Abstract
To search for effective cancer vaccines based on sTn, a sialylated tumor-associated carbohydrate antigen (sialo-TACA) expressed by a number of tumors, four unnatural N-acyl sTn derivatives, including 5′-N-p-methylphenylacetyl sTn (sTnNMePhAc), 5′-N-p-methoxylphenylacetyl sTn (sTnNMeOPhAc), 5′-N-p-acetylphenylacetyl sTn (sTnNAcPhAc), and 5′-N-p-chlorophenylacetyl sTn (sTnNClPhAc), as well as their protein conjugates, were synthesized by a highly convergent procedure. The immunological properties of these sTn derivatives in the form of keyhole limpet hemocyanin conjugate were evaluated in mice and compared to that of sTnNPhAc, a sTn derivative previously investigated as a vaccine candidate. It was shown that sTnNMePhAc, sTnNMeOPhAc, sTnNAcPhAc, and sTnNClPhAc are all much more immunogenic than sTnNPhAc and that they provoked strong T cell-dependent IgG1 immune responses useful for cancer immunotherapy. It was concluded that sTnNClPhAc is a promising candidate for cancer vaccine development and is worthy of further investigation.
Keywords: Cancer vaccine, immunology, tumor-associated carbohydrate antigen, sTn antigen, sTn derivative, glycoconjugate
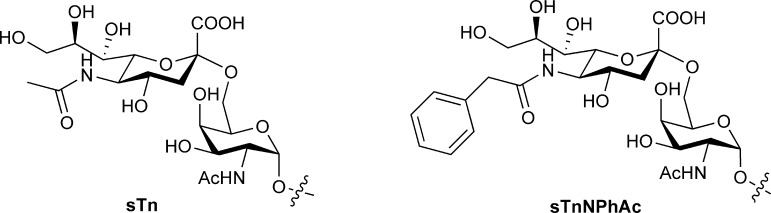
It is well established that tumor cells express some distinctive glycans, termed tumor-associated carbohydrate antigens (TACAs), of which many are sialooligosaccharides that carry N-acetyl sialic acid (NeuNAc) at the glycan nonreducing end.1−3 Sialo-TACAs are useful molecular templates in the development of cancer vaccines or cancer immunotherapies.4−6 For example, sialyl Tn (sTn, Figure 1), a sialo-disaccharide TACA abundantly expressed by a number of tumors such as breast, prostate, colorectal, ovarian, pancreatic, and gastric cancer, is a hot target.7−12 STn is relatively tumor-specific, and it is rarely observed in normal tissues.13,14 Moreover, tumor cell expression of sTn is usually associated with poor prognosis8−10,15,16 and is also a sign of malignant and metastatic progression.17,18 Consequently, an effective vaccine based on sTn can be very useful for the treatment of a variety of metastatic tumors. However, like most TACAs, sTn is poorly immunogenic and cannot provoke T cell-mediated immune response that is necessary for cancer immunotherapy.19 Attempts to overcome this problem by covalent linkage of sTn to carrier proteins, such as keyhole limpet hemocyanin (KLH), have achieved limited success. For instance, although sTn-KLH conjugate (Theratope) has been proved to be quite immunogenic and thereby evaluated as a therapeutic vaccine for metastatic breast cancer,17,20 unfortunately, it mainly elicited humoral immune responses in cancer patients and eventually failed at the phase III clinical trial.17
Figure 1.
Structures of sTn and sTnNPhAc antigens.
To deal with the poor immunogenicity problem of TACAs, we have recently developed a new strategy for cancer immunotherapy, which combines vaccination using vaccines made of chemically modified TACAs with metabolic engineering of TACAs on the cancer cell surface.21−25 We have demonstrated that unnaturally modified TACA derivatives are much more immunogenic than their natural counterparts.22−24 In particular, our immunological studies have shown that N-phenylacetyl sTn (sTnNPhAc, Figure 1) was the most immunogenic among a number of N-acyl sTn derivatives investigated thus far and that sTnNPhAc elicited robust T cell-dependent immune responses.23,24 Therefore, sTnNPhAc is a promising vaccine candidate.
