scholar]
- 5.Levin MJ. Bacon TH. Leary JJ. Resistance of herpes simplex virus infections to nucleoside analogues in HIV-infected patients. Clin Infect Dis. 2004;39(Suppl 5):S248–S257. doi: 10.1086/422364. [DOI] [PubMed] [Google Scholar]
- 6.Cermelli C. Orsi CF. Ardizzoni A. Lugli E. Cenacchi V. Cossarizza A. Blasi E. Herpes simplex virus type 1 dysregulates anti-fungal defenses preventing monocyte activation and downregulating toll-like receptor-2. Microbiol Immunol. 2008;52:575–584. doi: 10.1111/j.1348-0421.2008.00074.x. [DOI] [PubMed] [Google Scholar]
- 7.Cermelli C. Orsi CF. Cuoghi A, et al. Gene expression profiling of monocytes displaying herpes simplex virus 1 induced dysregulation of antifungal defences. J Med Microbiol. 2009;58:1283–1290. doi: 10.1099/jmm.0.011023-0. [DOI] [PubMed] [Google Scholar]
- 8.Mastino A. Sciortino MT. Medici MA, et al. Herpes simplex virus 2 causes apoptotic infection in monocytoid cells. Cell Death Differ. 1997;4:629–638. doi: 10.1038/sj.cdd.4400289. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad A. Sharif-Askari E. Fawaz L. Menezes J. Innate immune response of the human host to exposure with herpes simplex virus type 1: In vitro control of the virus infection by enhanced natural killer activity via interleukin-15 induction. J Virol. 2000;74:7193–7203. doi: 10.1128/jvi.74.16.7196-7203.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gosselin J. Flamand L. D'Addario M. Hiscott J. Menezes J. Infection of peripheral blood mononuclear cells by herpes simplex and Epstein-Barr viruses. Differential induction of interleukin 6 and tumor necrosis factor-alpha. J Clin Invest. 1992;89:1849–1856. doi: 10.1172/JCI115789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gosselin J. Flamand L. D'Addario M, et al. Modulatory effects of Epstein-Barr, herpes simplex, and human herpes-6 viral infections and coinfections on cytokine synthesis. A comparative study. J Immunol. 1992;149:181–187. [PubMed] [Google Scholar]
- 12.Potel C. Kaelin K. Gautier I. Lebon P. Coppey J. Rozenberg F. Incorporation of green fluorescent protein into the essential envelope glycoprotein B of herpes simplex virus type 1. J Virol Methods. 2002;105:13–23. doi: 10.1016/s0166-0934(02)00027-7. [DOI] [PubMed] [Google Scholar]
- 13.Menezes J. Bourkas AE. Herpesvirus-lymphoid cell interactions: comparative studies on the biology of herpes simplex virus-induced Fc receptors in B, T, and “null” lymphoid cell lines. J Virol. 1980;33:115–122. doi: 10.1128/jvi.33.1.115-122.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gómez-Segura J. Caballero S. Moreno V. Prieto MJ. Bosch A. Palladium(II) binding to N(7) of acyclovir: DNA interaction and herpes simplex virus (HSV-1) inhibitory activity. J Inorg Biochem. 2009;103:128–134. doi: 10.1016/j.jinorgbio.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad R. Knafo L. Xu J. Sindhu S. Menezes J. Ahmad A. Thrombin induces apoptosis in human tumor cells. Intern J Cancer. 2000;87:707–715. [PubMed] [Google Scholar]
- 16.Ahmad R. Menezes J. Knafo L. Ahmad A. Activated human platelets express Fas-L and induce apoptosis in Fas-positive tumor cells. J Leukoc Biol. 2001;69:123–128. [PubMed] [Google Scholar]
- 17.Mittelstadt PR. Ashwell JD. Cyclosporin A-sensitive transcription factor Egr-3 regulates Fas ligand expression. Mol Cell Biol. 1998;18:3744–3751. doi: 10.1128/mcb.18.7.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krysko DV. Vanden Berghe T. D'Herde K. Vandenabeele P. Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods. 2008;44:205–221. doi: 10.1016/j.ymeth.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Pagano JS. Datta AK. Perspectives on interactions of acyclovir with Epstein-Barr and other herpes viruses. Am J Med. 1982;73:18–26. doi: 10.1016/0002-9343(82)90057-2. [DOI] [PubMed] [Google Scholar]
- 20.Voss M. Lettau M. Paulsen M. Janssen O. Posttranslational regulation of Fas ligand function. Cell Commun Signal. 2008;6:11. doi: 10.1186/1478-811X-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruun T. Kristoffersen AK. Rollag H. Beck S. Degre M. Herpes simplex virus type 1 inhibits in vitro differentiation and selected functions of human blood-derived monocytes. APMIS. 1998;106:1194–1203. [PubMed] [Google Scholar]
- 22.Bruun T. Kristoffersen AK. Rollag H. Degre M. Interaction of herpes simplex virus with mononuclear phagocytes is dependent on the differentiation stage of the cells. APMIS. 1998;106:305–314. doi: 10.1111/j.1699-0463.1998.tb01351.x. [DOI] [PubMed] [Google Scholar]
