Abstract
Defining evolutionary origins is a means of understanding an organism’s position within the integrated web of living beings, and to not only to trace characteristics back in time, but also to project forward in an attempt to reveal relationships with more recently evolved forms. Both the vertebrates and arthropods possess condensed nervous systems, but this is dorsal in the vertbrates and ventral in the arthropods. Also, whereas the nervous system in the vertebrates develops from a neural tube in the embryo, that of the arthropods comes from an ectodermal plate. Despite these apparently fundamental differences, it is now generally accepted that life-long neurogenesis, the generation of functionally integrated neurons from progenitor cells, is a common feature of the adult brains of a variety of organisms, ranging from insects and crustaceans to birds and mammals. Among decapod crustaceans, there is evidence for adult neurogenesis in basal species of the Dendrobranchiata, as well as in more recent terrestrial, marine and fresh-water species. The widespread nature of this phenomenon in decapod species may relate to the importance of the adult-born neurons, although their functional contribution is not yet known. The many similarities between the systems generating neurons in the adult brains of decapod crustaceans and mammals, reviewed in this paper, suggest that adult neurogenesis is governed by common ancestral mechanisms that have been retained in a phylogenetically broad group of species.
Keywords: deutocerebral organ, stem cell niche, decapod, bromodeoxyuridine, glutamine synthetase, migration
1. Introduction
The process of adult neurogenesis includes the proliferation of precursor cells, migration of daughter cells to the locations where the new neurons will reside, and acquisition of neuronal properties appropriate to a cell’s location. Similarities in these processes even between phylogenetically distant species such as crustaceans and mammals, suggest that common ancestral mechanisms direct the production and integration of new neurons in the brains of diverse adult organisms. In addition, the prevalence of adult neurogenesis indicates that adult-born neurons play important, although thus far undefined, roles in the crustacean nervous system.
The embryonic nervous system of decapods is generated by the repeated, asymmetric division of neuroblasts, a pattern shared with other arthropods (e.g., insects) (Scholtz and Dohle, 1996; Harzsch, 2001). Neuroblasts in crayfish embryos, identified as large cells that divide asymmetrically, are often seen in histological preparations to be associated with a cluster of smaller daughter cells and, in the brain, associated with emerging cell clusters. The daughters of the neuroblast divisions (ganglion mother cells), divide at least once to produce offspring that will differentiate into neurons. The sequence and timing of divisions of neuroblasts and ganglion mother cells are highly stereotyped, resulting in the production of predictable lineages of cells. At the end of embryonic life or early in postembryonic life, having produced the basic scaffold of the nervous system, the large asymmetrically dividing neuroblasts are no longer visible in the brains of crayfish (Cherax destructor, Procambarus clarkii), lobsters (Homarus americanus) and coconut crabs (Birgus latro), and are thought to undergo apoptosis as do the majority of neuroblasts in insects (Scholtz, 1992; Farris et al., 1999; Ganeshina et al., 2000; Benton and Beltz, 2002; Sandeman and Sandeman, 2003). A small number of neuroblasts in some insect species do survive into postembryonic stages and produce offspring that will become neurons in the mushroom body (Cayre et al., 1996, 2002; Dufour and Gadenne, 2006).
A hallmark of many decapod species is that growth persists throughout the sometimes very long lives of these animals (e.g. American lobsters, 100 years or more; Purves, 1988), punctuated by molts at longer intervals as the animals age. A lobster’s size, for instance, can increase by a factor of 105or more over this long lifespan, a degree of growth that rivals the largest mammals. Brain size in crustaceans also increases with growth, partly by the increased size of individual neurons as they extend and expand the numbers of processes, but also, in the olfactory and accessory lobes, by the addition of new neurons (Harzsch and Dawirs, 1996; Schmidt 1997; Schmidt and Harzsch, 1999; Harzsch et al., 1999). Because the neuroblasts that build the embryonic neuronal scaffold are reported to die during late embryonic or early postembryonic life (Scholtz, 1992; Sandeman and Sandeman, 2003), a major question has been how adult-born neurons are generated.
Neurogenesis in the brains of large, sexually mature decapod crustaceans was discovered by exposing the animals to the thymidine analogue, bromodeoxyuridene (BrdU). When present in high enough concentration during the S phase of the cell cycle, BrdU is incorporated into the newly synthesized DNA strands. An antibody to BrdU can then be used to detect which cells in the animal’s body passed through S phase during the BrdU exposure. Labeled cells that are known to be part of the olfactory system have been detected in areas of the deutocerebrum of many different adult decapods. Such labeled cells were first found amongst the already-differentiated projection interneurons in cell cluster 10 (CL10) and local olfactory interneurons in cell cluster 9 (CL9). (Nomenclature according to Sandeman et al., 1992). Nevertheless, as of a few years ago, the primary precursors of these new cells had still not been definitively determined. While new neurons also are known to be generated in soma clusters associated with the optic neuropils in the Australian crayfish Cherax destructor (Sullivan and Beltz, 2005a), nothing is known about the prevalence of this phenomenon in other decapod species, or the underlying mechanisms. The present paper will therefore focus on adult neurogenesis in the deutocerebral cell clusters of decapods.
Studies in the crayfish Procambarus clarkii revealed that the labeled cells in CL9 and CL10 were linked through lines of BrdU-labeled cells to a cluster of cells that surrounded a central “cavity”, suggesting that a distinct group of progenitor cells (whose origins are currently unknown), are the 1st-generation precursors of adult-born neurons (Sullivan et al., 2005, 2007a, b; Song et al., 2007; Zhang et al., 2009). These precursors undergo symmetrical divisions, unlike traditional neuroblasts, and their divisions are not self-renewing (Zhang et al., 2009; Benton et al., 2010). The cells are bipolar, a structural feature that is clearly distinct from neuroblasts found in the embryonic brain; it has been proposed that the processes of the bipolar cells guide the migration of the 2nd generation cells in the neural precursor lineage to sites of further proliferation and differentiation, proliferation zones in CL9 and CL10. The 1st generation progenitors therefore appear to serve as both precursor and support cells (Sullivan et al., 2007a; Zhang et al., 2009; but see also section 4.5 for the alternative hypothesis of Song et al., 2009).
In spiny lobsters (Panulirus argus), it has been suggested that a few neuroblasts survive the post-embryonic period to become neuronal stem cells in the adult brain (Schmidt, 2007a). These cells would presumably undergo self-renewing, asymmetric divisions, as they did in the embryonic nervous system. However, in contrast to the neuroblast divisions in the embryonic brain, adult neurogenesis is regulated by a multitude of environmental and endogenous signals (Beltz and Sandeman, 2003; Sullivan et al., 2007b) and neuroblasts would therefore also need to acquire new properties that would allow their division and those of their offspring to respond to these diverse signals.
In this paper, we begin with a review of the prevalence of adult neurogenesis in decapod species and then focus on the two, so far, most closely studied mechanisms in crayfish and spiny lobsters which, given the phylogenetic relationships involved, may reveal interesting comparisons. Spiny lobsters may be evolutionarily older than crayfish (Scholtz and Richter 1995), and could represent a situation closer to an earlier stage in the development of the mechanism found in the crayfish, or may have diverged early from the evolutionary line leading to the crayfish and other reptants (Dixon et al., 2003). We also relate the existing data to what is known concerning adult neurogenesis in evolutionarily higher organisms, and in particular mammalian species.
2. Prevalence of adult neurogenesis in decapod species
It has long been known that as decapods grow, their brains enlarge in parallel with their body size. However, largely because arthropods are notable for the stereotyped layout of individually identifiable neurons in their brains and segmental ganglia, brain growth was attributed to increases in size of individual neurons whose arborizations were expanded to accommodate the increasing numbers of afferents coming from the enlarging periphery. In some cases cell body counts were possible because of the large size and relatively low numbers of cells; the standard complement of neurons, achieved early in the development and growth of the animals, did not appear to change. However, counts of the very large numbers of neurons with small cell bodies that are associated with the olfactory (OLs) and, where present, accessory (ALs) lobes would have shown (as did later studies [Sandeman et al., 1998]) that as the animals enlarged, so did the number of cells in these clusters -- a sure sign that the number of neurons incorporated into the brains of these animals was being increased throughout their lives. These densely packed cell clusters (CL9 and 10) are associated with chemoreceptive input from the first antennae and higher-order processing of olfactory, visual and tactile information, respectively (Sandeman et al., 1995; Sullivan and Beltz, 2005b).
As a result of this history, the contribution of postembryonic neurogenesis to brain growth was not recognized until the late 1990s, when the work of Harzsch and Dawirs (1996; Hyas araneus) and Schmidt (1997; Carcinus maenas) demonstrated that new neurons are incorporated into the brains of a variety of decapod species after metamorphosis and into adult life. Indeed, estimates of the cell numbers in the two main clusters of small cells in the olfactory system (local interneurons in cluster 9 [CL9], and projection neurons in cluster 10 [CL10]) in crayfish demonstrated that the generation of new neurons revealed by BrdU labeling was accompanied by an increase in the overall numbers of cells in these areas (Sandeman et al., 1998). Since the early discoveries in H. araneus and C. maenas, the presence of BrdU-labeled cells has been demonstrated in a variety of adult, sexually mature decapods (Sandeman et al., 1998; Harzsch et al., 1999; Schmidt and Harzsch, 1999; Sullivan et al., 2007b). In every case, the BrdU label is restricted to the small proliferative areas in two cell clusters in the deutocerebrum (CL 9 and 10), although in several species labeling in CL10 appears to be more reliable and robust than in CL9. These studies have led to the acceptance of decapods as models for the study of basic mechanisms underlying adult neurogenesis (Figure 1) (Lindsey and Tropepe, 2006; Schmidt, 2007b; Sullivan, 2007b).
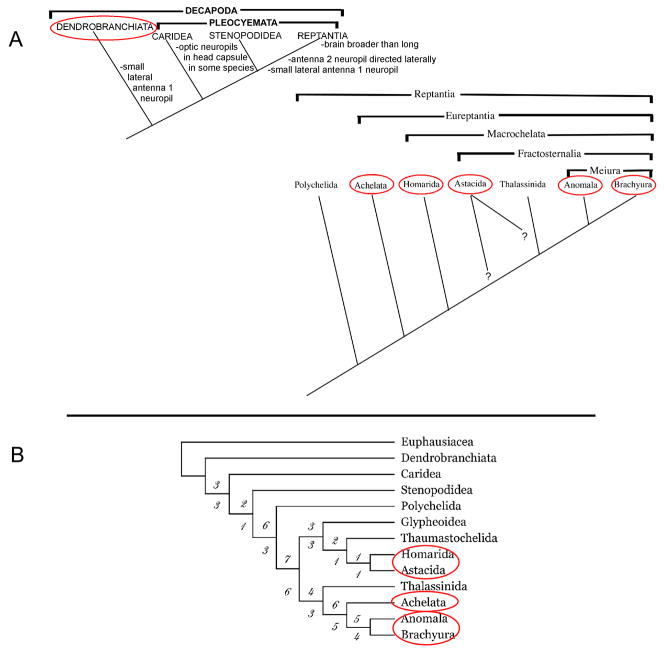
Figure 1.
An overview of the phylogenetic relationships among the decapod crustaceans, adapted from Sandeman et al., 1993 and Scholtz and Richter, 1995 (A) and Dixon et al., 2003 (B). The ellipses indicate those groups containing species in which studies have shown the presence of cell proliferation and the production of new neurons within sexually mature adults (see Schmidt and Harzsch, 1999). Evidence for the differentiation of newly born cells into neurons expressing the appropriate transmitters has been obtained only for the Astacida (Sullivan and Beltz, 2005a) and Achelata (Schmidt, 2001).
3. The deutocerebral organ
In 1968, a new organ was discovered and described in the deutocerebrum of C. maenas (Bazin and Demeusy, 1968). This first report was followed by an extensive comparative study of the deutocerebral organ in 14 species of decapod crustaceans taken from the Homarida, Astacida, Thalassinida, Achelata, Anomala, and Brachyura (Bazin, 1969a, b, 1970a, b). From these studies a general picture of the organization of the deutocerebral organ was gained, as well as cellular insights gleaned from histological and ultrastructural techniques. The deutocerebral organ consists of several basic components that are represented in all the species examined; although the disposition of these components varies between orders and species, the cellular components are all very similar and there is little doubt that the deutocerebral organ is a single structure, common to a large number of reptantian decapod crustaceans. Further, there is no doubt that the deutocerebral organ is the same structure that has more recently been described as the neurogenic niche (Figure 2) (Sullivan et al., 2005, 2007a; see section 4.1).