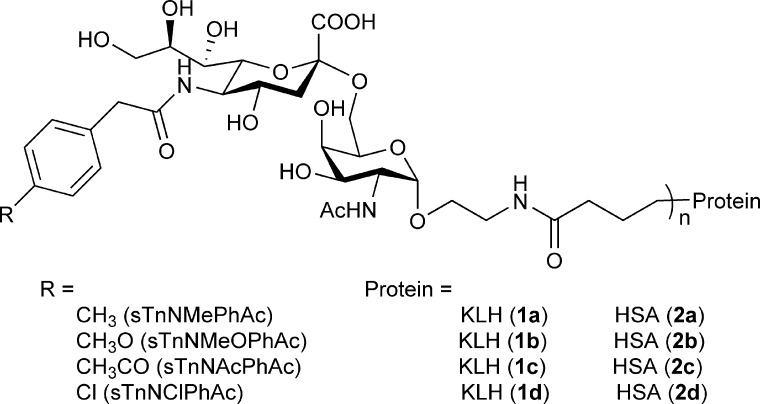
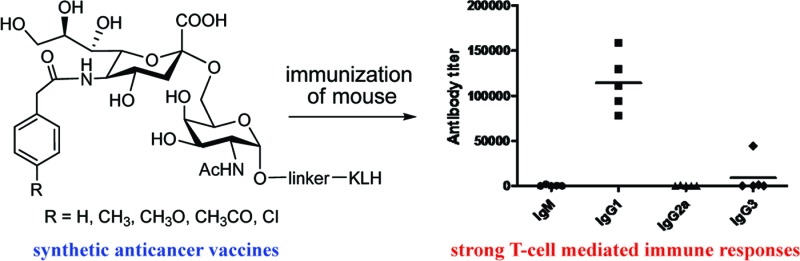
Encouraged by the results of sTnNPhAc, we aimed to evaluate in this research the influence of further chemical modifications of the phenyl ring of sTnNPhAc on the immunological properties of resultant sTn derivatives, so as to characterize the structure−reactivity relationship and identify more potent vaccine candidates. In this context, we designed and synthesized several 5′-N-(p-substituted phenyl)acetyl derivatives of sTn antigen, including 5′-N-p-methylphenylacetyl sTn (sTnNMePhAc), 5′-N-p-methoxylphenylacetyl sTn (sTnNMeOPhAc), 5′-N-p-acetylphenylacetyl sTn (sTnNAcPhAc), and 5′-N-p-chlorophenylacetyl sTn (sTnNClPhAc) (Figure 2), which contain electron-donating and electron-withdrawing substituents, respectively, coupled these derivatives to KLH and evaluated the immunological properties of the resultant glycoconjugates 1a−d (Figure 2).
Figure 2.
Structures of synthesized sTn derivatives and their protein glycoconjugates.
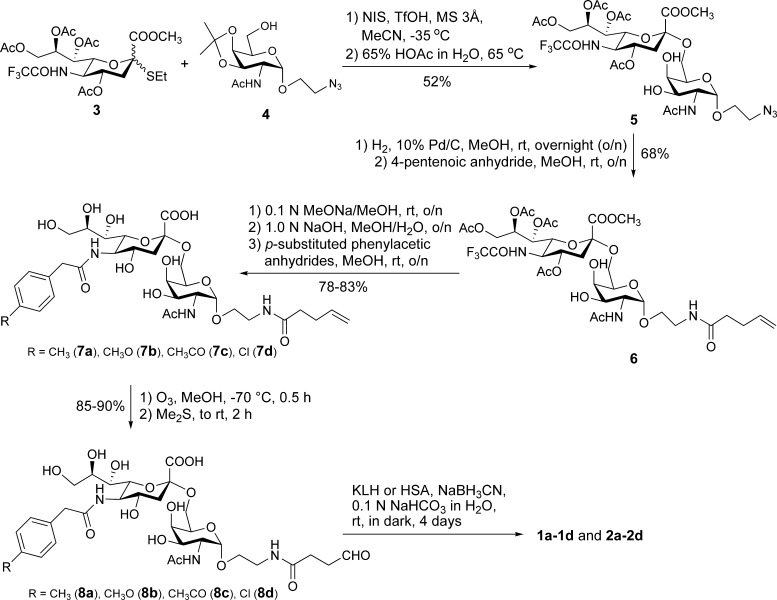
The chemical synthesis of the designed sTnNPhAc derivatives and their KLH and human serum albumin (HSA) conjugates 1a−d and 2a−d (Figure 2) is shown in Scheme 1. The sialylation reaction was realized with N-trifluoroacetyl thiosialoside 3(26) as the glycosyl donor, using N-iodosuccinamide (NIS) and triflic acid (TfOH) as promoters, to afford a mixture of two anomeric disaccharides in a good yield (66%) and in favor of the desired α anomer (α/β 3.7/1). However, the two anomers were difficult to separate by column chromatography. Only after their isopropylidene group was removed with 65% acetic acid in water were the resultant diols readily separated to offer the desired α-linked disaccharide 5 in a 52% yield (two steps overall). The α-configuration of the sialic acid residue in 5 was determined based on the observed downfield shift of the 1H NMR signal of H-3′e (δ 2.63, as compared to δ 2.42 of β-linked sialic acid). The strong correlation between C-1′ and H-3′a, but not H-3′e, in the HMBC spectrum of 5 further confirmed the α-configuration of the sialyl residue. Subsequently, a pentenoyl group was attached to the glycan reducing end following selective reduction of the azido group in 5 to form a primary amine and then selective acylation of the resultant amino group with pentenoic anhydride. The pentenoyl group was used as a molecular handle to facilitate the coupling of the carbohydrate antigens to carrier proteins. Eventually, the N-trifluoroacetyl and O-acetyl groups in 6 were removed by treatments with a methanolic solution of NaOMe and then a methanolic aqueous solution of NaOH, and the exposed amino group was selectively acylated in methanol via reactions with p-methylphenylacetic, p-methoxylphenylacetic, p-acetylphenylacetic, and p-chlorophenylacetic anhydrides, respectively, to afford the sTnNPhAc derivatives 7a−d. Products 7a−d were purified on a Biogel P2 column using deionized water as the eluent, and their structures were confirmed by NMR spectrometry and high resolution MS.
