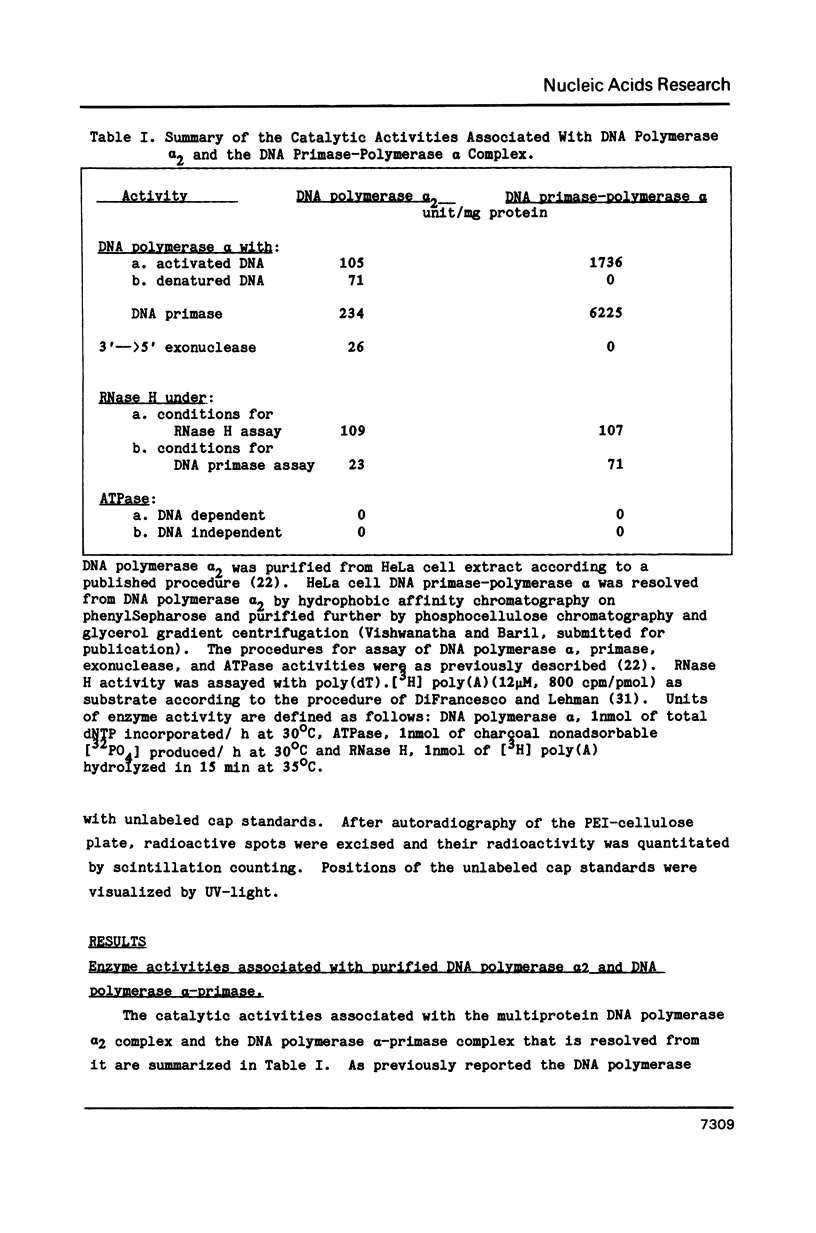
Abstract
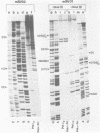
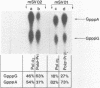
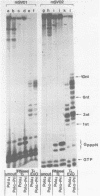
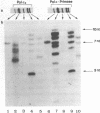
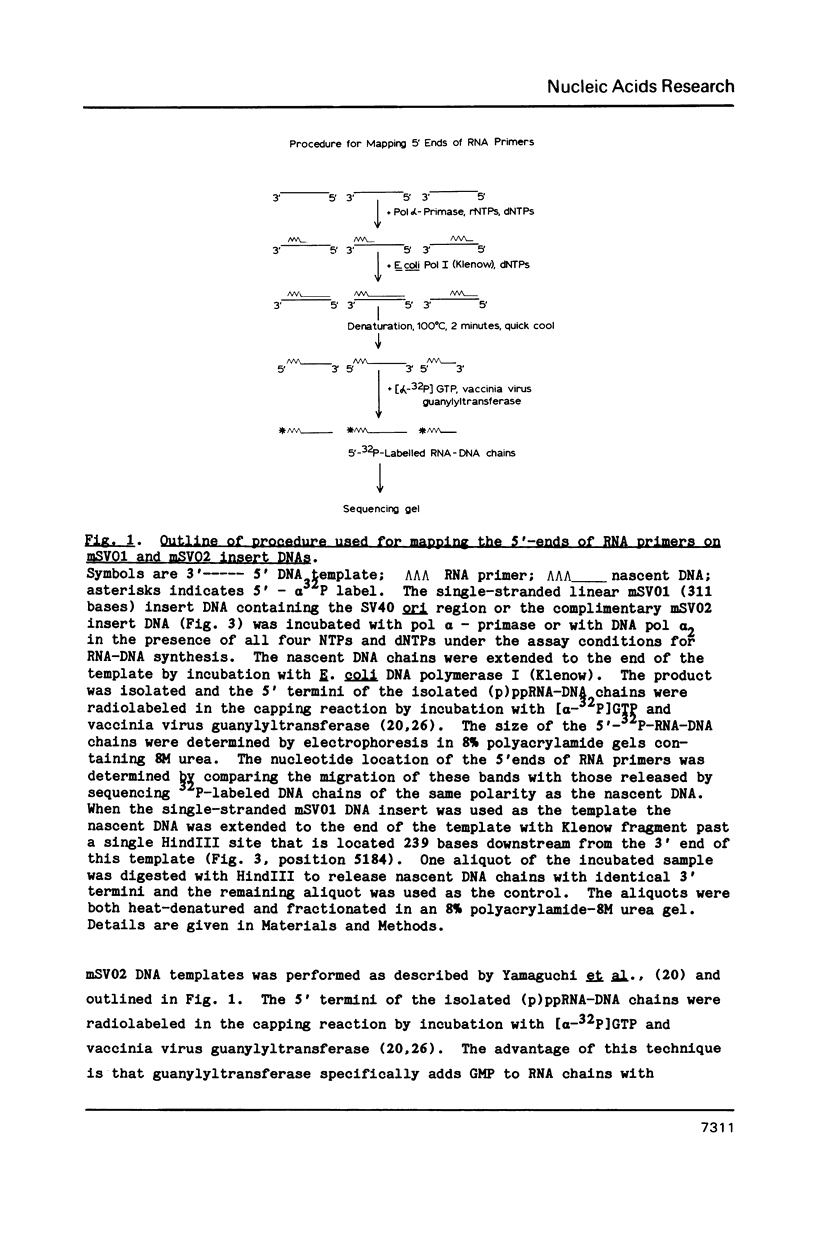
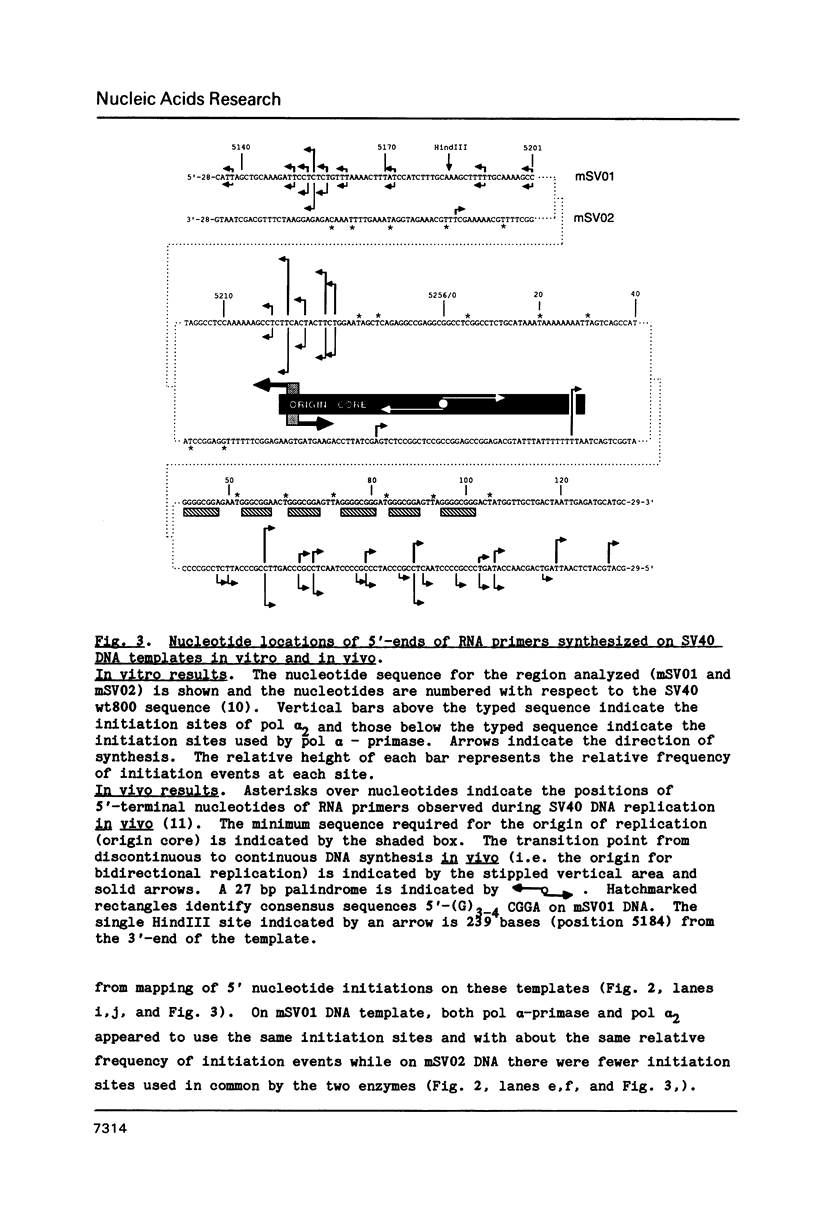
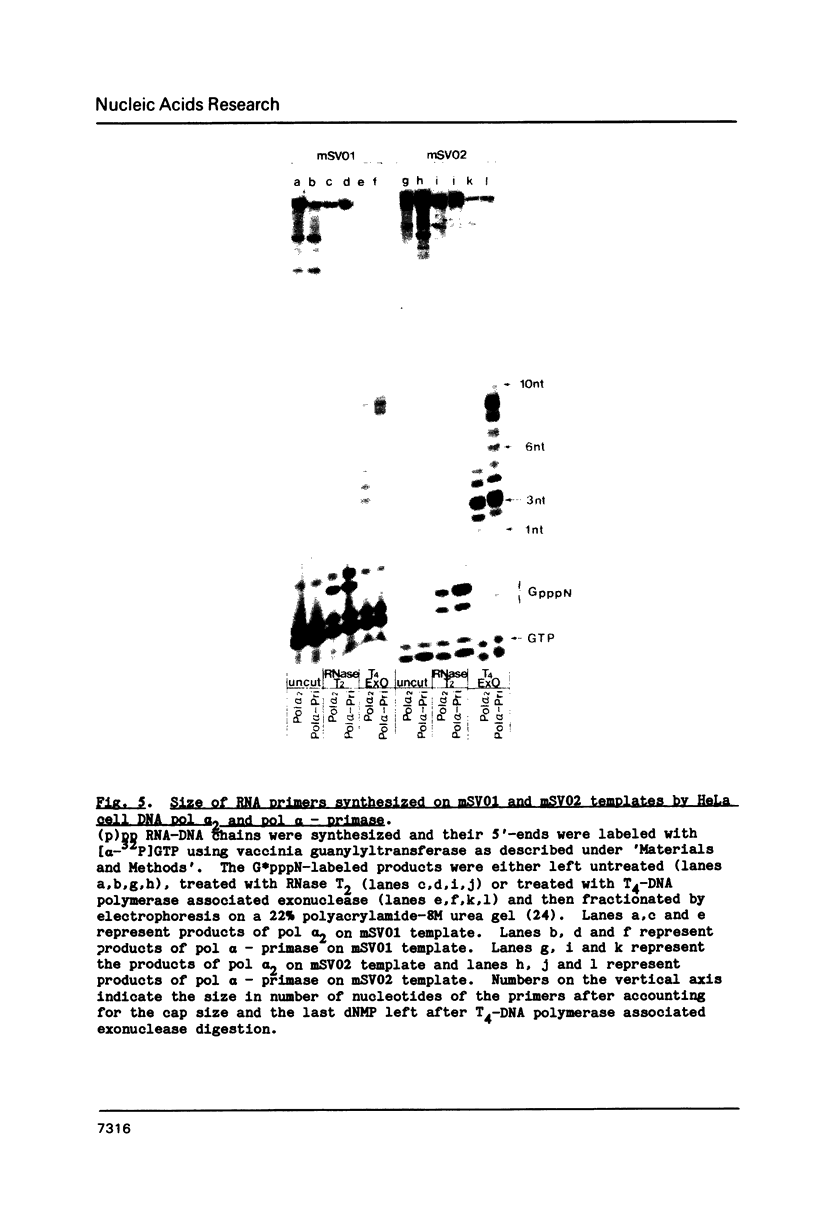
Synthesis of (p)ppRNA-DNA chains by purified HeLa cell DNA primase-DNA polymerase alpha (pol alpha-primase) was compared with those synthesized by a multiprotein form of DNA polymerase alpha (pol alpha 2) using unique single-stranded DNA templates containing the origin of replication for simian virus 40 (SV40) DNA. The nucleotide locations of 33 initiation sites were identified by mapping G*pppN-RNA-DNA chains and identifying their 5'-terminal ribonucleotide. Pol alpha 2 strongly preferred initiation sites that began with ATP rather than GTP, thus frequently preferring different initiation sites than pol alpha-primase, depending on the template examined. The initiation sites selected in vitro, however, did not correspond to the sites used during SV40 DNA replication in vivo. Pol alpha 2 had the greatest effect on RNA primer size, typically synthesizing primers 1-5 nucleotides long, while pol alpha-primase synthesized primers 6-8 nucleotides long. These differences were observed even at individual initiation sites. Thus, the multiprotein form of DNA primase-DNA polymerase alpha affects selection of initiation sites, the frequency at which the sites are chosen, and length of RNA primers.