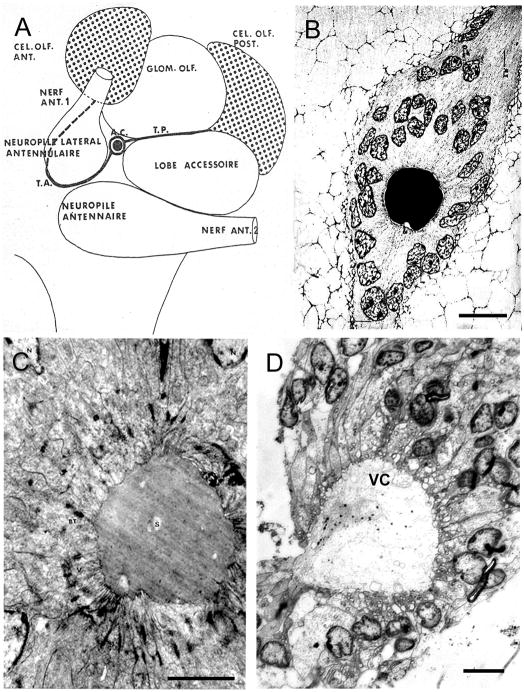
Figure 2.
A. Diagram of the left side of the brain of Astacus leptodactylus, seen from the ventral side and showing the disposition of the deutocerebral organ first described by Bazin (1969a, b). This organ consists of a central cluster of cells that surround a homogeneously staining cavity. The central cluster of cells are connected by thin strands of fibers that run laterally over the surface of the accessory lobe to end amongst the projection neurons in Cluster 10 and medially around the nerve root of the nerve from the antennule to end in the local olfactory neurons in Cluster 9. B. Light micrograph of a section through the cavity in the center of the deutocerebral organ cell cluster of A. leptodactylus showing the amorphous contents and the surrounding cells with characteristically dark granules in their nuclei. C. Electron micrograph of a section through the same area as in B of A. leptodactylus which shows the membranes of the cells surrounding the cavity to be sculpted into many finger like processes that contact the structureless contents. D. Light micrograph of a semi-thin, toluidine blue stained section through the neurogenic niche of Procambarus clarkii. The components of the neurogenic system of P. clarkii, with a central niche consisting of a cluster of cells surrounding a central cavity and linked by fiber strands to the local and projection olfactory neurons, is homologous with the deutocerebral organ of Astacus. Abbreviations: amas cellulaire, A. C.; groupe olfactif antérieur, CEL.OLF.ANT; groupe olfactif postérieur, CEL.OLF.POST.; glomérule olfactif, GLOM.OLF; tractus antérieur, T. A.; tractus postérieur, T. P.; terminal bar, BT; Cluster 9, CL9; Cluster 10, CL10; nucleus, N; substance in the cavity, S; villi, V; vascular cavity, VC. Scale bars: B, 20 μm; C, 15 μm; D, 20 μm. (A, from Bazin, 1969a; B and C are previously unpublished images from Bazin, 1969b; D from Zhang et al., 2009)
The deutocerebral organ is located on the ventral surface of the brain and close to the bilateral olfactory lobes (OLs) and, in those species where it is present, the accessory lobes (ALs) (Figure 2A). The most characteristic feature of the organ is a cluster of glial-like cells that surround a “cavity” which contains an apparently homogeneous matrix which in some cases appears to have some substructure (Figure 2B-D). A fibrous “tract” extends from the central cluster of cells laterally to the area containing the olfactory projection neurons (CL10) and medially to the olfactory local interneurons (CL9). Here, some variation occurs between species. In some, in particular the Astacida (see Figures 2A, 3A), the cavity and the surrounding cluster lies more or less midway between CL9 and CL10 and the cluster of cells around the cavity are polarized towards either the medial or lateral fiber tract. This pattern is also found in the anomalan Galathea squamifera (Figure 3B). In Pisidia longicornis, also an anomalan, the cell cluster and its cavity lie much closer to CL10 and are bound to it by a short tract; a much longer tract connects with CL9, but the overall system still maintains a bilateral nature (Figure 3C).
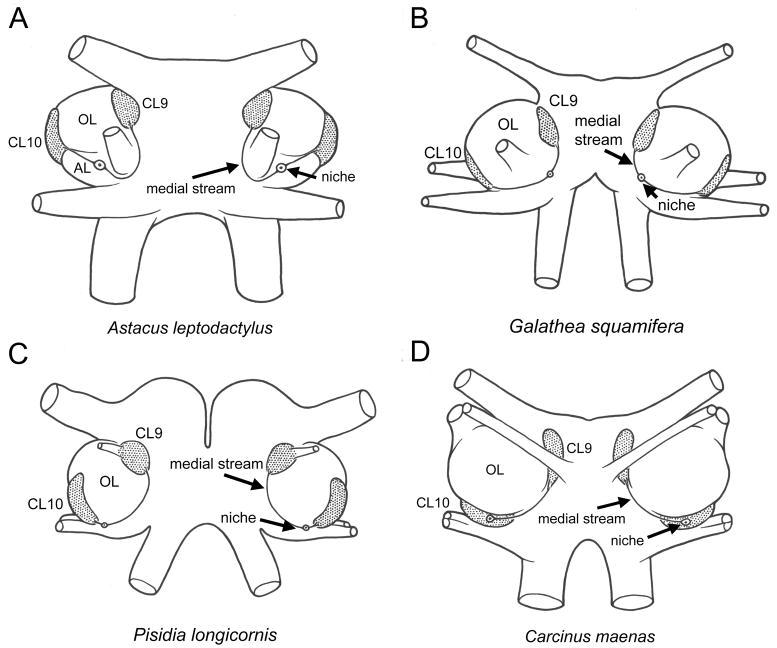
Figure 3.
Location of the components of the deutocerebral organ/neurogenic system on the ventral surface of the brains of the Astacida (A. leptodactylus), Anomala (G. squamifera, P. longicornus) and Brachyura (C. maenas). The central cell cluster/niche in Astacus leptodactylus lies on the ventral surface of the accessory lobe (AL) with a long medial migratory stream that curves around the nerve from the antennule and ends among the cells of the local olfactory interneurons in Cluster 9. The lateral migratory stream projects directly from the niche across the AL to end among the cells of the olfactory projection neurons in Cluster 10. B. Galathea squamifera is similar to A. leptodactylus, except that the niche lies more medial but is still separated from the local and projection neuron clusters by relatively long medial and lateral migratory streams. C. In Pisidia longicornis, the medial migatory stream is long, the lateral migratory stream very short and the niche is positioned close to the projection neuron cluster (CL10). D. The niche in Carcinus maenas is located within the olfactory projection neurons in Cluster 10. The accessory lobes in Galathea and Pisidia are relatively small in comparison with A. leptodactylus and reduced to a vestige in C. maenas. The migratory streams in these species therefore do not have to cross a large accessory lobe as in the astacids, and follow a path around the posterior and medial edges of the olfactory lobes (OL). Abbreviations: Cluster 9, CL9; Cluster 10, CL10; olfactory lobe, OL; accessory lobe, AL. Images are modified from Bazin 1969b. The diagrams are not to scale.
The brachyuran C. maenas, a representative of the latest order of the decapods to evolve, has the cell cluster and central cavity contained within CL10 and only a single tract which links it to CL9 (Figure 3D). Information for other members of the Brachyura is incomplete but suggests that they may have either 1 or 2 tracts, and hence a central cluster either contained within or just outside CL10. The different disposition of the deutocerebral organ and the more lateral location of the cell cluster and cavity in some species may be correlated with an unrelated anatomical feature, the accessory lobe, which decreased in size during the evolutionary progression from the Astacida to the Brachyura. The deutocerebral organ is always found on the ventral side of the brain, and in the Astacidae the tracts traverse the large accessory lobe that is characteristic of this group. The accessory lobe in the anomalans is considerably smaller, and in the brachyurans is represented by a very small cluster of glomeruli, completely dwarfed by the large OLs. Hence the difference in the lengths of the tracts and the location of the cavity and its surrounding cluster of cells may have no functional significance, but may simply reflect changes in overall brain architecture of the different species.
In Nephrops norvegicus, and Homarus gammarus, both homarids (marine clawed lobsters), Bazin’s deutocerebral organs consist of cells gathered around a unique medially situated central cavity containing an oval body that resembles an onion bulb in its layered structure. Although having the appearance of cuticle, no evidence has been supplied for a chitinous composition. Despite the curious inclusion and the medial location, this structure in these species was considered to be homologous with the deutocerebral organs of the other groups given the nature of the cells that surround the central cavity and the fiber tracts that appear to connect the structure to the cell body clusters 9 and 10 (Bazin, 1970). The relationship between this structure and the migratory streams and proliferation zones in Homarus americanus, defined by Sullivan et al. (2007b) is currently under investigation.
A very different organization is found in the anomalan Pagurus bernhardus. Here the cavity appears to be sac-like, and the surrounding cell cluster is associated with only one fiber tract; this variation is also true of Birgus latro and Coenobita clypeatus (see Section 6).
An indication of the functional significance of the deutocerebral organ is more likely to emerge from an understanding of the cellular nature of the components, and here the early descriptions show that the deutocerebral organs of all species share many common features. The cluster of cells surrounding a “cavity” is a common feature of the deutocerebral organ in all the species examined so far (although in some, e.g. Maia squinado, it is very small), and is unlike any other organ so far described in the crustaceans or in other arthropods. Indeed the closest parallel to this structure is found in the vertebrate neurogenic niche (see section 7).
The cells that surround the cavity appear to be of a single type in the astacids but an additional cell type is present in all the anomalan and brachyuran species that have been examined (Bazin, 1969a, b). Type 1 cells are arranged radially around the central cavity, and are generally fusiform. Proximally they lie tightly packed along the edge of the cavity where their cell boundaries contain fine invaginations and microvilli that extend into the cavity and terminal bars indicating a re-inforcement of the contacts between the cells (Bazin, 1969b). The distal extensions of the type 1 cells are drawn out into fine fibers that are aligned along the tracts. The nuclear envelope of these cells is crevassed and the nuclei contain abundant, irregularly distributed chromatin (Bazin, 1969a, b). Type 1 cells therefore closely resemble glial cells in their anatomy and fine structure (Bazin, 1969b). The type 2 cells in Galathea squamifera and other species are located at the periphery of the cell cluster and distinguished from the type 1 cells by having larger nuclei and found mainly at the point where the fiber tract leaves the cluster to extend to the proliferation zones in CL9 and CL10. Cell divisions in both cell types have been observed.
The cavity at the center of the cell cluster is still a puzzle. Some histological and immunocyto-chemical treatments show this to have a variable structure in different species. In most cases the contents of the cavity are reported to be “homogeneous” and attempts to define its nature using histochemical or immunocytochemical methods have not yet revealed what the cavity contains. There are also conflicting results concerning its connection to the vascular system. Bazin (1969) was unable to find any evidence of a connecting link between the cavity in Astacus leptodactylus and the vascular system. Song et al. (2009) were also unable to demonstrate a connection between the vascular system and the niche in P. clarkii. However, injection of a low molecular weight fluorescent dye into the perfused brain via the dorsal artery (Figure 4D) or directly into the pericardium of P. clarkii resulted in the clear presence of this dye within the cavity (Sullivan et al., 2007a; Benton et al., 2010), as did injections into the dorsal artery of Cherax destructor (Sandeman et al., 2009). Dye injection into the cavity itself also suggests some form of physical connection, as dye fills not only the cavity, but also nearby vessels (Sandeman and Beltz, unpublished results). Serial semi-thin sections and electron micrographs of P. clarkii (Figure 2D) have not revealed any direct connection to the blood system although in some images it is clear that the cavity is very closely apposed to a blood vessel, from which it is separated by what appears to be a retia-like complex of fine channels. This aspect of the system has not yet been satisfactorily clarified.