Scheme 1. Synthesis of the sTnNPhAc Derivatives and Their Protein Conjugates 1a−d and 2a−d.
The carbohydrate antigens were finally coupled to carrier proteins by an established protocol.27 First, 7a−d were converted into aldehydes 8a−d upon ozone treatment in methanol, and the products were purified on a Biogel P2 column and characterized with 1H NMR spectrometry, which showed the disappearance of the double bond signals and the appearance of the hydrated aldehyde signal at δ 5.2. Next, 8a−d were linked to KLH and HSA via reductive amination carried out in 0.1 N NaHCO3 buffer in the presence of NaBH3CN. The resultant glycoconjugates 1a−d and 2a−d were purified on a Biogel A0.5 column, and the first eluted component was the desired glycoconjugates, which were well separated from the corresponding aldehydes 8a−d that were used in excess. Column fractions containing conjugates 1a−d and 2a−d, which were tested to contain both carbohydrate and protein, were combined, dialyzed against deionized water, and then freeze-dried to afford 1a−d and 2a−d as white fluffy solids. Antigen loading levels of glycoconjugates 1a−d and 2a−d were determined based on their sialic acid contents analyzed by the resorcinol method as described in the literature.28 As shown in Table 1 in the Supporting Information, KLH and HSA conjugates 1a−d and 2a−d contained ca. 3−5 and 6−8% (w/w) of the carbohydrate antigens, respectively, which were comparable to that of glycoconjugates obtained previously.23,24
KLH conjugates 1a−d were evaluated as vaccines in C57BL/6 mouse and compared to the KLH conjugate of sTnNPhAc, while the corresponding HSA conjugates were utilized as capture antigens in enzyme-linked immunosorbent assays (ELISA) of the immunological activities of these vaccines. Animal immunizations followed the protocols reported previously.23,24 In brief, groups of five mice were individually immunized with intramuscular (im) injection of an emulsion (a total of 0.1 mL) of 1a−d or sTnNPhAc-KLH (containing 2 μg of a carbohydrate antigen) and Titermax Gold adjuvant on days 0, 14, 21, and 28, respectively. Mice were bled on day −1 prior to the initial immunization and on day 27 and day 37 after immunization. Blood samples collected at each time point were clotted for the preparation of antisera by protocols described in the literature,23,24 which were then stored at −80 °C. For ELISA, after plates were coated with sTn-HSA, sTnNPhAc-HSA, and 2a−d, respectively, they were used to detect the corresponding antigen-specific antibodies as reported.23,24 The titers of total antibody and individual antibody isotypes, such as IgM, IgG1, IgG2a, and IgG3, were analyzed with various anti-antibodies. The antibody titer, defined as the serum dilution number at which the optical density (OD) value of an ELISA experiment reaches 0.5, was obtained from the best-fit line by plotting the OD values against the dilution values.