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Kaufman G., DePamphilis M. L. RNA primers in SV40 DNA replication: identification of transient RNA-DNA covalent linkages in replicating DNA. Biochemistry. 1977 Nov 15;16(23):4990–4998. doi: 10.1021/bi00642a009. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Plevani P., Bollum F. J. Proteolytic degradation of calf thymus terminal deoxynucleotidyl transferase. J Biol Chem. 1982 May 25;257(10):5700–5706. [PubMed] [Google Scholar]
- Chang L. M., Rafter E., Augl C., Bollum F. J. Purification of a DNA polymerase-DNA primase complex from calf thymus glands. J Biol Chem. 1984 Dec 10;259(23):14679–14687. [PubMed] [Google Scholar]
- Conaway R. C., Lehman I. R. A DNA primase activity associated with DNA polymerase alpha from Drosophila melanogaster embryos. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2523–2527. doi: 10.1073/pnas.79.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L., Wassarman P. M. Replication of eukaryotic chromosomes: a close-up of the replication fork. Annu Rev Biochem. 1980;49:627–666. doi: 10.1146/annurev.bi.49.070180.003211. [DOI] [PubMed] [Google Scholar]
- DiFrancesco R. A., Lehman I. R. Interaction of ribonuclease H from Drosophila melanogaster embryos with DNA polymerase-primase. J Biol Chem. 1985 Nov 25;260(27):14764–14770. [PubMed] [Google Scholar]
- Eliasson R., Reichard P. Primase initiates Okazaki pieces during polyoma DNA synthesis. Nature. 1978 Mar 9;272(5649):184–185. doi: 10.1038/272184a0. [DOI] [PubMed] [Google Scholar]