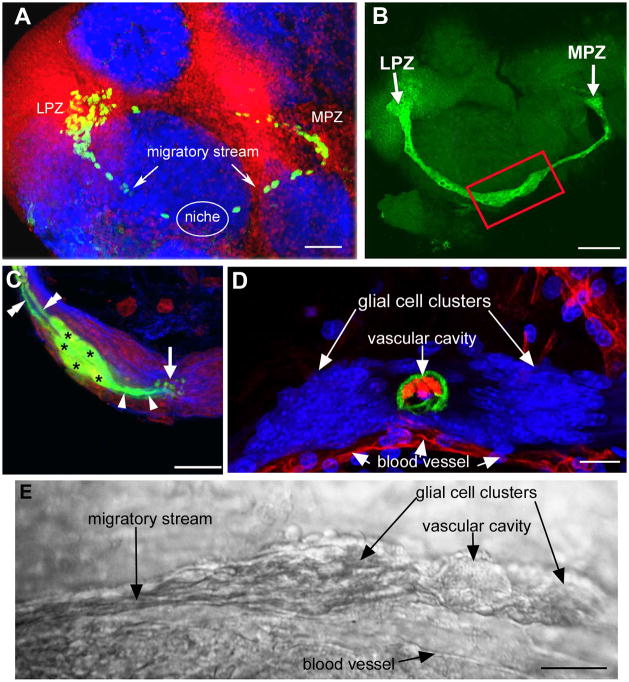
Figure 4.
The proliferative system maintaining adult neurogenesis in the central olfactory pathway of the crayfish Procambarus clarkii. A. Left side of the brain of P. clarkii labeled immunocytochemically for BrdU (green). Labeled cells are found in the lateral proliferation zone (LPZ) contiguous with cluster 10 and in the medial proliferation zone (MPZ) near cluster 9. The two zones are linked by a chain of labeled cells in a migratory stream that originates in the oval region labeled “niche”. Labeling for Drosophila synapsin (blue) and propidium iodide (red) is also shown. B. Both the LPZ and MPZ are contacted by the processes of a specialized population of glial cells immunoreactive to glutamine synthetase (green). The somata of these neurons form a cluster, the niche (red box), on the ventral surface of the brain. C. Glial cells (green, asterisks) in the niche labeled by intracellular injection of Lucifer yellow, have short processes (single arrow heads) that project to, and end in small swellings around the vascular cavity (arrow) and longer fibers (double arrow heads) that fasciculate together to form the tracts projecting to the LPZ and MPZ. (blue: glutamine synthetase; red: propidium iodide). D. The vascular cavity in the centre of the glial soma cluster in a brain in which the brain vascular system was filled by injecting a dextran dye solution into the cerebral artery. The cavity, outlined in green by its reactivity to an antibody to Elav, contains the dextran dye (red) which is also contained within a larger blood vessel that runs along beneath the niche. Propidium iodide (blue) labeling of the glial cell nuclei is also shown. E. Differential interference contrast image of a living niche dissected from the ventral surface of the brain. The glial clusters, vascular cavity, migratory stream and blood vessel shown in the labeled preparation are all clearly distinguishable in the living system. Scale bars: A, 100μm; B, 75μm; C and D, 20μm; E, 25μm. (Images from Sullivan et al., 2007b)
At the time of Bazin’s first description of the fiber tracts, the possibility that they could be migratory pathways was not determined. However, it was established that they are not axon bundles based on their anatomy and the fact that they do not begin or end in any neuropil (Bazin, 1969). It was found that the fiber tracts contained cells, the nuclei of which closely resembled those in the cells surrounding the cavity and that granulations within them were again characteristic of some glia cells; the cells along the tracts were also known to divide. The fiber tracts were no longer visible, however, once they enter CL9 or CL10.
It is interesting to note that in several arthropods (Opilion, Diplopodes, Solifuga) similar groups of cells around cavities are described as “organs neuraux intracerebraux” (Juberthie 1964, 1966; Juberthie-Jupeau 1966; Juberthie and Munoz-Cuevos 1970; Junqua 1966). In future studies is will be important to include these animals in an examination of arthropod species beyond the decapods and determine whether these structures are also involved in adult neurogenesis in these species.
4. The Crayfish Neurogenic System
The application of immunocytochemical techniques to the brain of P. clarkii during investigations of adult neurogenesis resulted in the rediscovery of the deutocerebral organ (Bazin, 1968) in these animals, and provided the first indication of its function (Sullivan et al., 2005, 2007a). The application of BrdU to label cells in the S phase of the cell cycle, was the key approach in these studies. That a slender trail of BrdU-labeled cells extended laterally from the proliferation zones in CL9 and medially from CL10 had been noted in many of the animals studied, but its relevance to migration was not appreciated. One reason for this may be that the stream is only observed in short-survival time BrdU studies; when the survival time after BrdU application is longer than a few days, migrating cells that would have been BrdU-labeled have completed their migration into Clusters 9 and 10, and so in these situations the migratory streams are not observed. The presence and appearance of the trail therefore was quite variable from one group of preparations to the next, even in a single species.
4.1. The neurogenic niche, aka the deutocerebral organ
Glutamine synthetase is localized in glial cells in vertebrates and crustaceans (Norenberg, 1979; Linser et al., 1997). An antibody against this molecule revealed the entire deutocerebral organ of Bazin for the first time since its original discovery (Figure 4B) (Sullivan et al., 2005). These studies, later published in their entirety in Sullivan et al., 2007a, demonstrated that the BrdU-labeled cells in CL9 and 10 (Figure 4A) are linked by a tract of fibers running across the ventral surface of the accessory lobe, to a cluster of spindle-shaped cells lying almost half-way between them (Figure 4C) and that the cluster of cells surrounded a “cavity” (Figure 4C–E). Immunocytochemical and ultrastructural studies on P. clarkii confirmed the earlier studies of Bazin on A. leptodactylus in every detail. While there is variation in terms of the disposition of the various components of the organ in the Astacida, P. clarkii and A. leptodactylus are very similar indeed and the immunocytochemical studies have added little to what was already known about the architecture of the organ, described above. The recent studies, though, extended the original anatomical work and have revealed the neurogenic function of the deutocerebral organ and the migratory nature of cells located along the fiber tracts.
The glutamine synthetase immunoreactivity of the fiber tract, the labeling of dividing cells in the tracts and olfactory interneuron clusters with BrdU, the staining of the cell cluster around the cavity with propidium iodide, and the apparent association of the central cavity with the blood vascular system (see Figure 4), led to the hypothesis that Bazin’s deutocerebral organ is a system for the generation of new neurons in the adult crayfish brain (Sullivan et al., 2007a, b; Zhang et al., 2009). This system could replace the embryonic neuroblasts when these die during late embryonic or early larval stages. A tentative model suggested that neuronal precursor cells in the central cluster of cells divide and that their daughters migrate medially and laterally from this cluster to the PZs in CL 9 and 10. A consistent feature of some of the BrdU-labeled cells in the tract, near where these fuse with CL9 and CL10, is that they often contain much larger nuclei than other labeled cells in the adjacent proliferation zones (PZs). The migrating cells and those that reach the proliferation zones divide, and those with large nuclei are likely to represent cells in the G2 phase of the cell cycle. For this model to be confirmed, evidence was needed that: 1. cells in the tracts indeed migrate from the central cluster to the lateral and medial PZs; 2. further division of these cells in the PZs leads to the production of cells that survive but no longer undergo division; 3. these cells differentiate into neurons. Finally, the origin of the migrating neuronal precursors needed to be tied to cells that divide within the central cluster of the niche. The methods available for assaying the properties of the deutocerebral organ/neurogenic niche components (cavity, central cluster, fiber tracts and PZs) were limited to immunocytochemical and histological methods in which labeling was restricted to specific time windows to provide a series of “snapshots” of a large number of individual animals that were subjected to different protocols (Figure 4).
4.2. Migration of neuronal precursor cells
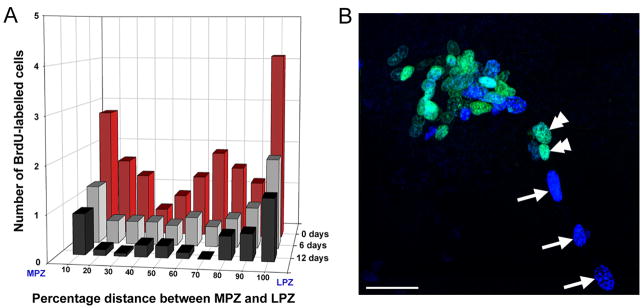
The hypothesis that the BrdU-labeled cells in the tracts were migratory was tested in two ways. First, animals were exposed to BrdU in the water in which they lived for 10 hours, then removed from this solution to fresh pond water. Each day for the next 12 days, several individuals were sacrificed and processed immunocytochemically for detection of BrdU. The locations of the labeled cells appearing along the tracts were plotted in terms of the distance between the central cluster and the PZs (Figure 5A). It was found that many cells were present all along the tracts for the first 2 to 3 days after which their numbers in the tract began to diminish; 12 days after the removal of the animals from the BrdU no labeled cells were found in the tracts. However, many cells within the PZs were labeled. The interpretation of this result was that BrdU remains available in the tissues of the animals for a few days (i.e., the “BrdU clearing time”; see Benton and Beltz, 2002), but that after this time the only cells to be labeled are those that were initially labeled as well as their progeny. That these were no longer present in the tracts after 12 days could only mean that they had migrated away or died.
Figure 5.
A. Distribution of BrdU-labelled cells within the glial tracts over time. B. The glial tracts are migratory pathways to the proliferation zones. BrdU (cyan) and IdU (blue) labelling in the LPZ and the adjacent region of the glial tract in a double-nucleoside labelling experiment in which crayfish were exposed initially to BrdU and then six days later to IdU. The double arrowheads show BrdU-labelled cells in the region of the glial tract immediately adjacent to the LPZ while the arrows indicate IdU-labelled occurring along regions of the tract closer to the glial soma cluster. Scale bars = 40 μm. (Images from Sullivan et al., 2007a)
A second, more sophisticated experiment that confirmed the migration of cells from the cell cluster towards the PZs involved a similar initial BrdU protocol, except that 6 days after treatment with BrdU, the animals were immersed in a solution of IdU (iododeoxyuridine) and then sacrificed. Two antibodies allowed discrimination between BrdU and IdU; one antibody labels only BrdU, and another BrdU and IdU. It was found that cells were labeled either green (BrdU only), cyan (BrdU and IdU) or blue (IdU only). The distribution of labeled cells in the central cluster, the tracts and the PZs after 6 days showed that only blue cells were contained in the central cluster and along the tracts, whereas the PZs contained green, cyan and blue cells (Figure 5B). The only interpretation of this result is that initially-labeled BrdU cells move out of the central cluster, along the tracts and end up in the PZs. Later dividing cells take up the IdU and are found at the source (the central cluster), along the tract and in the PZs. On these grounds we have renamed the tracts of the deutocerebral organ, the migratory streams.
Further evidence for the role of the niche cells and the tracts formed by their long fibers was obtained by examining the distribution of the highly conserved molecule Lissencephaly1 (LIS1), a dynein binding protein that is involved in neuronal migration in vertebrate systems (Morris et al., 1998; Tsai et al., 2005). In P. clarkii, LIS1 is expressed by cells in the niche and their processes in the streams (Figure 6) (Zhang et al., 2009). Further, the shape changes that migrating cells undergo are consistent with nucleokinetic migration, where a leading process is extended in the direction of migration, followed by the saltatory forward movement of the nucleus. This mode of movement is utilized by newborn cells in the cerebellum that migrate along radial glial processes, as well as by migrating cortical neurons (Samuels and Tsai, 2004; Martini and Valdeolmillos, 2010). Our current studies are examining the migration of neuronal precursors along the streams in the crustacean brain in real time, in order to extend our understanding of this process.
Figure 6.