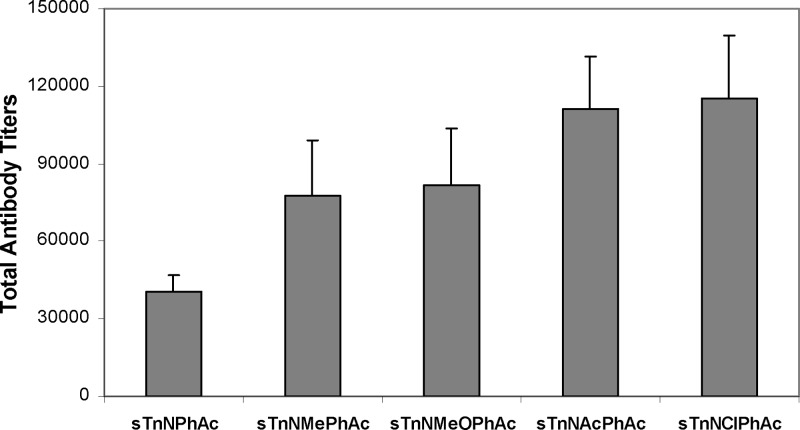
ELISA results showed that the antibody titers of the day 37 antisera were globally much higher than that of the day 27 antisera, reflecting the general trend of enhanced immune response to recurring exposure to the same antigens, which is desirable. Here, we only present and discuss in detail about the ELISA results of the day 37 antisera. Figure 3 shows the total antibody titers of the pooled antisera with regard to each specific antigen. Clearly, all of the new conjugates 1a−d elicited strong antigen-specific immune responses, stronger than that of sTnNPhA-KLH. Moreover, it seems that the sTnNPhAc derivatives with electron-withdrawing groups attached to the phenyl ring, including sTnNAcPhAc and sTnNClPhAc, are more immunogenic than those with electron-donating groups, such as sTnNMePhAc and sTnNMeOPhAc. Whether this observation has any general significance is unclear presently. sTnNClPhAc is especially interesting, because it contains the smallest substituent among the four newly designed sTn derivatives but is the most immunogenic. These results indicate that the immunogenicity of these sTn derivatives is related to the general property of the unnatural N-acyl groups rather than their steric bulk as compared to sTn or sTnNPhAc.
Figure 3.
Total antigen-specific antibody titers in the day 37 antisera obtained from mice inoculated with sTnNPhAc-KLH, sTnNMePhAc-KLH (1a), sTnNMeOPhAc-KLH (1b), sTnNAcPhAc-KLH (1c), and sTnNClPhAc-KLH (1d) conjugates, respectively. For ELISA, the corresponding HSA conjugates were used as the capture antigens for plate coating, and goat antimouse κ antibodies were used to detect antibodies bound to the plates. Each column represents the average antibody titer in the antiserum of five replicate animals.
