Abstract
Objective
Menopausal hot flashes can seriously disrupt the lives of symptomatic women. The physiological mechanisms of the hot flash efferent responses, particularly in the cutaneous circulation, are not completely understood. The aim of this study was to examine the mechanisms of increases in skin blood flow during the postmenopausal hot flash in symptomatic women.
Methods
Healthy postmenopausal women rested in a temperature controlled laboratory while responses prior to and during hot flashes were recorded for three unique protocols. Protocols 1 and 2: Women were locally pretreated with an intradermal injection of botulinum toxin A (BTX; blocks the release of neurotransmitters from sympathetic cholinergic nerves) in the forearm (protocol 1) and in the glabellar region (protocol 2). Protocol 3: Skin sympathetic nerve activity from the peroneal nerve was recorded, along with skin blood flow and sweating within the region innervated by that neural signal. Skin blood flow was indexed using laser-Doppler flowmetry at BTX-treated and adjacent untreated control sites. The onset of a hot flash was objectively identified as a transient and pronounced elevation of sternal sweat rate.
Results
The elevation in forearm (protocol 1) and glabellar skin blood flow (protocol 2) during hot flashes were attenuated at BTX sites relative to adjacent untreated sites (P<0.05 for both protocols). In protocol 3, skin sympathetic nerve activity significantly increased during hot flashes and returned to pre-flash levels following the hot flashes.
Conclusion
Elevations in skin blood flow during the postmenopausal hot flash are neurally mediated primarily through BTX sensitive nerves; presumably sympathetic cholinergic.
Keywords: Skin Blood Flow, Sympathetic Cholinergic, Menopause
Introduction
Hot flashes (or flushes) are a primary symptom of menopause that can seriously disrupt the lives of symptomatic women24. Approximately 70% of women experience hot flashes for 1–5 years following the onset of the menopause transition1, 24, 25, 29. The incidence and severity of symptoms are even higher in surgically-induced postmenopausal women and oncological female patients1, 4, 36. Hot flashes are typically defined as sudden subjective sensations of heat, frequently accompanied by skin flushing and perspiration13, 16, 24, 29. Symptomatic women also report a range of additional symptoms during a hot flash, such as anxiety, frustration, embarrassment, nausea and depression9, 10, 16, 24. Importantly, hot flashes can negatively affect concentration, sleep quality, sexual function, and result in fatigue and stress8, 24, 33, 34, 41, thereby significantly reducing the quality of life and overall health of afflicted women2, 6, 25, 34.
The physiological mechanisms associated with hot flashes, particularly in the cutaneous circulation, are not completely understood. We recently showed large increases in sternum and forearm skin blood flow during hot flashes of symptomatic postmenopausal women18, 27, confirming earlier speculations that skin blood flow increases during hot flashes5, 14, 15, 26. Such increases in blood flow of non-glabrous skin (i.e., hairy skin) could be achieved through the withdrawal of sympathetic vasoconstrictor activity, through the engagement of a separate sympathetic cholinergic active vasodilator system21, and/or through non-neural circulating factors, as previously proposed14, 39. We hypothesized that increases in skin blood flow during postmenopausal hot flashes are neurally mediated predominantly through the engagement of sympathetic cholinergic active vasodilation recognized to occur in human skin21. The aim of this study was, therefore, to examine the mechanisms responsible for increases in skin blood flow during the postmenopausal hot flash. This aim was achieved by recording skin blood flow responses during hot flashes at forearm and glabellar sites locally pretreated with botulinum toxin A (BTX) to block the release of neurotransmitters from sympathetic cholinergic nerves, as well as recording post-ganglionic sympathetic skin neural activity (SSNA), in postmenopausal symptomatic women.
Methodology
Subjects
Nineteen postmenopausal women participated in three separate but similar protocols. Not all subjects participated in each protocol. Their mean (±SD) age and body mass index (BMI) were 50 ± 5 yr (Range 41–60 yr) and 27 ± 6 kg.m2 (Range 21–42 kg.m2), respectively. All subjects had been amennorheic for at least 12 months and were experiencing at least 4 hot flashes a day (verified by a 7 day hot flash frequency journal completed prior to the study)33. Subjects were healthy and free of cardiovascular and metabolic diseases and were not taking hormone replacement therapy or any other treatments to alleviate hot flash symptoms. Subjects refrained from alcohol and exercise 24 h and caffeine 12 h before the study. Written, informed consent was obtained from all participants before they enrolled in the study. Procedures and consent forms were approved by the University of Texas Southwestern Medical Center at Dallas and Texas Health Presbyterian Hospital Dallas and were in agreement with the principles set by the Declaration of Helsinki.
