Abstract
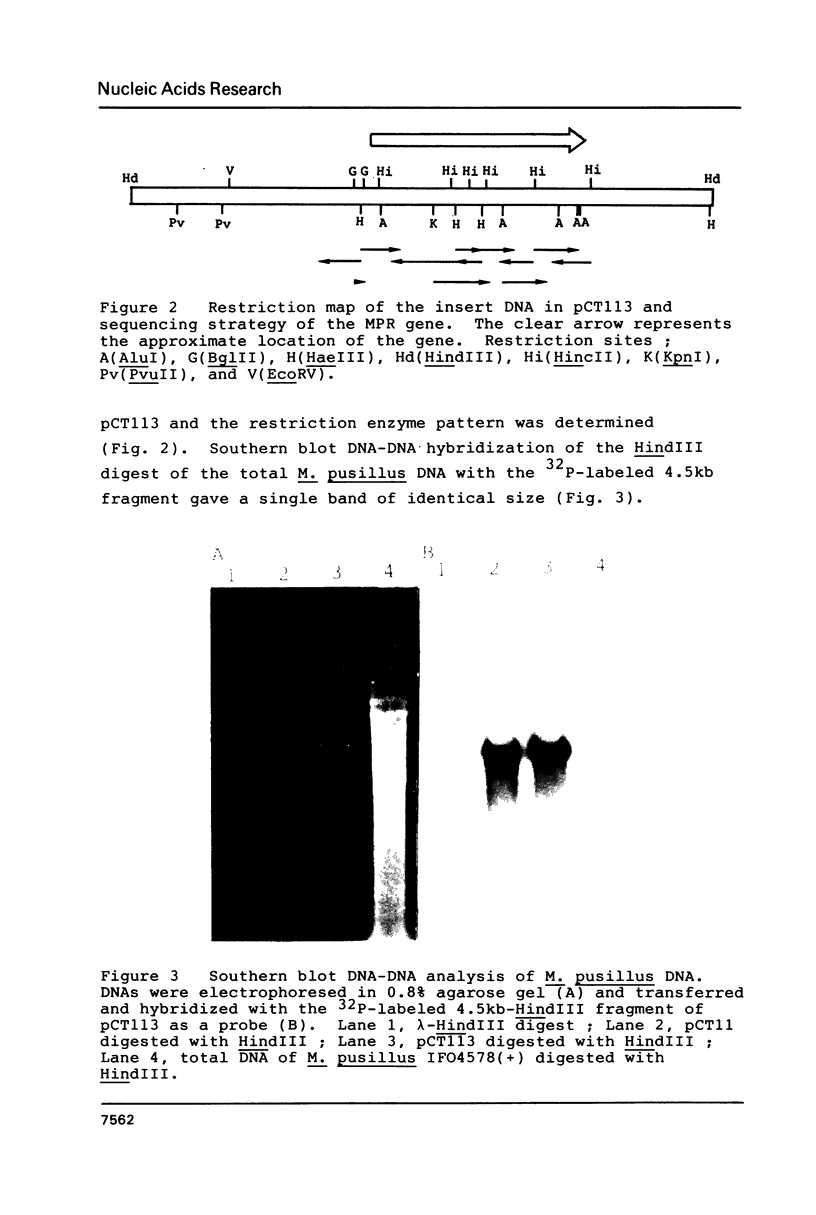
The aspartate protease of Mucor pusillus (Mucor pusillus rennin; MPR) is a milk-clotting enzyme used in the cheese industry. The partial amino acid sequence of MPR was determined and oligonucleotide probes were synthesized for cloning of the MPR gene. A clone giving positive hybridization with the probes was selected from the cosmid library. Sequencing of the cloned DNA revealed an open reading frame of 1281 bp without introns which encodes 361 amino acids for the expected MPR with an NH2-terminal extension of 66 amino acids. MPR seems to be synthesized as a prepro enzyme.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baudys M., Kostka V. Covalent structure of chicken pepsinogen. Eur J Biochem. 1983 Oct 17;136(1):89–99. doi: 10.1111/j.1432-1033.1983.tb07709.x. [DOI] [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Boel E., Hjort I., Svensson B., Norris F., Norris K. E., Fiil N. P. Glucoamylases G1 and G2 from Aspergillus niger are synthesized from two different but closely related mRNAs. EMBO J. 1984 May;3(5):1097–1102. doi: 10.1002/j.1460-2075.1984.tb01935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etoh Y., Shoun H., Arima K., Beppu T. Photo-oxidation of a histidyl residue of milk-clotting acid protease, Mucor rennin. J Biochem. 1982 Mar;91(3):747–753. doi: 10.1093/oxfordjournals.jbchem.a133761. [DOI] [PubMed] [Google Scholar]
- Etoh Y., Shoun H., Ogino T., Fujiwara S., Arima K., Beppu T. Proton magnetic resonance spectroscopy of an essential histidyl residue in a milk-clotting acid protease, Mucor rennin. J Biochem. 1982 Jun;91(6):2039–2046. doi: 10.1093/oxfordjournals.jbchem.a133897. [DOI] [PubMed] [Google Scholar]
- Foltmann B., Pedersen V. B., Jacobsen H., Kauffman D., Wybrandt G. The complete amino acid sequence of prochymosin. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2321–2324. doi: 10.1073/pnas.74.6.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson G. N., Vapnek D. A simple method of preparing large amounts of phiX174 RF 1 supercoiled DNA. Biochim Biophys Acta. 1973 Apr 11;299(4):516–520. doi: 10.1016/0005-2787(73)90223-2. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide T., Ikenaka T. Studies on soybean trypsin inhibitors. 1. Fragmentation of soybean trypsin inhibitor (Kunitz) by limited proteolysis and by chemical cleavage. Eur J Biochem. 1973 Feb 1;32(3):401–407. doi: 10.1111/j.1432-1033.1973.tb02622.x. [DOI] [PubMed] [Google Scholar]