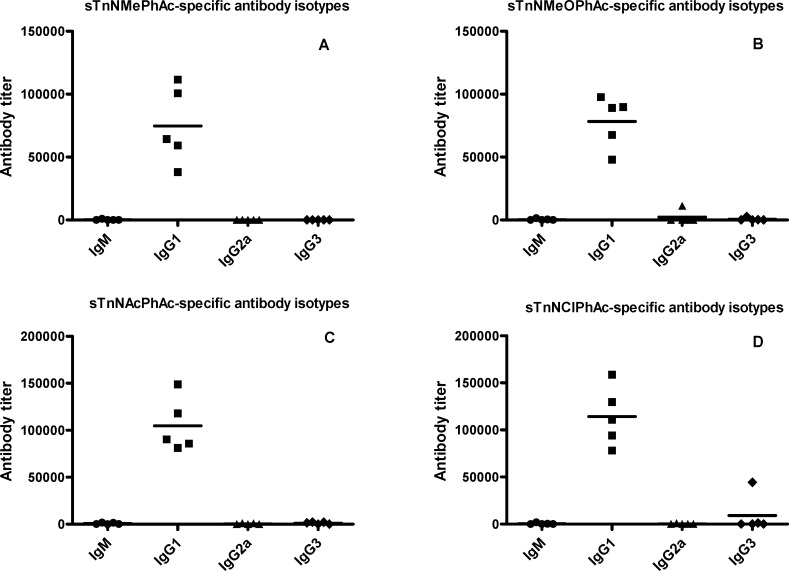
To evaluate the quality of the immune responses elicited by glycoconjugates 1a−d, we analyzed the titers of various antibody isotypes in the day 37 antisera by ELISA. The results shown in Figure 4 clearly demonstrated that all four conjugates provoked high titers of IgG1 antibodies in all of the mice uniformly, while the titers of other antibody isotypes, such as IgM, IgG2a, and IgG3 antibodies, were very low. In fact, the IgG1 antibody titers in Figure 4 match the total antibody titers in Figure 3 well, which further proved that 1a−d elicited mainly IgG1 antibodies. The relatively low (but still significant) titers of IgM antibodies and substantially elevated levels of IgG1 antibodies elicited by conjugates 1a−d, as compared to sTnNPhAc-KLH, suggest potential antibody isotype switching, and improved T cell-mediated immune responses to the new sTn analogues. It is documented that, after antigen processing, the carrier protein of glycoconjugates can provide peptides that will interact noncovalently with class II MHC molecules and be presented to helper T cells. The interactions of helper T cells with carbohydrate antigen-specific B cells, which can present the carrier-derived peptides to T cells, elicit the switching of antibody production from IgM to IgG1 via CD154-CD40 engagement.29 Antibody isotype switching to IgG also means potentially improved immunological memorization, antibody maturation, and cell-mediated cytotoxicity,29 which are very important and useful for cancer immunotherapy. Similar to the results of total antibody analysis, we observed much higher levels of IgG1 antibodies in the antisera of 1c and 1d, which further proved that these two glycoconjugates are more immunogenic than 1a and 1b and that the N-acyl group of sTn antigen has an important impact on the immunological properties.
Figure 4.
Titers of various isotypes of antigen-specific antibodies in the day 37 antisera obtained with glycoconjugates 1a−d (panels A−D, respectively) as determined by ELISA. Each dot represents the ELISA result of an individual mouse, and each black line represents the average antibody level of a group of five mice.
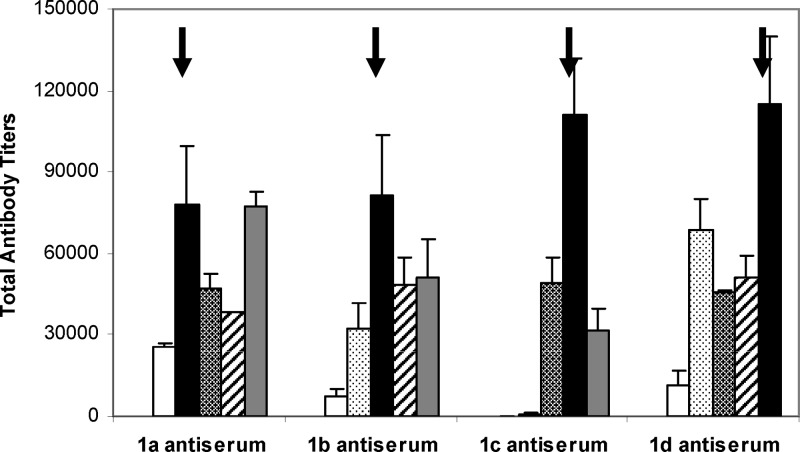
We also examined the reactivity of the antisera obtained with 1a−d to natural sTn and to all of the synthetic sTn derivatives discussed above, including sTnNPhAc, sTnNMePhAc, sTnNMeOPhAc, sTnNAcPhAc, and sTnNClPhAc by ELISA (Figure 5). In these experiments, the ELISA plates were first coated with sTn-HSA, sTnNPhAc-HSA, and conjugates 2a−d, respectively, and then treated with the day 37 antisera. It was revealed that all of the antisera had essentially no reactivity to natural sTn but showed certain levels of reactivity to sTnNPhAc and other sTnNPhAc analogues. Specifically, the antiserum of 1a (sTnNMePhAc) showed very strong cross-reactivity to sTnNPhAc and all of other derivatives including sTnNMeOPhAc, sTnNAcPhAc, and sTnNClPhAc. Essentially the antiserum of 1a had the same reactivity to sTnNMePhAc and sTnNClPhAc. The antisera of 1b (sTnNMeOPhAc) and 1d (sTnNClPhAc) had relatively low reactivity to sTnNPhAc but reacted strongly with other sTnNPhAc derivatives. Most distinctively, the antiserum of 1c (sTnNAcPhAc) did not show obvious reactivity to either sTnNPhAc or sTnNMePhAc, but it did have significant cross-reactivity with both sTnNMeOPhAc and