Experimental Design
Experiments were performed in a temperature controlled laboratory (26 ± 1 °C) in the morning or early afternoon at least 2 h postprandial. For protocols 1 and 2, at least 3 days prior to experimentation, subjects received an intradermal injection of botulinum toxin type A (BTX, 10 units in 0.15 ml normal saline), to locally abolish sympathetic cholinergic neural transmission previously shown to be responsible for heat stressed induced cutaneous vasodilation22, 30, 31. This dosage and timeframe were used in order to be consistent with previous studies from our lab and others that used the same procedures and showed a consistent and clear BTX blockade of hyperthermia-induced elevations in skin blood flow23, 30, 31. BTX was injected in the forearm in protocol 1 (8 subjects) and in the glabellar region of the forehead in protocol 2 (4 subjects). Protocol 2 was conducted to confirm the findings of Protocol 1 but from an area of the body where hot flash symptoms are particularly prominent in symptomatic women33. In Protocol 3 (10 subjects), multifiber recordings of SSNA were obtained from subjects during hot flashes to provide additional insight/confirmation regarding a neural origin of observations from Protocols 1 and 2.
For all protocols, subjects rested in the semi-recumbent position for ~90 min while waiting for a hot flash to occur. The onset of a hot flash was objectively identified as a transient and pronounced elevation of sweat rate11, 12, 27. During this period 34 °C water was perfused through the tube-lined suit. Subjects were then exposed to a mild heat stress by perfusing 43–48 °C water through the suit for ~60 min. This mild heating was used to provoke hot flashes11, 24 as well as to verify the effectiveness of the BTX, which was confirmed by an absence of substantial cutaneous vasodilation at the BTX-treated site, relative to the adjacent control site, upon increase in the subjects' internal temperature of at least 0.4 °C22, 30, 31.
Instrumentation and Measurements
Subjects were placed in a tube-lined suit (jacket and/or pants; Med-Eng, Ottawa, Canada) that permitted the control of skin temperature by changing the temperature of the water perfusing the suit. In protocols 1 and 3, the tube-lined suit did not cover the forearm that received the BTX injection or the lower limb from which SSNA was obtained, respectively. Heart rate was obtained from an electrocardiogram (SpaceLabs, Redmond, WA) interfaced with a cardiotachometer (CWE, Ardmore, PA). Continuous beat-by-beat arterial blood pressure was recorded from a finger (Finometer, The Netherlands). Intermittent arterial blood pressure was also measured from the brachial artery by electrosphygmomanometry (SunTech, Raleigh, NC). Mean skin temperature was measured via the electrical average of six thermocouples attached to the skin37. Core temperature was measured from an ingestible pill telemetry system (HTI Technologies, Palmetto, FL) that was swallowed at least 2 h before data collection began. Cutaneous blood flow was indexed using multifiber laser-Doppler flowmetry probes (Perimed, North Royalton, OH). For protocols 1 and 2, laser-Doppler flow probes were placed over each injection site, as well as over adjacent untreated areas (i.e., control sites) that did not receive BTX. Sweat rate was continuously recorded using the ventilated capsule technique coupled with capacitance hygrometry (Viasala, Woburn, MA) or was indexed using galvanic skin conductance (Biopac, Santa Barbara CA). Subjects also indicated their subjective onset and offset of a hot flash by manually triggering a switch.
For protocol 3, multifiber recordings of SSNA were obtained using a tungsten microelectrode positioned in the common peroneal nerve. A reference electrode was placed subcutaneously ~2–3 cm from the recording electrode. The position of the recording electrode was adjusted until a site was attained in which bursts of SSNA were identified using previously established criteria7, 38. Nerve signals were amplified, passed through a bandpass filter with a bandwidth of 700–2000 Hz, integrated with a time constant of 0.1 s (Iowa Bioengineering, Iowa City, IA, USA), and were routed to an oscilloscope and a loudspeaker for monitoring throughout the study. Sweat rate was continuously recorded using capacitance hygrometry, together with skin blood flow, within the region of innervation of the recorded nerve (i.e., primarily dorsal foot).
Data Analysis
Data were sampled at 50 Hz in Protocols 1 and 2 and at 200 Hz for Protocol 3 via a data-acquisition system (Biopac System, Santa Barbara, CA). Because of the variance in the length of hot flashes, each hot flash was divided into 8 equal segments, with each segment representing 12.5% of hot flash duration. Five second periods of data at the end of each segment, and over a period of 2 min prior to and up to 5 min after the hot flash, were used in the statistical analysis. Cutaneous vascular conductance was calculated from the ratio of skin blood flow to arterial blood pressure. Cutaneous vascular responses were expressed as a percent increase in cutaneous vascular conductance from pre-flash baseline. Thirty seconds of SSNA data were analyzed during the following time points: 2–3 min prior to a hot flash (e.g., non hot flash), the peak of SSNA during the hot flash, and 2–3 min after the hot flash (e.g., post hot flash). SSNA was normalised relative to the pre-hot flash period, with mean SSNA of that period being assigned a value of 100 and subsequent changes expressed relative to that normalized baseline value.