- Konigsberg W., Godson G. N. Evidence for use of rare codons in the dnaG gene and other regulatory genes of Escherichia coli. Proc Natl Acad Sci U S A. 1983 Feb;80(3):687–691. doi: 10.1073/pnas.80.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange A., Paquet D., Alais C. Comparative study of two Mucor miehei acid proteinases. Purification and some molecular properties. Int J Biochem. 1980;11(5):347–352. doi: 10.1016/0020-711x(80)90304-3. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Morávek L., Kostka V. Complete amino acid sequence of hog pepsin. FEBS Lett. 1974 Jul 15;43(2):207–211. doi: 10.1016/0014-5793(74)81001-x. [DOI] [PubMed] [Google Scholar]
- Norgard M. V., Keem K., Monahan J. J. Factors affecting the transformation of Escherichia coli strain chi1776 by pBR322 plasmid DNA. Gene. 1978 Jul;3(4):279–292. doi: 10.1016/0378-1119(78)90038-0. [DOI] [PubMed] [Google Scholar]
- Nunberg J. H., Meade J. H., Cole G., Lawyer F. C., McCabe P., Schweickart V., Tal R., Wittman V. P., Flatgaard J. E., Innis M. A. Molecular cloning and characterization of the glucoamylase gene of Aspergillus awamori. Mol Cell Biol. 1984 Nov;4(11):2306–2315. doi: 10.1128/mcb.4.11.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottesen M., Rickert W. The isolation and partial characterization of an acid protease produced by Mucor miehei. C R Trav Lab Carlsberg. 1970;37(14):301–325. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechtman M. G., Yanofsky C. Structure of the trifunctional trp-1 gene from Neurospora crassa and its aberrant expression in Escherichia coli. J Mol Appl Genet. 1983;2(1):83–99. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Vapnek D., Hautala J. A., Jacobson J. W., Giles N. H., Kushner S. R. Expression in Escherichia coli K-12 of the structural gene for catabolic dehydroquinase of Neurospora crassa. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3508–3512. doi: 10.1073/pnas.74.8.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Hirose T., Miyake T., Kawashima E. H., Itakura K. The use of synthetic oligonucleotides as hybridization probes. II. Hybridization of oligonucleotides of mixed sequence to rabbit beta-globin DNA. Nucleic Acids Res. 1981 Feb 25;9(4):879–894. doi: 10.1093/nar/9.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]