sTnNClPhAc. As the antisera of 1a−d did not cross-react with natural sTn, the phenylacetyl group in sTnNPhAc, sTnNMePhAc, sTnNMeOPhAc, sTnNAcPhAc, and sTnNClPhAc must have played an important role in the augmentation of antigen−antibody binding specificity and affinity. On the other hand, the extensive cross-reactivity of the antisera of 1a−d with sTnNPhAc and other sTn analogues suggests that at least a part of the antibodies elicited by each glycoconjugate could recognize and bind to sTn analogues carrying differently substituted phenylacetyl groups, which can be explained by nonspecific hydrophobic interactions instead of steric or electronic effect. Nevertheless, these results have demonstrated that the majority of antibodies elicited by 1a−d were specific to the individual sTn derivatives. Furthermore, we believed that the specific immune responses elicited by conjugates 1a−d were primarily against the whole sTn antigen instead of the modifying phenylacetyl group, because our previous studies have shown that antibodies induced by sTnNPhAc-KLH reacted to the whole carbohydrate antigen instead of the linker or the independent PhAc group.23,24
Figure 5.
Titers of antibodies in the day 37 antisera of 1a−d reactive to sTnNPhAc-HSA (white bars), sTnNMePhAc-HSA (dotted bars, 2a), sTnNMeOPhAc-HSA (hatched bars, 2b), sTnNAcPhAc-HSA (diagonally striped bars, 2c), and sTnNClPhAc-HSA (gray bars, 2d), respectively. The arrows (→) highlight the antibody titers of the antisera, where the sTnNPhAc derivative employed to immunize animals was used to coat ELISA plates as well. In all of these experiments, pooled antisera from five replicate animals immunized with the indicated conjugates were used to obtain total antibody titers by ELISA.
In conclusion, we have synthesized via a convergent method several N-phenylacetyl derivatives of sTn antigen, including sTnNMePhAc, sTnNMeOPhAc, sTnNAcPhAc, and sTnNClPhAc, as well as their KLH conjugates 1a−d. The immunological properties of conjugates 1a−d as vaccines were evaluated in C57BL/6 mouse and compared to that of sTnNPhAc. It was disclosed that sTnNMePhAc, sTnNMeOPhAc, sTnNAcPhAc, and sTnNClPhAc were more immunogenic than sTnNPhAc and that they induced almost exclusively T cell-mediated IgG1 antibody response, which is useful for cancer immunotherapy. Among the four derivatives investigated, conjugates 1c and 1d provoked obviously more robust immune responses than 1a and 1b. Moreover, even though the antisera of 1a−d showed significant reactivity to sTnNPhAc and to all other derivatives, they had no reactivity to natural sTn. Therefore, the immune responses provoked by 1a−d were specific to the whole modified form of sTn antigen not only to the unnatural N-acyl groups. Putting all together, we identify sTnNClPhAc as the most promising candidate for cancer vaccine development for our new cancer immunotherapeutic strategy. First, the KLH conjugate of sTnNClPhAc turned out to be the most immunogenic to induce high titers of IgG1 antibodies, and second, among the four derivatives, sTnNClPhAc contains the smallest substituent; thus, its precursor may be more easily accepted by the biosynthetic machineries of sTn and other sialo-TACAs. With this regard, we are currently investigating the efficiency of N-p-chlorophenylacetyl mannosamine and N-p-chlorophenylacetyl sialic acid to metabolically engineer cancer cells.