Differences in cutaneous vascular conductance at the control and BTX sites prior to, during, and after the hot flash were evaluated using a repeated measures two-way ANOVA with main effects of time and site. Thermoregulatory, hemodynamic, and SSNA responses prior to, during, and after the hot flash were evaluated using a repeated measures one way ANOVA (main effect of time). Differences in steady-state thermoregulatory and hemodynamic responses, as well as the skin blood flow responses at control and BTX treated sites, between normothermia and at the end of the mild heat stress were assessed using paired t-tests or a repeated measures two-way ANOVA (main effects of time and site), where appropriate. All values are reported as means (±SD) unless indicated. P values of < 0.05 were considered statistically significant.
Results
Hot Flash Responses
Protocol 1
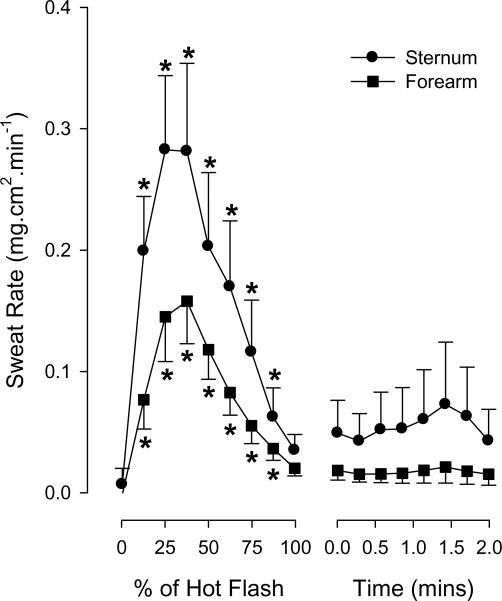
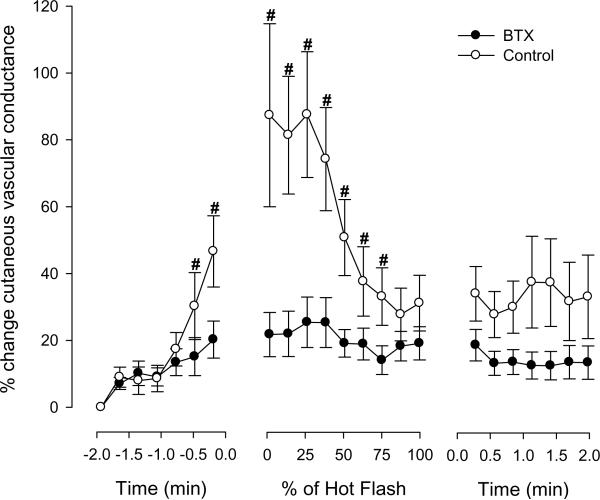
Eighteen hot flashes were recorded during the experimental sessions for protocol 1, with an average duration being 3.2 ± 1.6 min (range 1.5–7.4 min). Sternal and forearm sweat rate increased at the onset of each hot flash and then returned to baseline by the end of the flash (both P < 0.001; see Figure 1). Prior to the hot flashes, skin blood flow, as indicated by cutaneous vascular conductance, was similar between the control and BTX treated sites (0.55 ± 0.31 vs 0.59 ± 0.35 AU.mm Hg−1, respectively, P = 0.59). Relative to the non-hot flash state, forearm cutaneous vascular conductance at the untreated sites significantly increased ~45 sec prior to and throughout the first half of the hot flash (P < 0.001; see Figure 2). In contrast, although cutaneous vascular conductance at the BTX sites also increased during the hot flash (P < 0.05), this increase was greatly attenuated relative to the untreated sites (P < 0.001 for interaction effect of site and time; Figure 2). No detectable change in core temperature was identified during the 2 min period prior to the hot flash, or throughout the hot flash; thereafter, core temperature slightly decreased 1–2 min after the hot flash period (−0.04 ± 0.10 °C, P < 0.05). Mean skin temperature slightly increased during the initial stages of the hot flash (0.07 ± 0.09 °C, P < 0.05). Mean arterial blood pressure decreased during the hot flashes (9 ± 9 mmHg, P < 0.05), while heart rate increased (11 ± 7 beats.min−1, P < 0.05).
Figure 1.
Changes in sternal and forearm (at control site) sweat rate during the hot flashes from protocol 1. *P < 0.05 relative to pre hot flash indicated as 0% of Hot Flash.