- Faust E. A., Nagy R., Davey S. K. Mouse DNA polymerase alpha-primase terminates and reinitiates DNA synthesis 2-14 nucleotides upstream of C2A1-2(C2-3/T2) sequences on a minute virus of mice DNA template. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4023–4027. doi: 10.1073/pnas.82.12.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronostajski R. M., Field J., Hurwitz J. Purification of a primase activity associated with DNA polymerase alpha from HeLa cells. J Biol Chem. 1984 Aug 10;259(15):9479–9486. [PubMed] [Google Scholar]
- Hay R. T., DePamphilis M. L. Initiation of SV40 DNA replication in vivo: location and structure of 5' ends of DNA synthesized in the ori region. Cell. 1982 Apr;28(4):767–779. doi: 10.1016/0092-8674(82)90056-3. [DOI] [PubMed] [Google Scholar]
- Hay R. T., Hendrickson E. A., DePamphilis M. L. Sequence specificity for the initiation of RNA-primed simian virus 40 DNA synthesis in vivo. J Mol Biol. 1984 May 15;175(2):131–157. doi: 10.1016/0022-2836(84)90471-6. [DOI] [PubMed] [Google Scholar]
- Kaufmann G., Anderson S., DePamphilis M. L. RNA primers in Simian virus 40 DNA replication. II. Distribution of 5' terminal oligoribonucleotides in nascent DNA. J Mol Biol. 1977 Nov 5;116(3):549–567. doi: 10.1016/0022-2836(77)90083-3. [DOI] [PubMed] [Google Scholar]
- Lamothe P., Baril B., Chi A., Lee L., Baril E. Accessory proteins for DNA polymerase alpha activity with single-strand DNA templates. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4723–4727. doi: 10.1073/pnas.78.8.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson G., Pigiet V., Winnacker E. L., Abrams R., Reichard P. RNA-linked short DNA fragments during polyoma replication. Proc Natl Acad Sci U S A. 1973 Feb;70(2):412–415. doi: 10.1073/pnas.70.