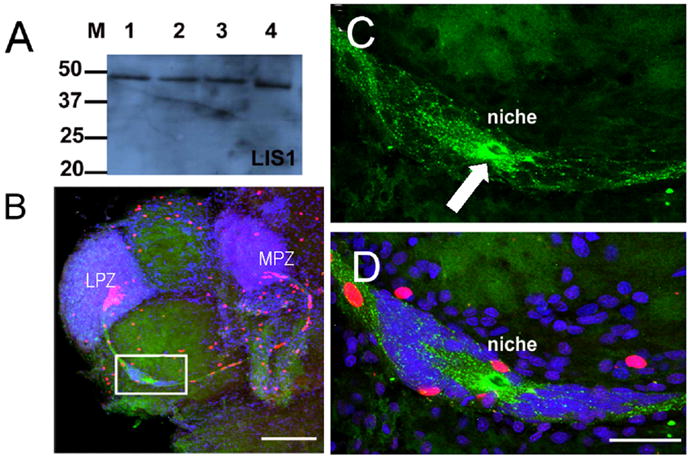
Precursor cells in the niche express LIS1 protein. A. Western blot for LIS1 on protein preparations from the P. clarkii brain (lane 1, 35mm carapace length (CL); lane 2, 16mm CL; lane 3, 8mm CL and adult mouse brain (lane 4). B. Whole mount brains were double-labeled for BrdU (red) and LIS1 (green) and counterstained with propidium iodide (blue), and optically sectioned with the confocal microscope. The white box outlines the niche, which is magnified in C and D. C. LIS1 staining is found throughout the niche cells and their fibers that form the stream, but is particularly strong immediately around the vascular cavity (white arrow). D. The same magnification as in C, but projecting all three channels in a single image. Scare bars: B, 200μm; C, D, 50 μm. (Images from Zhang et al., 2009)
4.3. Division of the cells in the proliferation zones
The BrdU labeling of cells in the PZs of CL9 and 10 was the first indication that neurogenesis was occurring in the brains of postembryonic and adult crustaceans (Harzsch and Dawirs, 1996; Schmidt, 1997). Since these initial studies, many assays of the numbers of cells that are produced and their survival over time have been undertaken. Such studies have revealed a great deal of information about the temporal dynamics of the neurogenetic process, and the influence of both internal (e.g., serotonin, nitric oxide) and external (e.g., photoperiod, seasonality, diet, environmental quality, social interaction) factors (reviewed in Beltz and Sandeman, 2003; Sullivan et al., 2007b). Evidence suggests that some of these factors influence the rate of proliferation (e.g., circadian signals, diet; Goergen et al., 2002; Beltz et al., 2007), while others (e.g., locomotory activity; Sandeman et al., in preparation) influence only the rate of cell survival in the PZs. Studies in which the presence of BrdU-labeled cells was demonstrated many months after their initial pulse labeling showed not only that many labeled cells were still present in the CL9 and 10, but that these had moved away from the PZs and resided among unlabeled, mature neurons (Beltz et al., 2001; Schmidt, 2001), suggesting that these labeled neurons had become integrated into the brain.
4.4 Differentiation of the cells in the proliferation zones
The persistence of labeled cell bodies in CL9 and 10, while highly suggestive, is not firm evidence that the cells have differentiated into functional neuronal elements integrated into the brain. More persuasive evidence for this comes from the injection of a fluorescent dextran dye (which is taken up by presynaptic endings of functional neurons) into the olfactory or accessory lobes of crayfish (Cherax destructor) that had been pulse labeled with BrdU and had survived for 4 months, resulted in double-labeling of some cell bodies with BrdU and dextran (Figure 7). These results provide strong evidence that BrdU-labeled cells in CL9 and 10, whose precursors originated in the neurogenic niche, develop branches in the appropriate neuropils of the brain (Sullivan and Beltz, 2005a).
Figure 7.
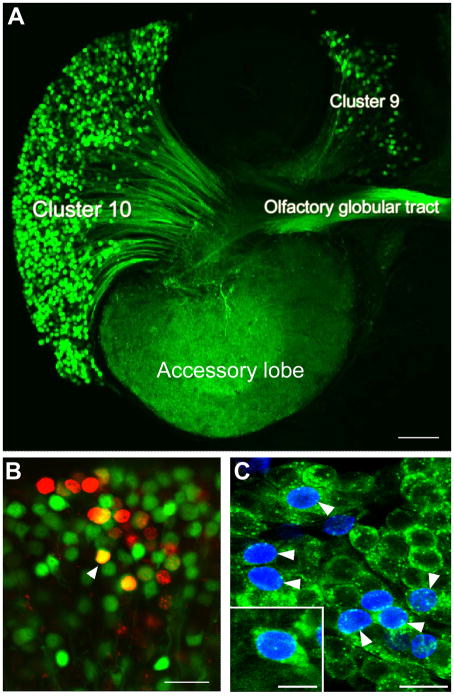
A. The left side of a brain of P. clarkii in which dextran was applied to the accessory lobe. The dextran (green) enters neurons that have their terminals in the accessory lobe and labels the corresponding cell bodies and axons. From this it is clear that both projection neurons in cluster 10, and local interneurons in cluster 9, have their terminals in the accessory lobes and the axons from the projection neurons lie in the olfactory globular tract. B. Cluster 10 cell bodies from an animal that was exposed to BrdU for 12 days and then maintained in fresh pond water for 4 months. At this stage the animal was killed and dextran fluorescein 3000 MW was applied to the accessory lobe (Sullivan and Beltz 2005c). Cells labeled red indicate that they passed through the cell cycle in the presence of BrdU. Cells labeled green indicate that they have terminals in the accessory lobe but did not pass through a cell cycle in the presence of BrdU. Double-labeled cells (orange) are cells that passed through a cell cycle in the presence of BrdU and have differentiated into neurons with their terminals in the accessory lobe. C. Cluster 10 cell bodies with BrdU (blue) and crustacean-SIFamide (green) label six months after being exposed to BrdU. Double-labeled cells, green and blue (arrowheads). Crustacean-SIFamide immunoreactivity is known to be expressed in olfactory interneurons in P. clarkii (Yasuda-Kamatani and Yasuda, 2006) and the presence of double labeling indicates that these cells were born in the adult animal and have differentiated into olfactory interneurons. Scale bars: A, 10μm; B, C, 20μm; C insert, 10μm.
Confirmatory evidence comes from a second approach in which the BrdU labeling was combined with immunohistochemical assays for transmitters (Sullivan et al, 2007a). The projection neurons in the brains of crayfish are immunoreactive to antibodies raised against SIFamide, while the local interneurons in CL9 label with antibodies raised against allatostatin and orkokinin. The double labeling of CL9 and 10 cells in animals that had been pulse labeled several months earlier with BrdU, demonstrated that newborn neurons express signaling molecules appropriate for the neurons in their respective cell clusters, again confirming that adult-born cells have differentiated properties consistent with their final locations.
4.5 The source of the neuronal precursors
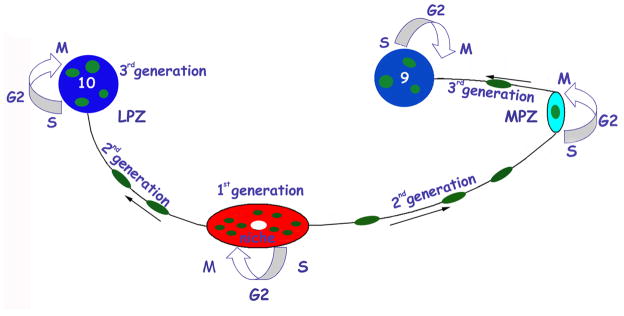
The studies cited above have therefore demonstrated that the newborn cells in CL9 and 10 in the crayfish originate from primary (1st generation) neuronal precursors located in the neurogenic niche, the central cluster of cells of the deutocerebral organ. Their daughters, the 2nd generation precursors, migrate from the neurogenic niche towards proliferation zones in CL9 and 10; here they divide again, perhaps several times, and differentiate into neurons that are a functional part of the olfactory system (Figure 8). It is reasonable to assume that the 1st-generation neuronal precursor cells in the central cluster are stem cells that undergo continuous, self-renewing divisions, as do functionally analogous cells in the vertebrate brain. Such divisions could be geometrically asymmetric, as are typical of neuroblast divisions, or geometrically symmetrical but with asymmetric cell fates: one cell that remains in the central cluster would be self-renewing and part of an immortal (or long-lived) lineage, while the second daughter cell would produce descendants that would differentiate into neurons.
Figure 8.
Model summarizing our current view of events leading to the production of new olfactory interneurons in adult crayfish. Neuronal precursor (1st generation) cells reside within a neurogenic niche where they divide symmetrically. Their daughters (2nd generation precursors) migrate along tracts created by the fibers of the niche cells, towards either the LPZ or the MPZ. At least one more division will occur in the LPZ and MPZ before the progeny (3rd and subsequent generations) differentiate into neurons.
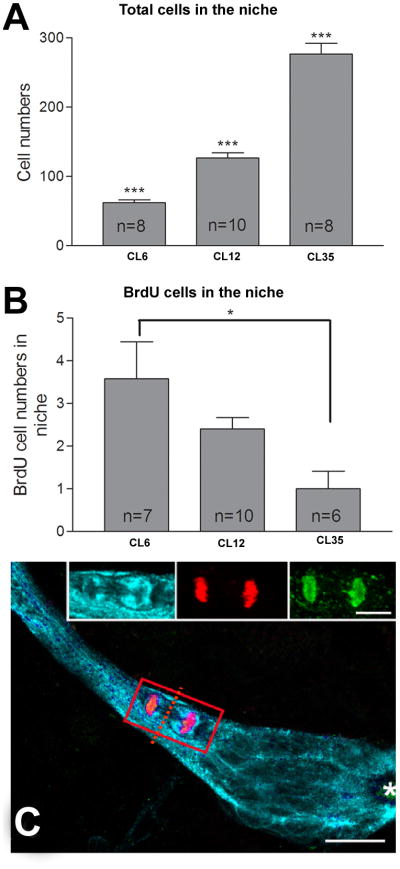
However, a large number of experiments utilizing the crayfish niche and streams do not support the above scenario. Tests of the self-renewal capacity of the niche cells using double-nucleoside labeling with BrdU followed by EdU (5-ethynyl-2′-deoxyuridine) demonstrate that in no case does a single BrdU-labeled daughter cell remain on its own in the niche. Instead cells in the niche label only with the EdU that was applied after the BrdU and show that all the BrdU labeled cells must have left the niche (Benton et al., 2010). In support of these functional studies, anatomical asymmetry of dividing cells has never been observed among the niche cells in many hundreds of cases; second, both daughters of the cell divisions within the central cluster are positioned together along the migratory pathway, leaving no labeled cell behind in the cluster (e.g., see Figure 9C). These observations have some interesting consequences. From pulse BrdU experiments it is possible to calculate the time needed for the migratory stream to become clear of BrdU labeled cells which in turn provides an estimate of the rate at which new cells migrate away from the central cluster. If both daughter cells of the divisions in the central cluster move away, the population of cells residing in the central cluster would be expected to be depleted over time. However, careful counts of the cells in the central cluster shows this not to be the case; indeed, in larger animals the central cluster contains more cells than in smaller animals (Figure 9A, B). Our histological studies and those of Bazin in astacidan species do not show the presence of consistently located large cells among the niche cell population. We have therefore hypothesized that all the niche cells may have the capacity to become neuronal precursors, and that the large BrdU-labeled cell profiles observed represent niche cells whose nuclei are enlarged during DNA replication, and whose cytoplasmic processes have been retracted. Several lines of previously published evidence support this theory. Firstly, all niche cells label with the G1-phase marker MCM2-7, except when they are progressing through S to M phase (Sullivan et al., 2007a). This indicates that the niche cells are actively in the cell cycle although resting in G1, suggesting that these are not terminally differentiated cells. Secondly, all niche cells, including those in M phase, label immunocytochemically for glutamine synthetase, a glial marker; this indicates common molecular properties shared among all the niche cells (Sullivan et al., 2007a). Thirdly, examination of semi-thin sections shows that the vast majority of niche cells have identical cellular characteristics (Zhang et al., 2009). Finally, the number of S-phase precursors in the niche is regulated by environmental enrichment, suggesting that there is a quiescent pool of precursor cells that can be recruited into the cell cycle in response to local conditions.
Figure 9.