Figure 2.
Changes in forearm cutaneous vascular conductance (CVC) at control and botulinum toxin type A (BTX) treated sites during hot flashes from protocol 1. #signifies significant difference between control and BTX-treated areas (P < 0.05), Data are expressed as a percentage change from pre-hot flash baseline.
Protocol 2
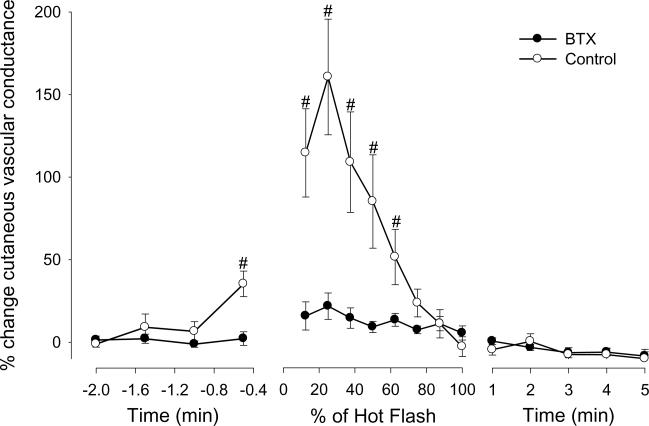
Eight hot flashes were recorded during the experimental sessions of protocol 2 (average duration 3.7 ± 1.7 min, range 1.8–6.4 min). Glabellar skin blood flow, indicated by cutaneous vascular conductance, was not different prior to the hot flashes between the control and BTX sites (0.93 ± 0.60 vs 0.84 ± 0.24 AU.mm Hg−1, respectively, P = 0.70). Similar to the forearm data of protocol 1, glabellar skin blood flow and cutaneous vascular conductance at the control sites increased just prior to and during the hot flash (P < 0.001). While subtle increases in these variables occurred at the BTX site during the hot flash (P<0.05), they were largely blocked by BTX treatment (P < 0.01 for interaction effect of site and time, see Figure 3). Other thermal and hemodynamic responses were similar to that observed in protocol 1.
Figure 3.
Changes in glabellar cutaneous vascular conductance (CVC) at control and botulinum toxin type A (BTX) treated sites during hot flashes from protocol 2. #signifies significant difference between control and BTX-treated areas (P < 0.05). Data are expressed as a percentage change from pre-hot flash baseline.
Mild heating after both protocols 1 and 2 caused expected increases in mean skin and core temperatures of ~3 and ~0.5 °C, respectively (see Table 1). This level of heating significantly increased skin blood flow and cutaneous vascular conductance from the non-treated sites (P < 0.01), whereas neither skin blood flow nor cutaneous vascular conductance changed at the BTX treated sites (P > 0.50; P < 0.01 for the interaction effect of time and site), thereby confirming the effectiveness of the BTX treatment21.
Table 1.
Mean ± SD steady-state thermoregulatory and cardiovascular responses during normothermia and heat stress for protocols 1 and 2. Mean arterial blood pressure data are from brachial artery electrosphygmomanometry.
| Normothermia | Heat stress | P | |
|---|---|---|---|
| Core temperature (°C) | 37.13 ± 0.19 | 37.64 ± 0.24 | <0.001 |
| Mean skin temperature (°C) | 34.67 ± 0.35 | 37.08 ± 0.60 | <0.001 |
| Heart rate (beats·min−1) | 69 ± 7 | 86 ± 8 | <0.001 |
| Mean arterial blood pressure (mm Hg) | 90 ± 7 | 84 ± 5 | 0.025 |
| Cutaneous Vascular Conductance | |||
| Forearm Control Site (AU.mm Hg−1) | 0.53 ± 0.44 | 1.06 ± 0.63 | 0.007 |
| Forearm BTX Site (AU.mm Hg−1) | 0.51 ± 0.41 | 0.57 ± 0.32 | 0.508 |
| Δ Sternal sweat rate (mg·cm2·min−1) | 0 | 0.70 ± 0.61 | <0.001 |
| Δ Forearm (control) sweat rate (mg·cm2·min−1) | 0 | 0.50 ± 0.60 | <0.001 |
AU: Arbitrary perfusion units from laser-Doppler flowmetry.