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Pigiet V., Eliasson R., Reichard P. Replication of polyoma DNA in isolated nuclei. 3. The nucleotide sequence at the RNA-DNA junction of nascent strands. J Mol Biol. 1974 Mar 25;84(1):197–216. doi: 10.1016/0022-2836(74)90222-8. [DOI] [PubMed] [Google Scholar]
- Pritchard C. G., DePamphilis M. L. Preparation of DNA polymerase alpha X C1C2 by reconstituting DNA polymerase alpha with its specific stimulatory cofactors, C1C2. J Biol Chem. 1983 Aug 25;258(16):9801–9809. [PubMed] [Google Scholar]
- Pritchard C. G., Weaver D. T., Baril E. F., DePamphilis M. L. DNA polymerase alpha cofactors C1C2 function as primer recognition proteins. J Biol Chem. 1983 Aug 25;258(16):9810–9819. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Tseng B. Y., Ahlem C. N. DNA primase activity from human lymphocytes. Synthesis of oligoribonucleotides that prime DNA synthesis. J Biol Chem. 1982 Jul 10;257(13):7280–7283. [PubMed] [Google Scholar]
- Tseng B. Y., Ahlem C. N. Mouse primase initiation sites in the origin region of simian virus 40. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2342–2346. doi: 10.1073/pnas.81.8.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng B. Y., Goulian M. DNA synthesis in human lymphocyts: intermediates in DNA synthesis, in vitro and in vivo. J Mol Biol. 1975 Dec 5;99(2):317–337. doi: 10.1016/s0022-2836(75)80149-5. [DOI] [PubMed] [Google Scholar]
- Vishwanatha J. K., Coughlin S. A., Wesolowski-Owen M., Baril E. F. A multiprotein form of DNA polymerase alpha from HeLa cells. Resolution of its associated catalytic activities. J Biol Chem. 1986 May 15;261(14):6619–6628. [PubMed] [Google Scholar]