Comparison of precursor cell total numbers and BrdU-labeled cells in the niches and the streams in different sizes of crayfish. The x-axis indicates the average carapace length (CL) in mm of the animals. Animals were exposed to BrdU for 8 hours prior to fixation, then immunolabeled for BrdU and glutamine synthetase and counterstained with propidium iodide. Total cell numbers and BrdU-labeled cells in the niches and streams were counted. Histograms A and B show the total numbers of cells and BrdU-positive cells in the niche respectively. Note a significant decrease in the numbers (B) of BrdU-labeled cells with increasing size. Significant difference between groups (Tukey multiple comparison) are marked with single (P<0.05), double (P< 0.01) or triple (P<0.001) asterisks. n = numbers of niches assayed in A-B. C. Triple-labeled M-phase cell close to the niche that has almost completed cytokinesis, immunolabeled with GS (cyan), phosphohistone-H3 (green) and BrdU (red). Images show a small area of cytoplasm that is still shared between the two emerging, geometrically symmetrical daughter cells. The cytoplasm of the dividing cells is GS-positive, a characteristic feature of the cells residing in the niche, thus confirming the ancestry of these cells. The asterisk marks the vascular cavity. Scale bar: C, 20μm; insets, 10μm.
Our conclusion from the studies described above is that the niche cell population is not self-renewing, and that “pre-stem” cells must be recruited into the niche cluster from outside the neurogenic system (Zhang et al., 2009). Similarities in the cytological features and size of cells in the vasculature and the niche precursors have been noted in both crayfish and lobsters (Schmidt, 2007a; Zhang et al., 2009), indicating a potential association between cells of hematopoietic origin and the niche precursors. Access to the niche by such cells could be via the blood and vascular cavity, or through the extracellular milieu, as the niche is not membrane-bounded. One caveat that could influence these conclusions would be the possibility that chromosomes might segregate non-randomly during divisions, so that the older “parental” DNA is retained in daughter stem cells, while the new strands are relegated to the daughter that will differentiate. This immortal strand hypothesis proposed by Cairns (1975; see also 7.1) predicts that the stem cell DNA will not be labeled with pulsed nucleotide analogs after their first division; hence these markers would be ineffective in revealing the 1st-generation neuronal precursors. Could such a situation apply to the crustacean niche precursor cells? We believe not because of the numbers of mitotic cells that we have observed in the niche; in all cases these have been geometrically symmetrical divisions and segregation of BrdU-labeled DNA into both daughters was observed in all telophase cells (e.g., Fig. 9C). Further, the functional data and other observations presented here do not support the proposal by Song et al. (2009), who suggest that by virtue of the presence of large BrdU-labeled cells in the crayfish niche, the 1st generation niche precursors are persistent neuroblasts with self-renewal capabilities. Determining the developmental origins of the niche precursor cells is critical to our understanding of their relationship, if any, to the system of neuroblasts that generates the embryonic scaffold of the nervous system.
Given the proposal that the 1st-generation neuronal precursors in the niche are not self-renewing, can these be considered stem cells, and can the deutocerebral organ be compared to a mammalian stem cell niche? In recent publications, we have altered our references to the niche cells and now generally refer to these as “1st generation neuronal precursors”, to avoid semantic confusion and to emphasize their critical position in the neuronal lineage. And, given that the deutocerebral organ has many features that are shared with mammalian stem cell niches (Sullivan et al., 2007a; Schmidt, 2007), and that the 1st-generation neuronal precursors are housed, maintained and regulated within this structure, we believe the analogy with a stem cell niche is appropriate.
5. The deutocerebral organs and neurogenesis in the Achelata
Deutocerebral organs are also present in members of the Achelata (Bazin, 1970). In the spiny lobster Panulirus regius and the slipper lobster Scyllarus arctus, these are somewhat different in their location and general organization to those of the other members of the reptants, in that they possess two separate deutocerebral organs on each side of the brain and these are located separately amongst the cells of CL9 and CL10. However, the cellular components of the organs are virtually the same as those found in the evolutionarily later forms. A central cavity filled with an apparently amorphous substance is surrounded by a radially-arranged cluster of cells with the same glial-like appearance found in all the other species examined. The deutocerebral organ in CL9 is smaller than that in CL10, but otherwise is no different in its structure. A short tract links the deutocerebral organ in CL10 to the nearby proliferation zone but no tract was found that linked the two separate deutocerebral organs (Bazin, 1969b,Bazin, 1970).
Adult neurogenesis has been thoroughly investigated in the olfactory system of Panulirus argus, including studies of the survival and differentiation of newly-added neurons (Schmidt 2001; 2007a, b). The neurogenic structures in P. argus appear to be identical to the deutocerebral organs in P. regius and Scylla arctus. In P. argus they are found in both CL9 and CL10, and are described as a “clump” of cells located among the neuronal cell bodies of CL9 and CL10. The cells in the clump are glial-like and radially arranged around a central region containing material that labels lightly with methylene blue. BrdU labeling shows the clump of cells to be located close to the proliferation zones in CL9 and CL10. Of considerable interest is the recorded appearance in the central region of the clump of a large cell undergoing cell division, a feature not previously observed in any of the central cavities in the many species studied. The presence of the large cell contained within the cell clump led to the hypothesis that “large putative NBs (neuroblasts) in the vicinity of proliferation zones act as primary neuronal stem cells. By asymmetric cell divisions, these self-renew and generate daughter cells migrating into the proliferation zone. Here they divide once symmetrically and both daughter cells are pushed out of the proliferation zone and either differentiate into neurons (N) or die by apoptosis (very few). In essence, this process resembles very closely neurogenesis in the nervous system of embryonic and larval crustaceans and insects. One important difference is that the putative adult NBs are associated with specialized clumps of cells that may represent stem cell niches critically important for the unusual, life-long self-renewal and proliferative capacity of the putative adult NBs” (see diagrammatic representation, Figure 10) (Schmidt 2007a). Evidence for apoptosis using the TUNEL method has been obtained in both crayfish (Sandeman et al 1998) and clawed lobster brains (Harzsch et al 1999), and pycnotic nuclei were identified in spiny lobsters and taken to indicate the presence of apoptosis in those animals as well (Schmidt 2001). In P. argus, fibrous material extending between putative neuroblasts and the proliferation centers has been noted and could represent the short tract described by Bazin. The association between the blood vascular system and the cell clump and cavity in P. argus is evident with arterioles coming in contact with the organ but with no demonstrable continuity between the lumen of the blood vessel and the cavity (Schmidt, 2007a).
Figure 10.
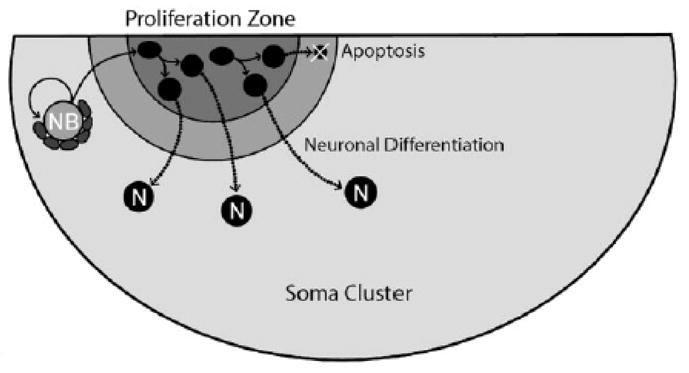
Schematic representation of the cellular basis of adult neurogenesis in the olfactory midbrain of Panulirus argus. Large putative adult neuroblasts (NB) in the vicinity of proliferation zones act as primary neuronal stem cells. By rapid asymmetric cell divisions they self-renew and generate daughter cells that migrate into the proliferation zone and there divide once symmetrically on a much slower time scale. After this mitosis, both daughter cells are pushed out of the proliferation zone and either differentiate into neurons (the vast majority) or die by apoptosis (very few). In essence this process resembles closely neurogenesis in the nervous system of embryonic and larval crustaceans and insects, where large neuroblasts undergo a series of asymmetric divisions in which they self-renew and generate smaller ganglion mothers cells, which in turn divide once symmetrically generating two neurons. One significant difference is that the putative adult neuroblasts (NB) are associated with specialized cellular aggregates that likely are critical for their unusual life-long capacity to self-renew and proliferate. (Image and legend adapted from Schmidt, 2007; request for permission to reprint is pending with John Wiley and Son, Inc.).
6. Neurogenesis in decapod crustaceans
Taken together, the initial anatomical studies on the deutocerebral organ and the later studies on the neurogenic system reveal an organ that is clearly homologous at the cellular and functional level, in a variety of decapod species. It is of interest to consider the possible evolutionary development of this neurogenic system (or complex, Song et al., 2009). Among the species that have been studied there are several separate “models” in which the location of the cellular components vary. If, in fact, the Achelata are the oldest of the reptant species considered here, they could represent the earliest form of the niche in which persistent neuroblasts responsible for the initial production of the cells in CL9 and CL10, each give rise to a separate niche in CL9 and CL10 which in adult life continue to supply new neurons to the growing populations of olfactory local and projection neurons (Schmidt, 2007a). In freshwater astacids, anomalans and brachyurans, the tendency appears to be the gradual movement of the niche along the migratory stream, away from CL9 and towards CL10, until in the brachyurans the niche is located in amongst the cells of CL10. A significant departure from this possible evolutionary line is found in hermit crabs. In Pagurus bernhardus, Birgus latro and Coenobita clypeatus there is only one niche and this is sac-like with the central cavity having a short “canal” leading towards the proliferation zone in CL9 (Bazin, 1969a, b; Harzsch, in preparation).
Three basic “models” of the neurogenic system according to phylogenetic position emerge from these studies of deutocerebral organs (Bazin, 1969b) and neurogenic niches (Sullivan et al., 2005,Sullivan et al., 2007; Schmidt, 2007a):
The Achelata in which CL9 and CL10 each have their own niches and there is no link between them;
The (homaridan), astacidan, anomalan and brachyuran models in which a niche is situated either between CL9 and CL10, or within CL10, from which cells migrate along fiber tracts or streams, either medially or laterally to the PZs in cell clusters 9 and 10 (e.g., see Figure 3); and
The anomalans (hermit crabs) in which the niche is sac-like and the cavity equipped with a canal leading to the PZ in CL9.
From a cellular and functional point of view, the niches in the various models are fundamentally no different. In all cases the niches are either located at, or linked to, cell clusters of neurons that are associated with olfactory processing. The differences in the organization of the neurogenic system may simply reflect the distinctive anatomical architectures of the brains and life styles of the different species. In the Achelata, CL9 and CL10 are widely separated from one another across a large and medially situated accessory lobe. The brain is also, unlike the other reptants, “folded” so that the olfactory lobes lie dorsal to the accessory lobes. The accessory lobe in the Astacida is large and long tracts are required to deliver the new cells from the niche to the PZs in CL9 and CL10. The accessory lobes of some of the anomalans (Galathea squamifera) are reduced in size but are still large enough to create a separation between CL9 and CL10 across which the precursor cells emerging from the niche have to traverse to reach their respective PZs. The accessory lobe in the brachyurans is extremely small, and, correlated with this, the tract that would have transferred the cells across the AL to CL10 is absent and the niche is now found within CL10. In the hermit crabs, anomalans, the AL is also very small, but these animals have a highly developed olfactory system, much enlarged olfactory lobes and often large hemiellipsoid bodies, also most likely associated with the olfactory system. The niche of these animals has a unique structure and, perhaps correlated with the emphasis on the olfactory sense, appears to deliver new neurons only to the olfactory local interneurons in CL9. Hence the variations in the location of the neurogenic system components may be traced to an original form in an evolutionarily basal species and a changing brain architecture in which animals become specialized in terms of a particular sensory modality.
An alternative to the above interpretation stems from a consideration of the cladogram shown in Figure 1B (Dixon et al., 2003). The end-points of the evolutionary lines leading to the five groups that we consider here reveal affinities between them (i.e. Homarida and Astacida, Anomala and Brachyura, and the Achelata on their own) that match the three different “models” of the deutocerebral neurogenic niches. In his first description of the deutocerebral organs, Bazin (1970) was lead to consider that the Homarida could represent the earliest form of the niche. To search for an early form of the niche and to test this hyothesis, the Dendrobranchiata, where it is known that neurons are added in the adult brain (Schmidt and Harzsch, 1999), is an obvious group of animals to explore.