- 23.Stewart DR. Anaraki F. Leary K. Analysis of the basis for persistence of herpes simplex virus type 1 in undifferentiated U937 cells. Viral Immunol. 1992;5:173–184. doi: 10.1089/vim.1992.5.173. [DOI] [PubMed] [Google Scholar]
- 24.Sit MF. Tenney DJ. Rothstein JL. Morahan PS. Effect of macrophage activation on resistance of mouse peritoneal macrophages to infection with herpes simplex virus types 1 and 2. J Gen Virol. 1988;69(Pt 8):1999–2010. doi: 10.1099/0022-1317-69-8-1999. [DOI] [PubMed] [Google Scholar]
- 25.Feng CP. Kulka M. Aurelian L. NF-kappa B-binding proteins induced by HSV-1 infection of U937 cells are not involved in activation of human immunodeficiency virus. Virology. 1993;192:491–500. doi: 10.1006/viro.1993.1065. [DOI] [PubMed] [Google Scholar]
- 26.Sciortino MT. Medici MA. Marino-Merlo F, et al. Involvement of gD/HVEM interaction in NF-kB-dependent inhibition of apoptosis by HSV-1 gD. Biochem Pharmacol. 2008;76:1522–1532. doi: 10.1016/j.bcp.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 27.Badley AD. McElhinny JA. Leibson PJ. Lynch DH. Alderson MR. Paya CV. Upregulation of Fas ligand expression by human immunodeficiency virus in human macrophages mediates apoptosis of uninfected T lymphocytes. J Virol. 1996;70:199–206. doi: 10.1128/jvi.70.1.199-206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoves S. Niller HH. Krause SW, et al. Decreased T cell stimulatory capacity of monocyte-derived human macrophages following herpes simplex virus type 1 infection. Scand J Immunol. 2001;54:93–99. doi: 10.1046/j.1365-3083.2001.00956.x. [DOI] [PubMed] [Google Scholar]
- 28a.Dockrell DH. Badley AD. Villacian JS, et al. The expression of Fas Ligand by macrophages and its upregulation by Human Immunodeficiency Virus infection. J Clin Invest. 1998;101:2394–2405. doi: 10.1172/JCI1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishikawa T. Yamada H. Oyamada A. Goshima F. Nishiyama Y. Yoshikai Y. Protective role of Fas-FasL signaling in lethal infection with herpes simplex virus type 2 in mice. J Virol. 2009;83:11777–11783. doi: 10.1128/JVI.01006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raftery MJ. Behrens CK. Muller A. Krammer PH. Walczak H. Schonrich G. Herpes simplex virus type 1 infection of activated cytotoxic T cells: Induction of fratricide as a mechanism of viral immune evasion. J Exp Med. 1999;190:1103–1114. doi: 10.1084/jem.190.8.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian H. Atherton S. Apoptosis and increased expression of Fas ligand after uniocular anterior chamber (AC) inoculation of HSV-1. Curr Eye Res. 2003;26:195–203. doi: 10.1076/ceyr.26.3.195.14897. [DOI] [PubMed] [Google Scholar]
- 32.Ennaciri J. Menezes J. Proulx F. Toledano BJ. Induction of apoptosis by herpes simplex virus-1 in neonatal, but not adult, neutrophils. Pediatr Res. 2006;59:7–12. doi: 10.1203/01.pdr.0000191816.57544.b4. [DOI] [PubMed] [Google Scholar]
- 33.Kather A. Raftery MJ. Devi-Rao G. Lippmann J. Giese T. Sandri-Goldin RM. Schonrich G. Herpes simplex virus type 1 (HSV-1)-induced apoptosis in human dendritic cells as a result of downregulation of cellular FLICE-inhibitory protein and reduced expression of HSV-1 antiapoptotic latency-associated transcript sequences. J Virol. 2010;84:1034–1046. doi: 10.1128/JVI.01409-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller DB. Raftery MJ. Kather A. Giese T. Schonrich G. Frontline: Induction of apoptosis and modulation of c-FLIPL and p53 in immature dendritic cells infected with herpes simplex virus. Eur J Immunol. 2004;34:941–951. doi: 10.1002/eji.200324509. [DOI] [PubMed] [Google Scholar]
- 35.Galvan V. Roizman B. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc Natl Acad Sci USA. 1998;95:3931–3936. doi: 10.1073/pnas.95.7.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodkin ML. Morton ER. Blaho JA. Herpes simplex virus infection and apoptosis. Int Rev Immunol. 2004;23:141–172. doi: 10.1080/08830180490265574. [DOI] [PubMed] [Google Scholar]
- 37.Sanfilippo CM. Chirimuuta FN. Blaho JA. Herpes simplex virus type 1 immediate-early gene expression is required for the induction of apoptosis in human epithelial HEp-2 cells. J Virol. 2004;78:224–239. doi: 10.1128/JVI.78.1.224-239.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta A. Gartner JJ. Sethupathy P. Hatzigeorgiou AG. Fraser NW. Anti-apoptotic function of a microRNA encoded by the HSV-1 latency-associated transcript. Nature. 2006;442:82–85. doi: 10.1038/nature04836. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen ML. Blaho JA. Apoptosis during herpes simplex virus infection. Adv Virus Res. 2007;69:67–97. doi: 10.1016/S0065-3527(06)69002-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.