Acknowledgments
We thank Drs. B. Shay and L. Hryhorczuk for HRMS measurements and Dr. B. Ksebati for some NMR measurement.
This work was supported by a research grant from NIH/NCI (R01 CA95142).
Supporting Information Available
Synthetic procedures, analytical and spectral characterization data of all synthesized compounds, immunization, and assay protocols. This material is available free of charge via the Internet at http://pubs.acs.org.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Troy F. A. 2nd Polysialylation: From bacteria to brains. Glycobiology 1992, 2, 5–23. [DOI] [PubMed] [Google Scholar]
- Fukuda M. Possible roles of tumor-associated carbohydrate antigens. Cancer Res. 1996, 56, 2237–44. [PubMed] [Google Scholar]
- Scheidegger E. P.; Lackie P. M.; Papay J.; Roth J. In vitro and in vivo growth of clonal sublines of human small cell lung carcinoma is modulated by polysialic acid of the neural cell adhesion molecule. Lab. Invest. 1994, 70, 95–106. [PubMed] [Google Scholar]
- Hakomori S.; Zhang Y. Glycosphingolipid antigens and cancer therapy. Chem. Biol. 1997, 4, 97–104. [DOI] [PubMed] [Google Scholar]
- Helling F.; Shang A.; Calves M.; Zhang S.; Ren S.; Yu R. K.; Oettgen H. F.; Livingston P. O. GD3 vaccines for melanoma: Superior immunogenicity of keyhole limpet hemocyanin conjugate vaccines. Cancer Res. 1994, 54, 197–203. [PubMed] [Google Scholar]
- Danishefsky S. J.; Allen J. R. From the Laboratory to the Clinic: A Retrospective on Fully Synthetic Carbohydrate-Based Anticancer Vaccines Frequently used abbreviations are listed in the appendix. Angew. Chem., Int. Ed. Engl. 2000, 39, 836–863. [DOI] [PubMed] [Google Scholar]
- Yonezawa S.; Tachikawa T.; Shin S.; Sato E. Sialosyl-Tn antigen. Its distribution in normal human tissues and expression in adenocarcinomas. Am. J. Clin. Pathol. 1992, 98, 167–174. [DOI] [PubMed] [Google Scholar]
- Leivonen M.; Nordling S.; Lundin J.; von Boguslawski K.; Haglund C. STn and prognosis in breast cancer. Oncology 2001, 61, 299–305. [DOI] [PubMed] [Google Scholar]
- Itzkowitz S. H.; Bloom E. J.; Kokal W. A.; Modin G.; Hakomori S.; Kim Y. S. Sialosyl-Tn. A novel mucin antigen associated with prognosis in colorectal cancer patients. Cancer 1990, 66, 1960–1966. [DOI] [PubMed] [Google Scholar]
- Kobayashi H.; Terao T.; Kawashima Y. Serum sialyl Tn as an independent predictor of poor prognosis in patients with epithelial ovarian cancer. J. Clin. Oncol. 1992, 10, 95–101. [DOI] [PubMed] [Google Scholar]
- Kim G. E.; Bae H. I.; Park H. U.; Kuan S. F.; Crawley S. C.; Ho J. J.; Kim Y. S. Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology 2002, 123, 1052–1060. [DOI] [PubMed] [Google Scholar]
- Victorzon M.; Nordling S.; Nilsson O.; Roberts P. J.; Haglund C. Sialyl Tn antigen is an independent predictor of outcome in patients with gastric cancer. Int. J. Cancer 1996, 65, 295–300. [DOI] [PubMed] [Google Scholar]
- Kjeldsen T.; Clausen H.; Hirohashi S.; Ogawa T.; Iijima H.; Hakomori S. Preparation and characterization of monoclonal antibodies directed to the tumor-associated O-linked sialosyl-2−6 alpha-N-acetylgalactosaminyl (sialosyl-Tn) epitope. Cancer Res. 1988, 48, 2214–2220. [PubMed] [Google Scholar]
- Itzkowitz S. H.; Yuan M.; Montgomery C. K.; Kjeldsen T.; Takahashi H. K.; Bigbee W. L.; Kim Y. S. Expression of Tn, sialosyl-Tn, and T antigens in human colon cancer. Cancer Res. 1989, 49, 197–204. [PubMed] [Google Scholar]