7. Comparisons with adult neurogenesis in evolutionarily more recent species
Embryonic and adult growth phases are found in all non-vertebrate and vertebrate animals. In some species the adult growth is limited after they reach a “terminal” size at sexual maturity (insects, mammals); in others (crustaceans, reptiles), growth continues throughout their lives, although even in these the rate of growth slows with age and in some may cease entirely. Nevertheless in all animals the regeneration of some tissues either through continuous replacement, or to repair occasional damage, is a common feature requiring the presence of stem cells that live for many years and are the source of precursors for new cellular tissues. Longevity poses particular problems for such “immortal” cells because their exposure to environmental factors such as chemical mutagens or radiation could lead to mutation, thus degrading the fidelity of the genetic information they carry and, as stem cells, pass on.
Traditionally, neurons were excluded from the category of cells that could be either renewed or added to the adult complement. However, many examples have now been described of adult neurogenesis that cross the most widely separated phyletic boundaries and that exhibit remarkable similarities despite significant phylogenetic distance. The most common organ associated with the generation of new cells, and not only neurons, is the “niche”. These organs are small islands of tissue that may arise from embryonic tissue or de novo, are frequently associated with the blood vascular system and contain stem cells that are thought to undergo self-renewing divisions. Stem cells generally cycle slowly and infrequently, and so spend a high proportion of their time in the G1 phase of the cell cycle. One daughter of the division will retain stem cell properties, while the other daughter will undergo rapid division to expand the population, the “transit amplifying” cells (Doetsch, 2003). The descendants of these 2nd generation precursors leave the cell cycle and differentiate. Stem cell niches are found associated with the renewal of many tissues and organs, including cardiac, gastric epithelial, epidermal and brain tissues (Crisan et al., 2008; Popescu et al 2009; Bredemyer et al 2009; Fuchs 2009; Walker et al., 2009).
While neuronal stem cell niches in different organisms and in different locations do have unique properties, there are many parallels across the phylogenetic range. Among these are the glial characteristics of the primary neuronal precursor (stem) cells (but see the exception in zebrafish, below), a close association between the niche and the vasculature, the presence of a specialized extracellular matrix and basal lamina, directed migration of progeny, and regulation by environmental and endogenous cues. The remarkable similarities in neurogenic niches in so many different animals suggests either a very early and common mechanism as old as the nervous system itself or, as has happened in the evolution of many sensory organs, the convergence on a common mechanism as a prerequisite that accompanies longevity or some other ability provided by the adult-born neurons.
7.1 Primary neuronal precursors: the stem cells
The glial nature of neuronal stem cells in the adult mammalian brain is now well established (Doetsch et al., 1999; Kriegstein and Alvarez-Buylla, 2009). In contrast, the stem cells in zebrafish are not typical glia but retain neuroepithelial properties (Kaslin et al., 2009), and so variability in the cellular nature of stem cells may be observed as additional species are examined. The astrocytic precursors in mammals produce both glia and neurons (Gage, 2002), a feature that is distinct from the niche precursors in the crustacean brain, which appear to produce only neurons (Harzsch et al., 1999; Sullivan and Beltz, 2005). In the mammalian brain, the neural stem cells display typical astroglial markers such as GFAP and glutamine synthetase, in addition to stem cell markers such as nestin. As a group, however, the astroglia are a heterogeneous population, with some serving as neurogenic cells and others as quiescent stem cells (Ma et al., 2008; Riquelme et al., 2008; Kriegstein and Alvarez-Buylla, 2009). It has been proposed that the quiescent astroglia may interact, and may also retain the capacity to become neurogenic in the presence of appropriate signals (Nyfeler et al., 2005). In this regard, it is very interesting that the crustacean niche cells, which have a bipolar morphology (Fig. 4C), appear to serve as both as precursor and support cells (Sullivan et al., 2007a). As in the mammalian brain, it is not known whether all niche cells have the capacity to become neuronal precursors (see discussion of this issue in 4.5).
It is thought that the relatively infrequent divisions of stem cells in a variety of tissues may be one mechanism by which the fidelity of the DNA is preserved in these long-lived cells (Gage et al., 2008). Mutational dangers are a potential weakness for “immortal” cells that serve as the basis for regeneration and renewal of adult organs and tissues; these cells will accumulate mutations that occur during DNA replication, passing the errors to their descendants. Indeed, many cancers are thought to originate from such errant stem cells (Vescovi et al., 2006). The immortal strand hypothesis (Cairns, 1975; described in 4.5) suggests that the original genetic code would be preserved in the stem cells and replication-related mutations would be kept to a minimum. There is evidence both in favor of (Lark and Bird, 1965; Potten et al., 1978, 2002; Karpowicz et al., 2005; Rando, 2007) and against (Kiel et al., 2007; Lansdorp, 2007) the immortal strand hypothesis, with strong arguments on both sides. The current debate can only be settled with further testing in a variety of contexts and species, and in situations where the identity of the stem cells is not controversial.
7.2 The vascular association
The blood vascular system has been receiving increasing attention as a common feature of all niches, including those in the nervous system (Tavazoie et al., 2008). In both the subventricular (SVZ) and subgranular (SGZ) zones that support adult neurogenesis of olfactory bulb and hippocampal neurons, respectively, the stem cells lie in close proximity to a rich plexus of blood vessels (Figure 11). Endothelial cells, which are known to regulate stem cell self-renewal and neurogenesis via a cadre of secreted factors, are emerging as critical components of neural stem cell niches (Shen et al., 2004, 2008; Riquelme et al., 2008). Blood vessels also are conduits for circulating hormones and cytokines released from distant sources (Lennington et al., 2003). In addition, the associated extracellular matrix appears to provide a means of cell anchoring in the niche (Shen et al., 2008), as well as creating a supportive microenvironment and architecture (Riquelme et al., 2008). While details of how the blood vasculature interacts with the crustacean neurogenic niche are not known, communication via the vascular cavity has been demonstrated in two different crayfish species using both injection of labeled dextrans directly into the dorsal artery (Fig. 3D) that vascularizes the brain, as well as into the pericardial cavity (Benton et al., 2010). Hence, that substances introduced into the blood vascular system find their way rapidly into the cavity of the niche in crayfish is not in doubt, only that the actual pathway has not been discovered. Given that the cavity is tightly surrounded by the niche cell cluster, the confluence of the vascular system with the cavity is more likely to be via a system of narrow intercellular spaces than a single relatively large capillary. The blood system also is closely associated with the niche in Panulirus argus (Schmidt, 2007a). A variety of cues regulate the numbers of niche cells and the rate of neurogenesis in crustaceans, and ongoing studies are defining how the vascular connections with the niche may support these actions.
Figure 11.
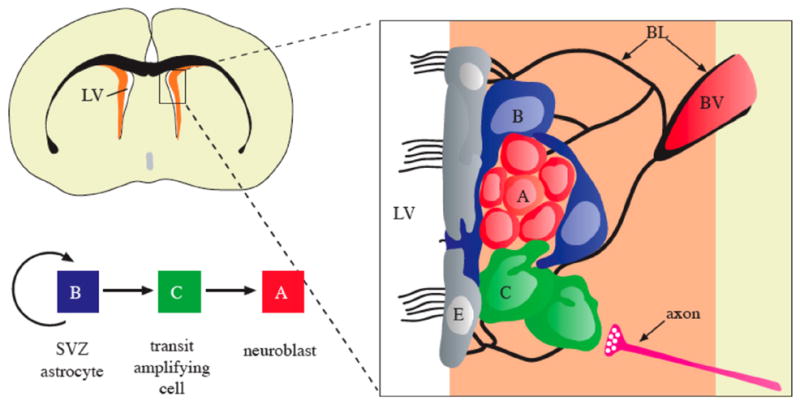
Cell types and anatomy of the adult SVZ niche. Schema of frontal section of the adult mouse brain showing the SVZ (orange) adjacent to the lateral ventricle (LV). SVZ astrocytes in this region (B, blue) are stem cells which generate migrating neuroblasts (A, red) destined for the olfactory bulb via a rapidly dividing transit-amplifying cell (C, green). Region in box is expanded at right to show the relationship of cells in this region and some elements of the SVZ niche. Multi-ciliated ependymal cells (E, grey) line the walls of the lateral ventricle. Chains of neuroblasts travel through tunnels formed by processes of SVZ astrocytes. Transit-amplifying cells are found in small clusters adjacent to the chains. Signals released from axons (pink) regulate proliferation and survival in this region. A specialized basal lamina (BL, black) extends from perivascular cells and contacts all cell types. Endothelial cells, blood vessels (BV) and the basal lamina are all likely key components of the niche. (Image and legend from Riquelme et al., 2008; permission to reprint granted by The Royal Society).
7.3 Directed migration of progeny
In the mammalian brain, the 3rd generation precursors (called neuroblasts) migrate away from the SVZ in the rostral migratory stream by means of chain migration; tens of thousands of cells travel this path to the olfactory bulb daily (Lois et al., 1996), along a route created by astrocytes that then communicate with the migrating cells and regulate their migration (Bolteus and Bordey, 2004). The rostral migratory stream is deeply embedded in the brain, and therefore it has been difficult to observe the migratory behavior of these cells in living organisms. Recently, MRI techniques in rats (Shapiro et al., 2006; Vreys et al., 2010) and two-photon time-lapse imaging in acute slices of mouse brain (Nam et al., 2007) have been used to examine the dynamic behavior of cells migrating in the RMS. These studies have shown that, while real-time observations of the RMS are possible, cell motility in the RMS is more complex than previously thought, involving multiple cell types, behaviors, speeds and directions (Nam et al., 2007). Neuronal precursors in the hippocampus also migrate, although over very short distances compared with the rostral migratory stream, which can be 5 mm in length in the adult mouse. In the crustacean brain, the primary neuronal precursor cells residing in the niche appear to serve as both procursor and support cells, with their long glutamine synthetase- and LIS1-labeled processes forming a tract along which the 2nd generation precursors travel. Like the RMS, the migratory streams in the crayfish brain are relatively long ---up to 1 mm in adult crayfish, and presumably even longer in larger species or in those where the placement of the niche relative to CL9 and 10 creates a particularly long route for precursors to reach the PZs. Unlike the RMS, however, the cells traversing the streams are few in number (<20 cells).
7.4 Regulation by environmental and endogenous cues
In all organisms, adult neurogenesis is highly regulated by a variety of signals, including living conditions (Kempermann and Gage, 1999; Sandeman and Sandeman, 2000; Scotto-Lomassese et al., 2000), hormonal cycles (Rasika et al., 1994; Harrison et al., 2001), physical activity (van Praag et al., 2005; van Praag, 2009), seasonality (Barnea and Nottebohm, 1994; Hansen and Schmidt, 2004) and the day-night cycle (Huang et al., 1998; Goergen et al., 2002; Jacobs, 2002), serotonin (Gould, 1999; Brezun and Daszuta, 2000; Beltz et al., 2001; Benton et al., 2008), and nitric oxide (Moreno-Lopez et al., 2004; Matarredona et al., 2005; Benton et al., 2007; Torroglosa et al., 2007). These factors influence the rate and timing of neuronal proliferation and survival in a variety of organisms, and the type of effect (up- or down-regulation) is generally consistent even between crustacean and mammalian species. These many parallels in the regulation of adult neurogenesis reinforce the suggestion that mechanisms controlling lifelong neurogenesis are conserved across a range of vertebrate and invertebrate species.
8. Summary and Conclusions
There are many parallels, as well as important differences, in the structures and mechanisms underlying the production of adult-born neurons in the brains of crustaceans and mammals. An understanding of shared properties will highlight fundamental aspects of adult neurogenesis that have been conserved or resulted from convergent mechanisms, while the differences will reveal areas where evolution may have transformed aspects of the pathway. For these reasons, non-vertebrate brains that undergo adult neurogenesis can provide a broad understanding of how evolutionary processes may have shaped the vertebrate/mammalian condition. Regarding adult neurogenesis in the crustacean brain, there are still many questions and challenges. What are the developmental origins of the niche and the neuronal precursor cells? What is the source of “pre-stem” cells that have been proposed to replenish the niche cell population in P. clarkii? What molecular changes are responsible for the reduction in cell proliferation with advancing age? Is the apparatus described by Bazin found beyond the decapods in other arthropod groups, or in other phyla? The original work of Bazin on the deutocerebral organ represents the first description in any organism of a neurogenic niche. Since then, the significance of these organs, their widespread occurrence, and the similarities across phylogenetic boundaries have been appreciated. The decapod deutocerebral neurogenic system is providing fresh insights into the production of adult-born neurons, and confirms that adult neurogenesis in these invertebrate species is a reliable phenomenon that has persisted in spite of evolutionary adaptations associated with varying life styles and habitats.