Protocol 3
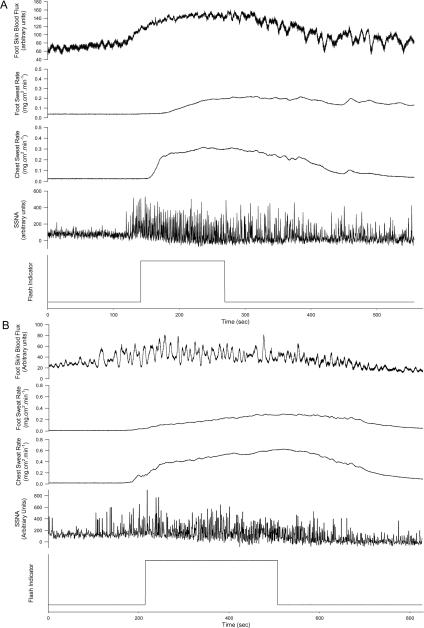
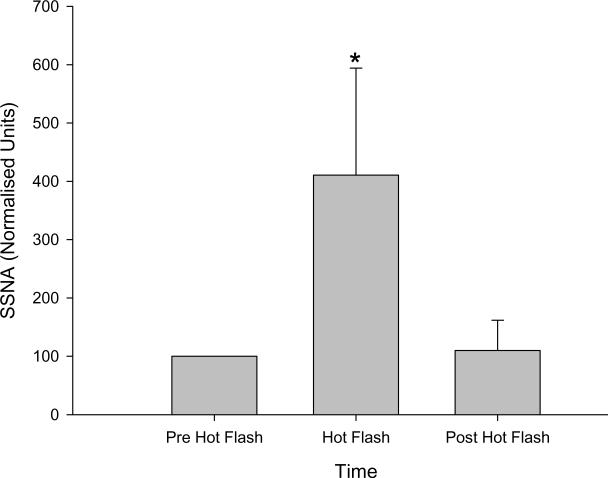
Seventeen hot flashes were recorded during the experimental sessions of protocol 3. SSNA increased by approximately four fold during these hot flashes (100 normalized units to 411 ± 183 normalised units, P < 0.001, see Figures 4 & 5) and returned to baseline after the hot flash (110 ± 52 normalised units, P = 0.99 relative to pre-flash baseline). Increases in SSNA were accompanied by increases in sweat rate (0.11 ± 0.05 to 0.16 ± 0.05 mg.cm2.min; P <0.05) and cutaneous vascular conductance (0.22 ± 0.13 to 0.44 ± 0.36 AU.mm Hg−1; P < 0.05) within the area of innervation of the recorded nerve.
Figure 4.
Representative data of dorsal foot skin blood flow, chest and dorsal foot sweat rate and skin sympathetic nerve activity (SSNA) recordings from two subjects (panels A and B) during hot flashes. The square wave on the lower panel of each tracing is when the subject indicated the subjective onset and offset of the hot flash.
Figure 5.
Mean skin sympathetic nerve activity (SSNA; normalized to pre hot flash activity, e.g., pre-hot flash SSNA assigned a value of 100 and subsequent changes expressed relative to that normalized baseline value) responses prior to, during, and after 17 flashes from 10 women. *signifies significant difference between both pre-and post-hot flash values (P < 0.001).
Discussion
The aim of this study was to examine possible mechanisms responsible for the increases in skin blood flow during the postmenopausal hot flash. In order to achieve this aim, skin blood flow at sites with and without pretreatment of BTX, along with SSNA, were evaluated prior to, during, and after hot flashes in symptomatic women. The primary findings are that increases in skin blood flow and cutaneous vascular conductance during a hot flash are blocked, or substantially inhibited by BTX, evident in both the forearm and glabellar regions. Furthermore, sympathetic neural activity to the skin significantly increases during, and returns to baseline after, the hot flashes. These data demonstrate that the increase in skin blood flow during the postmenopausal hot flash is predominantly a neurally-mediated event and thus is unlikely to be due to non-neural mechanisms as previously suggested14.
Despite the clear disruption that hot flashes cause to menopausal women's lives, the physiological mechanisms of a hot flash are not completely understood, particularly with regard to changes in skin blood flow. In the present study, skin blood flow increased ~2 fold at forearm and glabellar regions during the hot flash, in agreement with prior data from our laboratory18, 27. The mechanism(s) responsible for these increases in skin blood flow were previously unknown, although we have recently shown that nitric oxide is likely involved18. Elevations in skin blood flow from non-glabrous skin during a hot flash could be achieved through the withdrawal of sympathetic vasoconstrictor activity, increases in sympathetic cholinergic vasodilator activity, a combination of both neural mechanisms20, 21, and/or non-neural factors28 as previously proposed14. In the present study, intradermal injections of BTX were used to locally block the release of neurotransmitters from nerves responsible for cutaneous active vasodilation via sympathetic cholinergic nerves22, 30–32. The effectiveness of the blockade was confirmed via a lack of cutaneous vasodilation at these sites in response to whole-body heating (see Table 1). The increase in cutaneous vascular conductance during the hot flashes at untreated sites, coupled with little to no changes in cutaneous vascular conductance at the BTX sites, strongly suggest that elevations in skin blood flow during hot flashes are predominantly neurally mediated via sympathetic cholinergic nerves.