- Werther J. L.; Tatematsu M.; Klein R.; Kurihara M.; Kumagai K.; Llorens P.; Guidugli Neto J.; Bodian C.; Pertsemlidis D.; Yamachika T.; Kitou T.; Itzkowitz S. Sialosyl-Tn antigen as a marker of gastric cancer progression: an international study. Int. J. Cancer 1996, 69, 193–199. [DOI] [PubMed] [Google Scholar]
- Nakagoe T.; Sawai T.; Tsuji T.; Jibiki M. A.; Nanashima A.; Yamaguchi H.; Yasutake T.; Ayabe H.; Arisawa K.; Ishikawa H. Predictive factors for preoperative serum levels of sialy Lewis(x), sialyl Lewis(a) and sialyl Tn antigens in gastric cancer patients. Anticancer Res. 2002, 22, 451–458. [PubMed] [Google Scholar]
- Holmberg L. A.; Sandmaier B. M. Vaccination with Theratope (STn-KLH) as treatment for breast cancer. Expert Rev. Vaccines 2004, 3, 655–663. [DOI] [PubMed] [Google Scholar]
- Bresalier R. S.; Niv Y.; Byrd J. C.; Duh Q. Y.; Toribara N. W.; Rockwell R. W.; Dahiya R.; Kim Y. S. Mucin production by human colonic carcinoma cells correlates with their metastatic potential in animal models of colon cancer metastasis. J. Clin. Invest. 1991, 87, 1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuberan B.; Lindhardt R. J. Carbohydrate based vaccines. Curr. Org. Chem. 2000, 4, 653–677. [Google Scholar]
- Holmberg L. A.; Oparin D. V.; Gooley T.; Lilleby K.; Bensinger W.; Reddish M. A.; MacLean G. D.; Longenecker B. M.; Sandmaier B. M. Clinical outcome of breast and ovarian cancer patients treated with high-dose chemotherapy, autologous stem cell rescue and THERATOPE STn-KLH cancer vaccine. Bone Marrow Transplant. 2000, 25, 1233–1241. [DOI] [PubMed] [Google Scholar]
- Liu T.; Guo Z.; Yang Q.; Sad S.; Jennings H. J. Biochemical engineering of surface alpha 2−8 polysialic acid for immunotargeting tumor cells. J. Biol. Chem. 2000, 275, 32832–32836. [DOI] [PubMed] [Google Scholar]
- Pan Y.; Chefalo P.; Nagy N.; Harding C.; Guo Z. Synthesis and immunological properties of N-modified GM3 antigens as therapeutic cancer vaccines. J. Med. Chem. 2005, 48, 875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.; Guo Z. Improving the antigenicity of sTn antigen by modification of its sialic acid residue for development of glycoconjugate cancer vaccines. Bioconjugate Chem. 2006, 17, 1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.; Ekanayaka S. A.; Wu J.; Zhang J.; Guo Z. Synthetic and Immunological Studies of 5′-N-Phenylacetyl sTn to Develop Carbohydrate-Based Cancer Vaccines and to Explore the Impacts of Linkage between Carbohydrate Antigens and Carrier Proteins. Bioconjugate Chem. 2008, 19, 2060–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefalo P.; Pan Y.; Nagy N.; Guo Z.; Harding C. V. Efficient metabolic engineering of GM3 on tumor cells by N-phenylacetyl-d-mannosamine. Biochemistry 2006, 45, 3733–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meo C. D.; Demchenko A. V.; Boons G. J. A stereoselective approach for the synthesis of alpha-sialosides. J. Org. Chem. 2001, 66, 5490–5497. [DOI] [PubMed] [Google Scholar]
- Xue J.; Pan Y. B.; Guo Z. W. Neoglycoprotein cancer vaccines: Synthesis of an azido derivative of GM3 and its efficient coupling to proteins through a new linker. Tetrahedron Lett. 2002, 43, 1599–1602. [Google Scholar]
- Svennerholm L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim. Biophys. Acta 1957, 24, 604–611. [DOI] [PubMed] [Google Scholar]
- Casadevall A.; Pirofski L. A. Antibody-mediated regulation of cellular immunity and the inflammatory response. Trends Immunol. 2003, 24, 474–478. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.