- Wang T. S., Hu S. Z., Korn D. DNA primase from KB cells. Characterization of a primase activity tightly associated with immunoaffinity-purified DNA polymerase-alpha. J Biol Chem. 1984 Feb 10;259(3):1854–1865. [PubMed] [Google Scholar]
- Weaver D. T., DePamphilis M. L. The role of palindromic and non-palindromic sequences in arresting DNA synthesis in vitro and in vivo. J Mol Biol. 1984 Dec 25;180(4):961–986. doi: 10.1016/0022-2836(84)90266-3. [DOI] [PubMed] [Google Scholar]
- Yagura T., Kozu T., Seno T. Mouse DNA polymerase accompanied by a novel RNA polymerase activity: purification and partial characterization. J Biochem. 1982 Feb;91(2):607–618. doi: 10.1093/oxfordjournals.jbchem.a133732. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Hendrickson E. A., DePamphilis M. L. DNA primase-DNA polymerase alpha from simian cells. Modulation of RNA primer synthesis by ribonucleoside triphosphates. J Biol Chem. 1985 May 25;260(10):6254–6263. [PubMed] [Google Scholar]
- Yamaguchi M., Hendrickson E. A., DePamphilis M. L. DNA primase-DNA polymerase alpha from simian cells: sequence specificity of initiation sites on simian virus 40 DNA. Mol Cell Biol. 1985 May;5(5):1170–1183. doi: 10.1128/mcb.5.5.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoda K., Okazaki T. Primer RNA for DNA synthesis on single-stranded DNA template in a cell free system from Drosophila melanogaster embryos. Nucleic Acids Res. 1983 Jun 11;11(11):3433–3450. doi: 10.1093/nar/11.11.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]