Robust increases in SSNA during the flash further support the conclusion that cutaneous vasodilation during the hot flash is neurally mediated. The integrated SSNA signal is a composite of cutaneous vasoconstrictor, sudomotor and vasodilator neural activities, with the relative contribution predominantly dependent on the thermal status of the individual3, 17, 35. An increase in SSNA could therefore be a result of an elevation in one, or a combination, of these neural components. Given the observed elevations in skin blood flow and sweating within the region innervated by the recorded nerve in the present study, it is likely that the recorded neural signal contains sudomotor and cutaneous vasodilator neural activities, but probably not cutaneous vasoconstrictor neural activities. Within this context, it is important to emphasize that similar increases in SSNA are observed during whole-body heat stress that also increases cutaneous vascular conductance and sweating3, 40.
In contrast to the findings of the present study, Freedman et al.14 found that increases in blood flow during hot flashes persisted from a finger that received neural blockade via local lidocaine injection. These findings led to the suggestion that digit vasodilation during a hot flash may be due to circulating vasodilating substances (e.g. a non-neural mechanism). However, there is a key difference in the methodological approaches of that work and the present study. Neural control of skin blood flow over the majority of the skin's surface (i.e., non-glabrous hairy skin) is regulated by both a vasoconstrictor and an active vasodilator neural system, which is in contrast to glabrous skin of the finger which is solely controlled by a vasoconstrictor limb19. Therefore, the mechanisms responsible for increases in blood flow over the majority of the body's skin surface during a hot flash may be different relative to the finger, and as such should not be assumed to be interchangeable. Despite the use of BTX, subtle but significant increases in cutaneous vascular conductance were evident at both the forearm and forehead BTX sites. Such an observation raises the possibility that a component, albeit relatively minor, of the increase in skin blood flow during a hot flash occurs through withdrawal of sympathetic vasoconstrictor activity and/or non-neural factors14,39.
Limitations to the interpretation of the findings
Participants were not aware of the precise rationale for the use of BTX, although it is unlikely that they were naïve as to the use of BTX for cosmetic purposes. Regardless, it is very unlikely that even if participants were aware of the mechanisms of BTX, that this would have affected the interpretation of the data given the clear divergence of responses between the control and BTX sites.
Protocol 2 was conducted to confirm the findings of Protocol 1 from an area often associated with hot flash symptoms (glabellar region). We recognize, however, that the small number of subjects is a limitation given the possibility of a type I error. Nevertheless, since the differences in skin blood flow responses between the BTX and the control sites were so pronounced, coupled with similar findings from the forearm in protocol 1, we are quite confident with the presented interpretation of the data despite the small number of subjects in protocol 2.
The authors are not contending that, based upon the presented findings, BTX treatment would be of benefit to alleviate symptoms associated with hot flashes. BTX was administered solely for the purpose of identifying mechanisms responsible for cutaneous vascular responses during hot flashes.
Conclusion
The findings of this study show that increases in sympathetic nerve activity to the skin occur alongside with elevations in skin blood flow, cutaneous vascular conductance, and sweating during a hot flash. Furthermore, the elevations in cutaneous blood flow were substantially inhibited at forearm and glabellar sites locally pretreated with BTX. These findings strongly indicate that increases in skin blood flow during the postmenopausal hot flash are neurally mediated via the same, or related, mechanisms to cutaneous vasodilation and sweating necessary for thermoregulation in heat stressed individuals 22,30,31.
Acknowledgements
The authors would like to thank Erin Welch MD, Sarah Weitzul MD, and Michael Wells MD for their assistance with the botulinum toxin injections.
This work was supported by grants from the National Institute of Health's National Institute on Aging (AG030189).