Acknowledgments
The authors thank J. Benton and Y. Zhang for technical assistance, critical readings of this manuscript and for contributions of images for figures. We are grateful to William Thomas (Colby Sawyer College, NH) and Nia Jones (Wellesley College) for translations from French of the PhD thesis of Francois Bazin. Experimental work was supported by NIH R01 MH67157 and NSF IOS 0818259.
References Cited
- Barnea A, Nottebohm F. Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadees. Proceedings of the National Academy of Science. 1994;91:11217–21. doi: 10.1073/pnas.91.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazin F. Étude comparée d’un organe deutocérébral chez les Crustacés Décapodes Reptantia. Comptes Rendus de l’Académie des Sciences. 1969a;269:958–961. [Google Scholar]
- Bazin F. PhD Thesis. Laboratoire de Biologie Animale et du Laboratoire Maritime, Université de Caen; France: 1969b. L’organe deutocérébral chez les Crustacés Décapodes Reptantia. [Google Scholar]
- Bazin F. Étude comparée de l’organe deutocérébral des Macroures Reptantia et des Anomoures (Crustacés Décapodes) Archives De Zoologie Experimentale Et Generale. 1970a;111:245–264. [Google Scholar]
- Bazin F. Les organs deutocérébraux chez deux Crustacés Décapodes Macroures Reptantia: Panulirus regius de Brito Capello, Scyllarus arctus (L.) Bulletin de la Societe Zoologique de France. 1970b;96:87–92. [Google Scholar]
- Bazin F, Demeusy N. Existance d’organes intracérébraux énigmatiques chez le Crustacé Décapode Carcinus maenas (L.) Comptes Rendus de l’Académie des sciences. 1968;267:356–358. [Google Scholar]
- Beltz BS, Sandeman DC. Regulation of life-long neurogenesis in the decapod crustacean brain. Arthropod Structure & Development. 2003;32:39–60. doi: 10.1016/S1467-8039(03)00038-0. [DOI] [PubMed] [Google Scholar]
- Beltz BS, Benton JL, Sullivan JM. Transient uptake of serotonin by newborn olfactory projection neurons may mediate their survival. Proceedings of the National Academy of Science. 2001;98:12730–12735. doi: 10.1073/pnas.231471298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz BS, Tlusty MF, Benton JL, Sandeman DC. Omega-3 fatty acids upregulate adult neurogenesis. Neuroscience Letters. 2007;415:154–158. doi: 10.1016/j.neulet.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton JL, Beltz BS. Patterns of neurogenesis in the midbrain of embryonic lobsters differ from proliferation in the insect and the crustacean ventral nerve cord. Journal of Neurobiology. 2002;53:57–67. doi: 10.1002/neu.10110. [DOI] [PubMed] [Google Scholar]
- Benton JL, Zhang Y, Kirkhart CR, Sandeman DC, Beltz BS. Primary neuronal precursors in the crayfish brain: self-renewal or replenishment from another source? Society for Neuroscience Abstracts. 2010;36:233.1. [Google Scholar]
- Benton JL, Sandeman DC, Beltz BS. Nitric oxide in the crustacean brain: regulation of neurogenesis and morphogenesis in the developing olfactory pathway. Developmental Dynamics. 2007;236:3047–60. doi: 10.1002/dvdy.21340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. Journal of Neuroscience. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredemeyer AJ, Geahlen JH, Weis VG, Huh WJ, Zinselmeyer BH, Srivatsan S, Miller MJ, Shaw AS, Mills JC. The gastric epithelial progenitor cell niche and differentiation of the zymogenic (chief) cell lineage. Developmental Biology. 2009;325:211–224. doi: 10.1016/j.ydbio.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Serotonin may stimulate granule cell proliferation in the adult hippocampus, as observed in rats grafted with foetal raphe neurons. European Journal of Neuroscience. 2000;12:391–6. doi: 10.1046/j.1460-9568.2000.00932.x. [DOI] [PubMed] [Google Scholar]
- Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255:197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- Cayre M, Strambi C, Charpin P, Augier R, Meyer MR, Edwards JS, Strambi A. Neurogenesis in adult insect mushroom bodies. Journal of Comparative Neurology. 1996;371:300–310. doi: 10.1002/(SICI)1096-9861(19960722)371:2<300::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Cayre M, Malaterre J, Scotto-Lomassese S, Strambi C, Strambi A. The common properties of neurogenesis in the adult brain: from invertebrates to vertebrates. Comparative Biochemistry and Physiology B. 2002;132:1–15. doi: 10.1016/s1096-4959(01)00525-5. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Bühring HJ, Giacobino JP, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Dufour MC, Gadenne C. Adult neurogenesis in a moth brain. Journal of Comparative Neurology. 2006;495:635–643. doi: 10.1002/cne.20909. [DOI] [PubMed] [Google Scholar]
- Farris SM, Robinson GE, Davis RL, Fahrbach SE. Larval and pupal development of the mushroom bodies in the honey bee, Apis mellifera. Journal of Comparative Neurology. 1999;414:97–113. doi: 10.1002/(sici)1096-9861(19991108)414:1<97::aid-cne8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Finding one’s niche in the skin. Cell Stem Cell. 2009;4:499–502. doi: 10.1016/j.stem.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH. Neurogenesis in the adult brain. Journal of Neuroscience. 2002;22:612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH, Kempermann G, Song H, editors. Adult Neurogenesis. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E. Serotonin and hippocampal neurogenesis. Neuropsychopharmacology. 1999;21:46S–51S. doi: 10.1016/S0893-133X(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Ganeshina O, Schäfer S, Malun D. Proliferation and programmed cell death of neuronal precursors in the mushroom bodies of the honeybee. Journal of Comparative Neurology. 2000;417:349–365. [PubMed] [Google Scholar]
- Goergen E, Bagay LA, Rehm K, Benton JL, Beltz BS. Circadian control of neurogenesis. Journal of Neurobiology. 2002;53:90–95. doi: 10.1002/neu.10095. [DOI] [PubMed] [Google Scholar]
- Hansen A, Schmidt M. Neurogenesis in the central olfactory pathway of the adult shore crab Carcinus maenas is controlled by sensory afferents. Journal of Comparative Neurology. 2001;441:223–233. doi: 10.1002/cne.1408. [DOI] [PubMed] [Google Scholar]
- Hansen A, Schmidt M. Influence of season and environment on adult neurogenesis in the cenral olfactory pathway of the shore crab, Carcinus maenas. Brain Research. 2004;1025:85–97. doi: 10.1016/j.brainres.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Cate HS, Swanson ES, Derby CD. Postembryonic proliferation in the spiny lobster antennular epithelium: rate of genesis of olfactory receptor neurons is dependent on molt stage. Journal of Neurobiology. 2001;47:51–66. doi: 10.1002/neu.1015. [DOI] [PubMed] [Google Scholar]
- Harzsch S. Neurogenesis in the crustacean ventral nerve cord: homology of neuronal stem cells in Malacostraca and Branchiopoda. Evolution and Development. 2001;3:154–169. doi: 10.1046/j.1525-142x.2001.003003154.x. [DOI] [PubMed] [Google Scholar]
- Harzsch S, Dawirs RR. Neurogenesis in the developing crab brain: postembryonic generation of neurons persists beyond metamorphosis. Journal of Neurobiology. 1996;29:384–398. doi: 10.1002/(SICI)1097-4695(199603)29:3<384::AID-NEU9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Harzsch S, Miller J, Benton J, Dawirs RR, Beltz B. Neurogenesis in the thoracic neuromeres of two crustaceans with different types of metamorphic development. Journal of Experimental Biology. 1998;201:2465–2479. doi: 10.1242/jeb.201.17.2465. [DOI] [PubMed] [Google Scholar]
- Harzsch S, Miller J, Benton JL, Beltz BS. From embryo to adult: persistent neurogenesis and apoptotic cell death shape the lobster deutocerebrum. Journal of Neuroscience. 1999;19:3472–3485. doi: 10.1523/JNEUROSCI.19-09-03472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Sato S. Progenitor cells in the adult zebrafish nervous system express a Brn-1-related POU gene, tai-ji. Mechanisms of Development. 1998;71:23–35. doi: 10.1016/s0925-4773(97)00199-8. [DOI] [PubMed] [Google Scholar]
- Jacobs BL. Adult brain neurogenesis and depression. Brain, Behavior, and Immunity. 2002;16:602–9. doi: 10.1016/s0889-1591(02)00015-6. [DOI] [PubMed] [Google Scholar]
- Juberthie C. Recherches sur la biologie des Opilions. Ann Speleol. 1964;19:1–238. [Google Scholar]
- Juberthie-Jupeau L. Existence d’ organes neuraux intracerebraux chez les Glomeridia (Diplopodes) épigés et cavernicoles. CR Acad Sci. 1967;264:86–92. [PubMed] [Google Scholar]
- Juberthie C, Muńoz-Cuevas A. Rôled des organes neuraux d’un Opilion Gonyleptidae, Pachylus quinamavidensis, dans la formation des globuli des corpora pedunculata. CR Acad Sci. 1970;270:1028–1031. [Google Scholar]
- Junqua C. Mém Mus Hist Nat. XLIII. Paris: édit du Muséum; 1966. Recherches biologiques et histophysiologiques sur un solifuge saharien Othoes saharae Panouse. [Google Scholar]
- Karpowicz P, Morshead C, Kam A, Jervis E, Ramunas J, Cheng V, Van der Kooy D. Support for the immortal strand hypothesis: neural stem cells partition DNA asymmetrically in vitro. Journal of Cell Biology. 2005;170:721–732. doi: 10.1083/jcb.200502073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. Experience-dependent regulation of adult hippocampal neurogenesis: effects of long-term stimulation and stimulus withdrawal. Hippocampus. 1999;9:321–32. doi: 10.1002/(SICI)1098-1063(1999)9:3<321::AID-HIPO11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Kaslin J, Ganz J, Geffarth M, Grandel H, Hans S, Brand M. Stem cells in the adult zebrafish cerebellum: initiation and maintenance of a novel stem cell niche. Journal of Neuroscience. 2009;29:6142–6153. doi: 10.1523/JNEUROSCI.0072-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, He S, Ashkenazi R, Gentry SN, Teta M, Kushner JA, Jackson TL, Morrison SJ. Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature. 2007;449:238–242. doi: 10.1038/nature06115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annual Review of Neuroscience. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdorp PM. Immortal strands? Give me a break. Cell. 2007;129:1244–1247. doi: 10.1016/j.cell.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Lark KG, Bird RE. Segregation of the conserved units of DNA in Escherichia coli. Proceedings of the National Academy of Sciences U S A. 1965;54:1444–1450. doi: 10.1073/pnas.54.5.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennington JB, Yang Z, Conover JC. Neural stem cells and the regulation of adult neurogenesis. Reproductive Biology and Endocrinology. 2003:13–99. doi: 10.1186/1477-7827-1-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linser PJ, Trapido-Rosenthal HG, Orona E. Glutamine synthetase is a glial-specific marker in the olfactory regions of the lobster (Panulirus argus) nervous system. Glia. 1997;20:275–283. [PubMed] [Google Scholar]
- Lindsey BW, Tropepe V. A comparative framework for understanding the biological principles of adult neurogenesis. Progress in Neurobiology. 2006;80:281–307. doi: 10.1016/j.pneurobio.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- Ma D-K, Ming G-L, Gage FH, Song H. Adult Neurogenesis. Chapter 11. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2008. Neurogenic niches in the adult mammalian brain. [Google Scholar]