Footnotes
Conflicts of Interest/Disclosures: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bachmann GA. Vasomotor flushes in menopausal women. Am J Obstet Gynecol. 1999;180:S312–316. doi: 10.1016/s0002-9378(99)70725-8. [DOI] [PubMed] [Google Scholar]
- 2.Ballinger SE. Psychosocial stress and symptoms of menopause: a comparative study of menopause clinic patients and non-patients. Maturitas. 1985;7:315–327. doi: 10.1016/0378-5122(85)90055-6. [DOI] [PubMed] [Google Scholar]
- 3.Bini G, Hagbarth KE, Hynninen P, Wallin BG. Thermoregulatory and rhythm-generating mechanisms governing the sudomotor and vasoconstrictor outflow in human cutaneous nerves. J Physiol. 1980;306:537–552. doi: 10.1113/jphysiol.1980.sp013413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carpenter JS, Johnson D, Wagner L, Andrykowski M. Hot flashes and related outcomes in breast cancer survivors and matched comparison women. Oncol Nurs Forum. 2002;29:E16–25. doi: 10.1188/02.ONF.E16-E25. [DOI] [PubMed] [Google Scholar]
- 5.Cignarelli M, Cicinelli E, Corso M, Cospite MR, Garruti G, Tafaro E, Giorgino R, Schonauer S. Biophysical and endocrine-metabolic changes during menopausal hot flashes: increase in plasma free fatty acid and norepinephrine levels. Gynecol Obstet Invest. 1989;27:34–37. doi: 10.1159/000293612. [DOI] [PubMed] [Google Scholar]
- 6.Daly E, Gray A, Barlow D, McPherson K, Roche M, Vessey M. Measuring the impact of menopausal symptoms on quality of life. Bmj. 1993;307:836–840. doi: 10.1136/bmj.307.6908.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delius W, Hagbarth KE, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human skin nerves. Acta Physiol Scand. 1972;84:177–186. doi: 10.1111/j.1748-1716.1972.tb05168.x. [DOI] [PubMed] [Google Scholar]
- 8.Dennerstein L. Well-being, symptoms and the menopausal transition. Maturitas. 1996;23:147–157. doi: 10.1016/0378-5122(95)00970-1. [DOI] [PubMed] [Google Scholar]
- 9.Dormire SL. What we know about managing menopausal hot flashes: navigating without a compass. J Obstet Gynecol Neonatal Nurs. 2003;32:455–464. doi: 10.1177/0884217503255069. [DOI] [PubMed] [Google Scholar]
- 10.Finck G, Barton DL, Loprinzi CL, Quella SK, Sloan JA. Definitions of hot flashes in breast cancer survivors. J Pain Symptom Manage. 1998;16:327–333. doi: 10.1016/s0885-3924(98)00090-6. [DOI] [PubMed] [Google Scholar]
- 11.Freedman RR. Laboratory and ambulatory monitoring of menopausal hot flashes. Psychophysiology. 1989;26:573–579. doi: 10.1111/j.1469-8986.1989.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 12.Freedman RR. Physiology of hot flashes. Am J Hum Biol. 2001;13:453–464. doi: 10.1002/ajhb.1077. [DOI] [PubMed] [Google Scholar]
- 13.Freedman RR, Woodward S. Core body temperature during menopausal hot flushes. Fertil Steril. 1996;65:1141–1144. [PubMed] [Google Scholar]
- 14.Freedman RR, Woodward S, Mayes MM. Nonneural mediation of digital vasodilation during menopausal hot flushes. Gynecol Obstet Invest. 1994;38:206–209. doi: 10.1159/000292480. [DOI] [PubMed] [Google Scholar]
- 15.Ginsburg J, Swinhoe J, O'Reilly B. Cardiovascular responses during the menopausal hot flush. Br J Obstet Gynaecol. 1981;88:925–930. doi: 10.1111/j.1471-0528.1981.tb02230.x. [DOI] [PubMed] [Google Scholar]
- 16.Greendale GA, Lee NP, Arriola ER. The menopause. Lancet. 1999;353:571–580. doi: 10.1016/S0140-6736(98)05352-5. [DOI] [PubMed] [Google Scholar]
- 17.Hagbarth KE, Hallin RG, Hongell A, Torebjork HE, Wallin BG. General characteristics of sympathetic activity in human skin nerves. Acta Physiol Scand. 1972;84:164–176. doi: 10.1111/j.1748-1716.1972.tb05167.x. [DOI] [PubMed] [Google Scholar]
- 18.Hubing KA, Wingo JE, Brothers RM, del Coso J, Low DA, Crandall CG. Nitric oxide inhibition attenuates cutaneous vasodilation but not sweating during the post-menopausal hot flash. Menopause. 2010;17 doi: 10.1097/gme.0b013e3181d674d6. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson JM, Pergola PE, Liao FK, Kellogg DL, Jr., Crandall CG. Skin of the dorsal aspect of human hands and fingers possesses an active vasodilator system. J Appl Physiol. 1995;78:948–954. doi: 10.1152/jappl.1995.78.3.948. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. In: Blatteis C, Fregly M, editors. Handbook of Physiology: Adaptations to the Environment. American Physiological Society; Bethesda, MD: 1996. pp. 215–243. [Google Scholar]