- Matarredona ER, Murillo-Carretero M, Moreno-Lopez B, Estrada C. Role of nitric oxide in subventricular zone neurogenesis. Brain Research Brain Research Review. 2005;49:355–66. doi: 10.1016/j.brainresrev.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Martini FJ, Valdeolmillos M. Actomyosin contraction at the cell rear drives nuclear translocation in migrating cortical interneurons. Journal of Neuroscience. 2010;30:8660–8670. doi: 10.1523/JNEUROSCI.1962-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini-Morris SM, Albrecht U, Reiner O, Eichele G, Yu-Lee LY. The lissencephaly gene product LIS1, a protein involved in neuronal migration, interacts with a nuclear movement protein, NudC. Current Biology. 1998;8:603–606. doi: 10.1016/s0960-9822(98)70232-5. [DOI] [PubMed] [Google Scholar]
- Morris NR, Efimov VP, Xiang X. Nuclear migration, nucleokinesis and lissencephaly. Trends in Cell Biology. 1998;8:467–70. doi: 10.1016/s0962-8924(98)01389-0. [DOI] [PubMed] [Google Scholar]
- Moreno-Lopez B, Romero-Grimaldi C, Noval JA, Murillo-Carretero M, Matarredona ER, Estrada C. Nitric oxide is a physiological inhibitor of neurogenesis in the adult mouse subventricular zone and olfactory bulb. Journal of Neuroscience. 2004;24:85–95. doi: 10.1523/JNEUROSCI.1574-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam SC, Kim Y, Dryanovski D, Walker A, Goings G, Woolfrey K, Kang SS, Chu C, Chenn A, Erdelyi F, Szabo G, Hockberger P, Szele FG. Dynamic features of postnatal subventricular zone cell motility: a two-photon time-lapse study. Journal of Comparative Neurology. 2007;505:190–208. doi: 10.1002/cne.21473. [DOI] [PubMed] [Google Scholar]
- Norenberg MD. The distribution of glutamine synthetase in the rat central nervous system. Journal of Histochemistry and Cytochemistry. 1979;27:756–762. doi: 10.1177/27.3.39099. [DOI] [PubMed] [Google Scholar]
- Nyfelar Y, Kirch RD, Mantei N, Leone DP, Radtke F, Suter U, Taylor V. Jagged 1 signals in the postnatal subventricular zone are required for neural stem cell self-renewal. EMBO Journal. 2005;24:3504–3515. doi: 10.1038/sj.emboj.7600816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu LM, Gherghiceanu M, Manole CG, Faussone-Pellegrini MS. Cardiac renewing: interstitial Cajal-like cells nurse cardiomyocyte progenitors in epicardial stem cell niches. Journal of Cellular and Molecular Medicine. 2009;13:866–86. doi: 10.1111/j.1582-4934.2009.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS, Hume WJ, Reid P, Cairns J. The segregation of DNA in epithelial stem cells. Cell. 1978;15:899–906. doi: 10.1016/0092-8674(78)90274-x. [DOI] [PubMed] [Google Scholar]
- Potten CS, Owen G, Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. Journal of Cell Science. 2002;115:2381–2388. doi: 10.1242/jcs.115.11.2381. [DOI] [PubMed] [Google Scholar]
- Purves D. Body and Brain: a Trophic Theory of Neural Connections. Harvard University Press; Cambridge, MA: 1988. [DOI] [PubMed] [Google Scholar]
- Rando TA. The immortal strand hypothesis: segregation and reconstruction. Cell. 2007;129:1239–1243. doi: 10.1016/j.cell.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Rasika S, Nottebohm F, Alvarez-Buylla A. Testosterone increases the recruitment and/or survival of new high vocal center neurons in adult female canaries. Proceedings of the National Academy of Science. 1994;91:7854–8. doi: 10.1073/pnas.91.17.7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme PA, Drapeau E, Doetsch F. Brain micro-ecologies: neural stem cell niches in the adult mammalian brain. Philosophical Transactions of the Royal Society B: Biological Science. 2008;363:123–137. doi: 10.1098/rstb.2006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels BA, Tsai LH. Nucleokinesis illuminated. Nature Neuroscience. 2004;7:116901170. doi: 10.1038/nn1104-1169. [DOI] [PubMed] [Google Scholar]
- Sandeman DC, Sandeman RE, Derby C, Schmidt M. Morphology of the brain of crayfish, crabs, and spiny lobsters: a common nomenclature for homologous structures. Biological Bulletin. 1992;183:304–326. doi: 10.2307/1542217. [DOI] [PubMed] [Google Scholar]
- Sandeman D, Scholtz G, Sandeman RE. Brain evolution in decapod Crustacea. Journal of Experimental Zoology. 1993;265:112–133. [Google Scholar]
- Sandeman D, Beltz BS, Sandeman R. Crayfish brain interneurons that converge with serotonin giant cells in accessory lobe glomeruli. Journal of Comparative Neurology. 1995;352:263–279. doi: 10.1002/cne.903520209. [DOI] [PubMed] [Google Scholar]
- Sandeman DC, Kim FY, Beltz BS. Birth, survival and differentiation of neurons in the adult crustacean brain. doi: 10.1002/dneu.22156. In preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandeman R, Clarke D, Sandeman D, Manly M. Growth-related and antennular amputation-induced changes in the olfactory centers of crayfish brain. Journal of Neuroscience. 1998;18:6195–6206. doi: 10.1523/JNEUROSCI.18-16-06195.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandeman R, Sandeman D. “Impoverished” and “enriched” living conditions influence the proliferation and survival of neurons in crayfish brain. Journal of Neurobiology. 2000;45:215–26. [PubMed] [Google Scholar]
- Sandeman R, Sandeman D. Development, growth, and plasticity in the crayfish olfactory system. Microscopy Research and Technique. 2003;60:266–277. doi: 10.1002/jemt.10266. [DOI] [PubMed] [Google Scholar]
- Sandeman DC, Benton JL, Beltz BS. An identified serotonergic neuron regulates adult neurogenesis in the crustacean brain. Developmental Neurobiology. 2009;69:530–545. doi: 10.1002/dneu.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. Continuous neurogenesis in the olfactory brain of adult shore crabs, Carcinus maenas. Brain Research. 1997;762:131–143. doi: 10.1016/s0006-8993(97)00376-4. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Neuronal differentiation and long-term survival of newly generated cells in the olfactory midbrain of the adult spiny lobster, Panulirus argus. Journal of Neurobiology. 2001;48:181–203. doi: 10.1002/neu.1050. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Identification of putative neuroblasts at the base of adult neurogenesis in the olfactory midbrain of the spiny lobster, Panulirus argus. Journal of Comparative Neurology. 2007a;503:64–84. doi: 10.1002/cne.21366. [DOI] [PubMed] [Google Scholar]
- Schmidt M. The olfactory pathway of decapod crustaceans --an invertebrate model for life-long neurogenesis. Chemical Senses. 2007b;32:365–384. doi: 10.1093/chemse/bjm008. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Harzsch S. Comparative analysis of neurogenesis in the central olfactory pathway of adult decapod crustaceans by in vivo BrdU-labeling. Biological Bulletin. 1999;196:127–136. doi: 10.2307/1542558. [DOI] [PubMed] [Google Scholar]
- Scholtz G. Cell lineage studies in the crayfish Cherax destructor (Crustacea, Decapoda): germ band formation, segmentation, and early neurogenesis. Roux’s Archives for Developmental Biology. 1992;202:36–48. doi: 10.1007/BF00364595. [DOI] [PubMed] [Google Scholar]
- Scholtz G, Dohle W. Cell lineage and cell fate in crustacean embryos --a comparative approach. International Journal for Developmental Biology. 1996;40:211–220. [PubMed] [Google Scholar]
- Scotto Lomassese S, Strambi C, Strambi A, Charpin P, Augier R, Aouane A, Cayre M. Influence of environmental stimulation on neurogenesis in the adult insect brain. Journal of Neurobiology. 2000;45:162–71. [PubMed] [Google Scholar]
- Shapiro EM, Gonzalez-Perez O, Manuel Garcia-Verdugo J, Alvarez-Buylla A, Koretsky AP. Magnetic resonance imaging of the migration of neuronal precursors generated in the adult rodent brain. Neuroimage. 2006;32:1150–1157. doi: 10.1016/j.neuroimage.2006.04.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Sholtz G, Richter S. Phylogenetic systematics of the reptantian Decapoda (Crustacea, Malacostraca) Zoological Journal of the Linnean Society. 1995;113:289–328. [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CK, Johnstone LM, Edwards DH, Derby CD, Schmidt M. Cellular basis of neurogenesis in the brain of crayfish, Procambarus clarkii: Neurogenic complex in the olfactory midbrain from hatchlings to adults. Arthropod Structure & Development. 2009;38:339–360. doi: 10.1016/j.asd.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Song CK, Johnstone LM, Schmidt M, Derby CD, Edwards DH. Social domination increases neuronal survival in the brain of juvenile crayfish Procambarus clarkii. Journal of Experimental Biology. 2007;210:1311–1324. doi: 10.1242/jeb.02758. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Beltz BS. Newborn cells in the adult crayfish brain differentiate into distinct neuronal types. Journal of Neurobiology. 2005a;65:157–170. doi: 10.1002/neu.20195. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Beltz BS. Integration and segregation of inputs to higher-order neuropils in the crayfish brain. Journal of Comparative Neurology. 2005b;481:118–126. doi: 10.1002/cne.20346. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Sandeman DC, Beltz BS. Characterization of a putative stem/progenitor cell niche in the brain of an adult invertebrate, the crayfish Procambarus clarkii. Society for Neuroscience Abstracts. 2005;31:366.4. [Google Scholar]
- Sullivan JM, Benton JL, Sandeman DC, Beltz BS. Adult neurogenesis: a common strategy across diverse species. Journal of Comparative Neurology. 2007a;500:574–584. doi: 10.1002/cne.21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Sandeman DC, Benton JL, Beltz BS. Adult neurogenesis and cell cycle regulation in the crustacean olfactory pathway: from glial precursors to differentiated neurons. Journal of Molecular Histology. 2007b;38:527–542. doi: 10.1007/s10735-007-9112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torroglosa A, Murillo-Carretero M, Romero-Grimaldi C, Matarredona ER, Campos-Caro A, Estrada C. Nitric oxide decreases subventricular zone stem cell proliferation by inhibition of epidermal growth factor receptor and phosphoinositide-3-kinase/Akt pathway. Stem Cells. 2007;25:88–97. doi: 10.1634/stemcells.2006-0131. [DOI] [PubMed] [Google Scholar]
- Tsai LH, Gleeson JG. Nucleokinesis in neuronal migration. Neuron. 2005;46:383–388. doi: 10.1016/j.neuron.2005.04.013. [DOI] [PubMed] [Google Scholar]
- van Praag H. Exercise and the brain: something to chew on. Trends in Neuroscience. 2009;32:283–90. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. Journal of Neuroscience. 2005;25:8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nature Reviews Cancer. 2006;6:425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- Vreys R, Velde GV, Krylychkina O, Vellema M, Verhoye M, Timmermans JP, Baekelandt V, Van der Linder A. MRI visualization of endogenous neural progenitor cell migration along the RMS in the adult mouse brain: Validation of various MPIO labeling strategies. Neuroimage. 2010;49:2094–2013. doi: 10.1016/j.neuroimage.2009.10.034. [DOI] [PubMed] [Google Scholar]
- Walker MR, Patel KK, Stappenbeck TS. The stem cell niche. Journal of Pathology. 2009;217:169–180. doi: 10.1002/path.2474. [DOI] [PubMed] [Google Scholar]
- Yasuda-Kamatani Y, Yasuda A. Characteristic expression patterns of allatostatin-like peptide, FMRFamide-related peptide, orcokinin, tachykinin-related peptide, and SIFamide in the olfactory system of crayfish Procambarus clarkii. Journal of Comparative Neurology. 2006;496:135–147. doi: 10.1002/cne.20903. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Allodi S, Sandeman DC, Beltz BS. Adult neurogenesis in the crayfish brain: proliferation, migration, and possible origin of precursor cells. Developmental Neurobiology. 2009;69:415–436. doi: 10.1002/dneu.20717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Beltz BS. Glutamine synthetase, a functionally active enzyme in the crayfish brain. Society for Neuroscience Abstracts. 2010;36:737.14. [Google Scholar]