- 21.Kellogg DL., Jr. In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans during thermoregulatory challenges. J Appl Physiol. 2006;100:1709–1718. doi: 10.1152/japplphysiol.01071.2005. [DOI] [PubMed] [Google Scholar]
- 22.Kellogg DL, Jr., Pergola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res. 1995;77:1222–1228. doi: 10.1161/01.res.77.6.1222. [DOI] [PubMed] [Google Scholar]
- 23.Kellogg DL, Jr., Pérgola PE, Piest KL, Kosiba WA, Crandall CG, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve co-transmission. Circ. Res. 1995;77:1222–1228. doi: 10.1161/01.res.77.6.1222. [DOI] [PubMed] [Google Scholar]
- 24.Kronenberg F. Hot flashes: epidemiology and physiology. Ann N Y Acad Sci. 1990;592:52–86. doi: 10.1111/j.1749-6632.1990.tb30316.x. discussion 123–133. [DOI] [PubMed] [Google Scholar]
- 25.Kronenberg F. Hot flashes: phenomenology, quality of life, and search for treatment options. Exp Gerontol. 1994;29:319–336. doi: 10.1016/0531-5565(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 26.Kronenberg F, Carraway RE. Changes in neurotensin-like immunoreactivity during menopausal hot flashes. J Clin Endocrinol Metab. 1985;60:1081–1086. doi: 10.1210/jcem-60-6-1081. [DOI] [PubMed] [Google Scholar]
- 27.Low DA, Davis SL, Keller DM, Shibasaki M, Crandall CG. Cutaneous and hemodynamic responses during hot flashes in symptomatic postmenopausal women. Menopause. 2008;15:290–295. doi: 10.1097/gme.0b013e3180ca7cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 29.Shanafelt TD, Barton DL, Adjei AA, Loprinzi CL. Pathophysiology and treatment of hot flashes. Mayo Clin Proc. 2002;77:1207–1218. doi: 10.4065/77.11.1207. [DOI] [PubMed] [Google Scholar]
- 30.Shibasaki M, Davis SL, Cui J, Low DA, Keller DM, Durand S, Crandall CG. Neurally mediated vasoconstriction is capable of decreasing skin blood flow during orthostasis in the heat-stressed human. J Physiol. 2006;575:953–959. doi: 10.1113/jphysiol.2006.112649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shibasaki M, Durand S, Davis SL, Cui J, Low DA, Keller DM, Crandall CG. Endogenous nitric oxide attenuates neutrally mediated cutaneous vasoconstriction. J Physiol. 2007;585:627–634. doi: 10.1113/jphysiol.2007.144030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simpson LL. The origin, structure, and pharmacological activity of botulinum toxin. Pharmacol Rev. 1981;33:155–188. [PubMed] [Google Scholar]
- 33.Sloan JA, Loprinzi CL, Novotny PJ, Barton DL, Lavasseur BI, Windschitl H. Methodologic lessons learned from hot flash studies. J Clin Oncol. 2001;19:4280–4290. doi: 10.1200/JCO.2001.19.23.4280. [DOI] [PubMed] [Google Scholar]
- 34.Stein KD, Jacobsen PB, Hann DM, Greenberg H, Lyman G. Impact of hot flashes on quality of life among postmenopausal women being treated for breast cancer. J Pain Symptom Manage. 2000;19:436–445. doi: 10.1016/s0885-3924(00)00142-1. [DOI] [PubMed] [Google Scholar]
- 35.Sugenoya J, Iwase S, Mano T, Sugiyama Y, Ogawa T, Nishiyama T, Nishimura N, Kimura T. Vasodilator component in sympathetic nerve activity destined for the skin of the dorsal foot of mildly heated humans. J Physiol. 1998;507(Pt 2):603–610. doi: 10.1111/j.1469-7793.1998.603bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor M. Psychological consequences of surgical menopause. J Reprod Med. 2001;46:317–324. [PubMed] [Google Scholar]
- 37.Taylor WF, Johnson JM, O'Leary D, Park MK. Effect of high local temperature on reflex cutaneous vasodilation. J Appl Physiol. 1984;57:191–196. doi: 10.1152/jappl.1984.57.1.191. [DOI] [PubMed] [Google Scholar]
- 38.Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- 39.Wilkin JK. Why is flushing limited to a mostly facial cutaneous distribution? J Am Acad Dermatol. 1988;19:309–313. doi: 10.1016/s0190-9622(88)70177-2. [DOI] [PubMed] [Google Scholar]
- 40.Wilson TE, Cui J, Crandall CG. Absence of arterial baroreflex modulation of skin sympathetic activity and sweat rate during whole-body heating in humans. J Physiol. 2001;536:615–623. doi: 10.1111/j.1469-7793.2001.0615c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woodward S, Freedman RR. The thermoregulatory effects of menopausal hot flashes on sleep. Sleep. 1994;17:497–501. doi: 10.1093/sleep/17.6.497. [DOI] [PubMed] [Google Scholar]